Evodiamine Exerts an Anti-Hepatocellular Carcinoma Activity through a WWOX-Dependent Pathway
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Cultures
2.2. MTT Assay
2.3. Small Interfering Ribonucleic Acid Transfection
2.4. Western Blots
2.5. Hepa1-6 Hepatoma-Bearing Animal Model
2.6. Statistical Analysis
3. Results
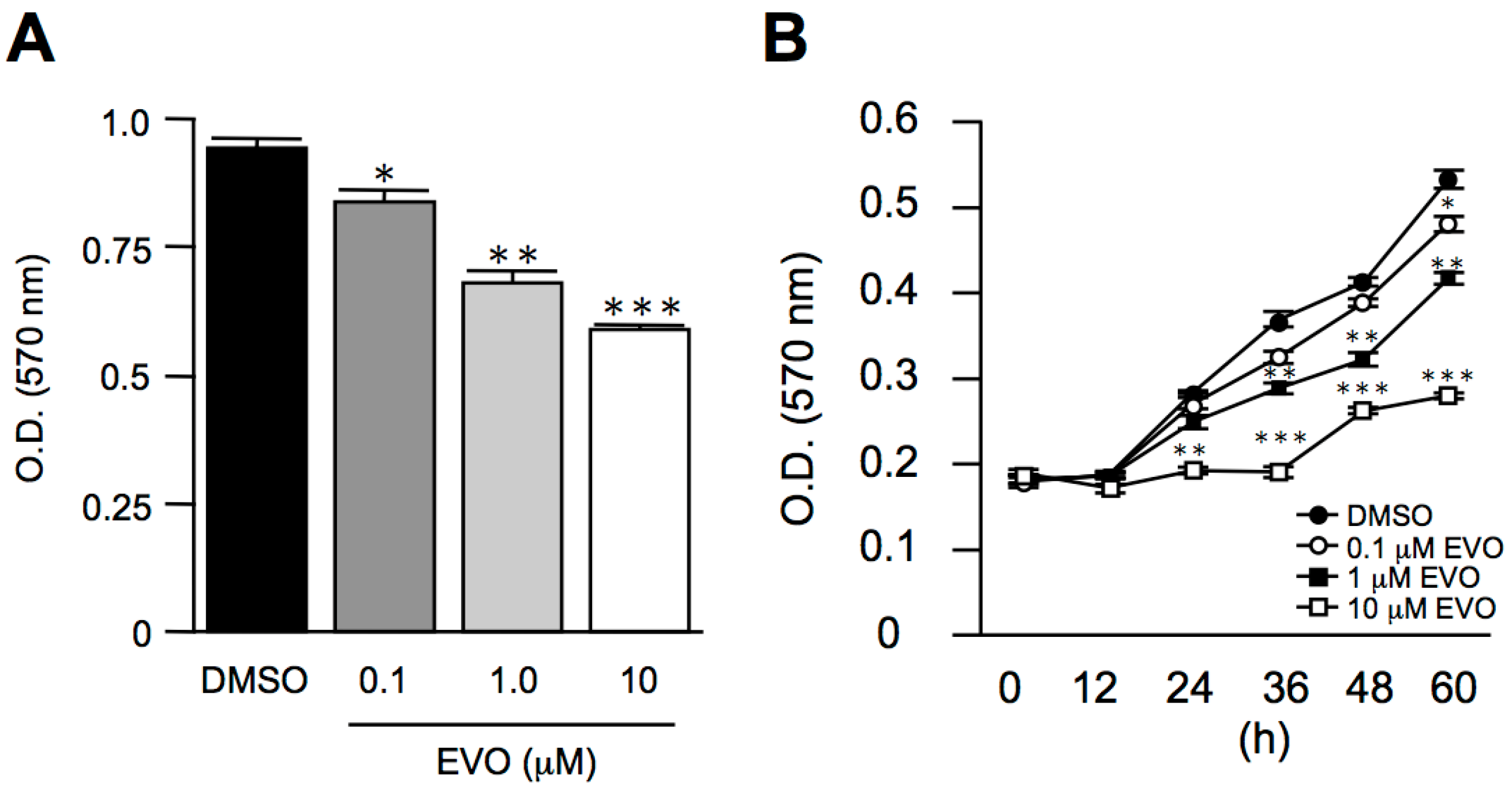
3.1. Evodiamine Dose- and Time-Dependently Inhibited Mus Musculus Hepatoma, Hepa1-6 Cell Proliferation
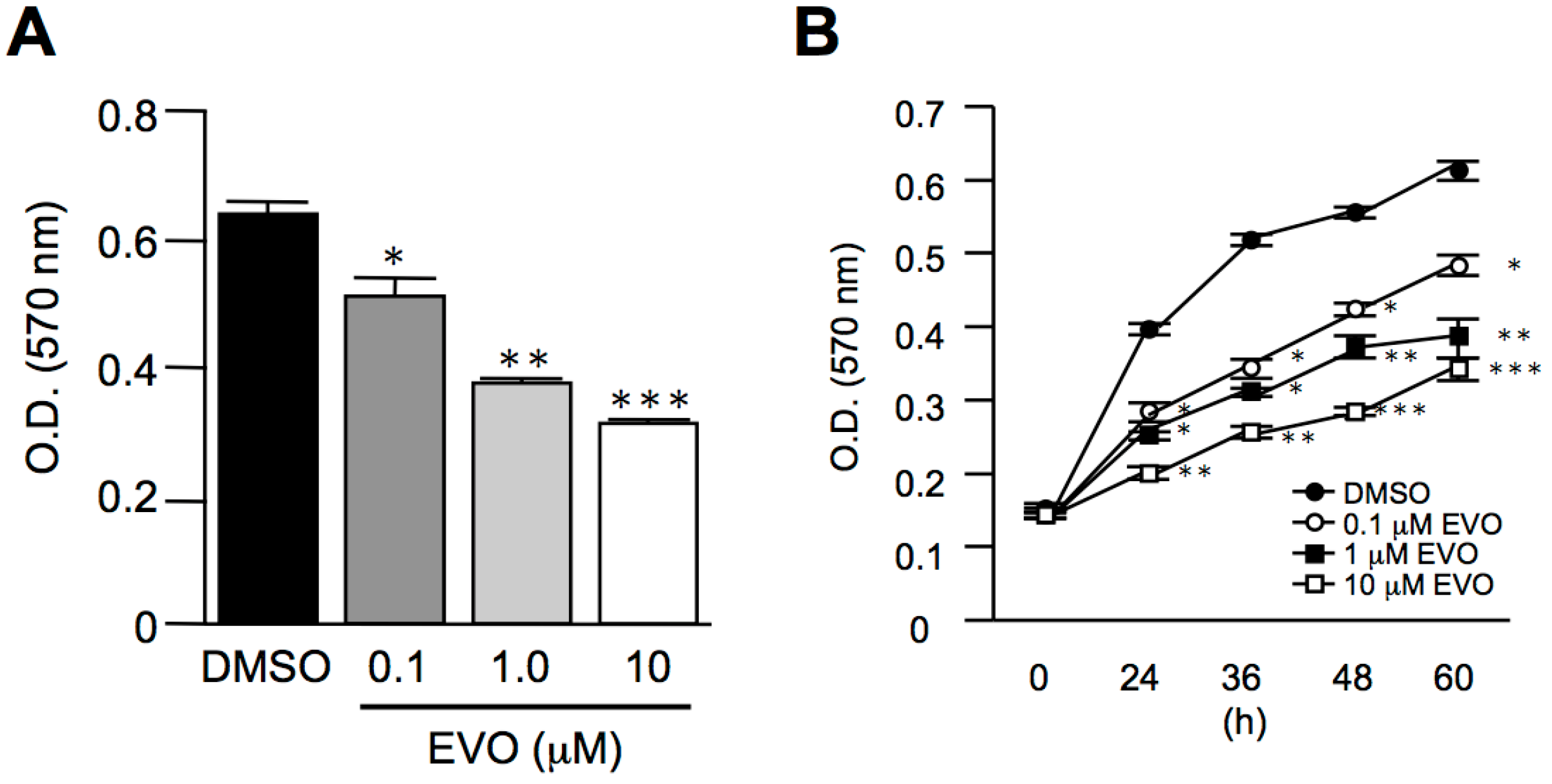
3.2. Evodiamine Dose- and Time-Dependently Inhibited Homo Sapiens Hepatocellular Carcinoma, HepG2 Cell Proliferation
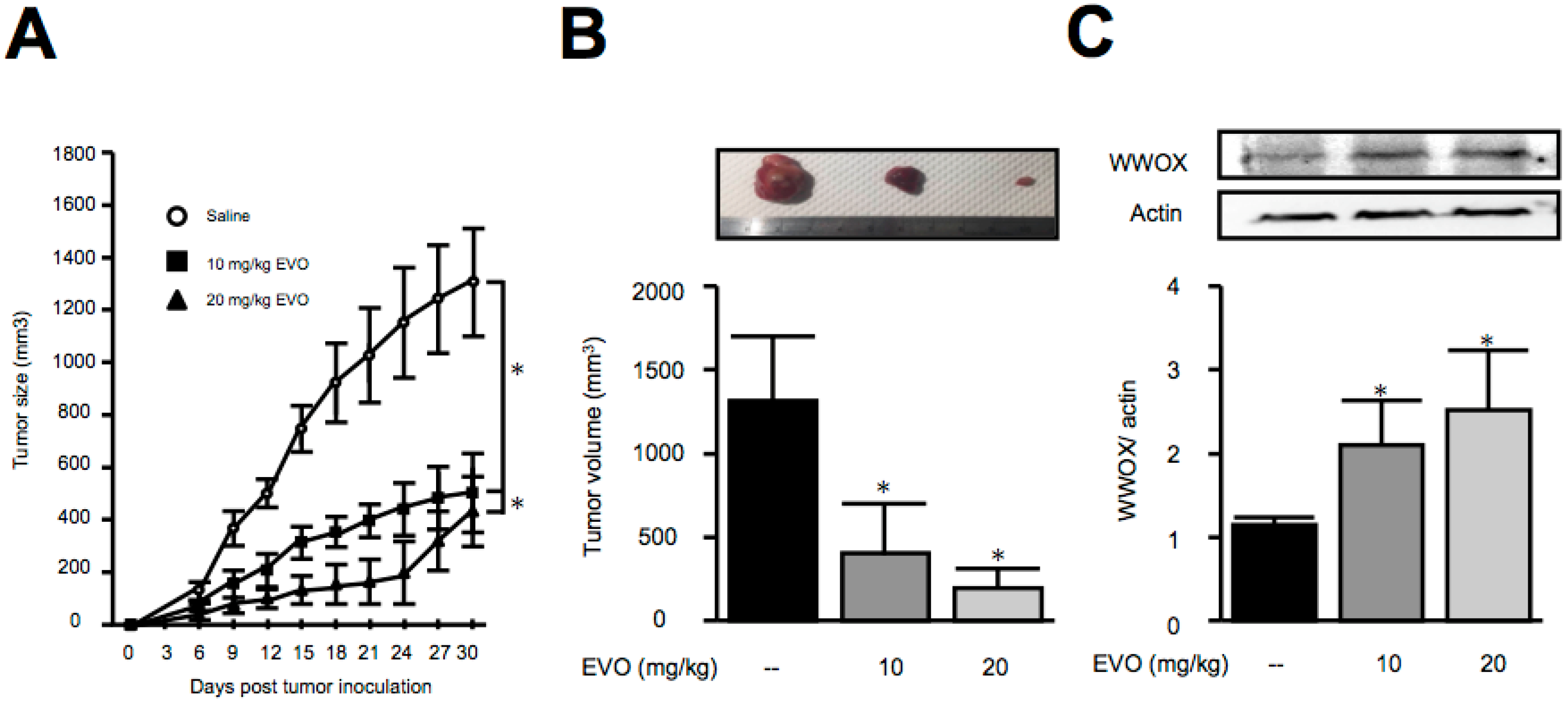
3.3. Treatment of Evodiamine Decreased Tumor Size in Hepa1-6 Hepatoma-Bearing Mice
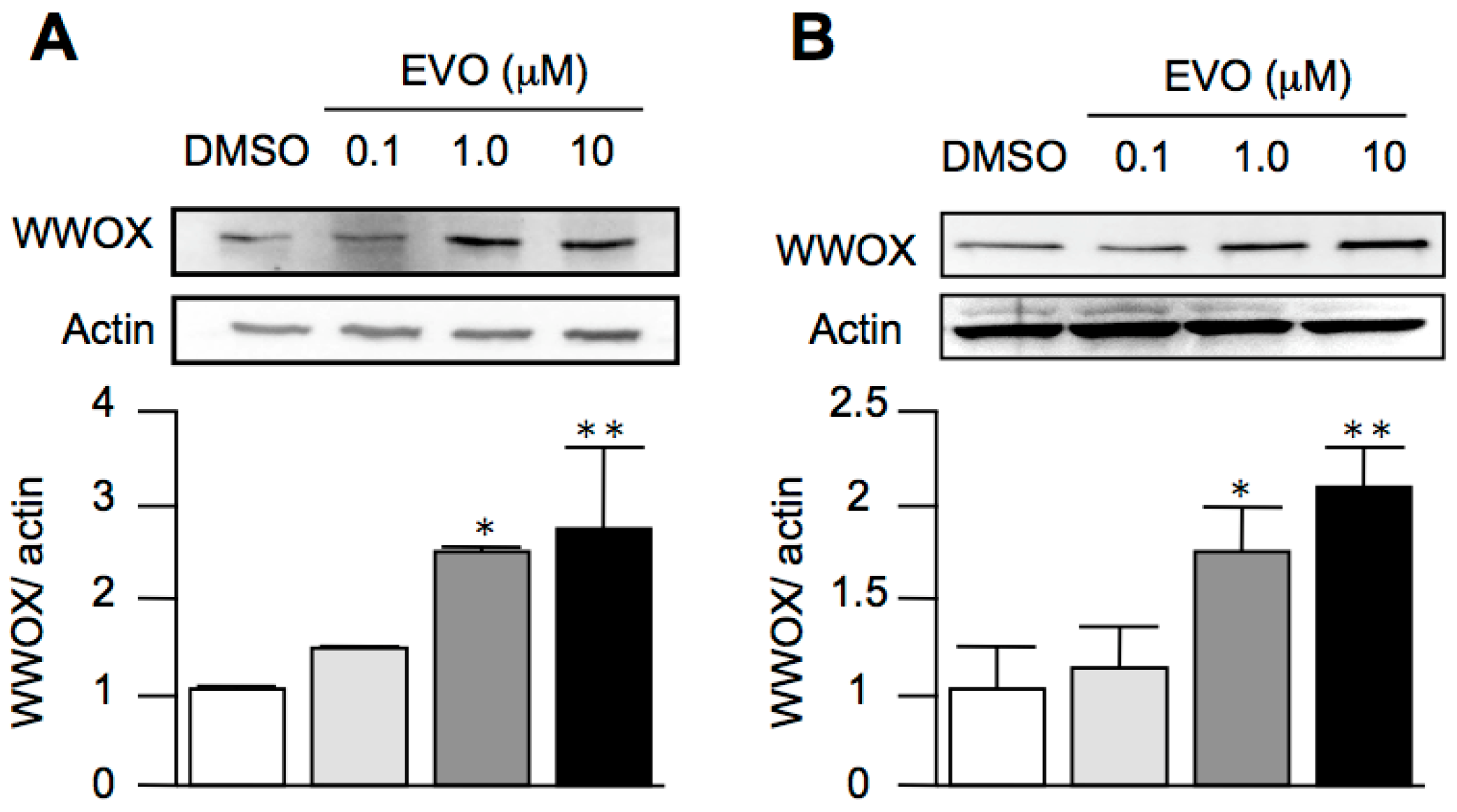
3.4. Evodiamine Dose-Dependently Increased the Expression of WWOX in Both HepG2 and Hepa1-6 Hepatoma Cells
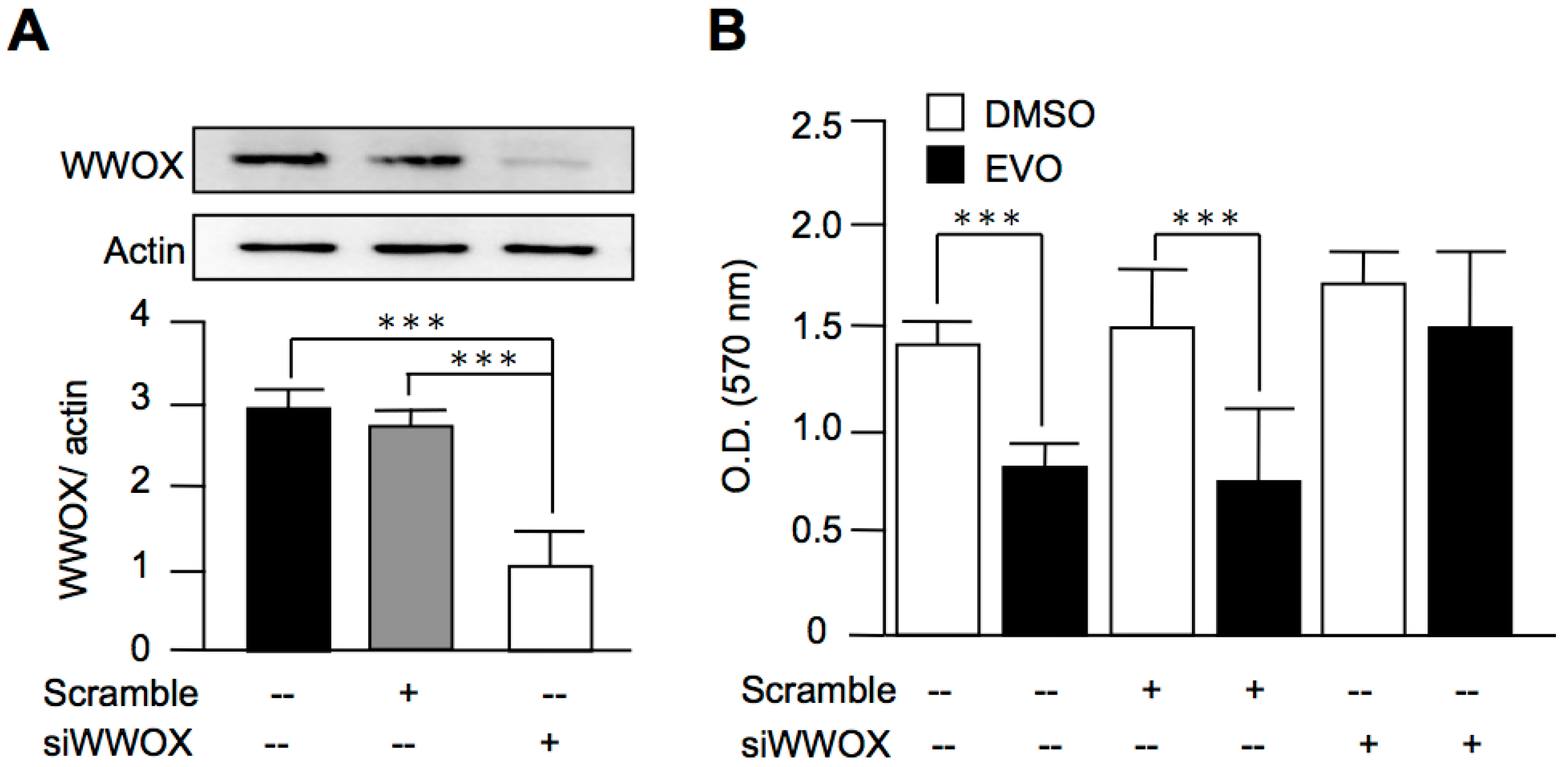
