Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis
Abstract
1. Introduction
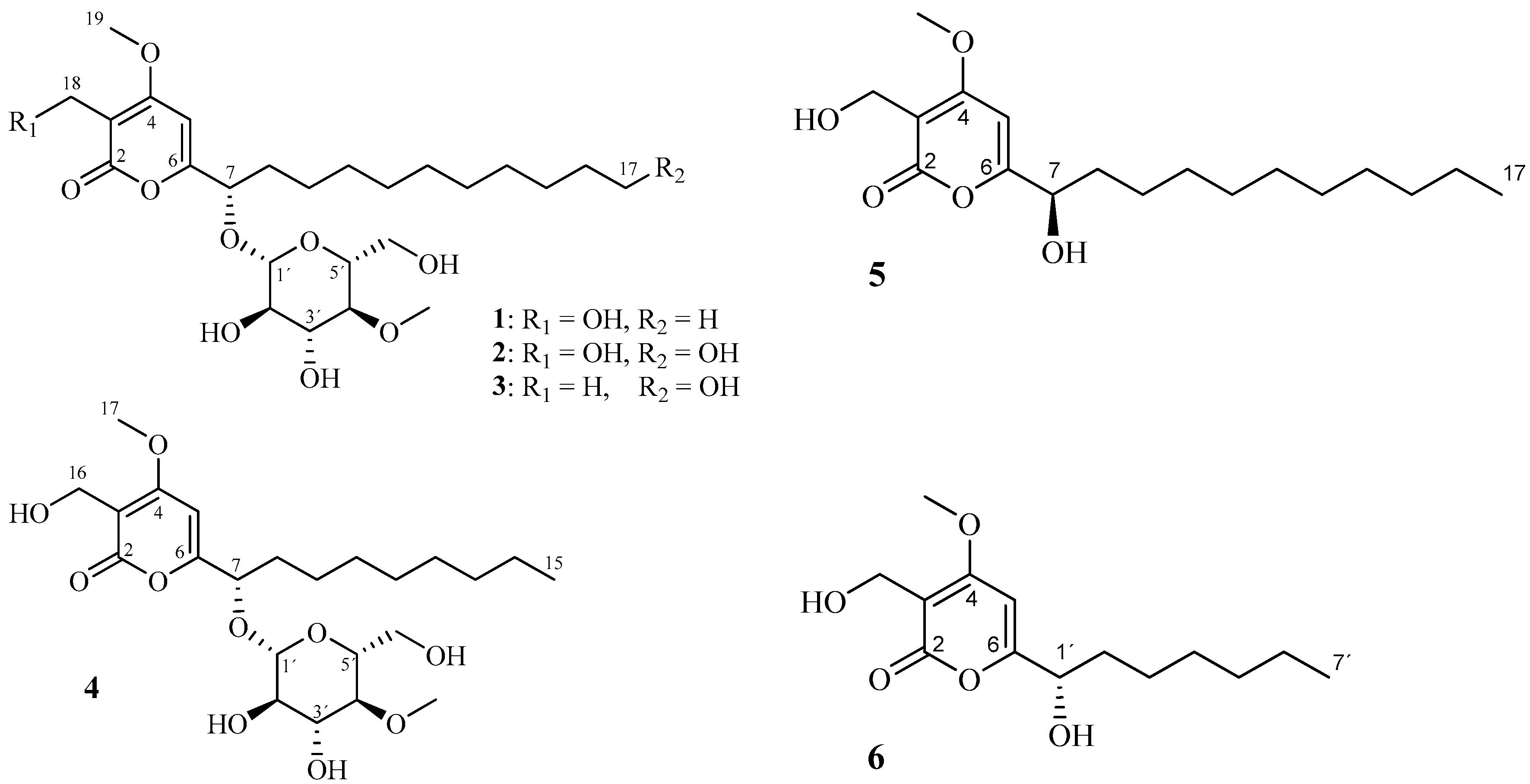
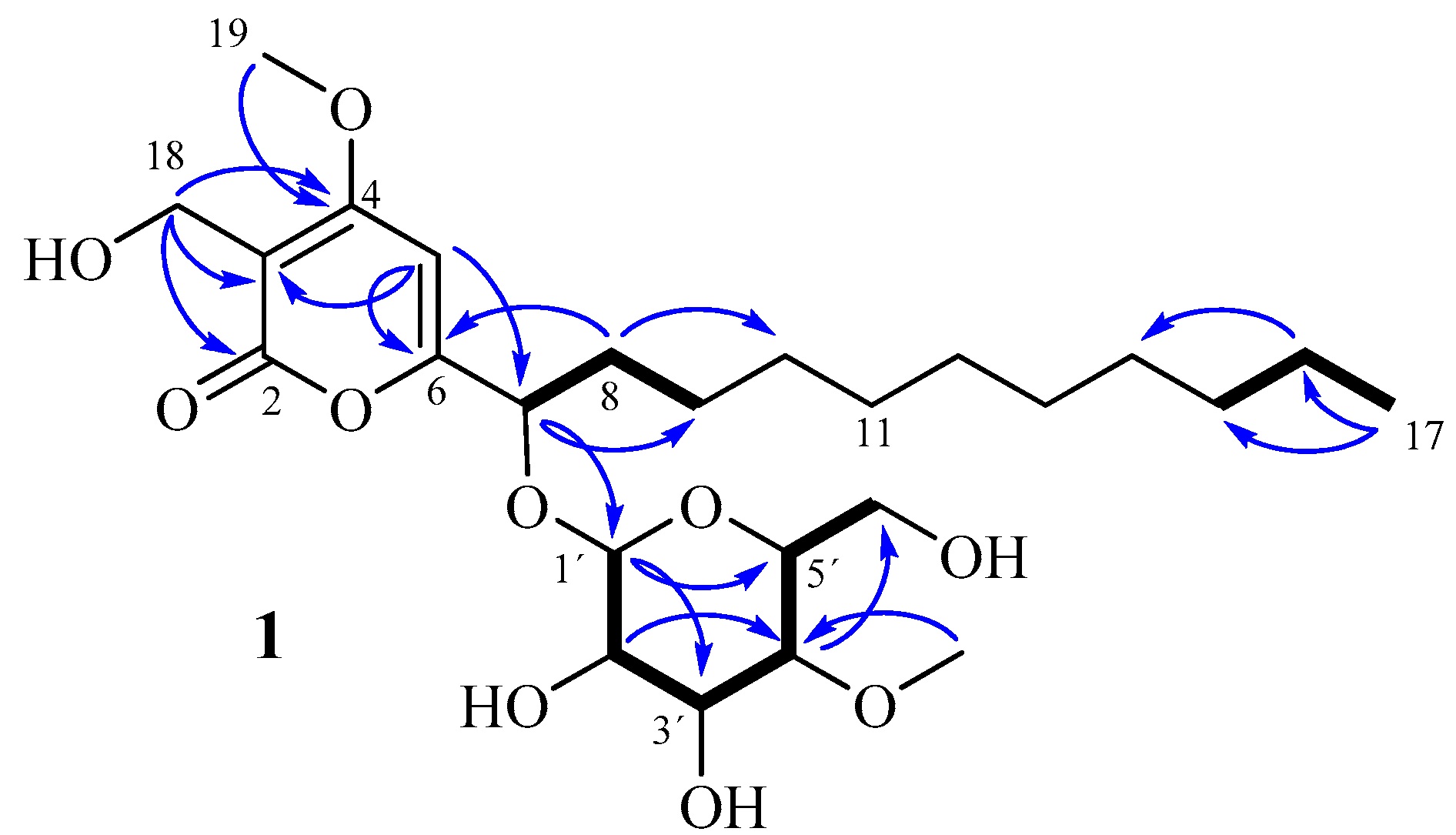
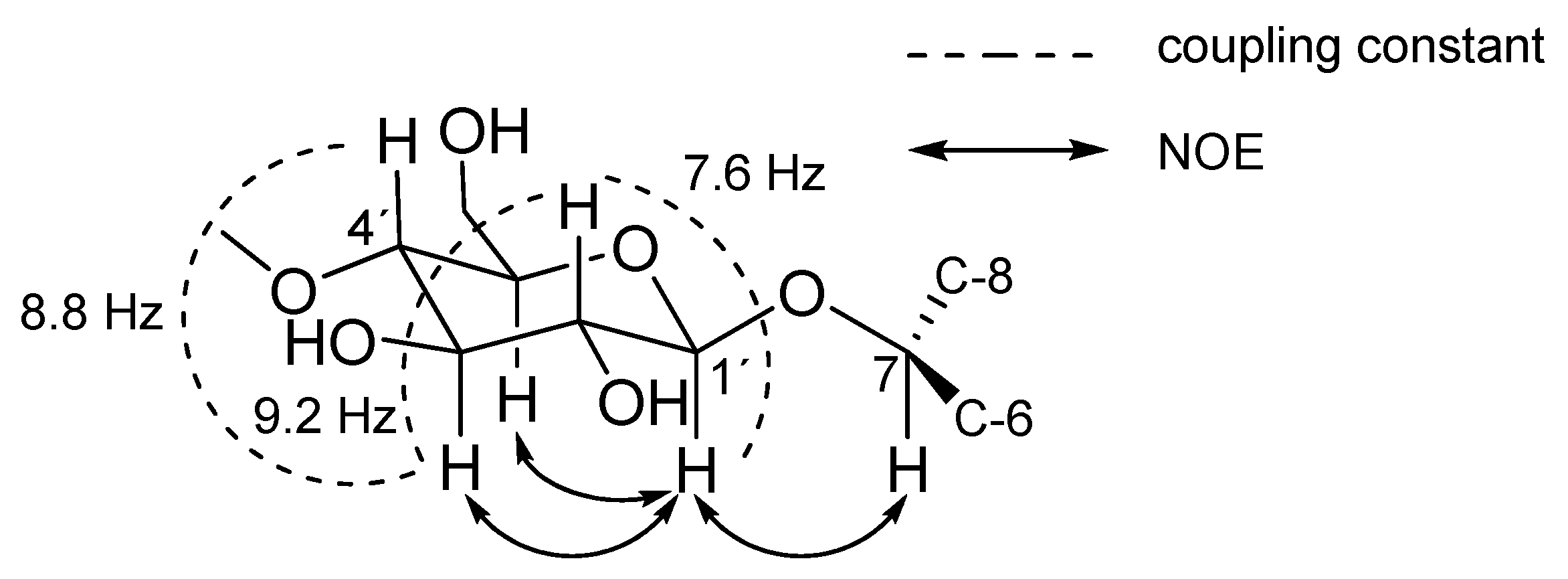
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation of Compounds 1–4
3.5. Hydrolysis of Akanthopyrone A (1)
3.6. Biological Activities
3.6.1. Antimicrobial Activity Assay
3.6.2. Cytotoxicity Activity Assay
3.6.3. Anti-biofilm Activity Assay
3.6.4. Nematicidal Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karwehl, S.; Stadler, M. Exploitation of Fungal Biodiversity for Discovery of Novel Antibiotics. Curr. Top. Microbiol. Immunol. 2016, 398, 303–338. [Google Scholar] [PubMed]
- Molnár, I.; Gibson, D.M.; Krasnoff, S.B. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat. Prod. Rep. 2010, 27, 1241–1275. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Kittakoop, P.; Kirtikara, K.; Hywel-Jones, N.L.; Thebtaranonth, Y. Bioactive substances from insect pathogenic fungi. Acc. Chem. Res. 2005, 38, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Humber, R.A. Evolution of entomopathogenicity in fungi. J. Invertebr. Pathol. 2008, 98, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Mains, E.B. Entomogenous Species of Akanthomyces, Hymenostilbe and Insecticola in North America. Mycol. Soc. Am. 1950, 42, 566–589. [Google Scholar] [CrossRef]
- Hsieh, L.S.; Tzean, S.S.; Wu, W.J. The genus Akanthomyces on spiders from Taiwan. Mycol. Soc. Am. 1997, 89, 319–324. [Google Scholar] [CrossRef]
- Hywel-Jones, N. Akanthomyces on spiders in Thailand. Mycol. Res. 1996, 100, 1065–1070. [Google Scholar] [CrossRef]
- Samson, R.A.; Brady, B.L. Akanthomyces novoguineensis sp. nov. Trans. Br. Mycol. Soc. 1982, 79, 571–572. [Google Scholar] [CrossRef]
- Samson, R.A.; Evans, H.C. Notes on entomogenous fungi from Ghana. II. The genus Akanthomyces. Plant Biol. 1974, 23, 28–35. [Google Scholar] [CrossRef]
