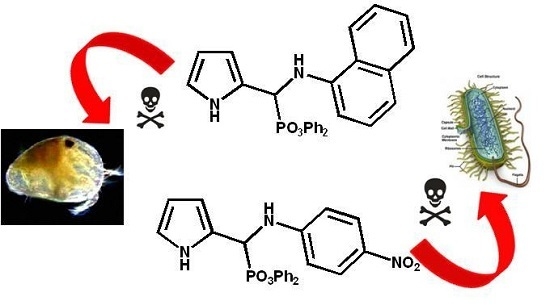
Novel N-Arylaminophosphonates Bearing a Pyrrole Moiety and Their Ecotoxicological Properties
Abstract
:1. Introduction
2. Results
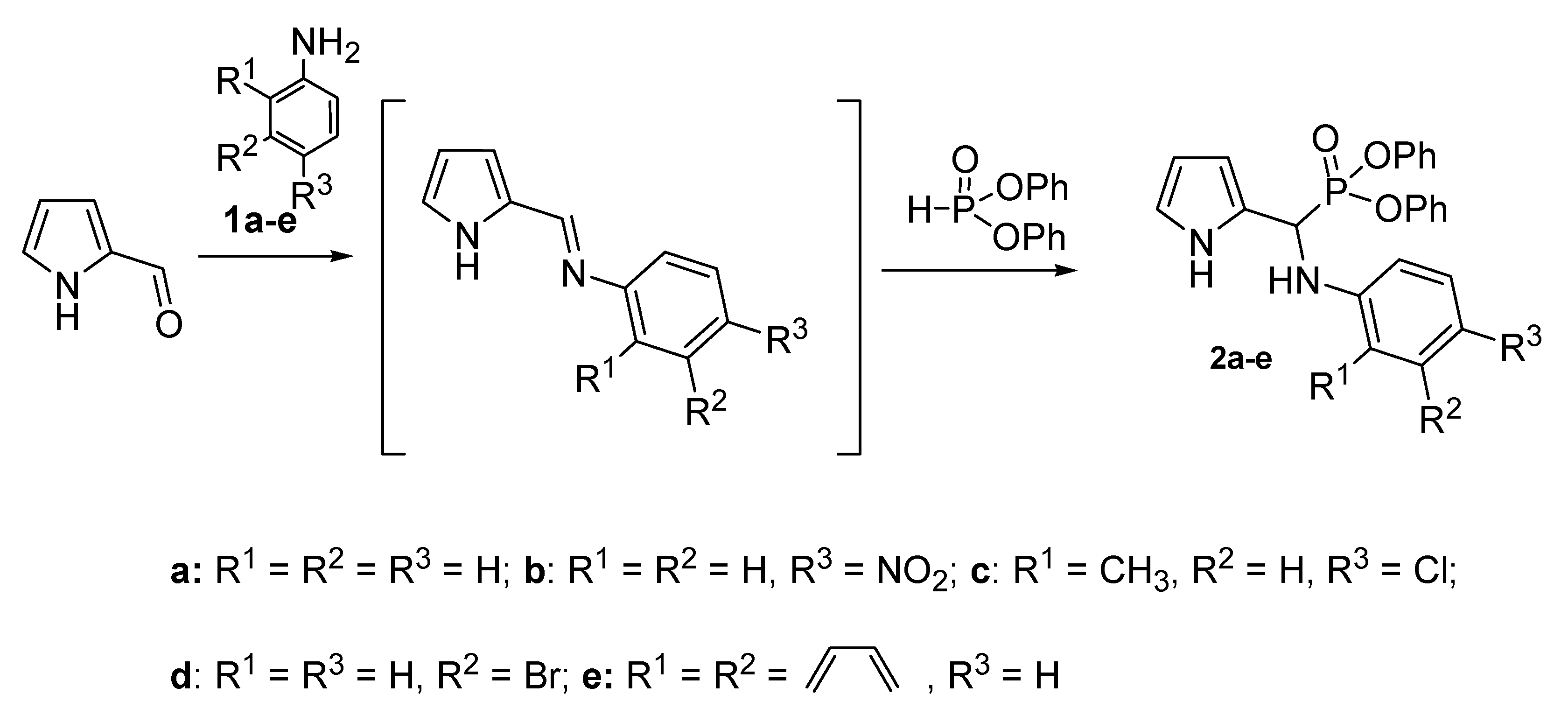
2.1. Preparation of Aminophosphonates 2a–e
2.2. Evaluation of Ecotoxicity of Compounds 2a–e
2.2.1. Ecotoxicological Effect of Compounds 2a–e on Aliivibrio fischeri Bacteria
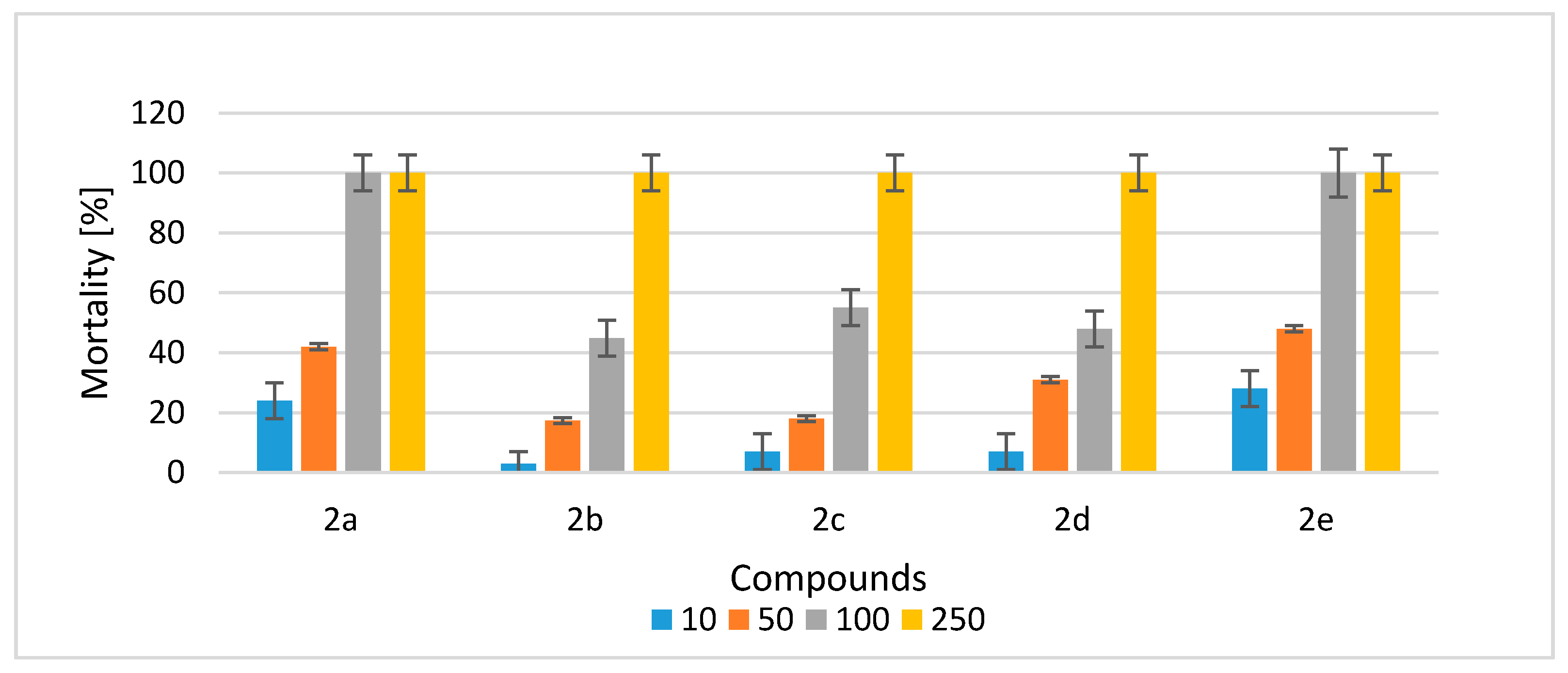
2.2.2. Ecotoxicological Impact of Compounds 2a–e on Heterocypris Incongruens Crustaceans
3. Discussion
4. Materials and Methods
4.1. Synthesis of Compounds 2a–e
4.1.1. General
4.1.2. Preparation of Diphenyl N-arylamino(pyrrol-2-yl)methylphosphonates 2a–e General Procedure
4.2. Evaluation of Ecotoxicity of Compounds 2a–e
4.2.1. Microtox® Toxicity Assay
4.2.2. Ostracod Test Kit
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kafarski, P.; Lejczak, B. Biological Activity of Aminophosphonic Acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193–215. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Aminophosphonic Acids of Potential Medical Importance. Curr. Med. Chem. Anticancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Berlicki, Ł.; Duò, M.; Dziędzioła, G.; Giberti, S.; Bertazzini, M.; Kafarski, P. Synthesis and Evaluation of Effective Inhibitors of Plant δ1-Pyrroline-5-carboxylate Reductase. J. Agric. Food Chem. 2013, 61, 6792–6798. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, A.; Berlicki, Ł.; Giberti, S.; Dziędzioła, G.; Kafarski, P.; Forlani, G. Effectiveness and mode of action of phosphonate inhibitors of plant glutamine synthetase. Pest Manag. Sci. 2010, 66, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hudson, H.R. Aminophosphonic and Aminophosphinic Acids and their Derivatives as Agrochemicals. In Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Kukhar, V.P., Hudson, H.R., Eds.; John Wiley and Sons: Chichester, UK, 2000; pp. 443–482. [Google Scholar]