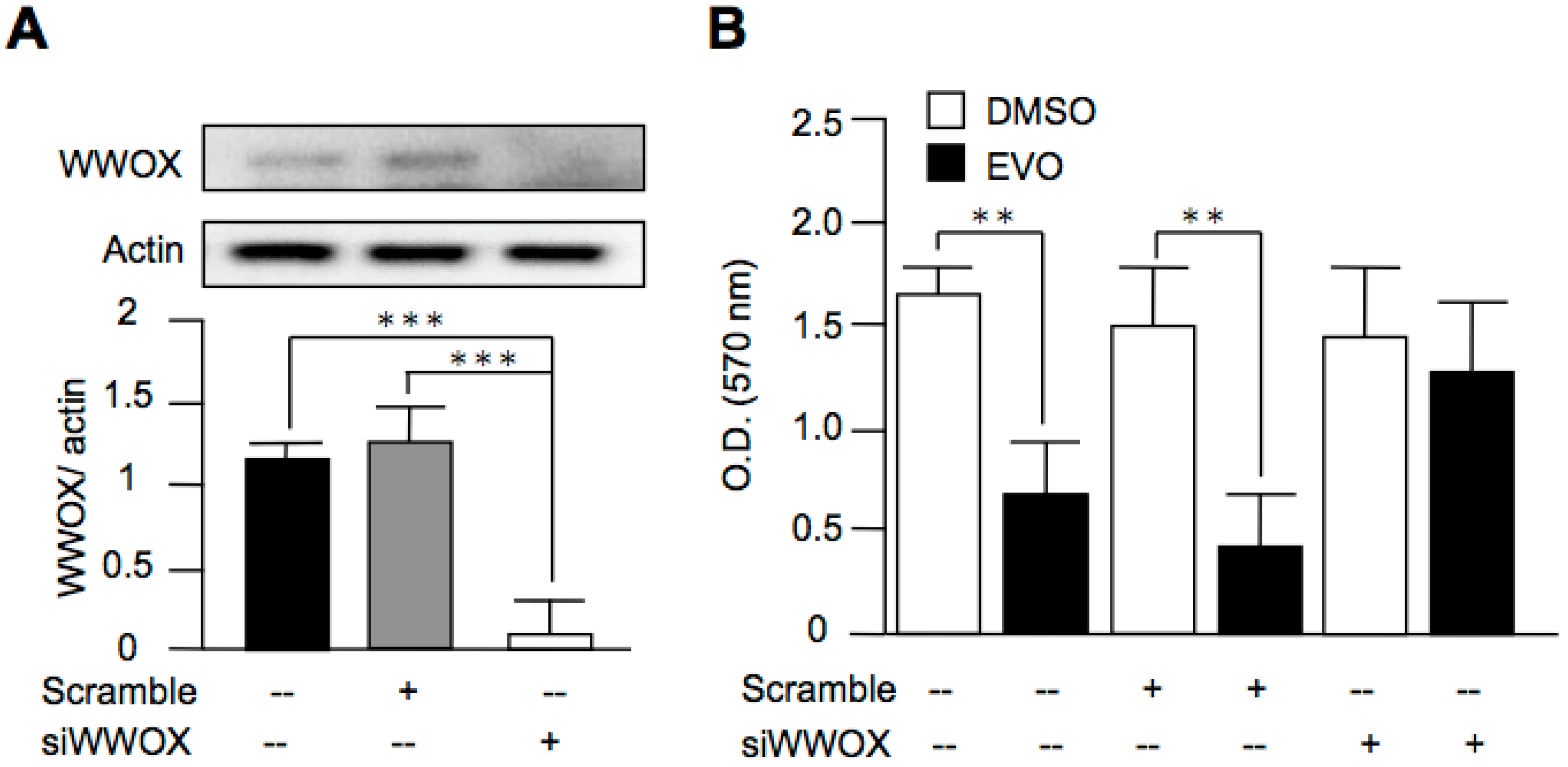
3.5. Evodiamine Exerted Anti-Cancer Activity through a WWOX-Dependent Pathway
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mittal, S.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma: Consider the population. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Schrock, M.S.; Huebner, K. WWOX: A fragile tumor suppressor. Exp. Biol. Med. 2015, 240, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Wu, C.C.; Chen, W.T.; Huang, Y.C.; Chai, C.Y. The expression and significance of WWOX and beta-catenin in hepatocellular carcinoma. APMIS 2013, 121, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.W.; Jiang, F.; Huang, Q.; Liao, Z.; Ding, G. MicroRNA-153 promotes Wnt/beta-catenin activation in hepatocellular carcinoma through suppression of WWOX. Oncotarget 2015, 6, 3840–3847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, Q.F.; Tian, Y.W.; Shen, Q.; Xue, H.Z.; Li, K. SENP2 