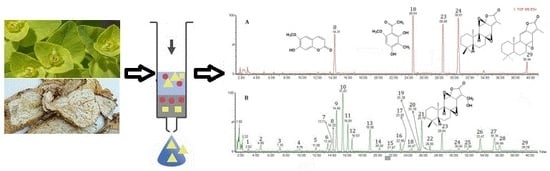
Development of a Matrix Solid-Phase Dispersion Extraction Combined with UPLC/Q-TOF-MS for Determination of Phenolics and Terpenoids from the Euphorbia fischeriana
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of MSPD Extraction Procedure
2.1.1. Selection of Dispersing Sorbent
2.1.2. Ratio of Dispersing Sorbent to Sample
2.1.3. Effect of Elution Solvents
2.2. Optimization of UPLC Conditions
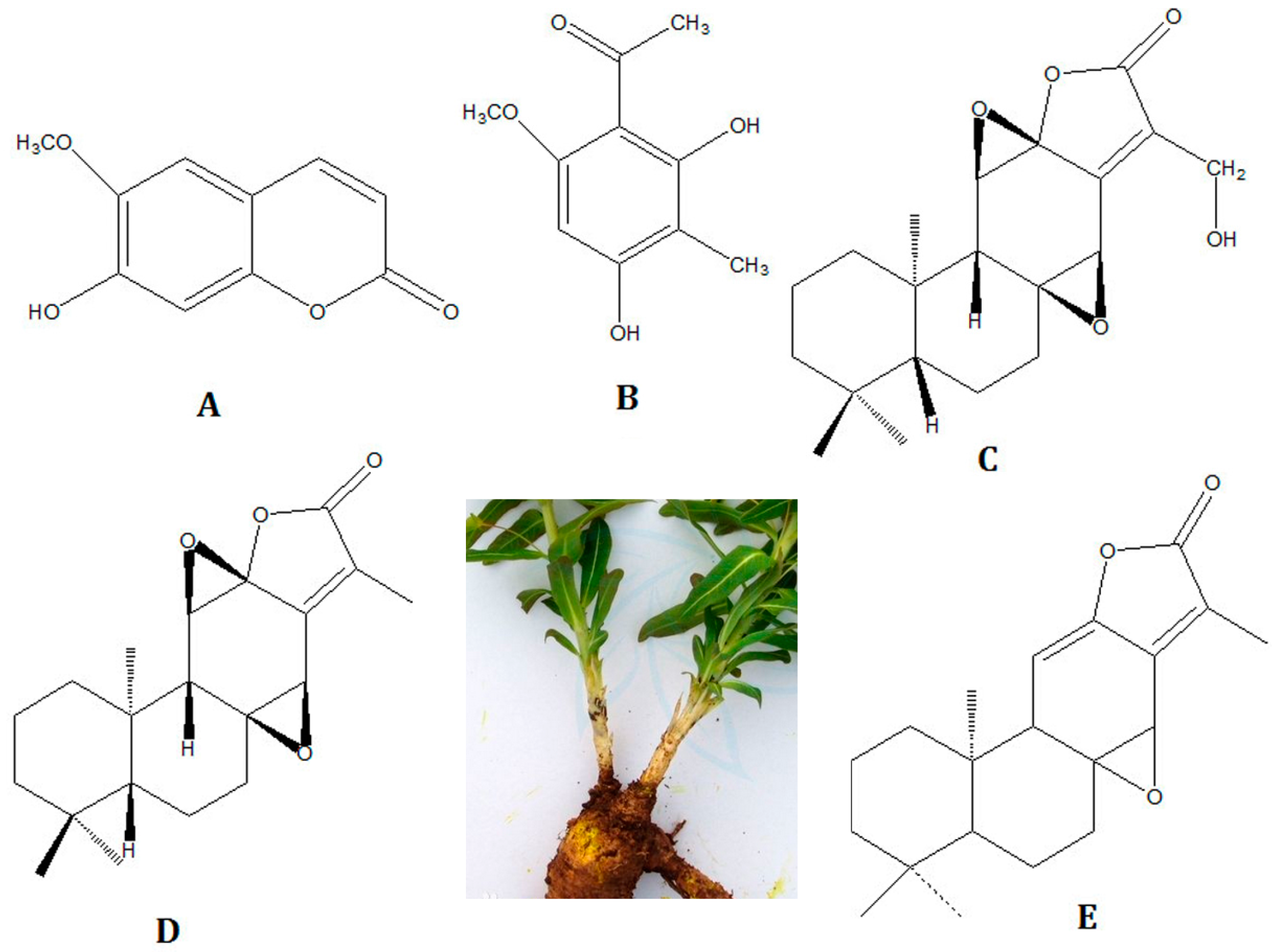
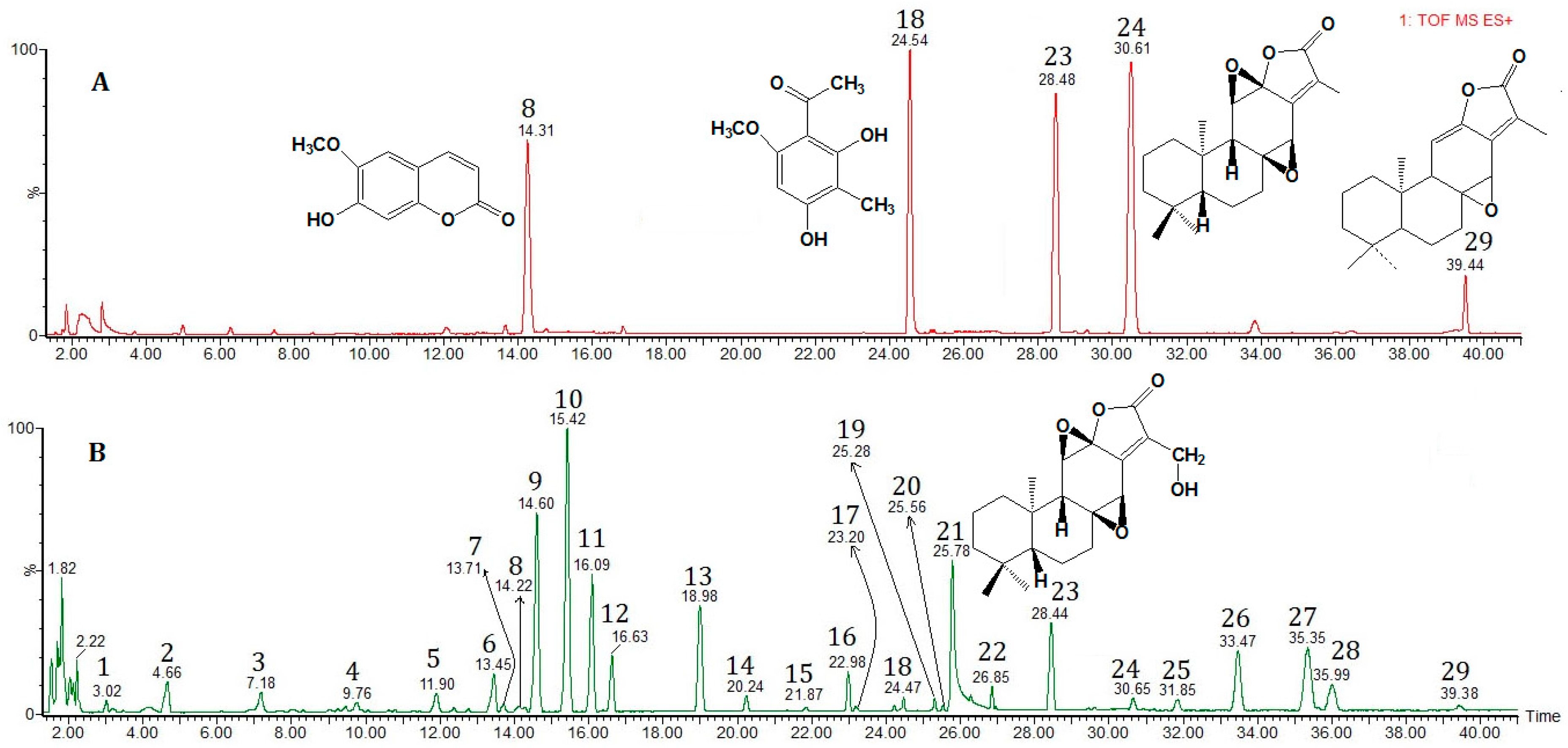
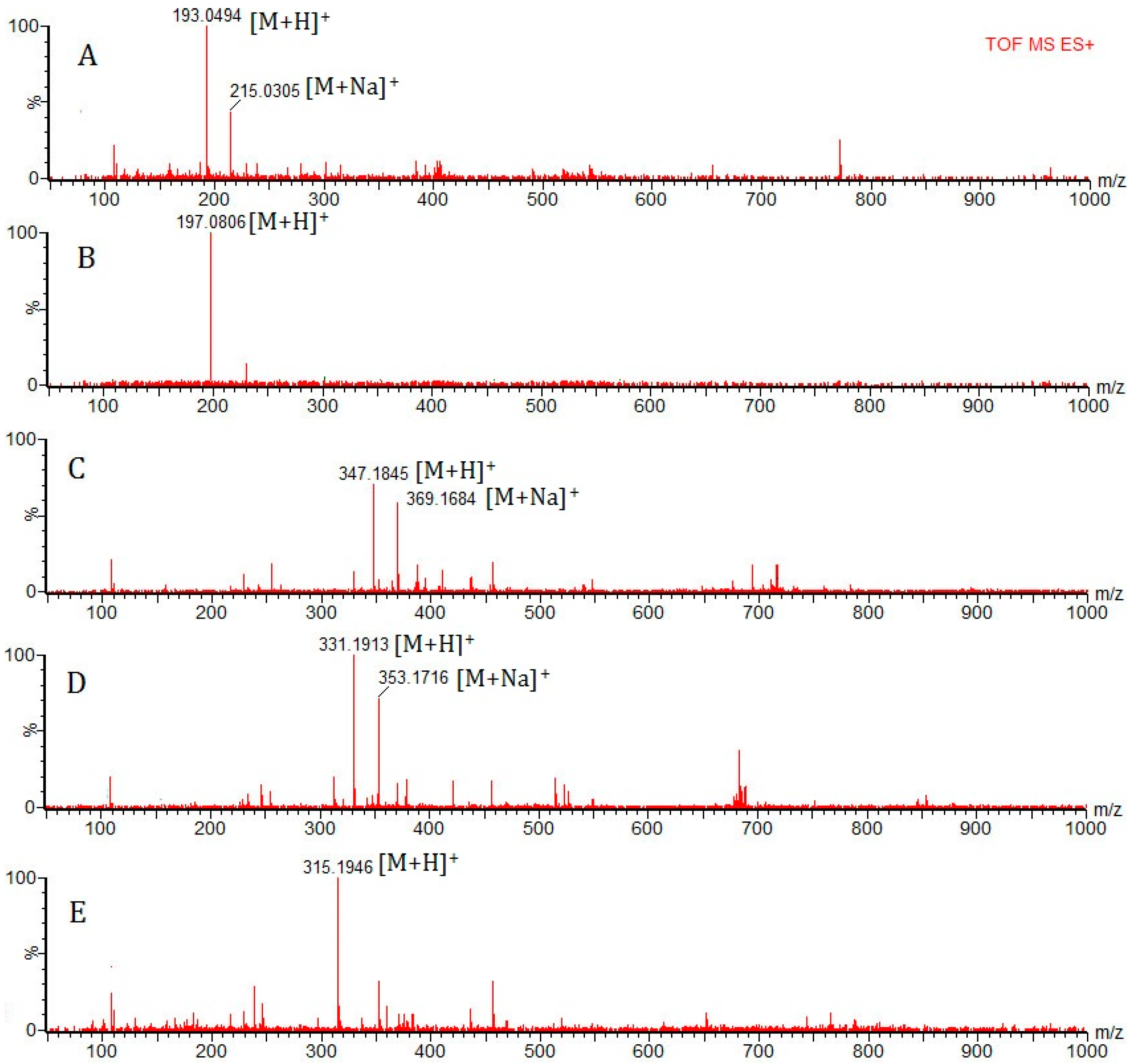
2.3. Procedure for Identification of the Components in Euphorbia fischeriana
2.4. Quantification Method Validation
2.5. Quantification of Five Compounds in the Euphorbia fischeriana
2.6. Comparison of MSPD, Ultrasonic and Reflux Extraction
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Standard Solution
3.4. Sample Preparation for LC-Q-TOF-MS
3.4.1. MSPD Extraction
3.4.2. Ultrasonic Extraction
3.4.3. Reflux Extraction
3.5. Analytical Method
3.6. Method Validation for Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barrero, R.A.; Chapman, B.; Yang, Y.; Moolhuijzen, P.; Gagnère, G.K.; Zhang, N.; Tang, Q.; Bellgard, M.I.; Qiu, D.Y. De novo assembly of Euphorbia fischeriana root transcriptome identifies prostratin pathway related genes. BMC Genom. 2011, 12, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.D.; Wan, R.D.; Yang, J.Y.; Wang, H.Q. Studies on the chemical constituents of Crassula argentea Thunb. J. Chin. Med. Mater. 2006, 29, 1184–1185. [Google Scholar]
- Sun, Y.X.; Liu, J.C. Chemical constituents and biological activities of Euphorbia fischeriana Steud. Chem. Biodiv. 2011, 8, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, Y.S.; Lin, S.X.; Tong, D.Y. Research on the optimize treating mechanism of Langdu extract for Lewis lung cancer. Chin. J. Can. Prev. Treat. 2012, 19, 1372–1376. [Google Scholar]
- Li, X.Q.; Chen, S.S.; Liu, S.L.; Li, M.H. Comparison of the inhibition to mycobacterium tuberculosis of different Euphorbia fischeriana extracts. Pharm. J. Chin. People Lib. Army 2006, 22, 153–155. [Google Scholar]