- Srinivasulu, K.; Raju, C.N.; Babu, Y.H.; Reddy, A.L.K. Synthesis and Antimicrobial Activity of Some New α-Aminophosphonates. S. Afr. J. Chem. 2007, 60, 47–51. Available online: http://hdl.handle.net/10520/EJC24418 (accessed on 7 July 2017).
- Rao, A.J.; Rao, P.V.; Rao, V.K.; Mohan, C.; Naga Raju, C.; Reddy, C.S. Microwave Assisted One-pot Synthesis of Novel α-Aminophosphonates and Their Biological Activity. Bull. Korean Chem. Soc. 2010, 31, 1863–1868. [Google Scholar] [CrossRef]
- Arigala, U.R.S.; Matcha, C.; Yoon, K.R. Zn(OAc)2 2H2O-Catalyzed Synthesis of α-Aminophosphonates under Neat Reaction. Heteroat. Chem. 2012, 23, 160–165. [Google Scholar] [CrossRef]
- Devineni, S.R.; Doddaga, S.; Donka, R.; Chamarthi, N.R. CeCl3·7H2O-SiO2: Catalyst promoted microwave assisted neat synthesis, antifungal and antioxidant activities of α-diaminophosphonates. Chin. Chem. Lett. 2013, 24, 759–763. [Google Scholar] [CrossRef]
- Hudson, H.R.; Lee, R.J. A Brief Review of the Anticancer Activity of α-Aminophosphonic Acid Derivatives and a Report on the in Vitro Activity of Some Dialkyl α-aryl- (or Heteroaryl)-α-(Diphenylmethylamino) Methanephosphonates. Phosphorus Sulfur Silicon Relat. Elements 2014, 189, 1149–1155. [Google Scholar] [CrossRef]
- Yan, S.; Valasani, K.R. Phosphonate Derivatives for Treatment of Alzheimer Disease. World Patent 173206, 21 November 2013. [Google Scholar]