regulated the stability of beta-catenin through WWOX in hepatocellular carcinoma cell. Tumour Biol. 2014, 35, 9677–9682. [Google Scholar] [CrossRef] [PubMed]
- Nejak-Bowen, K.N.; Monga, S.P. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin. Cancer Biol. 2011, 21, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.M.; Guh, J.H.; Huang, Y.T.; Chueh, S.C.; Chiang, P.C.; Teng, C.M. Induction of mitotic arrest and apoptosis in human prostate cancer pc-3 cells by evodiamine. J. Urol. 2005, 173, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.C.; Li, J.; Liao, K.; Luo, N.; Shi, Q.Q.; Feng, Z.Q.; Chen, D.L. Evodiamine Induces Apoptosis and Inhibits Migration of HCT-116 Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2015, 16, 27411–27421. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.F.; Huang, W.J.; Lin, L.C.; Wang, P.S. Inhibitory effects of evodiamine on the growth of human prostate cancer cell line LNCaP. Int. J. Cancer 2004, 110, 641–651. [Google Scholar] [CrossRef]
- Lin, E.J.D.; Sun, M.; Choi, E.Y.; Magee, D.; Stets, C.W.; During, M.J. Social overcrowding as a chronic stress model that increases adiposity in mice. Psychoneuroendocrinology 2015, 51, 318–330. [Google Scholar] [CrossRef]
- Yang, F.; Shi, L.; Liang, T.; Ji, L.; Zhang, G.; Shen, Y.; Zhu, F.; Xu, L. Anti-tumor effect of evodiamine by inducing Akt-mediated apoptosis in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 485, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S.; Chien, C.C.; Liu, K.H.; Chen, Y.C.; Chiu, W.T. Evodiamine Prevents Glioma Growth, Induces Glioblastoma Cell Apoptosis and Cell Cycle Arrest through JNK Activation. Am. J. Chin. Med. 2017, 45, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Li, J.M.; Chin, C.C.; Kuo, Y.H.; Lee, Y.R.; Huang, Y.C. Evodiamine Induces Cell Growth Arrest, Apoptosis and Suppresses Tumorigenesis in Human Urothelial Cell Carcinoma Cells. Anticancer Res. 2017, 37, 1149–1159. [Google Scholar] [PubMed]
