A Review of the Antiviral Role of Green Tea Catechins
Abstract
:1. Introduction
2. Inhibitory Effects of GTC on DNA Virus
2.1. GTC Inhibits HBV
2.2. Effect of GTCs on Herpes Simplex Virus
2.3. Effect of GTCs on the EBV
2.4. Effect of GTCs on Adenovirus
3. Inhibitory Effects of GTCs on RNA Virus
3.1. GTC Inhibits HIV
3.2. EGCG Inhibits HCV
3.3. Inhibitory Effects of GTCs on Influenza Virus
3.4. Effect of GTC on Some Arboviruses
3.5. Effect of GTCs on Human T-cell Lymphotropic Virus-1
3.6. Effect of GTCs on Rotaviruses and Enteroviruses
3.7. Effect of GTCs on EBOV
3.8. Effect of GTCs on Viruses Infecting Other Animals
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef] [PubMed]
- Narotzki, B.; Reznick, A.Z.; Aizenbud, D.; Levy, Y. Green tea: A promising natural product in oral health. Arch. Oral Biol. 2012, 57, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Schinzari, V.; Barnaba, V.; Piconese, S. Chronic hepatitis B virus and hepatitis C virus infections and cancer: Synergy between viral and host factors. Clin. Microbiol. Infect. 2015, 21, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, E.J.; Moon, S.; Lee, J.Y.; Kang, J.H. Systemic multi-organ involvement in chronic active Epstein-Barr virus disease. Pediatr. Int. 2015, 57, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Churchill, M.J.; Deeks, S.G.; Margolis, D.M.; Siliciano, R.F.; Swanstrom, R. HIV reservoirs: What, where and how to target them. Nat. Rev. Microbiol. 2016, 14, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Kawasaki, Y.; Kawakami, K.; Yamada, H. Anti-influenza virus effects of catechins: A molecular and clinical review. Curr. Med. Chem. 2016, 23, 4773–4783. [Google Scholar] [CrossRef] [PubMed]
- Fassina, G.; Buffa, A.; Benelli, R.; Varnier, O.E.; Noonan, D.M.; Albini, A. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. Aids 2002, 16, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Deng, F.; Hu, Z.; Wang, H. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antivir. Res. 2008, 78, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouille, Y.; Seron, K. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Liu, H.J.; Chang, C.D.; Chen, Y.C.; Chang, C.I.; Shih, W.L. Avian reovirus S1133-induced apoptosis is associated with Bip/GRP79-mediated Bim translocation to the endoplasmic reticulum. Apoptosis 2015, 20, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, S.; Li, C.; Yang, L.; Zu, Y. In vitro evaluation of the antiviral activity of the synthetic epigallocatechin gallate analog-epigallocatechin gallate (EGCG) palmitate against porcine reproductive and respiratory syndrome virus. Viruses 2014, 6, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Jusoh, S.A. Molecular docking and molecular dynamics simulation studies to predict flavonoid binding on the surface of DENV2 E protein. Interdiscip. Sci. Comput. Life Sci. 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Sliva, K.; von Rhein, C.; Kummerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Coffin, C.S.; Lee, S.S. New paradigms in hepatitis B management: Only diamonds are forever. Br. Med. Bull. 2015, 116, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Karamese, M.; Aydogdu, S.; Karamese, S.A.; Altoparlak, U.; Gundogdu, C. Preventive effects of a major component of green tea, epigallocathechin-3-gallate, on hepatitis-B virus DNA replication. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 4199–4202. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.Y.; Zhao, K.J.; Wang, J.B.; Ma, Z.J.; Xiao, X.H. Green tea polyphenol, epigallocatechin-3-gallate, possesses the antiviral activity necessary to fight against the hepatitis B virus replication in vitro. J. Zhejiang Uni. Sci. B 2014, 15, 533–539. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, L.X.; Liao, Q.J.; Liu, C.L.; Chen, X.L. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication-inducible cell line. World J. Gastroenterol. 2011, 17, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gu, W.; Li, C.; Li, X.; Xing, G.; Li, Y.; Song, Y.; Zheng, W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Natl. Med. 2016, 70, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Tao, M.H.; Hung, T.M.; Chen, J.C.; Lin, Z.J.; Huang, C. (−)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antivir. Res. 2014, 111, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Hu, J.; Shu, W.; Gao, B.; Xiong, S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015, 6, e1770. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.M.; Jia, J.H.; Rohan, L.; Corbo, C.; Di Maggio, V.; Jenkins, E.C., Jr.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Xu, W.; Merz, G.; Hillier, S.; Rohan, L.; Wen, G.Y. Digallate dimers of (−)-epigallocatechin gallate inactivate herpes simplex virus. Antimicrob. Agents Chemother. 2011, 55, 5646–5653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Jiang, J.; Zheng, R.; Pearl, H.; Dickinson, D.; Fu, B.; Hsu, S. A proprietary topical preparation containing EGCG-stearate and glycerin with inhibitory effects on herpes simplex virus: Case study. Inflamm. Allergy Drug Targets 2012, 11, 364–368. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, C.C.; Schang, L.M. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J. Virol. 2014, 88, 7806–7817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Zhao, X.R.; Luo, F.J.; Tang, M.; Cao, Y. Epigallocatechin-3-gallate interferes with EBV-encoding AP-1 signal transduction pathway. Zhonghua zhong liu za zhi (Chin. J. Oncol.) 2004, 26, 393–397. [Google Scholar]
