A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells
Abstract
:1. Introduction
2. Results
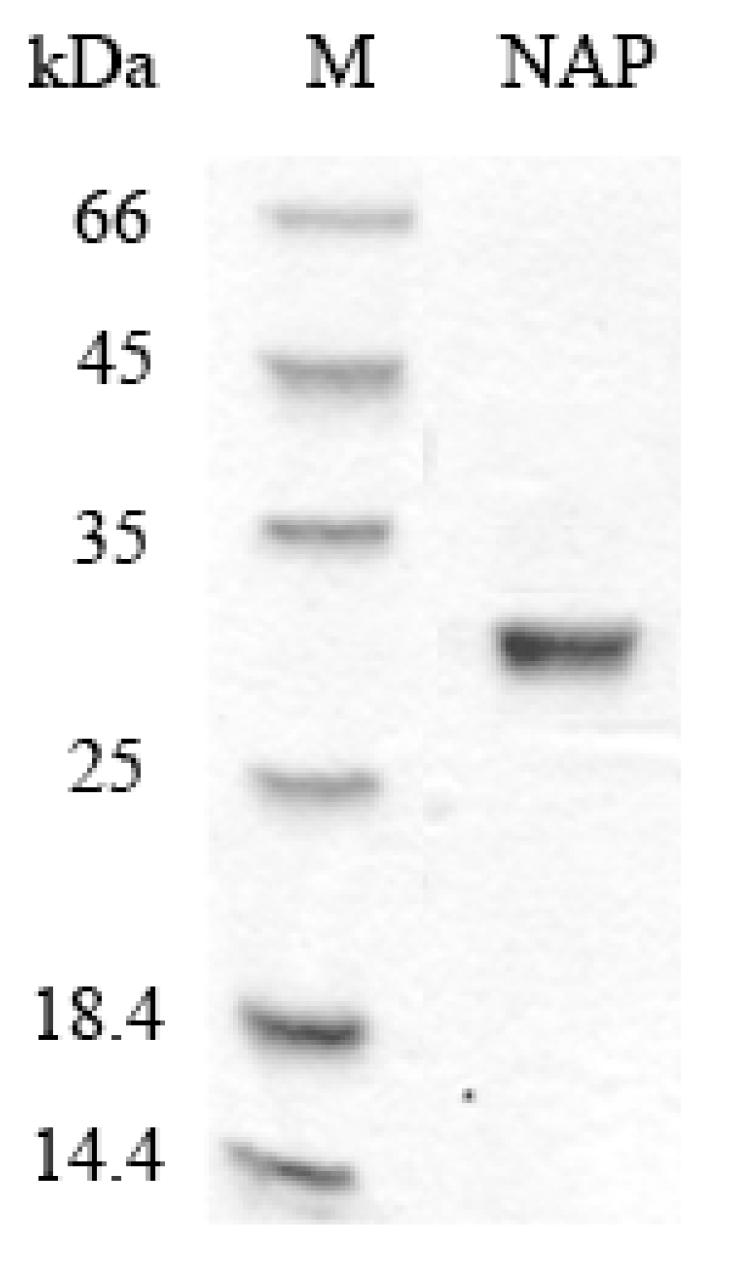
2.1. Purification of NAP
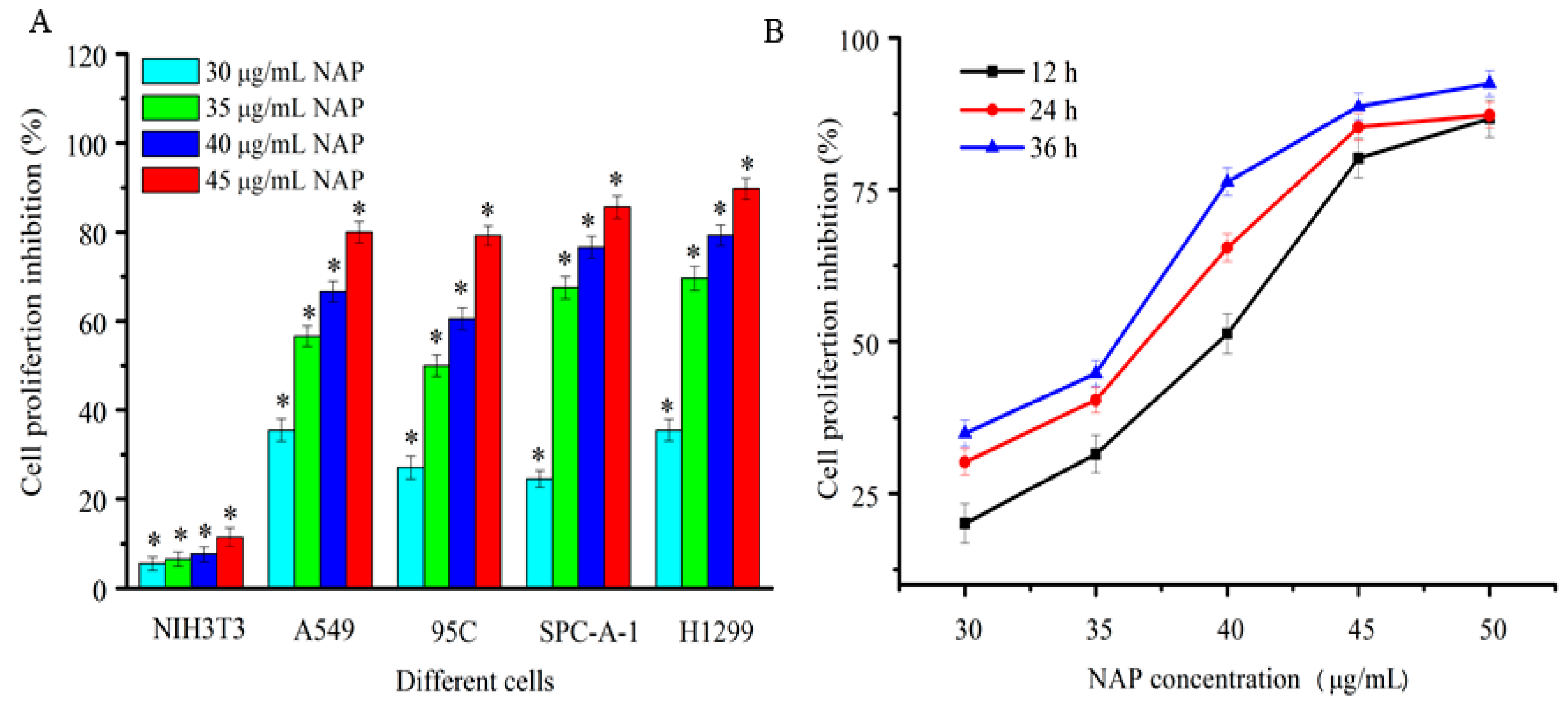
2.2. Anti-Proliferative Activity to Different Human Lung Cancer Cells
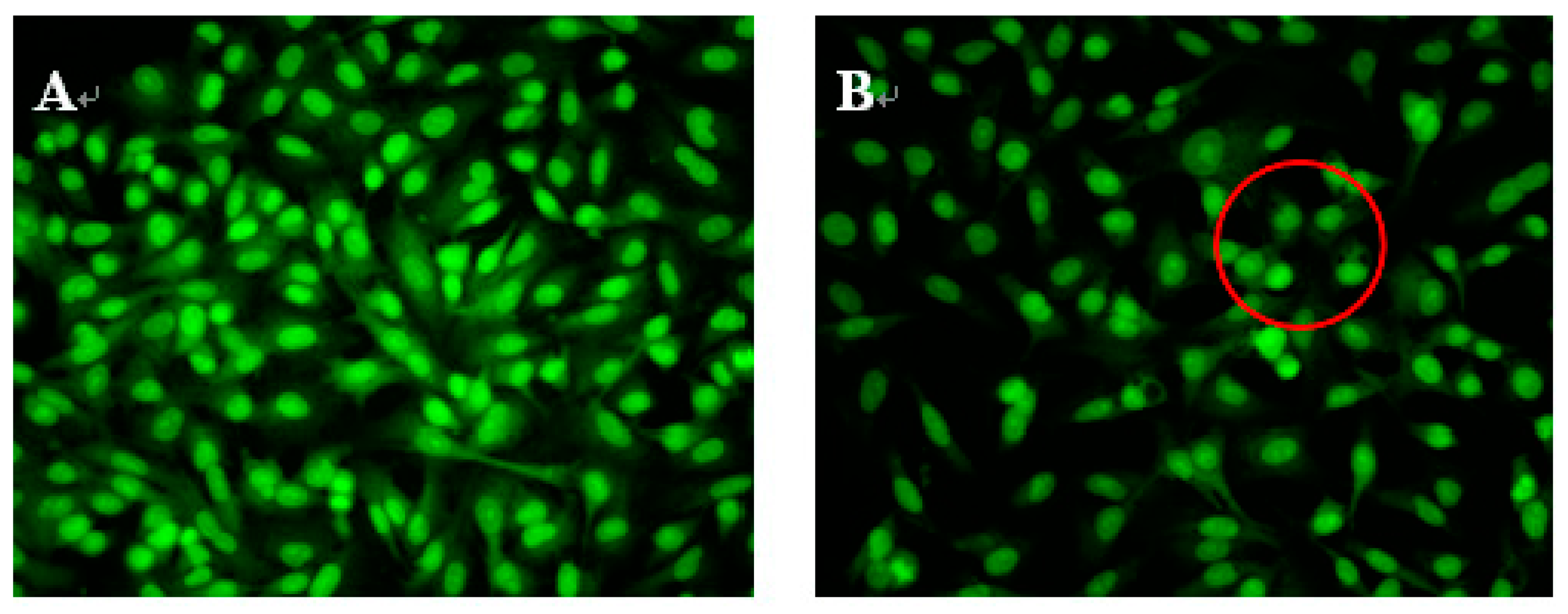
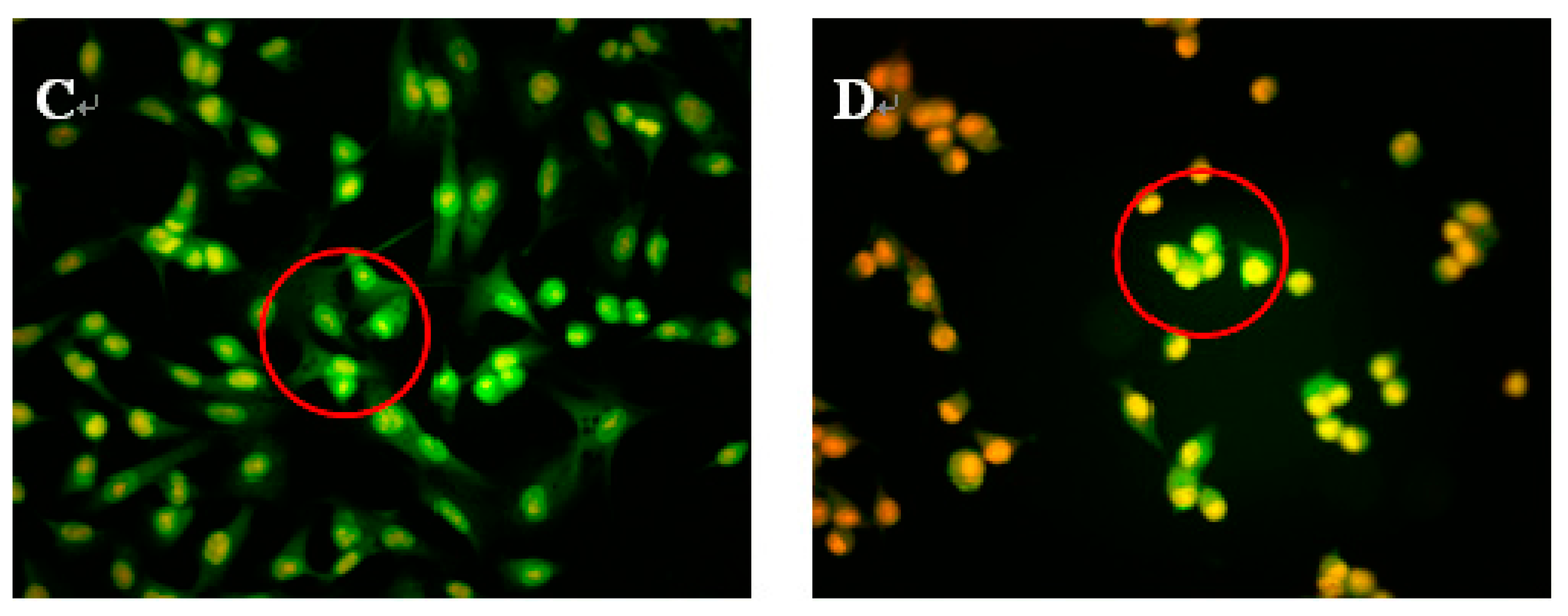
2.3. Morphological Observations
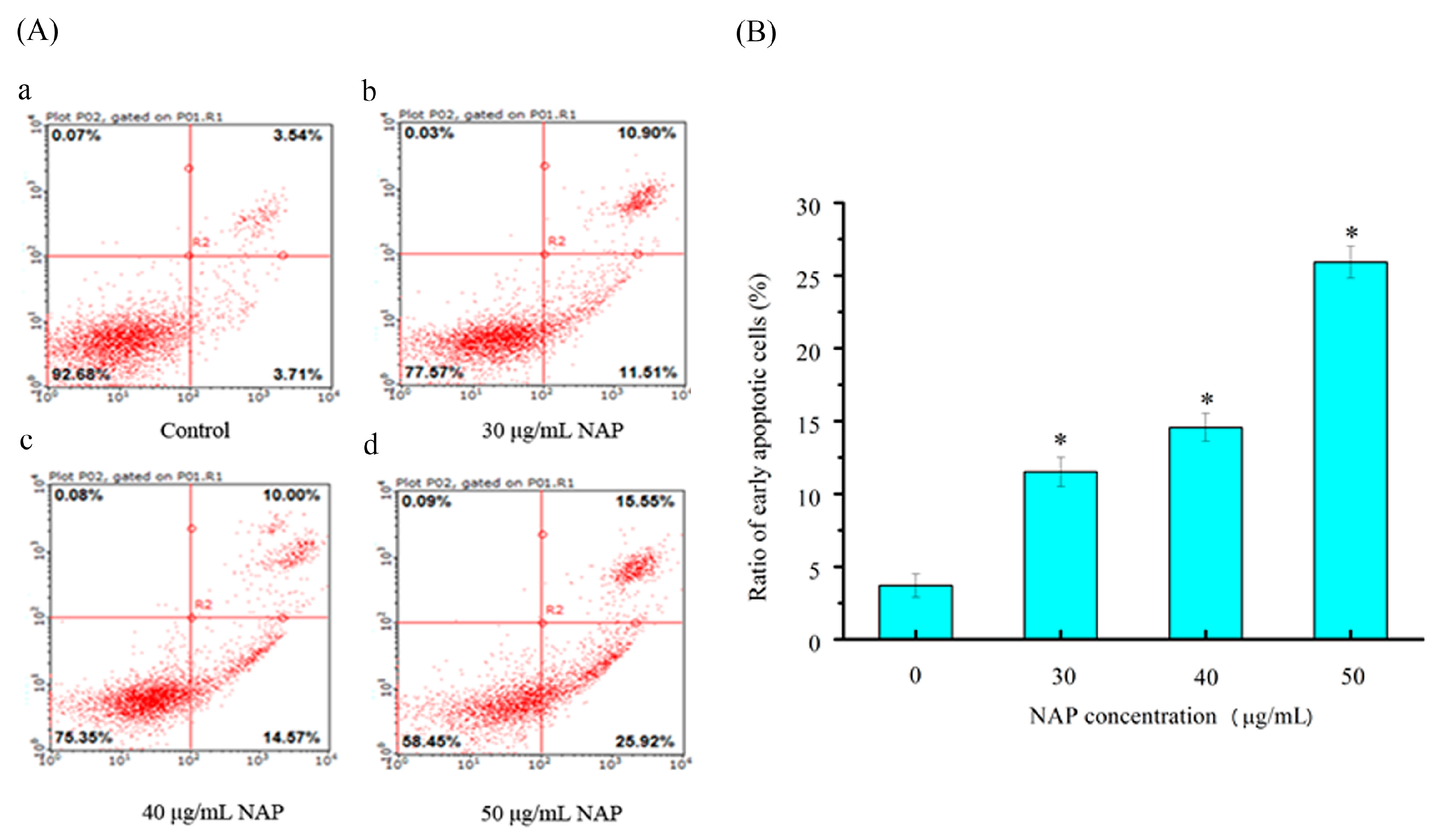
2.4. Cell Apoptotic Rate Detected by Flow Cytometry
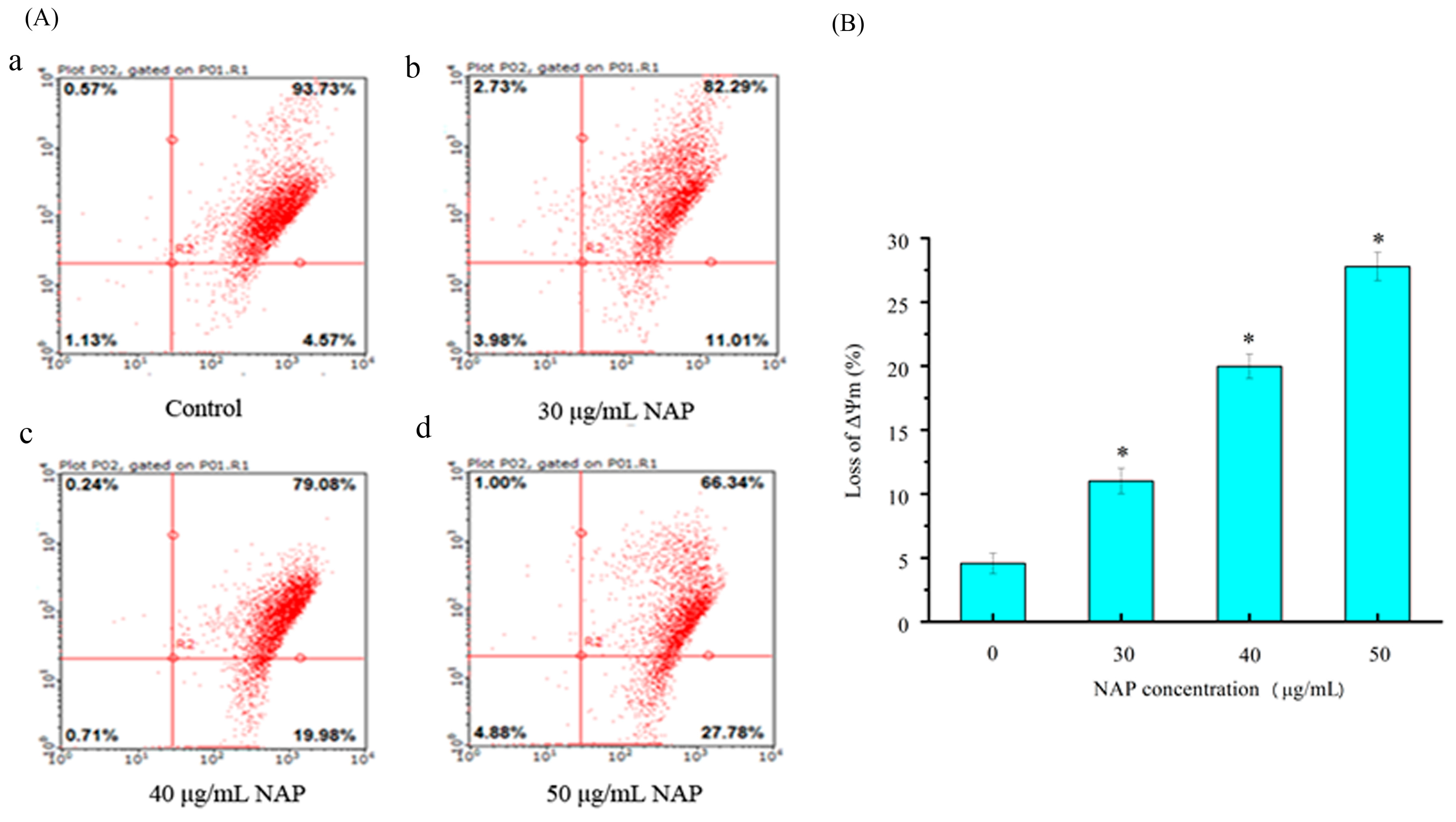
2.5. Mitochondrial Membrane Potential (ΔΨm) Change in H1299 Cells Treated with NAP
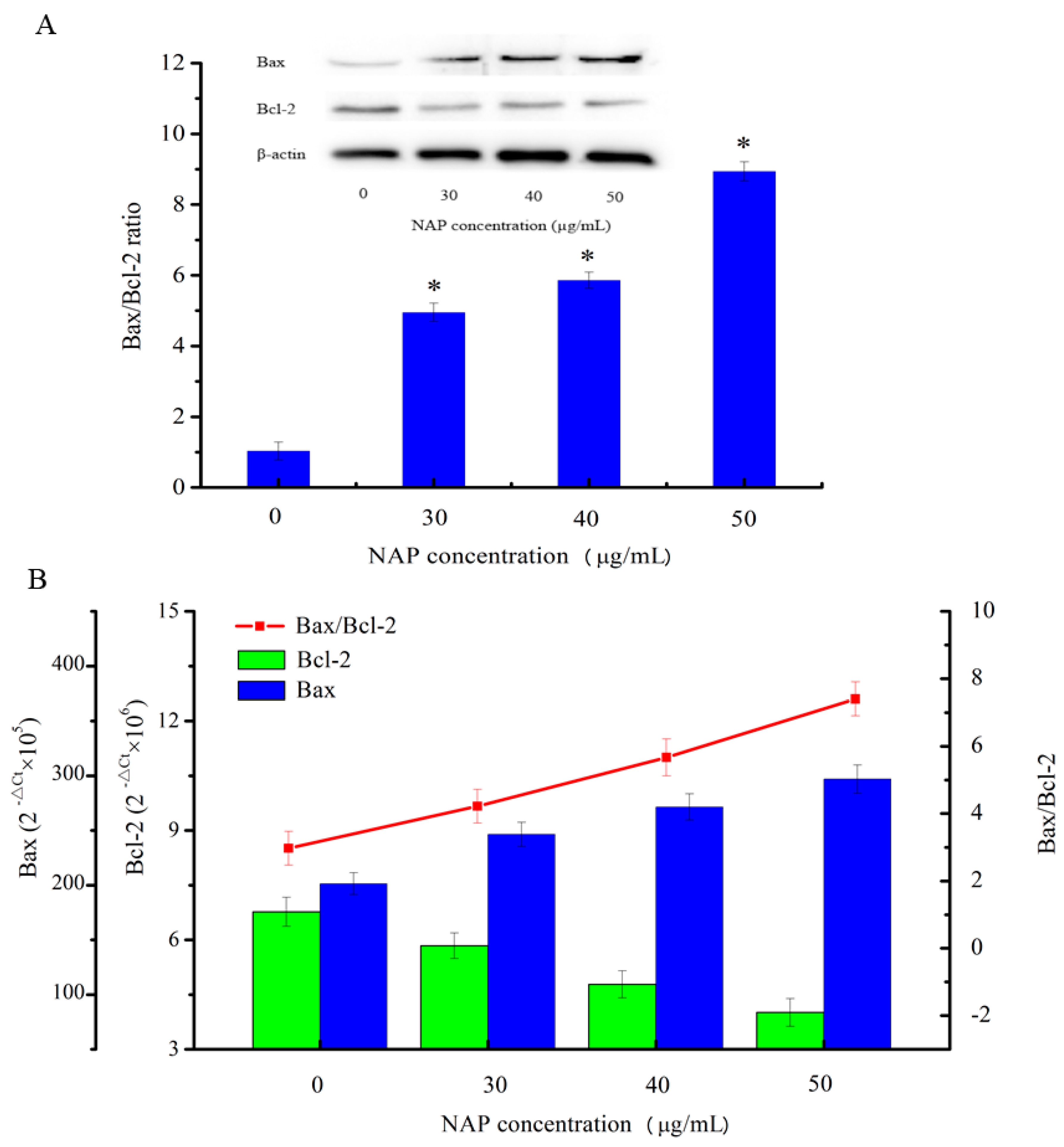
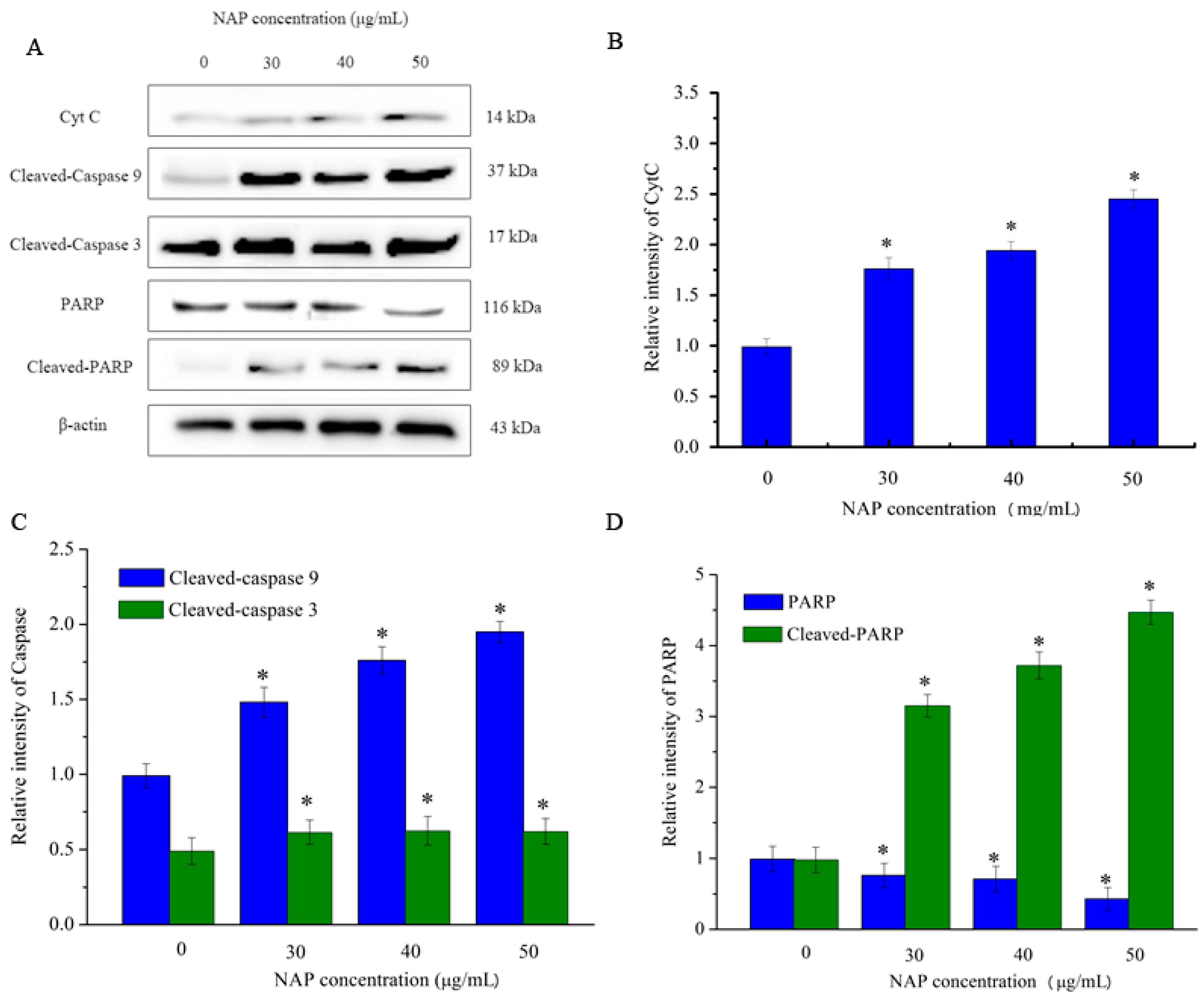
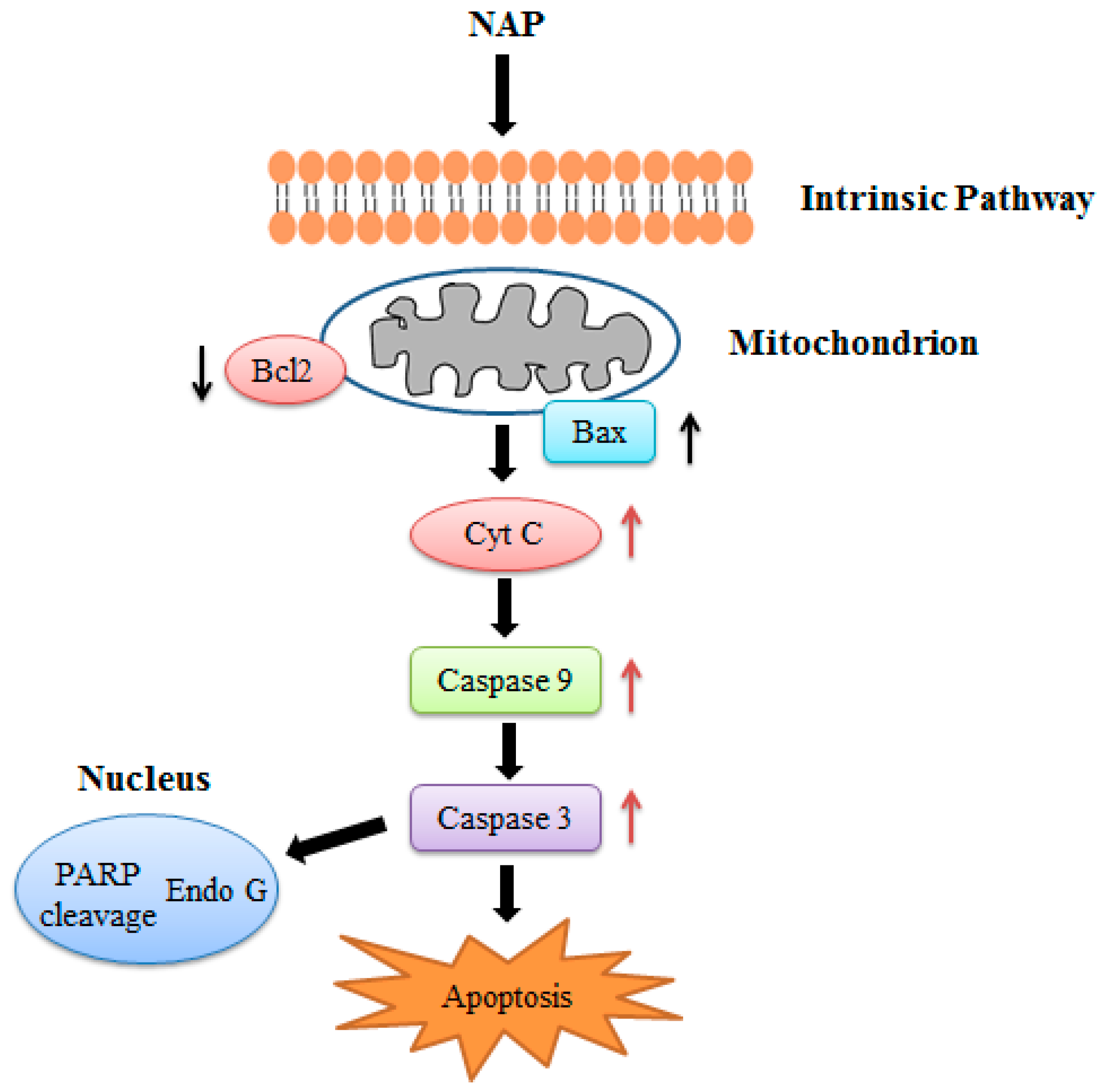
2.6. Effects of NAP on the Apoptosis-Related Proteins of H1299 Cells
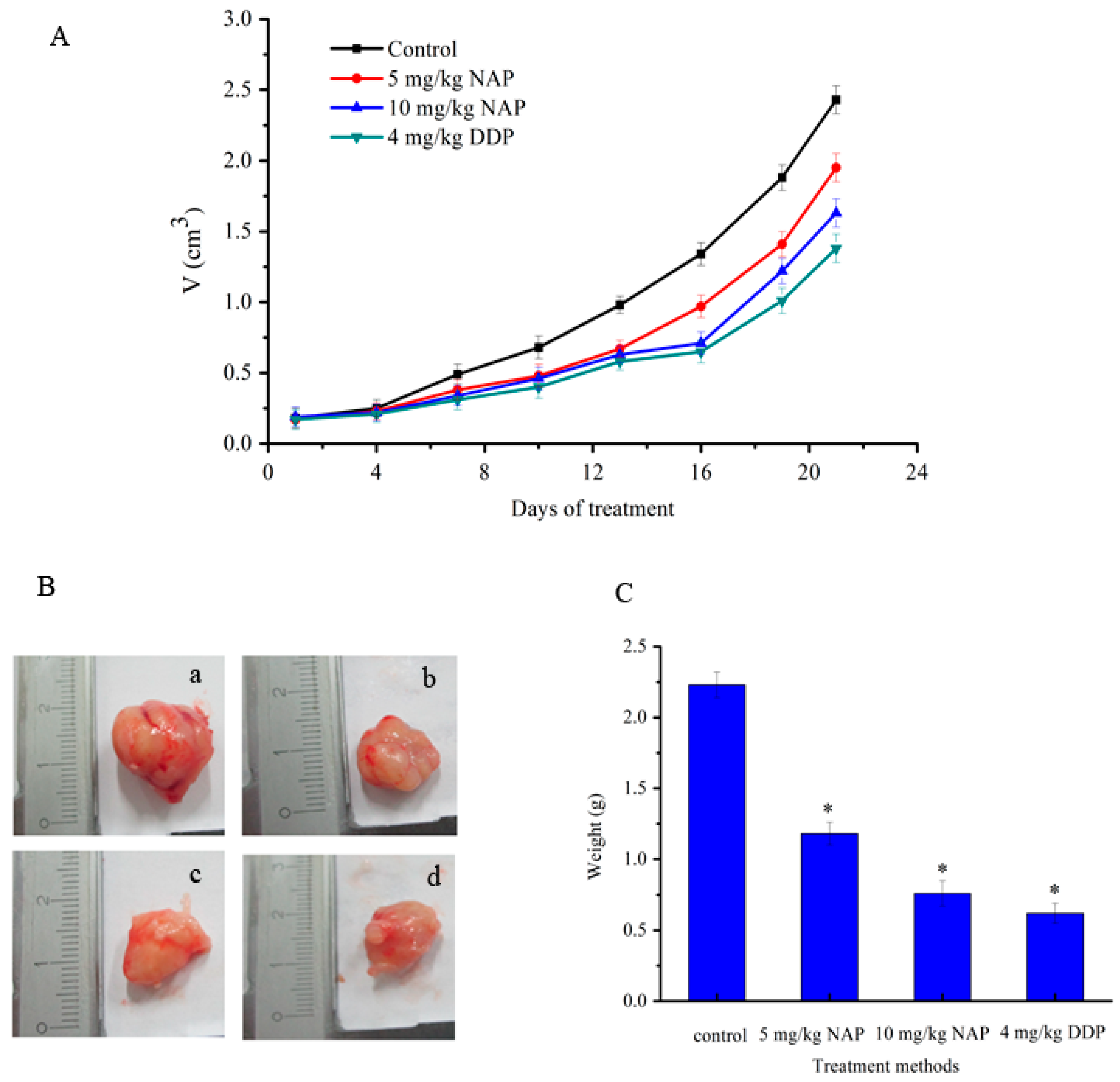
2.7. Effects of NAP on Tumor Growth in an in Animal Models
2.8. Effects of NAP on the Apoptosis-Related Proteins of H1299 Tumor Tissue
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines and Culture
4.3. Purification of NAP
4.4. Anti-Proliferative Activity to Different Human Lung Cancer Cells