- Acklin, P.; Allgeier, H.; Auberson, Y.; Ofner, S.; Veenstra, S.J. Substituted Aminoalkane Phosphonic Acids. U.S. Patent 6,117,873, 12 September 2000. [Google Scholar]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Estevez, V.; Villacampa, M.; Menendez, J.C. Multicomponent reactions for the synthesis of pyrroles. Chem. Soc. Rev. 2010, 39, 4402–4421. [Google Scholar] [CrossRef] [PubMed]
- Boduszek, B. Synthesis and Biological Activity of Heterocyclic Aminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 1999, 144, 433–436. [Google Scholar] [CrossRef]
- Balakrishna, A.; Veera Narayana Reddy, M.; Visweswara Rao, P.; Anil Kumar, M.; Siva Kumar, B.; Nayak, S.K.; Suresh Reddy, C. Synthesis and bio-activity evaluation of tetraphenyl(phenylamino) methylene bisphosphonates as antioxidant agents and as potent inhibitors of osteoclasts in vitro. Eur. J. Med. Chem. 2011, 46, 1798–1802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hao, G.; Pan, J.; Zhang, J.; Hu, D.; Song, B. Asymmetric Synthesis and Bioselective Activities of α-Aminophosphonates Based on the Dufulin Motif. J. Agric. Food Chem. 2016, 64, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Hudson, H.R.; Lee, R.J.; Matthews, R.W. 1-Amino-1-aryl- and 1-Amino-1-heteroarylmethanephosphonic Acids and Their N-Benzhydrylprotected Diethyl Esters: Preparation and Characterization. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 1691–1709. [Google Scholar] [CrossRef]
- Bedolla-Medrano, M.; Hernández-Fernández, E.; Ordóñez, M. Phenylphosphonic Acid as Efficient and Recyclable Catalyst in the Synthesis of α-Aminophosphonates under Solvent-Free Conditions. Synlett 2014, 25, 1145–1149. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, S.; Zhou, S.; Wei, Y.; Zhang, L.; Wang, F.; Feng, Z.; Guo, L.; Mu, X. Lanthanide Amido Complexes Incorporating Amino-Coordinate-Lithium Bridged Bis(indolyl) Ligands: Synthesis, Characterization, and Catalysis for Hydrophosphonylation of Aldehydes and Aldimines. Inorg. Chem. 2012, 51, 7134–7143. [Google Scholar] [CrossRef] [PubMed]