- Zhong, Z.F.; Tan, W.; Wang, S.P.; Qiang, W.A.; Wang, Y.T. Anti-proliferative activity and cell cycle arrest induced by evodiamine on paclitaxel-sensitive and -resistant human ovarian cancer cells. Sci. Rep. 2015, 5, 16415. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, F.; Luo, F.; Liu, Y.; Zhang, F.; Zou, M.; Liu, Q. Evodiamine exerts anti-tumor effects against hepatocellular carcinoma through inhibiting beta-catenin-mediated angiogenesis. Tumour Biol. 2016, 37, 12791–12803. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ren, L.; Wen, L.; Wang, Y.; Qi, J. Effect of evodiamine on the proliferation and apoptosis of A549 human lung cancer cells. Mol. Med. Rep. 2016, 14, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, C.; Wu, L.Y.; Wen, B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Oncotargets Ther. 2016, 9, 4121–4126. [Google Scholar]
- Hu, C.Q.; Gao, X.X.; Han, Y.Y.; Guo, Q.; Zhang, K.L.; Liu, M.M.; Wang, Y.G.; Wang, J. Evodiamine sensitizes BGC-823 gastric cancer cells to radiotherapy in vitro and in vivo. Mol. Med. Rep. 2016, 14, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chien, C.C.; Wu, M.S.; Chen, Y.C. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine 2016, 23, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, X.; Wu, D.; Zhang, M.; Ran, G.; Bi, Y.; Huang, H. Growth inhibition and induction of apoptosis in SGC7901 human gastric cancer cells by evodiamine. Mol. Med. Rep. 2014, 9, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Feng, S.; Wei, L.; Wang, Z.; Hong, D.; Wang, Q. Evodiamine, a novel inhibitor of the Wnt pathway, inhibits the self-renewal of gastric cancer stem cells. Int. J. Mol. Med. 2015, 36, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.-Y.; Wu, H.-T.; Su, Y.-C.; Lin, C.