Copper-Catalyzed Synthesis of Unsymmetrical Diorganyl Chalcogenides (Te/Se/S) from Boronic Acids under Solvent-Free Conditions †
Abstract
:1. Introduction
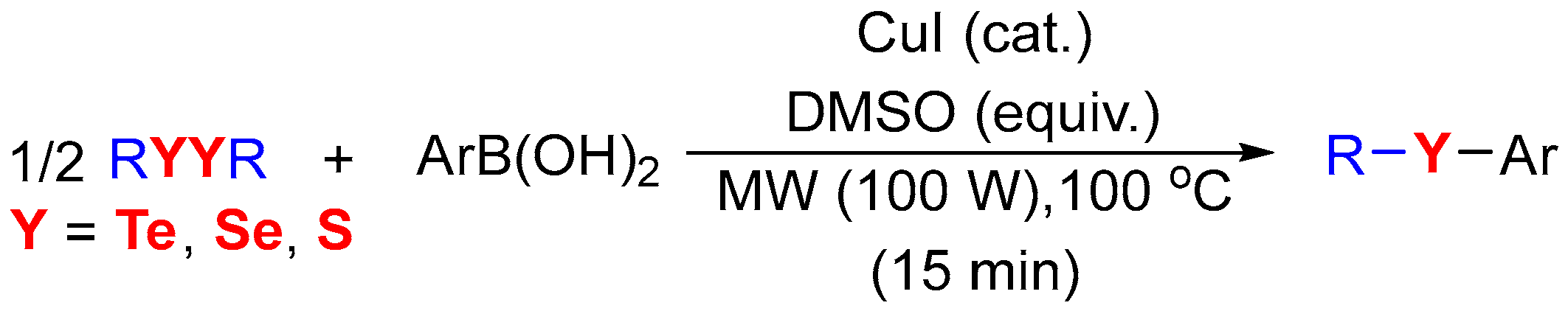
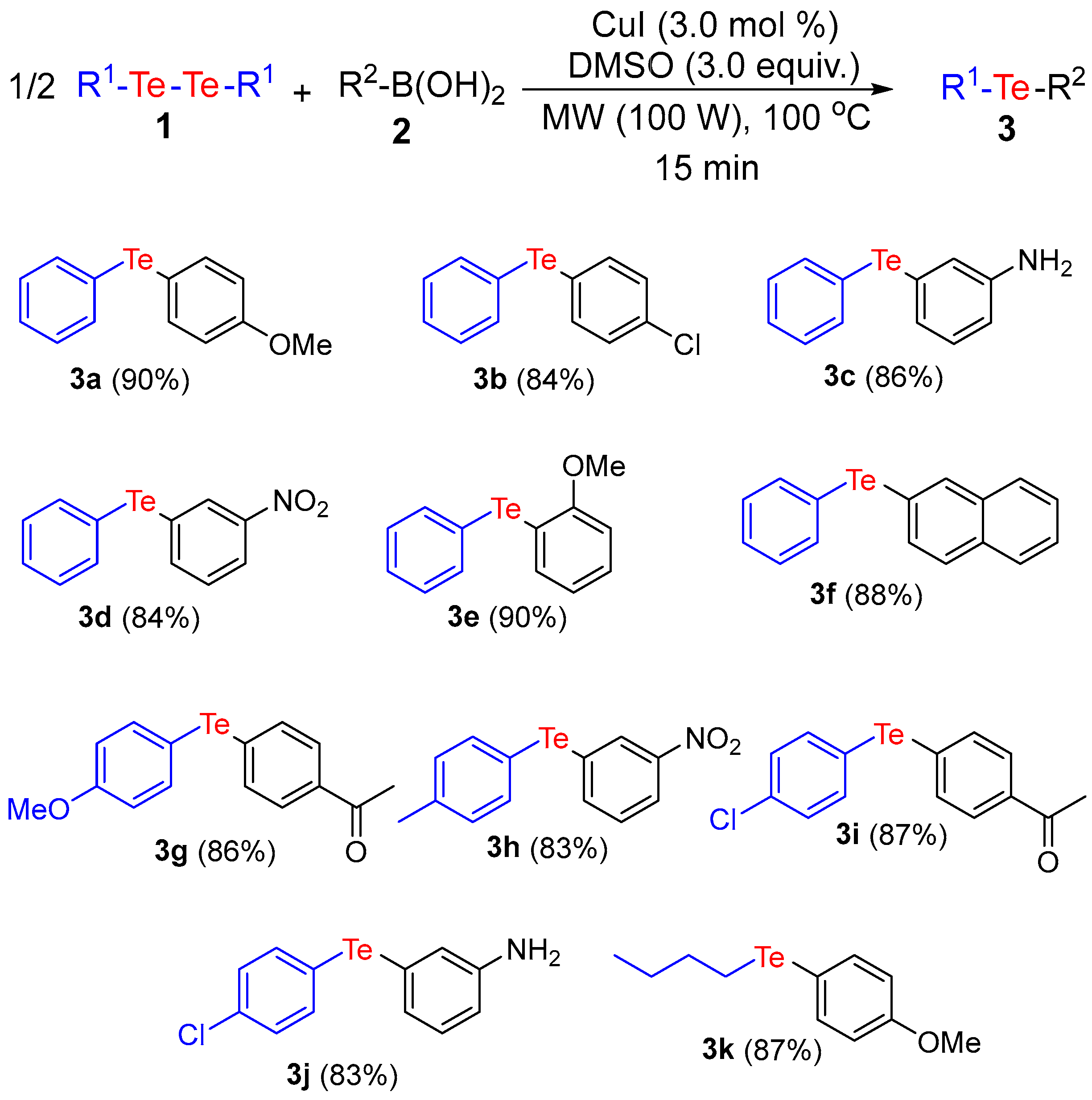
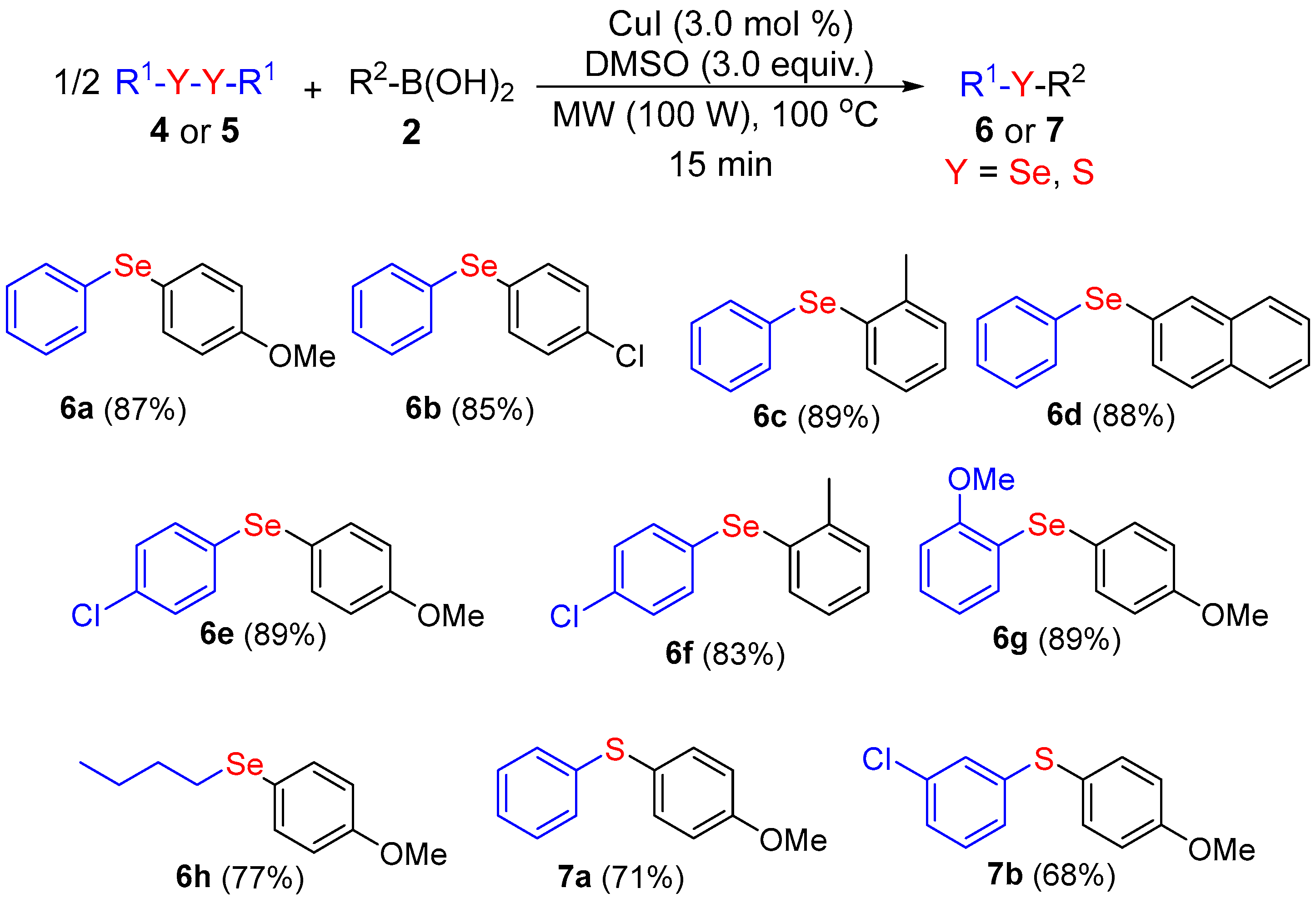
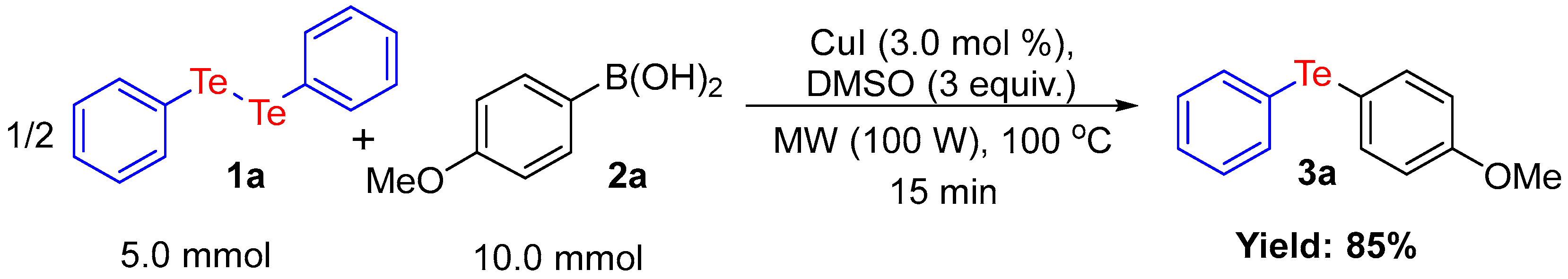
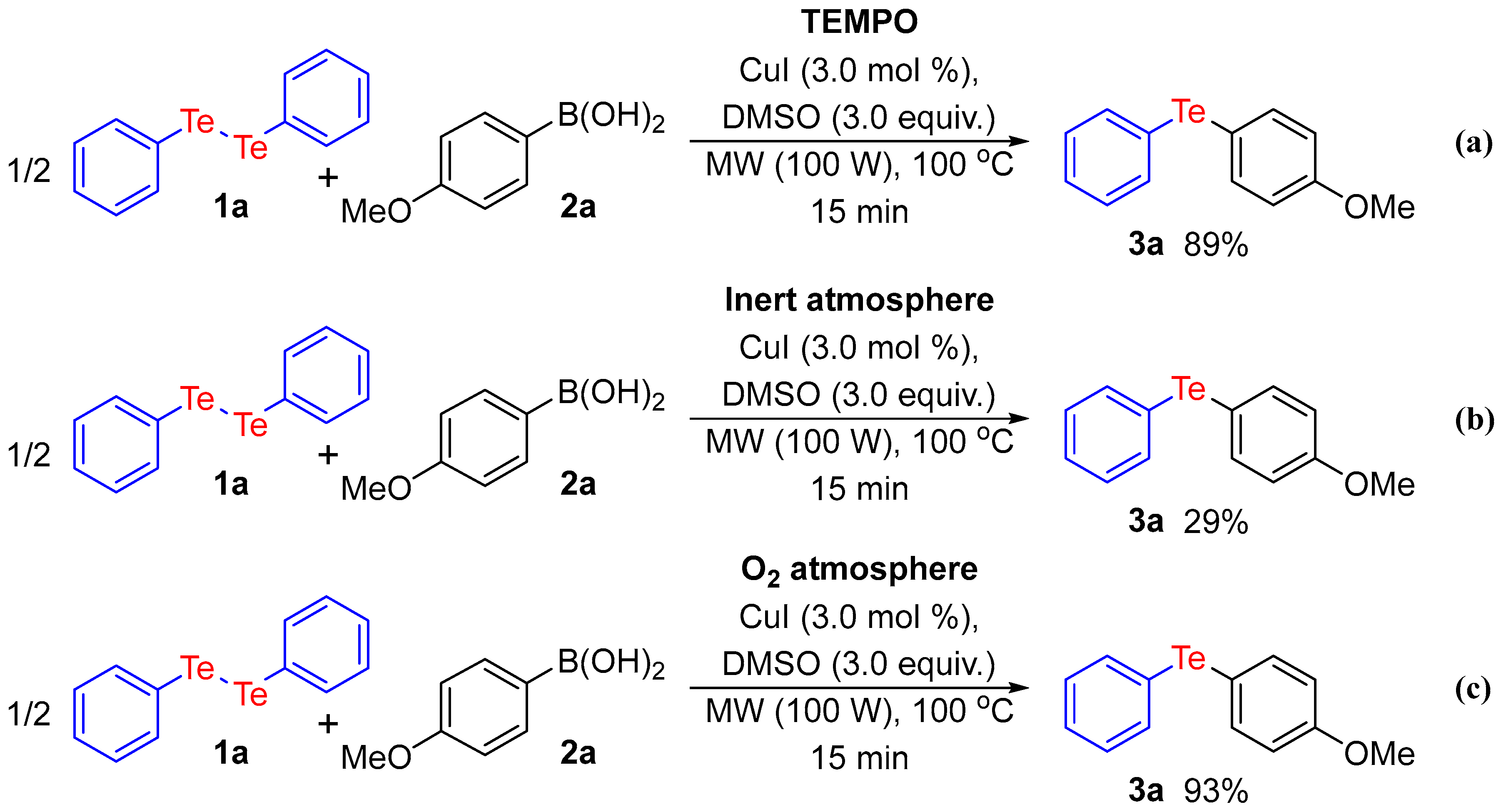
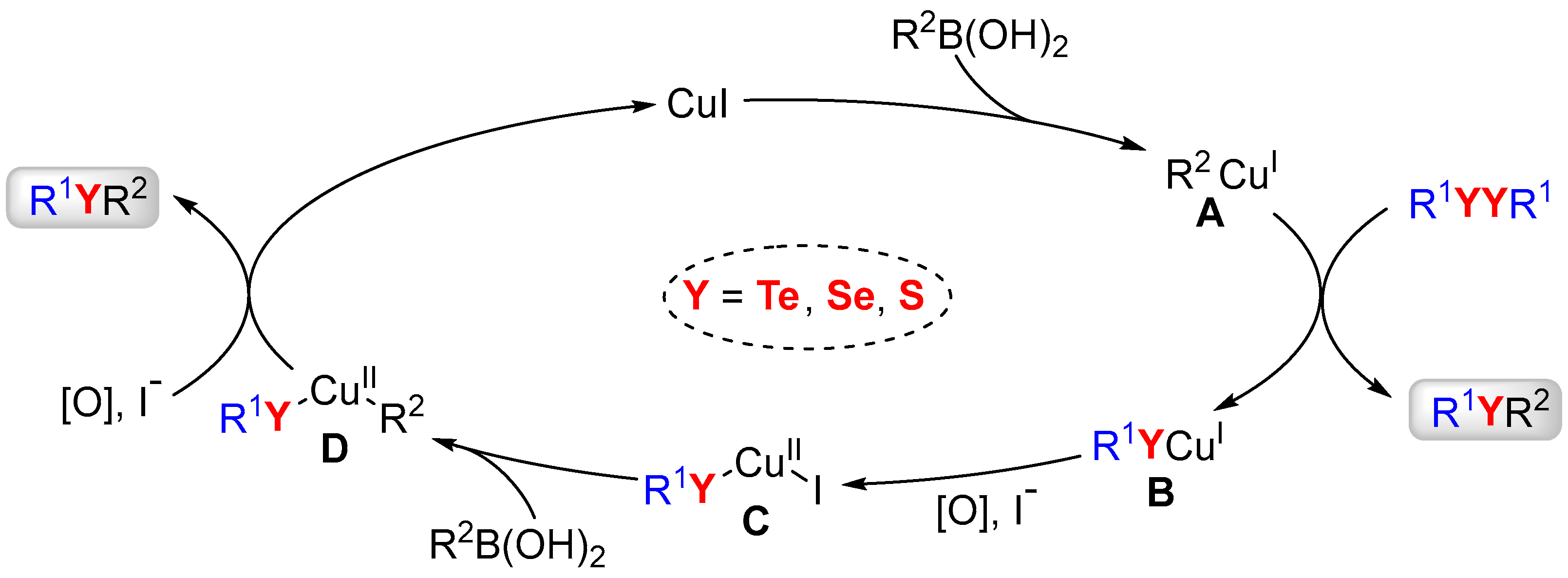
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Unsymmetrical Organochalcogenides under MW Irradiation
Analytical Data of Products 3a–k, 6a–h and 7a,b
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond formations between two nucleophiles: Transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, H. Transition-metal-catalyzed direct addition of unactivated C–H bonds to polar unsaturated bonds. Chem. Rev. 2015, 115, 3468–3517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nie, C.; Wang, H.; Li, X.; Verpoort, F.; Duan, C. A highly efficient method for the copper-catalyzed selective synthesis of diaryl chalcogenides from easily available chalcogen sources. Eur. J. Org. Chem. 2011, 2011, 7331–7338. [Google Scholar] [CrossRef]
- Sun, J.; Deng, L. Cobalt complex-catalyzed hydrosilylation of alkenes and alkynes. ACS Catal. 2017, 7, 631–651. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Wang, Z. Transition-metal-catalyzed hydrosulfoximination and oxidation reaction for the synthesis of sulfoximine derivatives. J. Org. Chem. 2016, 81, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Szostak, M. Twisted amides: From obscurity to broadly useful transtition-metal-catalyzed reaction by N-C amide bond Activation. Chem. Eur. J. 2017, 23, 7157–7173. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Shi, S.; Szostak, M. Cross-coupling of amides by N–C bond activation. Synlett 2016, 27, 2530–2540. [Google Scholar]
- Godoi, M.; Paixão, M.W.; Braga, A.L. Chiral organoselenium-transition-metal catalysts in asymmetric transformations. Dalton Trans. 2011, 40, 11347–11355. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, A.J.; Eey, S.T.-C.; Denmark, S.E. Catalytic, stereospecific syn-dichlorination of alkenes. Nat. Chem. 2015, 7, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, T.; Back, D.F.; Geni, Z. Iron(III) chloride and diorganyl diselenide-promoted nucleophilic closures of 1-benzyl-2-alkynylbenzenes in the preparation of 9-(organoselanyl)-5H-benzo[7]annulenes. Adv. Synth. Catal. 2016, 358, 1119–1129. [Google Scholar] [CrossRef]