- Hubert, C.; Oussaid, B.; Etemad-Moghadam, G.; Koenig, M.; Garrigues, B. Improved Synthesis of New α-Aminophosphonic Acids by Sonochemical Activation. Synthesis 1994, 1994, 51–55. [Google Scholar] [CrossRef]
- Joly, G.D.; Jacobsen, E.N. Thiourea-Catalyzed Enantioselective Hydrophosphonylation of Imines: Practical Access to Enantiomerically Enriched α-Amino Phosphonic Acids. J. Am. Chem. Soc. 2004, 126, 4102–4103. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Harika, P. An Efficient Green Synthesis of Novel α-Diaminophosphonates. Phosphorus Sulfur Silicon Relat. Elements 2015, 190, 1893–1900. [Google Scholar] [CrossRef]
- Boduszek, B. 1-Aminophosphonic Acids and Esters Bearing Heterocyclic Moiety. Part 2. Pyridine, Pyrrole and Imidazole Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 1996, 113, 209–218. [Google Scholar] [CrossRef]
- Ma, X.Y.; Wang, X.C.; Ngo, H.H.; Guo, W.; Wu, M.N.; Wang, N. Bioassay based luminescent bacteria: Interferences, improvements, and applications. Sci. Total Environ. 2014, 468–469, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Connon, R.E.; Geist, J.; Werner, I. Effect-Based Tools for Monitoring and Predicting the Ecotoxicological Effects of Chemicals in the Aquatic Environment. Sensors 2012, 12, 12741–12771. [Google Scholar] [CrossRef] [PubMed]
- MicroBioTests. Toxkit Advantages/Assets. Available online: http://www.microbiotests.be/information/toxkit-advantagesassets/ (accessed on 19 July 2016).