- Kinoshita, H.; Wongsuntornpoj, S.; Ihara, F.; Nihira, T. Anti-Rhodotorula activity of mycophenolic acid enhanced in the presence of polyene-antibiotic nystatin. Lett. Appl. Microbiol. 2016, 64, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kuephadungphan, W.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V. Antimicrobial activity of invertebrate-pathogenic fungi in the genera Akanthomyces and Gibellula. Mycoscience 2013, 55, 127–133. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nakajima, I.; Ihara, F.; Kinoshita, H.; Nihira, T. Cultivation of entomopathogenic fungi for the search of antibacterial compounds. Mycopathologia 2005, 160, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, M.M.; Gibson, D.M.; Clardy, J. Akanthomycin, a new antibiotic pyridone from the entomopathogenic fungus Akanthomyces gracilis. Org. Lett. 2002, 4, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Yamamoto, T.; Oshima, Y. Aromatic polyketide production in Cordyceps indigotica, an entomopathogenic fungus, induced by exposure to a histone deacetylase inhibitor. Org. Lett. 2012, 14, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Takahashi, N.; Oshima, Y. Novel aromatics bearing 4-O-methylglucose unit isolated from the oriental crude drug Bombyx Batryticatus. Tetrahedron Lett. 2004, 45, 367–370. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Supothina, S.; Komwijit, S.; Luangsa-Ard, J.J. Bioactive compounds from the scale insect pathogenic fungus Conoideocrella tenuis BCC 18627. J. Nat. Prod. 2011, 74, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Kornsakulkarn, J.; Thongpanchang, C.; Lapanun, S.; Srichomthong, K. Isocoumarin glucosides from the scale insect fungus Torrubiella tenuis BCC 12732. J. Nat. Prod. 2009, 72, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Kornsakulkarn, J.; Saepua, S.; Srichomthong, K.; Supothina, S.; Thongpanchang, C. New mycotoxins from the scale insect fungus Aschersonia coffeae Henn. BCC 28712. Tetrahedron 2012, 68, 8480–8486. [Google Scholar] [CrossRef]