4.5. Cell Morphologic Study
4.6. Cell Apoptosis Analysis
4.7. ΔΨm Change Effected by NAP
4.8. Western Blot Analysis
4.9. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT-PCR)
4.10. In Vivo Antitumor Activity
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, Q.; Chen, W.Z.; Man, S.L.; Teng, Y.O.; Meng, X.; Zhang, Y.M.; Yu, P.; Gao, W.Y. Paris saponin I inhibits proliferation and promotes apoptosis through down-regulating AKT activity in human non-small-cell lung cancer cells and inhibiting ERK expression in human small-cell lung cancer cells. RSC Adv. 2016, 6, 70816–70824. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Vaikundamoorthy, R.; Sundaramoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Marine steroid derived from Acropora formosa enhances mitochondrial-mediated apoptosis in non-small cell lung cancer cells. Tumor Biol. 2016, 37, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Zhang, S.; Zou, X.; Zhao, P.; He, J. Lung cancer incidence and mortality in China, 2009. Thorac. Cancer 2013, 4, 102–108. [Google Scholar] [CrossRef]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Chi, C.F.; Wang, B. Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B.; Gospodarowicz, M.; Curtis, R.E.; Clarke, E.A.; Andersson, M.; Glimelius, B.; Joensuu, T.; Lynch, C.F.; van Leeuwen, F.E.; Holowaty, E. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J. Natl. Cancer Inst. 2002, 94, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Anvarbatcha, R.; Rajendran, S.; Vaiyapuri, S.P.; Paul, P.; Suresh, S.; Mohammed, Z.; Hanumanthappa, K.; Sankaralingam, A.; Mohammad, A.A. Antiproliferative and apoptosis-induction studies of a metallosurfactant in human breast cancer cell MCF-7. RSC Adv. 2014, 4, 49953–49959. [Google Scholar]
- Taddia, L.; D’Arca, D.; Ferrari, S.; Marraccini, C.; Severi, L.; Ponterini, G.; Assaraf, Y.G.; Marverti, G.; Costi, M.P. Inside the biochemical pathways of thymidylate synthase perturbed by anticancer drugs: Novel strategies to overcome cancer chemoresistance. Drug Resist. Updat. 2015, 23, 20–54. [Google Scholar] [CrossRef] [PubMed]