- Rafique, J.; Canto, R.F.S.; Saba, S.; Barbosa, F.A.R.; Braga, A.L. Recent advances in the synthesis of biologically relevant selenium-containing 5-membered heterocycles. Curr. Org. Chem. 2016, 20, 166–188. [Google Scholar] [CrossRef]
- Manna, D.; Roy, G.; Mugesh, G. Antithyroid drugs and their analogues: Synthesis, structure, and mechanism of action. Acc. Chem. Res. 2013, 46, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.A.R.; Canto, R.F.S.; Saba, S.; Rafique, J.; Braga, A.L. Synthesis and evaluation of dihydropyrimidinone-derived selenoesters as multi-targeted directed compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2016, 24, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yan, J.; Poon, J.-F.; Singh, V.P.; Lu, X.; Ott, M.K.; Engman, L.; Kumar, S. Multifunctional antioxidants: Regenerable radical-trapping and hydroperoxide-decomposing ebselenols. Angew. Chem. Int. Ed. 2016, 55, 3729–3733. [Google Scholar] [CrossRef] [PubMed]
- Rafique, J.; Saba, S.; Canto, R.F.S.; Frizon, T.E.A.; Hassan, W.; Waczuk, E.P.; Jan, M.; Back, D.F.; Rocha, J.B.T.D.; Braga, A.L. Synthesis and biological evaluation of 2-picolylamide-based diselenides with non-bonded interactions. Molecules 2015, 20, 10095–10109. [Google Scholar] [CrossRef] [PubMed]
- Freudendahl, D.M.; Santoro, S.; Shahzad, S.A.; Santi, C.; Wirth, T. Green chemistry with selenium reagents: Development of efficient catalytic reactions. Angew. Chem. Int. Ed. 2009, 48, 8409–8411. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part A. Antioxidant compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley & Sons, Ltd.: West Sussex, UK, 2014; Volume 4, Chapter 13; pp. 989–1052. [Google Scholar]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley & Sons, Ltd.: West Sussex, UK, 2014; Volume 4, Chapter 14; pp. 1053–1118. [Google Scholar]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part C. Miscellaneous biological activities. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley & Sons, Ltd.: West Sussex, UK, 2014; Volume 4, Chapter 15; pp. 1119–1174. [Google Scholar]