- Chen, Y.L.; Tsai, H.L.; Peng, C.W. EGCG debilitates the persistence of EBV latency by reducing the DNA binding potency of nuclear antigen 1. Biochem. Biophys. Res. Commun. 2012, 417, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Chen, L.; Yang, L.; Li, L.; Tao, Y.; Li, W.; Li, Z.; Liu, H.; Tang, M.; et al. (−)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis 2013, 34, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.M.; Ruzindana-Umunyana, A.; Imbeault, L.; Sircar, S. Inhibition of adenovirus infection and adenain by green tea catechins. Antivir. Res. 2003, 58, 167–173. [Google Scholar] [CrossRef]
- Nakane, H.; Ono, K. Differential inhibitory effects of some catechin derivatives on the activities of human immunodeficiency virus reverse transcriptase and cellular deoxyribonucleic and ribonucleic acid polymerases. Biochemistry 1990, 29, 2841–2845. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Hsu, F.L.; Lin, J.Y. Inhibitory effects of polyphenolic catechins from Chinese green tea on HIV reverse transcriptase activity. J. Biomed. Sci. 1994, 1, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hattori, T.; Kodama, E.N. Epigallocatechin gallate inhibits the HIV reverse transcription step. Antivir. Chem. Chemother. 2011, 21, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Honda, M.; Ikigai, H.; Hara, Y.; Shimamura, T. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antivir. Res. 2002, 53, 19–34. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhao, Q.; He, Y.; Niu, J.; Debnath, A.K.; Wu, S.; Jiang, S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta 2005, 1723, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Zhan, C.G. How can (−)-epigallocatechin gallate from green tea prevent HIV-1 infection? Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors. J. Phys. Chem. B 2006, 110, 2910–2917. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.P.; McCormick, T.G.; Nance, C.L.; Shearer, W.T. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. J. Allergy Clin. Immunol. 2006, 118, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Nance, C.L.; Siwak, E.B.; Shearer, W.T. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J. Allergy Clin. Immunol. 2009, 123, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Obregon, D.; Hou, H.; Zeng, J.; Sun, N.; Nikolic, V.; Ehrhart, J.; Shytle, D.; Fernandez, F.; Tan, J. EGCG mitigates neurotoxicity mediated by HIV-1 proteins gp120 and Tat in the presence of IFN-gamma: Role of JAK/STAT1 signaling and implications for HIV-associated dementia. Brain Res. 2006, 1123, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Rrapo, E.; Zhu, Y.; Tian, J.; Hou, H.; Smith, A.; Fernandez, F.; Tan, J.; Giunta, B. Green Tea-EGCG reduces GFAP associated neuronal loss in HIV-1 Tat transgenic mice. Am. J. Trans. Res. 2009, 1, 72–79. [Google Scholar]
- Hauber, I.; Hohenberg, H.; Holstermann, B.; Hunstein, W.; Hauber, J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc. Natl. Acad. Sci. USA 2009, 106, 9033–9038. [Google Scholar] [CrossRef] [PubMed]
- Hartjen, P.; Frerk, S.; Hauber, I.; Matzat, V.; Thomssen, A.; Holstermann, B.; Hohenberg, H.; Schulze, W.; zur Wiesch, J.S.; van Lunzen, J. Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG. AIDS Res. Ther. 2012, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Ciesek, S.; von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, H.; Suzuki, T.; Wakita, T.; Murakami, Y. A cell-based, microplate colorimetric screen identifies 7,8-benzoflavone and green tea gallate catechins as inhibitors of the hepatitis C virus. Biol. Pharm. Bull. 2012, 35, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qiu, H.; Gong, J.; Liu, Q.; Xiao, H.; Chen, X.W.; Sun, B.L.; Yang, R.G. (−)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Arch. Virol. 2012, 157, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Sahuc, M.E.; Belouzard, S.; Pene, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wychowski, C.; et al. Polyphenols inhibit hepatitis C virus entry by a new mechanism of action. J. Virol. 2015, 89, 10053–10063. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Wu, Y.H.; Tseng, C.K.; Lin, C.K.; Chen, W.C.; Hsu, Y.C.; Lee, J.C. Green tea phenolic epicatechins inhibit hepatitis C virus replication via cycloxygenase-2 and attenuate virus-induced inflammation. PLoS ONE 2013, 8, e54466. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H. Inhibition of multiplication of influenza virus by extracts of tea. Proc. Soc. Exp. Biol. Med. 1949, 71, 84. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res. 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Imanishi, N.; Tuji, Y.; Katada, Y.; Maruhashi, M.; Konosu, S.; Mantani, N.; Terasawa, K.; Ochiai, H. Additional inhibitory effect of tea extract on the growth of influenza A and B viruses in MDCK cells. Microbiol. Immunol. 