- Torino, F.; Barnabei, A.; Paragliola, R.; Baldelli, R.; Appetecchia, M.; Corsello, S.M. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid 2013, 23, 1345–1366. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kim, Y.S.; Hwang, J.W.; Lee, J.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Purification and characterization of a novel anticancer peptide derived from Ruditapes philippinarum. Process Biochem. 2013, 48, 1086–1090. [Google Scholar] [CrossRef]
- Reed, J.C. Mechanisms of apoptosis. Am. J. Pathol. 2000, 157, 1415–1430. [Google Scholar] [CrossRef]
- Burz, C.; Berindanneagoe, I.; Balacescu, O.; Irimie, A. Apoptosis in cancer: Key molecular signaling pathways and therapy targets. Acta Oncol. 2009, 48, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Yang, Z.S.; Yu, D.; Wang, J.; Li, R.; Ding, G. Sepia ink oligopeptide induces apoptosis in prostate cancer cell lines via caspase-3 activation and elevation of Bax/Bcl-2 ratio. Mar. Drugs 2012, 10, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, J.; Zhang, R.; Wang, Y.; Hong, M. A novel fibrinolytic serine protease from the polychaete Nereis (Neanthes) virens (Sars): Purification and characterization. Biochimie 2007, 89, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wong, B.S.; Yea, S.H.; Lu, C.I.; Weng, S.H. Sinularin Induces Apoptosis through Mitochondria Dysfunction and Inactivation of the pI3K/Akt/mTOR Pathway in Gastric Carcinoma Cells. Mar. Drugs 2016, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Shiny, P.J.; Mukherjee, A.; Chandrasekaran, N. DNA damage and mitochondria-mediated apoptosis of A549 lung carcinoma cells induced by biosynthesised silver and platinum nanoparticles. RSC Adv. 2016, 6, 27775–27787. [Google Scholar]
- Jia, D.L.; Yang, H.; Tao, Z.; Wan, L.; Cheng, J.Q.; Lu, X.F. Preparation and characterization of a novel variant of human tumor necrosis factor-related apoptosis-inducing ligand from the rhesus monkey, Macaca mulatta. Appl. Microbiol. Biotechnol. 2016, 100, 3035–3047. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chang, J.; Li, Z.Y. A serine protease extracted from Trichosanthes kirilowii induces apoptosis via the PI3K/AKT-mediated mitochondrial pathway in human colorectal adenocarcinoma cells. Food Funct. 