- Liu, X.C.; Zhou, L.G.; Liu, Z.L. Evaluation of nematicidal activity of ethanol extracts of Euphorbiaceae plants and constituents from Euphorbia fischeriana to Meloidogyne incognita (Kofoid and White) Chitwood. J. Entomol. Zool. Stud. 2014, 2, 311–317. [Google Scholar]
- Wang, H.B.; Chen, W.; Zhang, Y.Y.; Wang, X.Y.; Liu, L.P.; Tong, L.J.; Chen, Y. Four new diterpenoids from the roots of Euphorbia fischeriana. Fitoterapia 2013, 91, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.X.; Bao, G.H.; Ma, Q.G.; Qin, G.W.; Che, C.T.; Lv, Y.; Wang, C.; Zheng, Q.T. Langduin C, a novel dimeric diterpenoid from the roots of Euphorbia fischeriana. Tetrahedron Lett. 2003, 44, 135–137. [Google Scholar] [CrossRef]
- Liu, W.Z.; He, F.L.; Ran, Z.Y.; Gu, X.F.; Wu, X.Y.; Qin, G.W. Studies on chemical constituents from Euphorbia fischeriana Steud. J. Chin. Mater. Med. 2011, 26, 180–188. [Google Scholar]
- Wang, X.Y.; Liu, L.P.; Kang, T.G.; Wang, H.B. Chemical constituents of Euphorbia fischeriana. Chin. J. Nat. Med. 2012, 10, 299–302. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Z.G.; Cao, Y.F.; Meng, D.L.; Hua, H.M. Chemotaxonomic and chemical studies on two plants from genus of Euphorbia: Euphorbia fischeriana and Euphorbia ebracteolata. Biochem. Syst. Ecol. 2014, 57, 345–349. [Google Scholar] [CrossRef]
- Liu, W.K.; Ho, J.C.K.; Qin, G.W.; Che, C.T. Jolkinolide B induces neuroendocrine differentiation of human prostate LNCaP cancer cell line. Biochem. Pharmacol. 2002, 63, 951–958. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, L.; Liu, J.C. The effect of 17-hydroxyjolkinolide B on proliferation and apoptosis of K562 cells. Chin. J. Exp. Tradit. Med. 2013, 19, 197–200. [Google Scholar]
- Xu, S.D. The antituberculosis effect of Langdu. Zhejiang J. Integr. Tradit. Chin. West Med. 2012, 22, 67–69. [Google Scholar]
- Tian, R.J. Inhibitory effect of leaf extract of Stellera chamaejasme on skin trichophyton mentagrophytes and staphylococcus aureus. Herald Med. 2014, 33, 729–732. [Google Scholar]