- Frizon, T.E.; Rafique, J.; Saba, S.; Bechtold, I.H.; Gallardo, H.; Braga, A.L. Synthesis of functionalized organoselenium materials: Selenides and diselenides containing cholesterol. Eur. J. Org. Chem. 2015, 2015, 3470–3476. [Google Scholar] [CrossRef]
- Brutchey, R.L. Diorganyl dichalcogenides as useful synthons for colloidal semiconductor nanocrystals. Acc. Chem. Res. 2015, 48, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Rafique, J.; Lee, J.; Lee, L.M.; Jenkins, H.A.; Britten, J.F.; Braga, A.L.; Vargas-Baca, I. Synthesis and structural characterisation of the aggregates of benzo-1,2-chalcogenazole 2-oxides. Dalton Trans. 2017, 46, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhao, Z.-Q.; Ding, Y.; Chen, H.-L.; Zhang, Y.-W.; Yan, C.-H. Liquid-phase syntheses and material properties of two-dimensional nanocrystals of rare earth-selenium compound containing planar se layers: RESe2 nanosheets and RE4O4Se3 nanoplates. J. Am. Chem. Soc. 2013, 135, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Godoi, B.; Schumacher, R.F.; Zeni, G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom. Chem. Rev. 2011, 111, 2937–2980. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mughesh, G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Zeni, G.; Braga, A.L.; Stefani, H.A. Palladium-catalyzed coupling of sp2-hybridized tellurides. Acc. Chem. Res. 2003, 36, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T. Organoselenium Chemistry: Synthesis and Reactions; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Rappoport, Z.; Liebman, J.F.; Marek, I.; Patai, S. The Chemistry of Organic Selenium and Tellurium Compounds; Wiley & Sons, Ltd.: West Sussex, UK, 2014; Volume 4. [Google Scholar]
- Rappoport, Z.; Liebman, J.F.; Marek, I.; Patai, S. The Chemistry of Organic Selenium and Tellurium Compounds; Wiley & Sons, Ltd.: West Sussex, UK, 2012; Volume 3. [Google Scholar]
- Zeni, G.; Lüdtke, D.S.; Panatieri, R.B.; Braga, A.L. Vinylic tellurides: From preparation to their applicability in organic synthesis. Chem. Rev. 2006, 106, 1032–1076. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; Singh, H.B. Heteroatom-directed aromatic lithiation: A versatile route to the synthesis of organochalcogen (Se, Te) compounds. Acc. Chem. Res. 2002, 35, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, H.B.; Wolmershäuser, G. Protection against peroxynitrite-mediated nitration reaction by intramolecularly coordinated diorganoselenides. Organometallics 2006, 25, 382–393. [Google Scholar] [CrossRef]