- Saepua, S.; Kornsakulkarn, J.; Choowong, W.; Supothina, S.; Thongpanchang, C. Bioxanthracenes and monomeric analogues from insect pathogenic fungus Conoideocrella luteorostrata Zimm. BCC 31648. Tetrahedron 2015, 71, 2400–2408. [Google Scholar] [CrossRef]
- Seephonkai, P.; Isaka, M.; Kittakoop, P.; Boonudomlap, U.; Thebtaranonth, Y. A novel ascochlorin glycoside from the insect pathogenic fungus Verticillium hemipterigenum BCC 2370. J. Antibiot. 2004, 57, 10–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Helaly, S.E.; Kuephadungphan, W.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V.; Stadler, M. Five unprecedented secondary metabolites from the spider parasitic fungus Akanthomyces novoguineensis. Molecules 2017, 22, 991. [Google Scholar] [CrossRef] [PubMed]
- Giner, J.L.; Feng, J.; Kiemle, D.J. NMR tube degradation method for sugar analysis of glycosides. J. Nat. Prod. 2016, 79, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Smith, F. The constitution of mesquite gum. Part III. The structure of the monomethyl glucuronic acid component. J. Chem. Soc. 1951, 2646–2652. [Google Scholar] [CrossRef]
- Pažoutová, S.; Follert, S.; Bitzer, J.; Keck, M.; Surup, F.; Šrůtka, P.; Holuša, J.; Stadler, M. A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013, 60, 107–123. [Google Scholar] [CrossRef]
- Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Metabolites from the endophytic mitosporic Dothideomycete sp. LRUB20. Phytochemistry 2009, 70, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Tomita, Y.; Tori, K.; Yoshimura, Y. Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1978, 100, 3331–3339. [Google Scholar] [CrossRef]
- McGlacken, G.P.; Fairlamb, I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Nat. Prod. Rep. 2005, 22, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, I.J.S.; Marrison, L.R.; Dickinson, J.M.; Lu, F.J.; Schmidt, J.P. 2-Pyrones possessing antimicrobial and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 4285–4299. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.; Schneider, K.; Nachtigall, J.; Vikineswary, S.; Tan, G.Y.A.; Zinecker, H.; Imhoff, J.F.; Süssmuth, R.D.; Fiedler, H.