- Manuele, M.G.; Ferraro, G.; Arcos, M.L.B.; López, P.; Cremaschi, G.; Anesini, C. Comparative immunomodulatory effect of scopoletin on tumoral and normal lymphocytes. Life Sci. 2006, 79, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Ding, Y.M. Studies on the active compounds B and C in the root of Euphorbia ebracteolata Hayata. J. Plant Res. Environ. 1992, 1, 6–9. [Google Scholar]
- Wang, Y.; Ma, X.; Yan, S.; Shen, S.; Zhu, H.; Gu, Y.; Wang, H.; Qin, G.; Yu, Q. 17-hydroxy-jolkinolide B inhibits signal transducers and activators of transcription 3 signaling by covalently cross-linking Janus kinases and induces apoptosis of human cancer cells. Cancer Res. 2005, 69, 7302–7310. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, A. Induction of apoptosis in K562 cells by jolkinolide B. Cancer J. Physiol. Pharm. 2006, 84, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, K.; Niu, H.Y.; Shu, L.H.; Yue, D.M.; Li, D.; He, P. Jolkinolide B from Euphorbia fischeriana Steud induces in human leukemic cells apoptosis via JAK2/STAT3 pathways. Int. J. Clin. Pharmacol. Ther. 2013, 51, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Fu, Y.Q.; Yang, Z.Q.; Zhao, H.Q.; Fan, X.M. Isolation and identification of antitumor constituents of diterpenoids lactone in Euphorbia fischeriana Steud. Chin. Acad. J. 1988, 13, 35–36. [Google Scholar]
- Wang, C.J.; Jiang, Y.Q.; Yan, X.H. Determination of four ent-abietane jolkinolides diterpene lactones from Euphorbia fischeriana Steud. by RP-HPLC. Chin. Tradit. Pat. Med. 2013, 10, 2196–2199. [Google Scholar]
- Wang, C.J.; Jiang, Y.Q.; Bi, F.J.; Lin, T.; Yan, X.H.; Liu, D.H. RP-HPLC determination of 2,4-Dihydroxy-6-methoxy-3-methylacetophenone and jolkinolide B in Euphorbiae Ebracteolatae Radix. Chin. J. Pharm. Anal. 2011, 31, 839–842. [Google Scholar]
- Su, X.L.; Lin, R.C.; Wong, S.K.; Tsui, S.K.; Kwan, S.Y. Identification and characterisation of the Chinese herb Langdu by LC-MS/MS analysis. Phytochem. Anal. 2003, 14, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Jiang, W.; Wu, Q.C.; Yu, L.; Zhang, L.; Tao, W.W.; Ding, A.W.; You, F.Q.; Duan, J.A. Comparative characteristic of the inflammatory diterpenes in the roots of Euphorbia fischeriana with different preparation method using HPLC-ELSD. Fitoterapia 2012, 83, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Long, A.R.; Short, C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. A 1989, 475, 353–361. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, W.Q.; Guan, H.; Liu, H.; Yang, W.Q.; Wang, H.R.; Cai, D.F. Development of a matrix solid-phase dispersion extraction combined with high-performance liquid chromatography for determination of five lignans from the Schisandra chinensis. J. Chromatogr. B 2016, 1011, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Sun, R.; Wang, Y.P.; Li, N.; Lei, L.; Yang, X.; Yu, A.M.; Qiu, F.P.; Zhang, H.Q. Determination of phenolic acids and flavonoids in raw propolisbysilica-supported ionic liquid-based matrix solid phase dispersion extraction high performance liquid chromatography-diodearray detection Zhibing. J. Chromatogr. B 2014, 969, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L. The study of chemical constituents and bioactivity of Euphorbia fischeriana Steud. Jilin Agric. Univ. 2011, 1, 15–19. [Google Scholar]
- Pan, L.L.; Fang, P.L.; Zhang, X.J.; Ni, W.; Li, L.; Yang, L.M.; Chen, C.X.; Zheng, Y.T.; Li, C.T.; Hao, X.J.; et al. Tigliane-type diterpenoid glycosides from Euphorbia fischeriana. J. Nat. Prod. 2011, 74, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Wang, Y.W.; Xu, H.H.; Wu, G.Q.; Zhao, S.H. Isolation and identification of extraction of Stellera chamaejasme (I). J. Anhui Agric. Univ. 2000, 27, 340–344. [Google Scholar]
- Wang, H.; Zhang, X.F.; Ma, Y.B.; Cai, X.H.; Wu, D.G.; Luo, X.D. Diterpenoids from Euphorbia wallichii. Chin. Tradit. Herb. Drugs 2004, 35, 611–614. [Google Scholar]
- Zou, H.Y.; Tu, P.F. Study on the chemical constituents of Lysimachia clethroides. Chin. Tradit. Herb. Drugs 2009, 40, 704–708. [Google Scholar]
- Su, X.F.; Lin, Q.; Huang, X.S.; Yang, J.X. Chemical constituents from the stem of Cleidiocarpon cavaleriei. Acta Bot. Boreali Occid. Sin. 2008, 28, 2339–2342. [Google Scholar]
- Wang, W.X.; Ding, X.B. Acetophenone derivatives from Euphorbia ebracteolata. Acta Pharm. Sin. 1999, 34, 514–517. [Google Scholar]