- Malysher, D.A.; Scott, N.M.; Marion, N.; Stevens, E.D.; Ananikov, V.P.; Beletskaya, I.P.; Nolan, S.P. Homogeneous nickel catalysts for the selective transfer of a single arylthio group in the catalytic hydrothiolation of alkynes. Organometallics 2006, 25, 4462–4470. [Google Scholar] [CrossRef]
- Cohen, R.J.; Fox, D.L.; Salvatore, R.N. A novel and highly efficient synthetic route to unsymmetrical organoselenides using cesium bases. J. Org. Chem. 2004, 69, 4265–4268. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.G.; Gujadhur, R.K.; Venkataraman, D. A general method for the formation of aryl-sulfur bonds using copper (I) catalysts. Org. Lett. 2002, 4, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, E.; van Klink, G.P.M.; de Vries, J.G.; van Koten, G. Ligand-free copper-catalyzed c-s coupling of aryl iodides and thiols. J. Org. Chem. 2008, 73, 5625–5628. [Google Scholar] [CrossRef] [PubMed]
- Tiecco, M.; Testaferri, L.; Bagnoli, L.; Mariani, F.; Temperini, A.; Tomassini, C.; Santi, C. Electrophilic 2-thienylselenenylation of thiophene. Preparation of oligo(seleno-2,5-thienylenes). Tetrahedron 2000, 56, 3255–3260. [Google Scholar] [CrossRef]
- Jin, W.; Zheng, P.; Wong, W.-T.; Law, G.-L. Efficient palladium-catalyzed direct C−H phenylselenylation of (hetero)arenes in water. Asian J. Org. Chem. 2015, 4, 875–878. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Yi, C.-L.; Liu, T.-J.; Lee, C.-F. Highly regioselective synthesis of aryl chalcogenides through C–H functionalization of arenes. Chem. Commun. 2012, 48, 8440–8442. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Yue, X.; Qing, F.-L. Cu (II)-mediated methylthiolation of aryl C−H bonds with DMSO. Org. Lett. 2010, 12, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dimitrijević, E.; Dong, V.M. Palladium-catalyzed C−H Bond functionalization with arylsulfonyl chlorides. J. Am. Chem. Soc. 2009, 131, 3466–3467. [Google Scholar] [CrossRef] [PubMed]
- Candeias, N.R.; Montlbano, F.; Cal, P.M.S.D.; Gois, P.M.P. Boronic acids and esters in the petasis-borono Mannich multicomponent reaction. Chem. Rev. 2010, 110, 6169–6193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Falck, J.R. Transition-Metal-Free ipso-Functionalization of Arylboronic Acids and Derivatives. Adv. Synth. Catal. 2014, 356, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G. Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011; Volume 2. [Google Scholar]