-P. Gombapyrones, new α-pyrone metabolites produced by Streptomyces griseoruber Acta 3662. J. Antibiot. 2009, 62, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.; Su, Y.-S.; Cheng, M.-J.; Liu, T.-W.; Chen, I.-S.; Wu, M.-D.; Chang, H.-S.; Yuan, G.-F. Chemical constituents from a soil-derived Actinomycete, Actinomadura miaoliensis BCRC 16873, and their inhibitory activities on lipopolysaccharide-induced tumor necrosis factor production. Chem. Biodivers. 2013, 10, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Noumeur, S.R.; Helaly, S.E.; Jansen, R.; Gereke, M.; Stradal, T.E.B.; Harzallah, D.; Stadler, M. Preussilides A–F, bicyclic polyketides from the endophytic fungus Preussia similis with antiproliferative activity. J. Nat. Prod. 2017, 80, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Luangsa-ard, J.J.; Mongkolsamrit, S.; Thanakitpipattana, D.; Khonsanit, A.; Tasanathai, K.; Noisripoom, W.; Humber, R.A. Clavicipitaceous entomopathogens: New species in Metarhizium and a new genus Nigelia. Mycol. Prog. 2017, 16, 369–391. [Google Scholar] [CrossRef]
- Phainuphong, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Bowornwiriyapan, K.; Muanprasat, C.; Srimaroeng, C.; Duangjai, A.; Sakayaroj, J. Lovastatin analogues from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. J. Nat. Prod. 2016, 79, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Helaly, S.E.; Thongbai, B.; Hyde, K.D.; Stadler, M. Pyristriatins A and B: Pyridino-cyathane antibiotics from the Basidiomycete Cyathus cf. striatus. J. Nat. Prod. 2016, 79, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Rahme, L.G.; Stevens, E.J.; Wolfort, S.F.; Shao, J.; Tompkins, R.G.; Ausubel, F.M. Common virulence factors for bacterial pathogenicity in plants and animals. Science 1995, 268, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Anke, H.; Arendholz, W.-R.; Hansske, F.; Anders, U.; Sterner, O.; Bergquist, K.-E. Lachnumon and lachnumol A, new metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. J. Antibiot. 1993, 46, 961–967. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |
| Pos | 1 a | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | |
| 2 | - | 165.1, C | - | 167.1, C | - | 167.9, C | - | 167.8, C |
| 3 | - | 104.4, C | - | 105.1, C | - | 102.1, C | - | 105.1, C |
| 4 | - | 167.4, C | - | 170.8, C | - | 169.0, C | - | 170.8, C |
| 5 | 6.76, s | 94.9, CH | 7.06, s | 96.6, CH | 7.00, s | 96.8, CH | 7.06, s | 96.6, CH |
| 6 | - | 165.3, C | - | 167.8, C | - | 164.9, C | - | 167.0, C |
| 7 | 4.51, b dd (7.3, 4.7) | 76.4, CH | 4.67, dd (7.3, 4.6) | 77.1, CH | 4.65, dd (7.5, 4.9) | 77.0, CH | 4.66, dd (7.3, 4.7) | 77.1, CH |
| 8 | 1.74, m; 1.84, m | 34.1, CH2 | 1.76, m; 1.86, m | 35.4, CH2 | 1.76, m; 1.85, m | 35.4, CH2 | 1.76, m; 1.86, m | 35.4, CH2 |
| 9 | 1.38, m | 24.9, CH2 | 1.44, m | 25.9, CH2 | 1.43, m | 25.9, CH2 | 1.43, m | 25.9, CH2 |
| 10 | 1.29, b m | 29.2, CH2 | 1.35, b m | 30.6, CH2 | 1.35, b m | 30.5, CH2 | 1.35, b m | 30.5, CH2 |
| 11 | 1.27, b m | 29.3,c CH2 | 1.32, b m | 30.7, c CH2 | 1.32, b m | 30.6,c CH2 | 1.32, b m | 30.6, c CH2 |
| 12 | 1.25, b m | 29.4,c CH2 | 1.30, b m | 30.9, c CH2 | 1.30, b m | 30.7,c CH2 | 1.30, b m | 30.7, c CH2 |
| 13 | 1.25, b m | 29.5,c CH2 | 1.30, b m | 30.8, c CH2 | 1.30, b m | 30.9,c CH2 | 1.30, b m | 33.2, CH2 |
| 14 | 1.25, b m | 29.6,c CH2 | 1.30, b m | 30.7, c CH2 | 1.30, b m | 30.8,c CH2 | 1.30, b m | 23.9, CH2 |
| 15 | 1.25, b m | 31.8, CH2 | 1.34, b m | 27.1, CH2 | 1.35, b m | 27.1, CH2 | 0.90, t (7.1) | 14.6, CH3 |
| 16 | 1.29, b m | 22.6, CH2 | 1.52, m | 33.8, CH2 | 1.53, m | 33.8, CH2 | 4.44, s | 54.2, CH2 |
| 17 | 0.87, t (6.9) | 14.1, CH3 | 3.54, t (6.7) | 63.2, CH2 | 3.54, t (6.8) | 63.2, CH2 | 3.99, s | 58.0, CH3 |
| 18 | 4.51, b s | 54.3, CH2 | 4.44, s | 54.2, CH2 | 1.87, s | 8.6, CH3 | - | - |
| 19 | 3.93, s | 56.9, CH3 | 3.99, s | 58.1, CH3 | 3.96, s | 57.7, CH3 | - | - |
| β-4-O-methyl-d-glucopyranose | ||||||||