- Wang, Y.B.; Huang, R.; Wang, H.B.; Jin, H.Z.; Lou, L.G.; Qin, G.W. Diterpenoids from the roots of Euphorbia fischeriana. J. Nat. Prod. 2006, 69, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Lou, Z.G. Studies on chemical constituents from Euphorbia fischeriana. Chin. Tradit. Herb. Drugs 1989, 20, 290–293. [Google Scholar]
- Liu, X.Q.; Jia, Z.J.; Liu, Z.M. Studies on the components of Sanguisorba officinalic L. Chem. J. Chin. Univ. 1992, 13, 767–769. [Google Scholar]
- Vitchu, L.; Kovit, C.; Kanchana, S.; Pichaet, W. Chemical constituents of Dianella ensifolia redoute. J. Sci. Soc. Thailand 1982, 8, 95–102. [Google Scholar]
- Wu, Q.C.; Tang, Y.P.; Ding, A.W.; You, F.Q.; Duan, J.A. Diterpenes and triterpenes from the roots of Euphorbia fischeriana. Chin. J. Nat. Med. 2010, 8, 101–102. [Google Scholar] [CrossRef]
- Wang, W.X.; Ding, X.B. Studies on diterpenoids from the roots of Euphorbia ebracteolata. Acta Pharmacol. Sin. 1998, 33, 128–131. [Google Scholar]
- Du, Z.Z.; He, H.P.; Wu, B.; Shen, Y.M.; Hao, X.J. Chemical Constituents from the Pericarp of Trewia nudiflora. Helv. Chim. Acta 2004, 87, 758–763. [Google Scholar] [CrossRef]
- Pan, Q.; Shi, M.F.; Min, Z.D. Studies on the 2D NMR spectra of Jolkinolide diterpenoids from Euphorbia fischeriana. J. Chin. Pharm. Univ. 2004, 35, 16–19. [Google Scholar]
- Zhang, Y.Y. Studies on Chemical Constituents from Roots of Euphorbia pekinensis Rupr. Master’s Thesis, Jilin University, Jilin, China, 2010. [Google Scholar]
- Yan, X.H.; Wang, C.J.; Bi, F.J.; Gu, L.H.; Jiang, Y.Q. Improvement of quality standard of Radix Euphorbia Fischeriana. Tradit. Chin. Drug Res. Clin. Pharmacol. 2012, 23, 321–324. [Google Scholar]
Sample Availability: Samples of the compounds Scopoletin and Jolkinolide B. are available from the authors. |
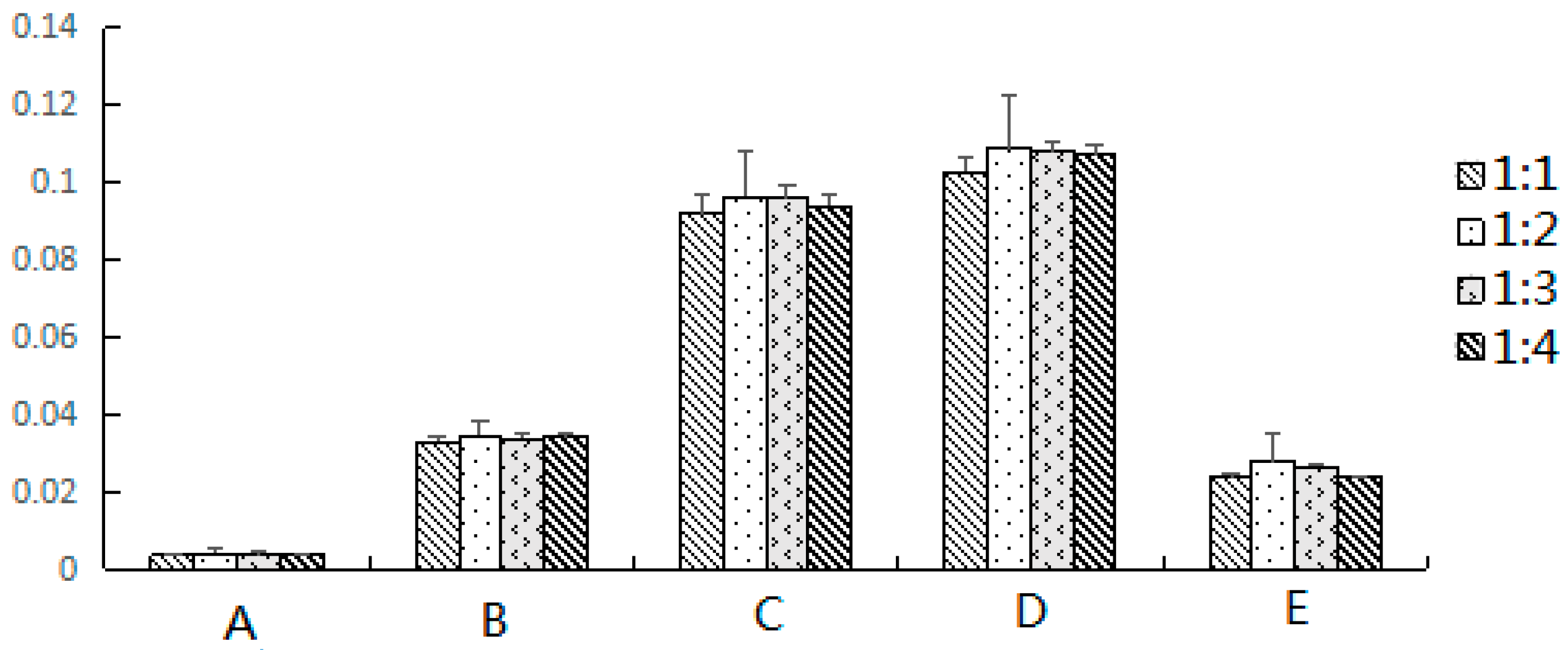
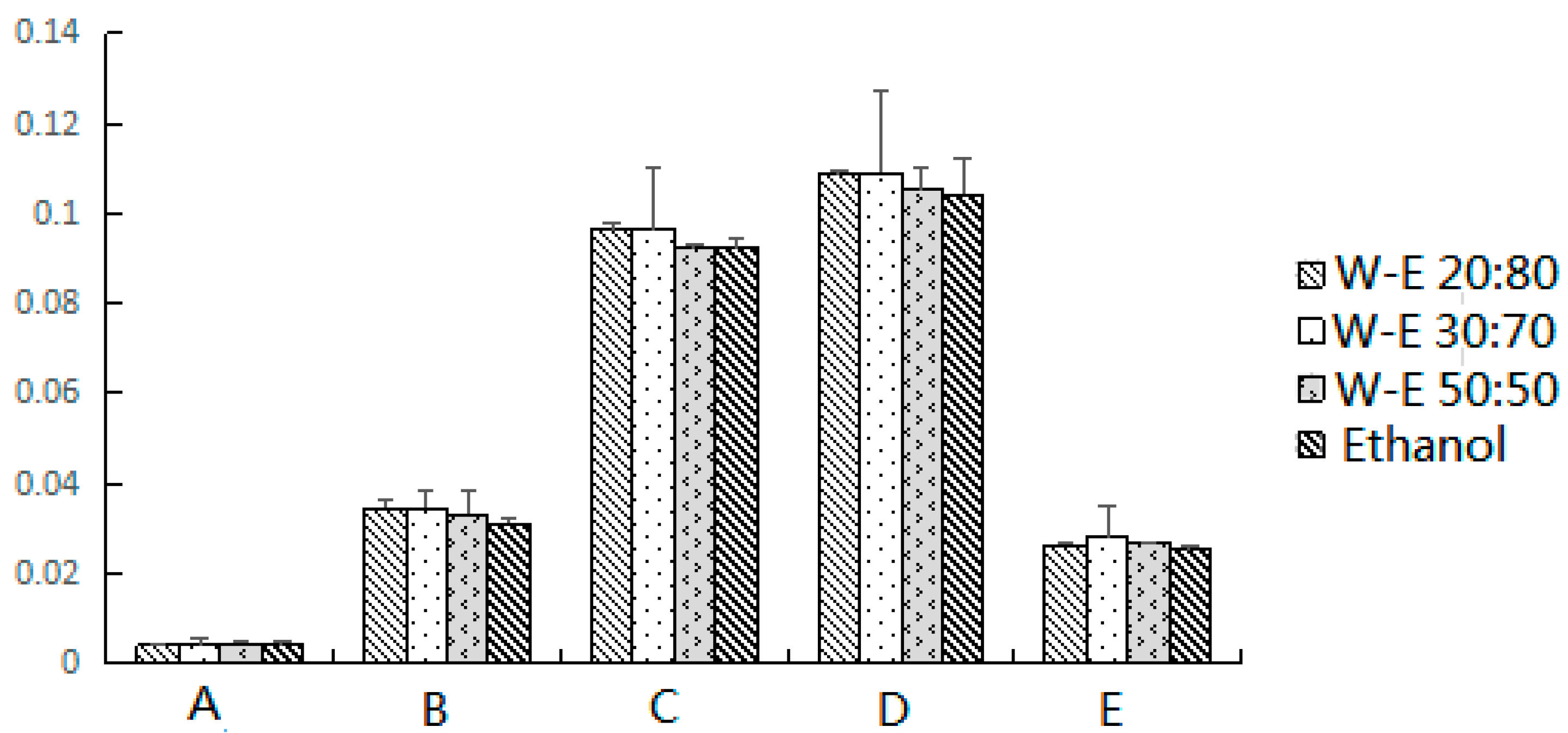
| Dispersion Adsorbents | Scopoletin (%) | 2,4-Dihydroxy-6-methoxy-3-methylacetophenone (%) | 17-Hydroxyjolkinolide B (%) | Jolkinolide B (%) | Jolkinolide A (%) |
|---|---|---|---|---|---|
| Silica gel | 0.0042 | 0.0346 | 0.0964 | 0.1089 | 0.0279 |
| florisil | 0.0034 | 0.0297 | 0.0678 * | 0.0822 | 0.0254 |