- Taniguchi, N. Aryl- or alkylation of diaryl disulfides using organoboronic acids and a copper catalyst. Synlett 2006, 14, 1351–1354. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Huang, F. A simple copper salt-catalyzed synthesis of unsymmetrical diaryl selenides and tellurides from arylboronic acids with diphenyl diselenide and ditelluride. Synlett 2005, 13, 2007–2010. [Google Scholar] [CrossRef]
- Ren, K.; Wang, M.; Wang, L. Lewis acid InBr3-catalyzed arylation of diorgano diselenides and ditellurides with arylboronic acids. Org. Biomol. Chem. 2009, 7, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, K.; Wang, L. Iron-catalyzed ligand-free carbon-selenium (or tellurium) coupling of arylboronic acids with diselenides and ditellurides. Adv. Synth. Catal. 2009, 351, 1586–1594. [Google Scholar] [CrossRef]
- Yu, J.-T.; Guo, H.; Yi, Y.; Fei, H.; Jiang, Y. The Chan-Lam reaction of chalcogen elements leading to aryl chalcogenides. Adv. Synth. Catal. 2014, 356, 749–752. [Google Scholar] [CrossRef]
- Zheng, B.; Gong, Y.; Xu, H.-H. Copper-catalyzed C–Se coupling of diphenyl diselenide with arylboronic acids at room temperature. Tetrahedron 2013, 69, 5342–5347. [Google Scholar] [CrossRef]
- Reddy, K.H.; Satish, G.; Rames, K.; Karnakar, K.; Nageswar, Y.V.D. Magnetically separable CuFe2O4 nanoparticle catalyzed C–Se cross coupling in reusable PEG medium. Chem. Lett. 2012, 41, 585–587. [Google Scholar] [CrossRef]
- Mohan, B.; Yoon, C.; Jang, S. Copper nanoparticles catalyzed Se(Te)-Se(Te) bond activation: A straightforward route towards unsymmetrical organochalcogenides from boronic acids. Chemcatchem 2015, 7, 405–412. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, Z.-J.; Da, Y.-X.; Zhang, Z.; Wang, X.-C. (2-Chlorobenzoyloxy)copper(I) catalyzed C-S cross-coupling of di(hetero)aryl disulfides with aryl boronic acis under base-free conditions. RSC Adv. 2015, 4, 45479–45483. [Google Scholar] [CrossRef]