2016, 7, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Liu, E.; Du, X.; Ge, R.; Liang, T.; Niu, Q.; Li, Q. Comparative toxicity and apoptosis induced by diorganotins in rat pheochromocytoma (PC12) cells. Food Chem. Toxicol. 2013, 60, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, F.; Lin, H.; Wang, X. Isolation and purification of a peptide from Bullacta exarata and its impaction of apoptosis on prostate cancer cell. Mar. Drugs 2013, 11, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Castedo, M.; Susin, S.A.; Zamzami, N.; Hirsch, T.; Macho, A.; Haeffner, A.; Hirsch, F.; Geuskens, M.; Kroemer, G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 1996, 184, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, T.; Newmeyer, D.D. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 2003, 15, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.; Cotter, T.G. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim. Biophys. Acta 2004, 1644, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Alevizakos, M.; Kaltsas, S.; Syrigos, K.N. The VEGF pathway in lung cancer. Cancer Chemother. Pharmacol. 2013, 72, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Shinkaruk, S.; Bayle, M.; Laïn, G.; Déléris, G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr. Med. Chem. Anticancer Agents 2003, 3, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Takahashi, S.; Imamura, M.; Okutani, E.; Zhang, Z.G.; Chayama, K.; Chen, B.A. Earthworm fibrinolytic enzyme: Anti-tumor activity on human hepatoma cells in vitro and in vivo. Chin. Med. J. 2007, 120, 898–904. [Google Scholar] [PubMed]
- Ge, X.; Bo, Q.Q.; Hong, X.Y.; Cui, J.Y.; Jiang, X.; Hong, M.; Liu, J.K. A novel acidic serine protease, ASPNJ inhibits proliferation, induces apoptosis and enhances chemo-susceptibility of acute promyelocytic leukemia cell. Leuk. Res. 2013, 37, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Bo, Q.Q.; Ge, X.; Cui, J.Y.; Jiang, X.; Liu, J.K.; Hong, M. Effects of Acidic Serine Protease ASPNJ on inhibition and injury of leukemia cell K562. Chin. J. Biochem. Pharm. 2012, 33, 736–739. [Google Scholar]
- Wen, Z.S.; Liu, L.J.; Ouyang, X.K.; Qu, Y.L.; Chen, Y.; Ding, G.F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Gene | Genebank Accession | Primer Sequence | Product Length (bp) | Annealing (°C) |