| neutral alumina | 0.0023 * | 0.0247 * | 0.0496 ** | 0.0466 * | 0.0198 |
| C18-bonded silica | 0.0038 | 0.0337 | 0.072 | 0.0923 | 0.0268 |
| Peak No. | tR (min) | Elemental Composition | Assigned Identity | Theoretical Mass (m/z) | Experimental Mass (m/z) | Error (m m/z Units) |
|---|---|---|---|---|---|---|
| 1 | 3.02 | 132.1028 | ||||
| 2 | 4.66 | 166.0867 | ||||
| 3 | 7.18 | 205.0510 | ||||
| 4 | 9.76 | C23H38O3 | 3β,16β,17-trihydroxy-ent-kaurane 16,17-acetonide [29,30] | 363.2899 [M + H]+ | 363.2848 | −5.1 |
| 5 | 11.90 | C28H40O12 | Fischerosides C [31] | 569.2598 [M + H]+ | 569.2621 | −2.3 |
| 6 | 13.45 | C30H24O10 | Chamechromone [32] | 567.4953 [M + Na]+ | 567.4905 | −4.8 |
| 7 | 13.71 | C20H34O3 | Ent-atisane-3β,16α,17-triol [33] | 345.2406 [M + Na]+ | 345.2386 | −2.0 |
| 8 | 14.22 | C10H8O4 | Scopoletin [34] | 193.0423 [M + H]+ 215.0320 [M + Na]+ | 193.0494, 215.0305 | 7.1, −1.5 |
| 9 | 14.60 | C20H30O3 | Kauranoic acid [35] | 341.2093 [M + Na]+ | 341.2021 | −7.2 |
| 10 | 15.42 | C16H22O9 | 2,4-Dihydroxy-6-methoxy-3-methylacetophenone-4-O-β-d-glucopyranoside [36] | 397.4388 [M + K]+ | 397.4324 | −6.4 |
| 11 | 16.09 | C29H50O | β-sitosterol [37] | 453.3499 [M + K]+ | 453.3437 | −6.2 |
| 12 | 16.63 | C26H36O9 | Fischeriana B [38] | 531.6569 [M + K]+ | 531.6565 | −0.4 |
| 13 | 18.89 | C28H40O11 | Fischerosides A [30] | 553.2649 [M + H]+ | 553.2646 | −0.3 |
| 14 | 20.24 | C35H44O15 | Fischerosides B [30] | 705.2758 [M + H]+ 727.2578 [M + Na]+ | 705.2756, 727.2574 | −0.2, −0.4 |
| 15 | 21.87 | C9H10O4 | 2,4-Dihydroxy-6-methoxy-acetophenone [39] | 183.0657 [M + H]+ | 183.0639 | −1.8 |
| 16 | 22.98 | C22H28O5 | 17-acetoxyjolknolide A [11] | 373.2015 [M + H]+ | 373.2004 | −1.1 |
| 17 | 23.20 | 353.2279 | ||||
| 18 | 24.49 | C10H12O4 | 2,4-Dihydroxy-6-methoxy-3-methylacetophenone [40] | 197.0814 [M + H]+ | 197.0806 | −0.8 |
| 19 | 25.28 | C20H32O3 | Ent-kaurane-3-oxo-16α, 17-diol [41] | 321.2430 [M + H]+ | 321.2401 | −2.9 |
| 20 | 25.56 | C20H28O4 | Ebracteolatanolide A [42] | 333.2066 [M + H]+ 355.1885 [M + Na]+ | 333.2062, 355.1890 | −0.4, 0.5 |