- Alves, D.; Santos, C.G.; Paixão, M.W.; Soares, L.C.; de Souza, D.; Rodrigues, O.E.D.; Braga, A.L. CuO nanoparticles: An efficient and recyclable catalyst for cross-coupling reactions of organic diselenides with aryl boronic acids. Tetrahedron Lett. 2009, 50, 6635–6638. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. A convenient and efficient copper-catalyzed synthesis of unsymmetrical and symmetrical diaryl chalcogenides from arylboronic acids in ethanol. Tetrahedron 2014, 70, 1763–1772. [Google Scholar] [CrossRef]
- Goldani, B.; Ricordi, V.G.; Seus, N.; Lenardao, E.J.; Schumacher, R.F.; Alves, D. Silver-catalyzed synthesis of diaryl selenides by reaction of diaryl diselenides with aryl boronic acids. J. Org. Chem. 2016, 81, 11472–11476. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Rafique, J.; Braga, A.L. Synthesis of unsymmetrical diorganyl chalcogenides under greener conditions: Use of an iodine/DMSO system, solvent- and metal-free approach. Adv. Synth. Catal. 2015, 357, 1446–1452. [Google Scholar] [CrossRef]
- Mohan, B.; Hwang, S.; Jang, S.; Park, K.H. Ultrasound-assisted transition-metal-free synthesis of diaryl tellurides from aryl boronic acids: A possible free-radical mechanism. Syntlett 2014, 25, 2078–2083. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Rosário, A.R.; Braga, A.L. Regioselective, solvent- and metal-free chalcogenation of imidazo[1,2-a]pyridines by employing I2/DMSO as the catalytic oxidation system. Chem. Eur. J. 2016, 22, 11854–11862. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Rafique, J.; Braga, A.L. DMSO/iodine-catalyzed oxidative C–Se/C–S bond formation: A regioselective synthesis of unsymmetrical chalcogenides with nitrogen- or oxygen-containing arenes. Catal. Sci. Technol. 2016, 6, 3087–3098. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Rosário, A.R.; Zeni, G.; Braga, A.L. K2CO3-mediated, direct C–H bond selenation and thiolation of 1,3,4-oxadiazoles in the absence of metal catalyst: An eco-friendly approach. RSC Adv. 2014, 4, 51648–51652. [Google Scholar] [CrossRef]
- Silva, L.T.; Azeredo, J.B.; Saba, S.; Rafique, J.; Bortoluzzi, A.J.; Braga, A.L. Solvent- and metal-free chalcogenation of bicyclic arenes using I2/DMSO as non-metallic catalytic system. Eur. J. Org. Chem. 2017. [Google Scholar] [CrossRef]