2002, 46, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Bai, L.P.; Huang, W.B.; Li, X.Z.; Zhao, S.S.; Zhong, N.S.; Jiang, Z.H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Oxford, J.S.; Lambkin, R.; Guralnik, M.; Rosenbloom, R.A.; Petteruti, M.P.; Digian, K.; Lefante, C. Preclinical in vitro activity of QR-435 against influenza A virus as a virucide and in paper masks for prevention of viral transmission. Am. J. Ther. 2007, 14, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Oxford, J.S.; Lambkin, R.; Guralnik, M.; Rosenbloom, R.A.; Petteruti, M.P.; Digian, K.; LeFante, C. In vivo prophylactic activity of QR-435 against H3N2 influenza virus infection. Am. J. Ther. 2007, 14, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorgan. Med. Chem. Lett. 2008, 18, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.G.; Lvov, D.K.; Botikov, A.G.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Effects of a nutrient mixture on infectious properties of the highly pathogenic strain of avian influenza virus A/H5N1. Biofactors 2008, 33, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Takuma, N.; Daimon, T.; Hara, Y. Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: A prospective clinical study. J. Altern. Complement. Med. 2006, 12, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complement. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Yashiki, S.; Sonoda, J.; Lou, H.; Ghosh, S.K.; Byrnes, J.J.; Lema, C.; Fujiyoshi, T.; Karasuyama, M.; Sonoda, S. Green tea polyphenols induce apoptosis in vitro in peripheral blood T lymphocytes of adult T-cell leukemia patients. Jpn. J. Cancer Res. 2000, 91, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Harakeh, S.; Diab-Assaf, M.; Azar, R.; Hassan, H.M.; Tayeb, S.; Abou-El-Ardat, K.; Damanhouri, G.A.; Qadri, I.; Abuzenadah, A.; Chaudhary, A.; et al. Epigallocatechin-3-gallate inhibits tax-dependent activation of nuclear factor kappa B and of matrix metalloproteinase 9 in human T-cell lymphotropic virus-1 positive leukemia cells. Asian Pac. J. Cancer Prev. 2014, 15, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Mukoyama, A.; Ushijima, H.; Nishimura, S.; Koike, H.; Toda, M.; Hara, Y.; Shimamura, T. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn. J. Med. Sci. Biol. 1991, 44, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Cheng, M.L.; Weng, S.F.; Leu, Y.L.; Chiu, D.T. Antiviral effect of epigallocatechin gallate on enterovirus 71. J. Agric. Food Chem. 2009, 57, 6140–6147. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.P.; Shurtleff, A.C.; Costantino, J.A.; Tritsch, S.R.; Retterer, C.; Spurgers, K.B.; Bavari, S. HSPA5 is an essential host factor for Ebola virus infection. Antivir. Res. 2014, 109, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Estepa, A.; Coll, J. Inhibition of SERPINe1 reduces rhabdoviral infections in zebrafish. Fish Shellfish Immunol. 2015, 47, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, W.; Yu, F.; Lu, L. Identification of (−)-epigallocatechin-3-gallate as a potential agent for blocking infection by grass carp reovirus. Arch. Virol. 2016, 161, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Tsatsos, M.; MacGregor, C.; Athanasiadis, I.; Moschos, M.M.; Hossain, P.; Anderson, D. Herpes simplex virus keratitis: An update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin. Exp. Ophthalmol. 2016, 44, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Dochez, V.; Ducarme, G. Acute herpesviridae hepatitis during pregnancy: A review. Presse Med. 2015, 44, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Munch, M.; Hvas, J.; Christensen, T.; Moller-Larsen, A.; Haahr, S. The implications of Epstein-Barr virus in multiple sclerosis—A review. Acta Neurol. Scand. Suppl. 1997, 169, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Sato, Y.; Kimura, H. Modes of infection and oncogenesis by the Epstein-Barr virus. Rev. Med. Virol. 2014, 24, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Barre-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Perkins, J.T.; Hennig, B. EGCG prevents PCB-126-induced endothelial cell inflammation via epigenetic modifications of NF-κB target genes in human endothelial cells. J. Nutr. Biochem. 2016, 28, 164–170. [Google Scholar] [CrossRef] [PubMed]
- HIV/AIDS Fact Sheet from WHO. Available online: http://www.who.int/mediacentre/factsheets/fs360/en/ (accessed on 17 July 2017).
- Ghosn, J.P.R.; Chaillon, A.; Boilet, V.; Néré, M-L.; Delobel, P.; Lutch, F.; Bouchaud, O.; Molinal, J-M.; Chaix, M-L.; Delaugerre, C.; et al. Viral rebound in semen after treatment interruption in a HIV therapeutic vaccine double-blind trial (VRI02/ANRS149-LIGHT). In Proceedings of the 9th IAS Conference on HIV Science, Paris, France, 23–26 July 2017; International AIDS Society: Geneva, Switzerland, 2017. [Google Scholar]
- Nath, S.; Bachani, M.; Harshavardhana, D.; Steiner, J.P. Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J. Neurovirol. 2012, 18, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.M.; Qiu, J.Y.; Tan, S.Y.; Liu, S.W.; Li, L. Semen-derived enhancer of viral infection—A key factor in sexual transmission of HIV. Chin. J. Virol. 2012, 28, 84–88. [Google Scholar]
- Hepatitis C Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs164/en/ (accessed on 17 July 2017).