- Urbanczyk, H.; Ast, J.C.; Higgins, M.J.; Carson, J.; Dunlap, P.V. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov. and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2823–2829. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M. Phytotoxkit/Phytotestkit and Microtoxs as tools for toxicity assessment of sediments. Ecotoxicol. Environ. Saf. 2013, 98, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Weltens, R.; Deprez, K.; Michiels, L. Validation of Microtox as a first screening tool for waste classification. Waste Manag. 2014, 34, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zuo, J.; Tang, X.; Li, R.; Li, Z.; Zhang, F. Toxicity evaluation of pharmaceutical wastewaters using the alga Scenedesmus obliquus and the bacterium Vibrio fischeri. J. Hazard. Mater. 2014, 266, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Rosado, D.; Usero, J.; Morillo, J. Assessment of heavy metals bioavailability and toxicity toward Vibrio fischeri in sediment of the Huelva estuary. Chemosphere 2016, 153, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Montalban, M.G.; Hidalgo, J.M.; Collado-Gonzalez, M.; Banos, F.G.D.; Víllora, G. Assessing chemical toxicity of ionic liquids on Vibrio fischeri: Correlation with structure and composition. Chemosphere 2016, 155, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Bonnemoy, F.; Charvy, J.-C.; Bohatier, J.; Mallet, C. Toxicity assessment of the maize herbicides S-metolachlor, benoxacor, mesotrione and nicosulfuron, and their corresponding commercial formulations, alone and in mixtures, using the Microtox test. Chemosphere 2013, 93, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzani, R.; Ceretti, E.; Zerbini, I.; Casale, R.; Gozio, E.; Bertanza, G.; Gelatti, U.; Donato, F.; Feretti, D. Biodegradability, toxicity and mutagenicity of detergents: Integrated experimental evaluations. Ecotoxicol. Environ. Saf. 2012, 84, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.C.; Pereira, J.L.; Cachada, A.; Duarte, A.C.; Goncalves, P.; Sousa, J.P.; Pereira, R. Structural effects of the bioavailable fraction of pesticides in soil: Suitability of elutriate testing. J. Hazard. Mater. 2010, 184, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Burga Perez, K.F.; Charlatchka, R.; Sahli, L.; Ferard, J.-F. New methodological improvements in the Microtox® solid phase assay. Chemosphere 2012, 86, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Stronkhorst, J.; Ciarelli, S.; Schipper, C.A.; Postma, J.F.; Dubbeldam, M.; Vangheluwe, M.; Brils, J.M.; Hooftman, R. Inter-laboratory comparison of five marine bioassays for evaluating the toxicity of dredged material. Aquat. Ecosyst. Health Manag. 2004, 7, 147–159. [Google Scholar] [CrossRef]