- Rocha, M.S.T.; Rafique, J.; Saba, S.; Azeredo, J.B.; Back, D.; Godoi, M.; Braga, A.L. Regioselective hydrothiolation of terminal acetylene catalyzed by magnetite (Fe3O4) nanoparticles. Synth. Commun. 2017, 47, 291–298. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Schneider, A.R.; Franco, M.S.; Silva, S.M.; Braga, A.L. Metal- and solvent-free approach to access 3-Se/S-chromones from the cyclization of enaminones in the presence of dichalcogenides catalyzed by KIO3. ACS Omega 2017, 2, 2280–2290. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Gayduk, K.A.; Belestskaya, I.P.; Khrustalve, V.N.; Antipin, M.Y. Remarkable ligand effect in Ni- and Pd-catalyzed bisthiolation and bisselenation of terminal alkynes: Solving the problem of stereoselective dialkyldichalcogenide addition to the C≡C bond. Chem. Eur. J. 2008, 14, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N. Diarylation of chalcogen elements using arylboronic acids via copper- or palladium-catalyzed oxidative coupling. Tetrahedron 2016, 72, 5818–5823. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Y.; Chen, Q.; Cai, M. A highly efficient and reusable MCM-41-immobilized bipyridine copper (I) catalyst for the C–Se coupling of organoboronic acids with diaryl diselenides. New J. Chem. 2015, 39, 2106–2115. [Google Scholar] [CrossRef]
- Taniguchi, N. Convenient synthesis of unsymmetrical organochalcogenides using organoboronic acids with dichalcogenides via cleavage of the S−S, Se−Se, or Te−Te bond by a copper catalyst. J. Org. Chem. 2007, 72, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Cella, R.; Cunha, R.L.O.R.; Reis, A.E.S.; Pimenta, D.C.; Klitzke, C.F.; Stefani, H.A. Suzuki-Miyaura cross-coupling reactions of aryl tellurides with potassium aryltrifluoroborate salts. J. Org. Chem. 2006, 71, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, V.G.; Freitas, C.S.; Perin, G.; Lenardão, E.J.; Jacob, R.G.; Savegnago, L.; Alves, D. Glycerol as a recyclable solvent for copper-catalyzed cross-coupling reactions of diaryl diselenides with aryl boronic acids. Green Chem. 2012, 14, 1030–1034. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a–k, 6a–h and 7a–b are available from the authors. |
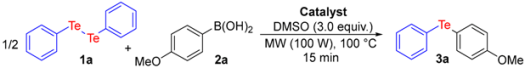
| Entry | Catalyst | Amount | Yield (%) b,c |
|---|---|---|---|
| 1 | CuI | 1.0 mol % | 43 |
| 2 | CuI | 2.0 mol % | 71 |
| 3 | CuI | 3.0 mol % | 90 |
| 4 | CuI | 4.0 mol % | 90 |
| 5 | CuBr | 3.0 mol % | 56 |
| 6 | CuCl | 3.0 mol % | 67 |