-H.; Chang, C.-J.; Wu, C.-L. Evodiamine Exerts an Anti-Hepatocellular Carcinoma Activity through a WWOX-Dependent Pathway. Molecules 2017, 22, 1175. https://doi.org/10.3390/molecules22071175
Hu C-Y, Wu H-T, Su Y-C, Lin C-H, Chang C-J, Wu C-L. Evodiamine Exerts an Anti-Hepatocellular Carcinoma Activity through a WWOX-Dependent Pathway. Molecules. 2017; 22(7):1175. https://doi.org/10.3390/molecules22071175
Chicago/Turabian StyleHu, Che-Yuan, Hung-Tsung Wu, Yu-Chu Su, Ching-Han Lin, Chih-Jen Chang, and Chao-Liang Wu. 2017. "Evodiamine Exerts an Anti-Hepatocellular Carcinoma Activity through a WWOX-Dependent Pathway" Molecules 22, no. 7: 1175. https://doi.org/10.3390/molecules22071175
APA StyleHu, C.-Y., Wu, H.-T., Su, Y.-C., Lin, C.-H., Chang, C.-J., & Wu, C.-L. (2017). Evodiamine Exerts an Anti-Hepatocellular Carcinoma Activity through a WWOX-Dependent Pathway. Molecules, 22(7), 1175. https://doi.org/10.3390/molecules22071175