| 21 | 25.78 | C21H34O3 | 17-dihydroxy-ent-atisan-19-oic acid methyl ester [43] | 335.2586 [M + H]+ | 335.2592 | 0.6 |
| 22 | 26.85 | C20H28O5 | Langduin A [13] | 371.1834 [M + Na]+ | 371.1821 | −1.3 |
| 23 | 28.44 | C20H26O5 | 17-hydroxyjolkinolide B [44] | 347.1859 [M + H]+ 369.1678 [M + Na]+ | 347.1845, 369.1684 | −1.4, 0.6 |
| 24 | 30.65 | C20H26O4 | Jolkinolide B [44] | 331.1909 [M + H]+ 353.1729 [M + Na]+ | 331.1913, 353.1716 | 0.4, −1.3 |
| 25 | 31.85 | C20H28O3 | Ent-11β-hydroxyabieta-8 (14), 13(15)-dien-16-12β-olide [11] | 317.2117 [M + H]+ 339.1936 [M + Na]+ | 317.2112, 339.1930 | −0.5, −0.6 |
| 26 | 33.48 | C20H26O4 | 17-hydroxyjolkinolide A [44] | 331.1909 [M + H]+ 353.1729 [M + Na]+ | 331.1906, 353.1723 | −0.3, −0.6 |
| 27 | 35.35 | C20H26O4 | Fischeriana A [38] | 369.1468 [M + K]+ | 369.1432 | −3.6 |
| 28 | 35.99 | C16H22O4 | Dibutyl phthalate [45] | 279.1596 [M + H]+, 301.1416 [M + Na]+ | 279.1604, 301.1421 | −0.8, 0.5 |
| 29 | 39.38 | C20H26O3 | Jolkinolide A [44] | 315.1960 [M + H]+ | 315.1946 | −1.4 |
| Compounds | Regression Equation | Confidence Intervals | R2 | Linear Range (μg/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| Scopoletin | Y = 239.93X − 7.6326 | 223.31–256.56 | 0.9964 | 0.625–50 | 23.67 | 78.12 |
| 2,4-Dihydroxy-6-methoxy-3-methylacetophenone | Y = 27.32X + 0.1943 | 25.85–28.80 | 0.9978 | 2.5–200 | 75.76 | 250.00 |
| 17-Hydroxyjolkinolide B | Y = 127.9X + 19.289 | 120.18–135.6 | 0.9973 | 1.25–100 | 47.35 | 156.25 |
| Jolkinolide B | Y = 29.571X − 3.6311 | 27.65–31.49 | 0.9968 | 2.5–200 | 94.70 | 312.50 |
| Jolkinolide A | Y = 45.632X + 6.3342 | 44.03–47.24 | 0.9991 | 0.625–50 | 18.94 | 62.50 |
| Compound | Precision RSD (%) | Repeatability (n = 6) | Stability (48 h, n = 3) | ||||
|---|---|---|---|---|---|---|---|
| Concentration (μg/mL) | Intraday (n = 6) | Interday (n = 3) | Content (%) | RSD (%) | Content (%) | RSD (%) | |
| Scopoletin | 6.25 | 1.12 | 2.23 | 0.0032 | 2.29 | 0.0029 | 2.05 |
| 2,4-Dihydroxy-6-methoxy-3-methylacetophenone | 25 | 0.93 | 1.21 | 0.0243 | 2.98 | 0.0241 | 2.57 |
| 17-Hydroxyjolkinolide B | 12.5 | 1.29 | 1.43 | 0.0585 | 4.02 | 0.0581 | 2.98 |