| 1′ | 4.29, d (7.6) | 100.6, CH | 4.26, d (7.9) | 102.7, CH | 4.25, d (7.9) | 102.6, CH | 4.25, d (7.9) | 102.7, CH |
| 2′ | 3.43, dd (7.6, 9.2) | 73.6, CH | 3.29, dd (7.9, 9.2) | 75.3, CH | 3.28, dd (7.9, 9.2) | 75.4, CH | 3.28, dd (7.9, 9.2) | 75.4, CH |
| 3′ | 3.54, dd (9.2, 8.8) | 76.6, CH | 3.43, dd (9.2, 8.8) | 78.3, CH | 3.43, dd (9.1, 9.1) | 78.3, CH | 3.43, dd (9.1, 9.1) | 78.4, CH |
| 4′ | 3.21, dd (8.8, 9.5) | 79.1, CH | 3.09, dd (9.2, 9.5) | 81.1, CH | 3.09, dd (9.1, 9.5) | 81.1, CH | 3.09, dd (9.1, 9.5) | 81.1, CH |
| 5′ | 3.26, m | 75.4, CH | 3.23, m | 77.4, CH | 3.22, m | 77.4, CH | 3.22, m | 77.4, CH |
| 6′ | 3.74, dd (12.2, 4.3) 3.89, dd (12.2, 2.4) | 61.8, CH2 | 3.68, dd (11.8, 5.3) 3.84, dd (11.8, 2.0) | 62.5, CH2 | 3.68, dd (11.8, 5.4) 3.83, dd (11.8, 2.0) | 62.5, CH2 | 3.68, dd (11.8, 5.4) 3.83, dd (11.8, 2.0) | 62.5, CH2 |
| OMe | 3.57, s | 60.8, CH3 | 3.56, s | 60.9, CH3 | 3.55, s | 60.9, CH3 | 3.55, s | 61.0, CH3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuephadungphan, W.; Helaly, S.E.; Daengrot, C.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V.; Stadler, M. Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis. Molecules 2017, 22, 1202. https://doi.org/10.3390/molecules22071202
Kuephadungphan W, Helaly SE, Daengrot C, Phongpaichit S, Luangsa-ard JJ, Rukachaisirikul V, Stadler M. Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis. Molecules. 2017; 22(7):1202. https://doi.org/10.3390/molecules22071202
Chicago/Turabian StyleKuephadungphan, Wilawan, Soleiman E. Helaly, Charuwan Daengrot, Souwalak Phongpaichit, Janet Jennifer Luangsa-ard, Vatcharin Rukachaisirikul, and Marc Stadler. 2017. "Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis" Molecules 22, no. 7: 1202. https://doi.org/10.3390/molecules22071202
APA StyleKuephadungphan, W., Helaly, S. E., Daengrot, C., Phongpaichit, S., Luangsa-ard, J. J., Rukachaisirikul, V., & Stadler, M. (2017). Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis. Molecules, 22(7), 1202. https://doi.org/10.3390/molecules22071202