- Halegoua-De Marzio, D.; Kraft, W.K.; Daskalakis, C.; Ying, X.; Hawke, R.L.; Navarro, V.J. Limited sampling estimates of epigallocatechin gallate exposures in cirrhotic and noncirrhotic patients with hepatitis C after single oral doses of green tea extract. Clin. Ther. 2012, 34, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Hirooka, Y.; Abe, A.; Sugata, Y.; Ueda, M.; Murakami, K.; Suzuki, T.; Tanaka, K.; Kan, T. Concise synthesis of dideoxy-epigallocatechin gallate (DO-EGCG) and evaluation of its anti-influenza virus activity. Bioorg. Med. Chem. Lett. 2007, 17, 3095–3098. [Google Scholar] [CrossRef] [PubMed]
- Jariwalla, R.J.; Roomi, M.W.; Gangapurkar, B.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Suppression of influenza A virus nuclear antigen production and neuraminidase activity by a nutrient mixture containing ascorbic acid, green tea extract and amino acids. Biofactors 2007, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Jariwalla, R.J.; Kalinovsky, T.; Roomi, N.; Niedzwiecki, A.; Rath, M. Inhibition of cellular invasive parameters in influenza A virus-infected MDCK and Vero cells by a nutrient mixture. Biofactors 2008, 33, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Tesh, R.B. Insect-specific viruses and their potential impact on arbovirus transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Beckham, J.D.; Tyler, K.L. Arbovirus infections. Continuum 2015, 21, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.B.; Thomas, S.J.; Endy, T.P. The emergence of Zika virus: A narrative review. Ann. Int. Med. 2016, 165, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, P.; Matsuoka, M. Human T-cell leukemia virus type 1 and Foxp3 expression: Viral strategy in vivo. Int. Immunol. 2014, 26, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.B.; Taylor, G.P. Treatment of adult T-cell leukaemia/lymphoma: Is the virus a target? Curr. Opin. Infect. Dis. 2015, 28, 583–588. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizition. Ebola Virus Disease. Available online: http://www.who.int/mediacentre/factsheets/fs103/en/ (accessed on 17 July 2017).
- Gomez-Mascaraque, L.G.; Sanchez, G.; Lopez-Rubio, A. Impact of molecular weight on the formation of electrosprayed chitosan microcapsules as delivery vehicles for bioactive compounds. Carbohydr. Polym. 2016, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Toyazaki, H.; Yoshioka, Y.; Yokoi, N.; Yamasaki, T. Structural characteristics for superoxide anion radical scavenging and productive activities of green tea polyphenols including proanthocyanidin dimers. Chem. Pharm. Bull. 2010, 58, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Mori, T.; Ichikawa, T.; Kaku, M.; Kusaka, K.; Uekusa, Y.; Akagawa, M.; Aihara, Y.; Furuta, T.; Wakimoto, T.; et al. Structural characteristics of green tea catechins for formation of protein carbonyl in human serum albumin. Bioorgan. Med. Chem. 2010, 18, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Thakur, V.S.; Paul, R.K.; Feng, G.S.; Qu, C.K.; Mukhtar, H.; Agarwal, M.L. SHP-2 tyrosine phosphatase inhibits p73-dependent apoptosis and expression of a subset of p53 target genes induced by EGCG. Proc. Natl. Acad. Sci. USA 2007, 104, 5419–5424. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.H.; Xu, J.; Hu, F.L.; Wan, X.C.; Deng, S.X.; Barasch, J. EGCG inhibit chemical reactivity of iron through forming an Ngal-EGCG-iron complex. Biometals 2013, 26, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Suarez, M.; Torres, J.L.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014, 42, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Devi, A.; Mishra, S. Molecular docking and molecular dynamics studies reveal structural basis of inhibition and selectivity of inhibitors EGCG and OSU-03012 toward glucose regulated protein-78 (GRP78) overexpressed in glioblastoma. J. Mol. Model. 2015, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Vivekanandan, S.; Brender, J.R.; Abe, Y.; Naito, A.; Ramamoorthy, A. NMR characterization of monomeric and oligomeric conformations of human calcitonin and its interaction with EGCG. J. Mol. Biol. 2012, 416, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.G.; Landis-Piwowar, K.R.; Chen, D.; Wan, S.B.; Chan, T.H.; Dou, Q.P. Methylation of green tea polyphenols affects their binding to and inhibitory poses of the proteasome β5 subunit. Int. J. Mol. Med. 2006, 18, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, O.; Nakamura, A.; Nakamura, T.; Iwamoto, H.; Hiroshi, M.; Inoue, K.; Torimura, T.; Ueno, T.; Sata, M. Methylated-(3″)-epigallocatechin gallate analog suppresses tumor growth in Huh7 hepatoma cells via inhibition of angiogenesis. Nutr. Cancer 2014, 66, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Ma, N.J.; Sang, S.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J. Agric. Food Chem. 2012, 60, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kaihatsu, K.; Nishino, K.; Ogawa, M.; Kato, N.; Yamaguchi, A. Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate. Front. Microbiol. 2012, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Sharma, M.; Sharma, P.D.; Singh, T.V. Design, semisynthesis, and evaluation of O-acyl derivatives of (−)-epigallocatechin-3-gallate as antitumor agents. J. Agric. Food Chem. 2007, 55, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Saito, A.; Horikawa, K.; Nakajima, N.; Tanaka, A.; Yoshida, H.; Matsubara, K. Acylated catechin derivatives: Inhibitors of DNA polymerase and angiogenesis. Front. Biosci. 2011, 3, 1337–1348. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Zhong, Y.J.; Perera, N.; Shahidi, F. Antiglycation activity of lipophilized epigallocatechin gallate (EGCG) derivatives. Food Chem. 2016, 190, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Nadim, M.; Auriol, D.; Lamerant-Faye, L.N.; Lefevre, F.; Dubanet, L.; Redziniak, G.; Kieda, C.; Grillon, C. Improvement of polyphenol properties upon glucosylation in a UV-induced skin cell ageing model. Int. J. Cosmet. Sci. 2014, 36, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Tan, C.; Zhang, X.; Feng, B.; Xia, S. Fabrication of epigallocatechin-3-gallate nanocarrier based on glycosylated casein: Stability and interaction mechanism. J. Agric. Food Chem. 2014, 62, 4677–4684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Hu, J.M.; Huang, Y.W.; Wu, X.Y.; Zi, C.T.; Wang, X.J.; Sheng, J. Synthesis and biological testing of novel glucosylated epigallocatechin gallate (EGCG) derivatives. Molecules 2016, 21, 620. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ma, F.; Yang, Y.; Xie, M.; Zhang, C.; Xu, Y.; Zeng, X. Antioxidant nanocomplexes for delivery of epigallocatechin-3-gallate. J. Agric. Food Chem. 2016, 64, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ha, J.; Zou, T.; Gu, L. Fabrication of coated bovine serum albumin (BSA)-epigallocatechin gallate (EGCG) nanoparticles and their transport across monolayers of human intestinal epithelial Caco-2 cells. Food Funct. 2014, 5, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ting, Y.; Yang, X.; Tang, W.; Zeng, X.; Huang, Q. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem. Commun. 2012, 48, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S. Compounds Derived from Epigallocatechin-3-Gallate (EGCG) as a Novel Approach to the Prevention of Viral Infections. Inflamm. Allergy Drug Targets 2015, 14, 13–18. [Google Scholar] [CrossRef] [PubMed]
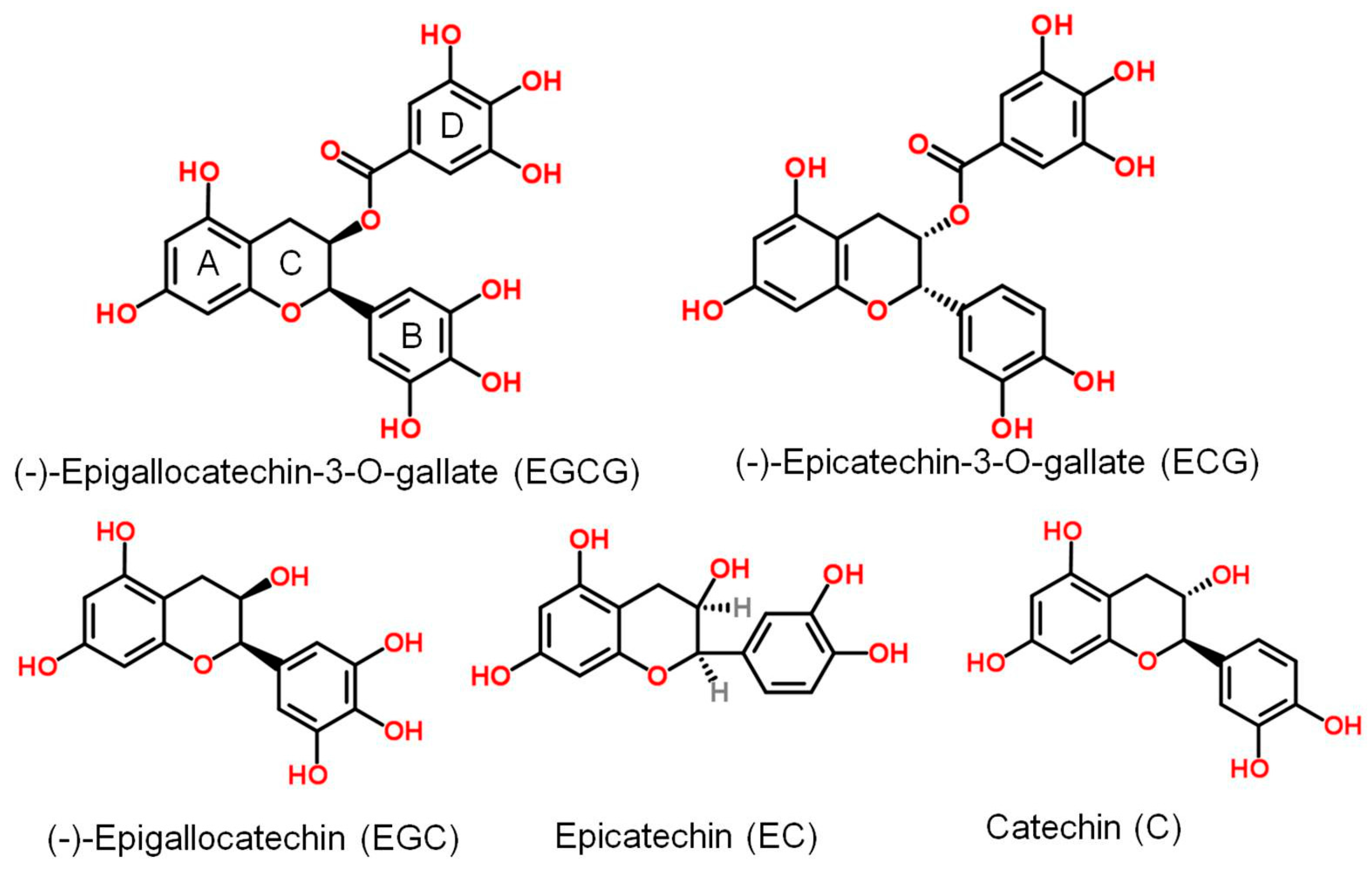
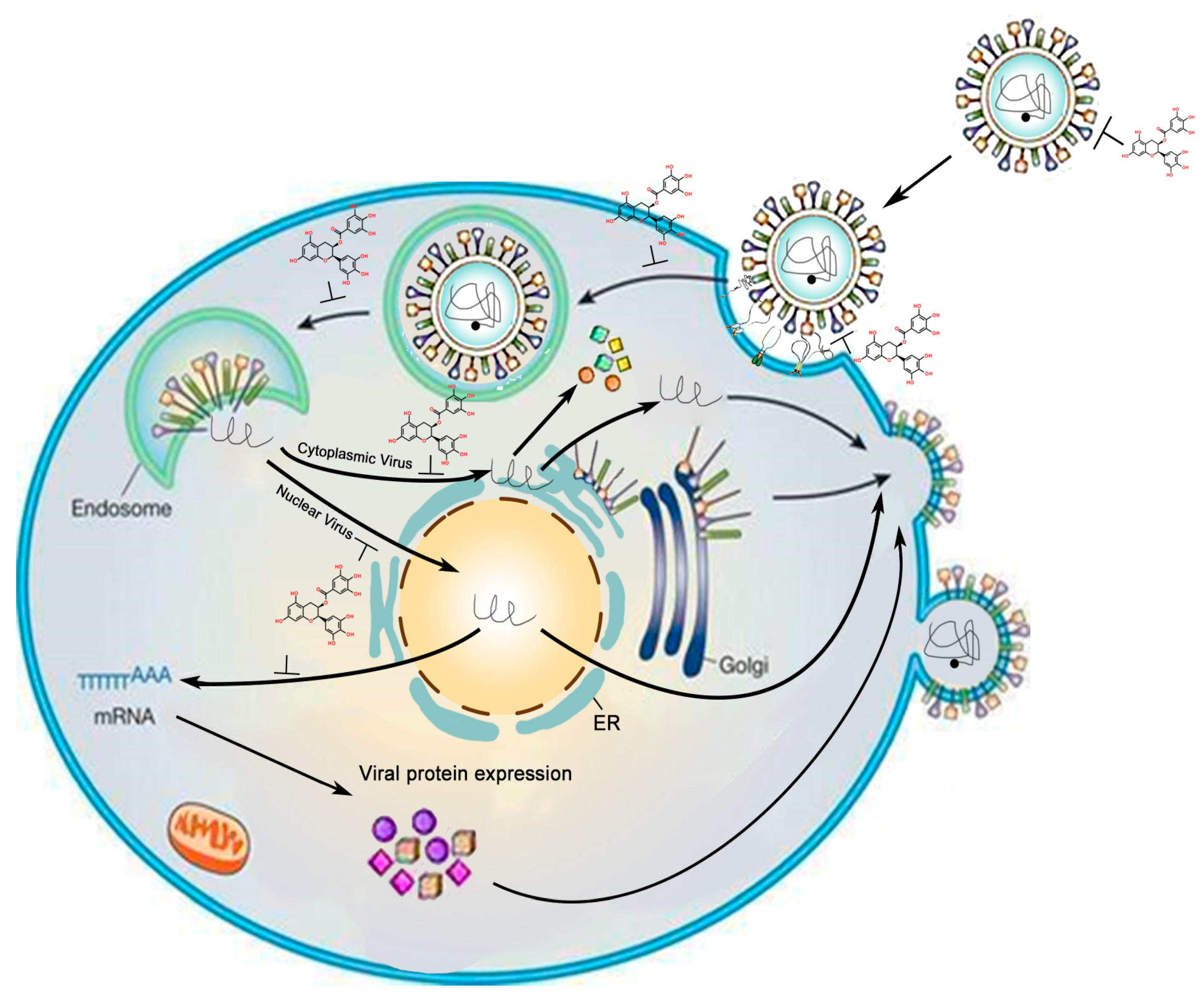
| Virus | Family | Genome | Effect | Effective Dosage or Concentration, Year (Reference) |
|---|---|---|---|---|
| HBV | Hepadnaviridae | Partially double-stranded and circular DNA | Inhibition of HBV RNA, DNA, and cccDNA synthesis and antigen expression EGCG targets replicative intermediates of DNA synthesis Interference with transcription of the HBV core promoter Inhibition of different genotypes of HBV entry into cells Reduction of HBV replication by opposing HBV-induced incomplete autophagy | EC50 (GTE, HBsAg) = 5.02 mg/mL, on, EC50 (GTE, HBeAg) = 5.681 mg/mL, EC50 (GTE, HBV DNA) = 19.81 mg/mL, 2008 [9]; EGCG (25, 50, 100 μM), 2011 [20] EGCG (25, 50, 100 μM), 2016 [21] EGCG (10, 20, 50 μM), 2014 [22] EGCG (25, 50 μM), 2015 [23] |
| HSV | Herpesviridae | Double-stranded linear DNA | Strong anti-HSV activity Inactivating clinical isolates of HSV by destruction of the virion structure Improved anti-HSV effectiveness of modified EGCG Inhibition of HSV-1 attachment by interacting with the virion surface | EC50 (EGCG,EGC,EC) HSV-1 = 2.5 µM, EC50 (ECG) HSV-1 = 4 µM, EC50 (EC) HSV-2 = 35 µM, EC50 (ECG) HSV-2 = 63 µM, 2005 [11] IC99 (EGCG) HSV-1 = 16–49 µM, IC99 (EGCG) HSV-2 = 12.5 µM, IC99 (EGCG) Lab strain HSV-1 = 72.3 µM, 2008 [24]; Digallate dimers of EGCG (100 μM), 2011 [25]; EGCG-stearate in 100% glycerin USP, 2012 [26], palmitoyl-EGCG, 2013 [27] EGCG (0.01–200 µM), 2014 [28] |