- Doe, K.; Scroggins, R.; Mcleay, D.; Wohlgeschaffen, G. Solid-phase test for sediment toxicity using the luminescent bacterium Vibrio fischeri. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.F., Eds.; Springer: Dordrecht, Holland, 2005; Volume 1, pp. 107–136. [Google Scholar]
- The International Organization for Standardization (ISO). Water Quality—Determination of Fresh Water Sediment Toxicity to Heterocypris incongruens (Crustacea, Ostracoda), 1st ed.; ISO 14371:2012; The International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Boduszek, B. An efficient synthesis of 1-aminophosphonic acids and esters bearing heterocyclic moiety. Phosphorus Sulfur Silicon Relat. Elem. 1995, 104, 63–70. [Google Scholar] [CrossRef]
- Lewkowski, J.; Malinowski, Z.; Matusiak, A.; Morawska, M.; Rogacz, D.; Rychter, P. The Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants: A Seedling Emergence and Growth Test. Molecules 2016, 21, 694. [Google Scholar] [CrossRef] [PubMed]
- Chrostowska, A.; Darrigan, C.; Khayar, S.; Baylère, P.; Lewkowski, J.; Krzyczmonik, A.; Tokarz, P.; Ślepokura, K.; Lis, T. The Diastereoselective Synthesis of Tetraalkyl (R,R)-1,2-Cyclohexylene-diamino-di-phosphonates Bearing Thiophene, Furan and Pyrrole Moieties. Tetrahedron 2015, 71, 2561–2571. [Google Scholar] [CrossRef]
- Matusiak, A.; Lewkowski, J.; Rychter, P.; Biczak, R. Phytotoxicity of New Furan-derived Aminophosphonic Acids, N-Aryl Furaldimines and 5-Nitrofuraldimine. J. Agric. Food Chem. 2013, 61, 7673–7678. [Google Scholar] [CrossRef] [PubMed]
- Lewkowski, J.; Morawska, M.; Rychter, P.; Rogacz, D.; Lewicka, K.; Dobrzyński, P. Evaluation of Ecotoxicological Impact of New Pyrrole-derived Aminophosphonates Using Selected Bioassay Battery. Ecotoxicology 2017. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; García-Lorenzo, M.L.; Martínez-López, S.; Bech, J.; García-Tenorio, R.; Bolívar, J.P. Use of bioassays for the assessment of areas affected by phosphate industry wastes. J. Geochem. Explor. 2014, 147, 130–138. [Google Scholar] [CrossRef]
- Gouider, M.; Feki, M.; Sayadi, S. Bioassay and use in irrigation of untreated and treated wastewaters from phosphate fertilizer industry. Ecotoxicol. Environ. Saf. 2010, 73, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Cvancarova, M.; Kresinova, Z.; Cajthaml, T. Influence of the bioaccessible fraction of polycyclic aromatic hydrocarbons on the ecotoxicity of historically contaminated soils. J. Hazard. Mater. 