| Jolkinolide B | 25 | 0.31 | 1.39 | 0.0594 | 3.21 | 0.0591 | 2.12 |
| Jolkinolide A | 6.25 | 1.09 | 1.87 | 0.0112 | 3.99 | 0.0114 | 3.09 |
| No. | Origins | Average Content (%) (n = 3) | ||||
|---|---|---|---|---|---|---|
| Scopoletin | 2,4-Dihydroxy-6-methoxy-3-methylacetophenone | 17-Hydroxyjolkinolide B | Jolkinolide B | Jolkinolide A | ||
| 1 | Qiqihar | 0.0032 | 0.0343 | 0.0943 | 0.1045 | 0.0112 |
| 2 | Harbin | 0.0043 | 0.0285 | 0.0885 | 0.0594 | 0.0205 |
| 3 | Mudanjiang | 0.0031 | 0.0453 | 0.0534 | 0.0454 | 0.0284 |
| 4 | Baoding | 0.0028 | 0.0293 | 0.0524 | 0.0506 | 0.0124 |
| 5 | Changchun | 0.0038 | 0.0405 | 0.0875 | 0.0498 | 0.0213 |
| Extraction Yield (%) | MSPD | Ultrasonic | Reflux |
|---|---|---|---|
| Scopoletin | 0.0043 | 0.0039 | 0.0042 |
| 2,4-Dihydroxy-6-methoxy-3-methylacetophenone | 0.0343 | 0.0327 | 0.0324 |
| 17-Hydroxyjolkinolide B | 0.0971 | 0.0937 | 0.0963 |
| Jolkinolide B | 0.1056 | 0.0973* | 0.1051 |
| Jolkinolide A | 0.0283 | 0.0264 | 0.0279 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Lin, Y.; Wang, Y.; Hong, B. Development of a Matrix Solid-Phase Dispersion Extraction Combined with UPLC/Q-TOF-MS for Determination of Phenolics and Terpenoids from the Euphorbia fischeriana. Molecules 2017, 22, 1524. https://doi.org/10.3390/molecules22091524
Li W, Lin Y, Wang Y, Hong B. Development of a Matrix Solid-Phase Dispersion Extraction Combined with UPLC/Q-TOF-MS for Determination of Phenolics and Terpenoids from the Euphorbia fischeriana. Molecules. 2017; 22(9):1524. https://doi.org/10.3390/molecules22091524
Chicago/Turabian StyleLi, Wenjing, Yu Lin, Yuchun Wang, and Bo Hong. 2017. "Development of a Matrix Solid-Phase Dispersion Extraction Combined with UPLC/Q-TOF-MS for Determination of Phenolics and Terpenoids from the Euphorbia fischeriana" Molecules 22, no. 9: 1524. https://doi.org/10.3390/molecules22091524
APA StyleLi, W., Lin, Y., Wang, Y., & Hong, B. (2017). Development of a Matrix Solid-Phase Dispersion Extraction Combined with UPLC/Q-TOF-MS for Determination of Phenolics and Terpenoids from the Euphorbia fischeriana. Molecules, 22(9), 1524. https://doi.org/10.3390/molecules22091524