| EBV | Herpesviridae | Double-stranded linear DNA | Reduction of EBV lytic protein expression Interference with transduction of the AP-1 signal pathway Decreasing binding activity of DNA and nuclear antigen 1 and blocking EBV lysis by down regulating RNA synthesis of viral immediate-early genes Blocking EBV spontaneous lytic infection by interfering with the MEK/ERK1/2 and PI3-K/Akt pathways | EGCG > 50 µM, 2003 [29] EGCG (25, 50, 100, 200 µM), 2004 [29] EGCG (10, 30, 50 µM), 2012 [30] IC50 (EGCG) = 20 µM, 2013 [31] |
| Adenovirus | Adenoviridae | Double-stranded linear DNA | Inhibition of viral titers of adenovirus and inactivation of purified adenovirions and adenain Inhibition of viral attachment by interacting with virion surface proteins | IC50 (EGCG,Effect on infectious virus production) = 25 µM, IC50 (EGCG,Inactivation of adenovirus) = 250 µM, IC50 (EGCG, Effect on adenain) = 109 µM, 2003 [32] EGCG (0.01–200 µM), 2014 [28] |
| HIV | Retroviridae | +ssRNA | Inhibition of HIV RT Inhibition of viral entry into target cells by interfering with the interaction of receptors with the HIV envelope Inhibition of p24 antigen production Attenuation of neuronal damage mediated by HIV infection Counteraction of semen-mediated enhancement of HIV infection | EC50 (EGC, EGCG) = 21.8–65.3 μM (0.01–0.02 mg/mL), 1990[33]; EC50 (EGC) = 25.5 μM (7.8 mg/L), EC50 (ECG) = 0.72 μM (0.32 mg/L), EC50 (EGCG) = 1.48 μM (0.68 mg/L), 1994 [34]; EC50 (EGCG) = 1.6~2.0 μM, 2011 [35] EGCG (10~100 μM, 2002 [36]; IC50 (EGCG) = 3.44, IC50 (GCG) = 2.45, 2005 [37]; IC50 (EGCG) ≈ 100 μM, 2006 [38]; EGCG (0.2 μM), 2006 [39]; IC50 ≈ 4.5 μM, 2009 [40] EGCG (20 μM in vitro; 50 mg/kg, mouse model), 2006 [41]; EGCG (300 mg/kg/day, mouse model), 2009 [42] EGCG (1~20 mM), 2009 [43]; EGCG (0.4 mM), 2012 [44] |
| HCV | Flaviviridae | +ssRNA | Inhibition of the HCV entry pathway, prevention of cell-to-cell transmission Supression of HCV RNA replication steps Impairment of viral attachment by altering viral particle structure Interference with HCV replication by down regulating a COX-2 inhibitor Targeting the HCV virion to prevent attachment to heparan sulfate) | IC50 (EGCG, Cell-culture–derived HCV entry) = 2.5 μg/mL, IC50 (EGCG, binding of HCV to cells, with or without) = 9.7 μg/mL or 17.2 μg/mL, 2011 [45]; EGCG (0.625~10 μM), 2012 [46]; EC50 (EGCG) = 17.9 μM, 2012 [47]; IC50 (EGCG) = 10.6 ± 2.9 μM, IC50 (delphinidin) = 3.7 ± 0.8 μM, 2015 [48] EC isomers (25, 50, 75 μM), 2013 [49] EGCG (0.01–200 µM), 2014 [28] |