| 7 | CuCl2 | 3.0 mol % | 72 |
| 8 | Cu(OAc)2 | 3.0 mol % | 46 |
| 9 | CuO | 3.0 mol % | 61 |
| 10 | nano-CuO | 3.0 mol % | 65 |
| 11 | I2 | 3.0 mol % | 69 |
| 12 | - | - | Traces |
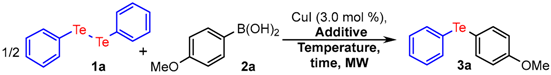
| Entry | Additive (equiv.) | MW (W) | T (°C) | Time (min) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | DMSO (3.0) | 100 | 100 | 15 | 90 |
| 2 | CH3CN (3.0) | 100 | 100 | 15 | 35 |
| 3 | EtOH (3.0) | 100 | 100 | 15 | 25 |
| 4 | Dioxane (3.0) | 100 | 100 | 15 | 40 |
| 5 | H2O (3.0) | 100 | 100 | 15 | - |
| 6 | TBHP (3.0) | 100 | 100 | 15 | - |
| 7 | - | 100 | 100 | 15 | 10 |
| 8 c | DMSO (3.0) | 100 | 100 | 15 | 29 |
| 9 | DMSO (2.0) | 100 | 100 | 15 | 78 |
| 10 | DMSO (4.0) | 100 | 100 | 15 | 90 |
| 11 | DMSO (3.0) | 100 | 100 | 10 | 73 |
| 12 | DMSO (3.0) | 100 | 100 | 20 | 88 |
| 13 | DMSO (3.0) | 100 | 80 | 15 | 68 |
| 14 | DMSO (3.0) | 100 | 120 | 15 | 77 |
| 15 | DMSO (3.0) | 120 | 100 | 15 | 86 |
| 16 | DMSO (3.0) | 80 | 100 | 15 | 70 |
| 17 d | DMSO (3.0) | - | 100 | 24 h | 57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saba, S.; Botteselle, G.V.; Godoi, M.; Frizon, T.E.A.; Galetto, F.Z.; Rafique, J.; Braga, A.L. Copper-Catalyzed Synthesis of Unsymmetrical Diorganyl Chalcogenides (Te/Se/S) from Boronic Acids under Solvent-Free Conditions. Molecules 2017, 22, 1367. https://doi.org/10.3390/molecules22081367
Saba S, Botteselle GV, Godoi M, Frizon TEA, Galetto FZ, Rafique J, Braga AL. Copper-Catalyzed Synthesis of Unsymmetrical Diorganyl Chalcogenides (Te/Se/S) from Boronic Acids under Solvent-Free Conditions. Molecules. 2017; 22(8):1367. https://doi.org/10.3390/molecules22081367
Chicago/Turabian StyleSaba, Sumbal, Giancarlo Vaccari Botteselle, Marcelo Godoi, Tiago Elias Allievi Frizon, Fábio Zazyki Galetto, Jamal Rafique, and Antonio L. Braga. 2017. "Copper-Catalyzed Synthesis of Unsymmetrical Diorganyl Chalcogenides (Te/Se/S) from Boronic Acids under Solvent-Free Conditions" Molecules 22, no. 8: 1367. https://doi.org/10.3390/molecules22081367
APA StyleSaba, S., Botteselle, G. V., Godoi, M., Frizon, T. E. A., Galetto, F. Z., Rafique, J., & Braga, A. L. (2017). Copper-Catalyzed Synthesis of Unsymmetrical Diorganyl Chalcogenides (Te/Se/S) from Boronic Acids under Solvent-Free Conditions. Molecules, 22(8), 1367. https://doi.org/10.3390/molecules22081367