|---|---|---|---|---|
| Bcl-2 | NM-000657.2 | F:5′-CCCGTTGCTTTTCCTCTGG-3′ | 1207 | 62.6 |
| R:5′-ATCCCACTCGTAGCCCCTCT-3′ | ||||
| Bax | NM-138764.3 | F:5′-GACGAACTGGACAGTAACATGGA-′3 | 849 | 61.4 |
| R:5′-GCAAAGTAAAAGGGCGACA-′3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Yu, F.; Zhang, G.; Yang, Z.; Huang, F.; Ding, G. A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells. Molecules 2017, 22, 1123. https://doi.org/10.3390/molecules22071123
Tang Y, Yu F, Zhang G, Yang Z, Huang F, Ding G. A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells. Molecules. 2017; 22(7):1123. https://doi.org/10.3390/molecules22071123
Chicago/Turabian StyleTang, Yunping, Fangmiao Yu, Guomei Zhang, Zuisu Yang, Fangfang Huang, and Guofang Ding. 2017. "A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells" Molecules 22, no. 7: 1123. https://doi.org/10.3390/molecules22071123
APA StyleTang, Y., Yu, F., Zhang, G., Yang, Z., Huang, F., & Ding, G. (2017). A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells. Molecules, 22(7), 1123. https://doi.org/10.3390/molecules22071123