| Influenza virus | Orthomyxoviridae | −ssRNA | Antiviral activity of tea extracts against influenza virus Infectivity reduction of IAV and IBV by preventing viral absorption to cell surface and inhibiting acidification of endosomes and lysosomes Activity reduction of viral neuraminidase and RNA synthesis of viral genome Inhibitory effects of influenza virus by EGCG analogs, derivatives and compounds Inhibitory effects of different nutrient mixtures of natural EGCG Clinical trials of EGCG as an influenza virus restriction factor | 1949 [50] EGCG (1–16 μM), 1993 [51]; GTE (1:20, 1:40, 1:80 dilutions), EGC (400 µg/mL), 2002 [52] EC50 (EGCG) = 22–28 μM, EC50 (ECG) = 22–40 μM, EC50 (EGC) = 309–318 μM, 2005 [53]; IC50 (GTC tested, IAV) = 16.2–56.5 µg/mL, IC50 (GTC tested, IBV) = 9.0–49.7 µg/mL, 2014 [54] QR-435, 2007 [55,56]; Fatty acid (3-O-acylcatechins), 2008 [57] 2007 [58], 2008 [58] Gargling with tea catechin extracts solution (200 µg/mL catechins, ECGC composes 60% of catechins), 2006 [59], GTC (378 mg/day), 2011 [60] |
| DENV, JEV,TBEV ZIKV | Flaviviridae | +ssRNA | Docking into the binding pocket of E protein Destruction of the virus particle by interacting with the lipid envelope | 2016 [14] EC50 (EGCG) = 21.4 µM, 2016 [16] |
| CHIKV | Togaviridae | +ssRNA | Blocking CHIKV entry into target cells | IC50 (EGCG) = 14.3 µM (6.54 µg/mL), 2015 [15] |
| HTLV-1 | Retroviridae | +ssRNA | Suppressing HTLV-I pX and Tax gene expression | EGCG or GTP (6.5–60 µM, 3–27 µg/mL), 2000 [61]; EGCG (25, 50, 75 µM) effective in C91-PL cells, EGCG (125, 225, 325 µM) effective in HuT-102 cells, 2014 [62] |
| Rotavirus | Reoviridae | dsRNA | Interference with virus adsorption | 1991 [63] |
| Enterovirus EV71 | Picornaviridae | +ssRNA | Interference with virus adsorption Inhibition of production of progeny virus by reducing ROS generation | 1991 [63] EGCG (25 μM), 2009 [64] |
| EBOV | Filoviridae | −ssRNA | As an inhibitor of HSPS5, EGCG reduced the production of new viruses via its action on HSPS5 | EGCG (10–100 μM), [65] |
| PRRSV | Arteriviridae | +ssRNA | Inhibition of viral adsorption and cell intrusion of PRRSV by EGCG palmitate | 10TCID50: EC50 (EGCG,pretreated) = 8.53 µM, EC50 (EGCGpalmitate, pretreated) = 0.58 µM, EC50 (EGCG,post-treated) = 9.18 µM, EC50 (EGCGpalmitate, post-treated) = 0.68 µM, 2014 [13] |
| VHSV, IHNV, SVCV | Rhabdoviridae | −ssRNA | Reduction of rhabdovirus infections by inhibitingSERPINe1 activity | EGCG (10, 100 µM), 2015 [66] |
| GCRV | Reoviridae | dsRNA | Interference with interaction of GCRV particles with laminin receptor | IC80 (EGCG) = 21.8 µM (10 µg/mL), 2016 [67] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. https://doi.org/10.3390/molecules22081337
Xu J, Xu Z, Zheng W. A Review of the Antiviral Role of Green Tea Catechins. Molecules. 2017; 22(8):1337. https://doi.org/10.3390/molecules22081337
Chicago/Turabian StyleXu, Jun, Zhao Xu, and Wenming Zheng. 2017. "A Review of the Antiviral Role of Green Tea Catechins" Molecules 22, no. 8: 1337. https://doi.org/10.3390/molecules22081337
APA StyleXu, J., Xu, Z., & Zheng, W. (2017). A Review of the Antiviral Role of Green Tea Catechins. Molecules, 22(8), 1337. https://doi.org/10.3390/molecules22081337