2013, 254–255, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Hollert, H. Comparison of sewage sludge toxicity to plants and invertebrates in three different soils. Chemosphere 2011, 83, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Płaza, G.; Nałęcz-Jawecki, G.; Ulfig, K.; Brigmon, R.L. The application of bioassays as indicators of petroleum-contaminated soil remediation. Chemosphere 2005, 59, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zenno, S.; Koike, H.; Tanokura, M.; Saigo, K. Gene cloning, purification, and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri. J. Biochem. 1996, 120, 736–744. Available online: https://www.jstage.jst.go.jp/article/biochemistry1922/120/4/120_4_736/_article (accessed on 6 July 2017). [CrossRef] [PubMed]
- Yanto, Y.; Hall, M.; Bommarius, A.S. Nitroreductase from Salmonella typhimurium: Characterization and catalytic activity. Org. Biomol. Chem. 2010, 8, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Sihtmäe, M.; Mortimer, M.; Kahru, A.; Blinova, I. Toxicity of five anilines to crustaceans, protozoa and bacteria. J. Serbian Chem. Soc. 2010, 75, 1291–1302. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2a–e are available from the authors. |
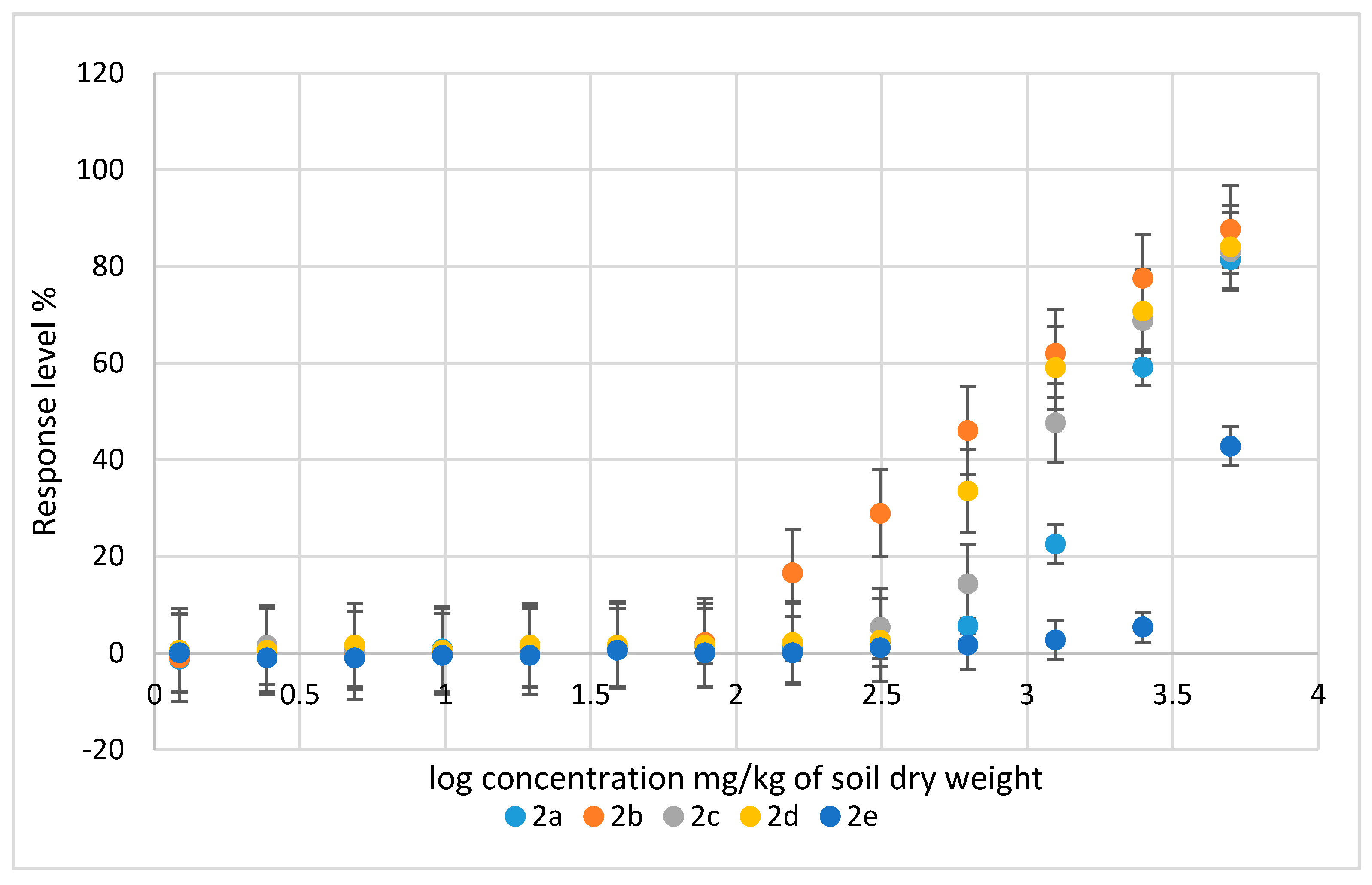
| Compounds | EC50 (Lower Limit; Upper Limit) | Coefficient of Determination (R2) |
|---|---|---|
| 2a | 2262 (1967; 2602) | 0.9715 |
| 2b | 750.9 (685.6; 810.5) | 0.9918 |
| 2c | 1656 (1465; 1871) | 0.9785 |
| 2d | 1141 (846.5; 1537) | 0.8998 |
| 2e | 5365 (4232; 6801) | 0.9796 |
| Concentration of the Compound [mg/kg of Soil d.w.] | Growth Inhibition [%] | ||||
|---|---|---|---|---|---|
| 2a | 2b | 2c | 2d | 2e | |
| Control | 0 | 0 | 0 | 0 | 0 |
| 10 | 16 ± 2 | 2 ± 1 | 3 ± 1 | 2 ± 1 | 19 ± 2 |
| 50 | NM | 21 ± 2 | 31 ± 2 | 26 ± 3 | NM |
| 100 | NM | NM | NM | NM | NM |
| 250 | NM | NM | NM | NM | NM |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewkowski, J.; Morawska, M.; Kaczmarek, A.; Rogacz, D.; Rychter, P. Novel N-Arylaminophosphonates Bearing a Pyrrole Moiety and Their Ecotoxicological Properties. Molecules 2017, 22, 1132. https://doi.org/10.3390/molecules22071132
Lewkowski J, Morawska M, Kaczmarek A, Rogacz D, Rychter P. Novel N-Arylaminophosphonates Bearing a Pyrrole Moiety and Their Ecotoxicological Properties. Molecules. 2017; 22(7):1132. https://doi.org/10.3390/molecules22071132
Chicago/Turabian StyleLewkowski, Jarosław, Marta Morawska, Anna Kaczmarek, Diana Rogacz, and Piotr Rychter. 2017. "Novel N-Arylaminophosphonates Bearing a Pyrrole Moiety and Their Ecotoxicological Properties" Molecules 22, no. 7: 1132. https://doi.org/10.3390/molecules22071132
APA StyleLewkowski, J., Morawska, M., Kaczmarek, A., Rogacz, D., & Rychter, P. (2017). Novel N-Arylaminophosphonates Bearing a Pyrrole Moiety and Their Ecotoxicological Properties. Molecules, 22(7), 1132. https://doi.org/10.3390/molecules22071132