Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin
Abstract
:1. Introduction
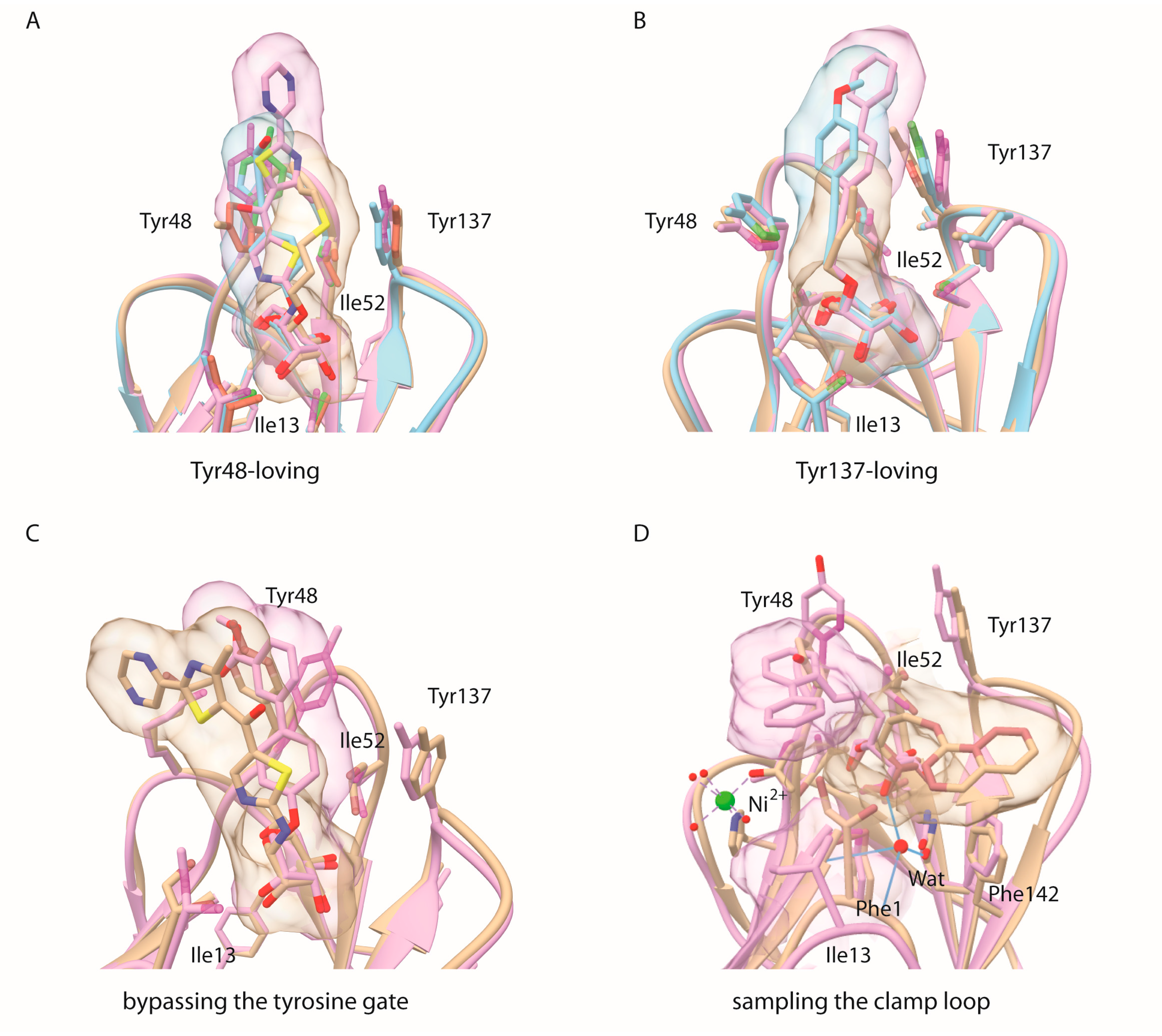
2. Results
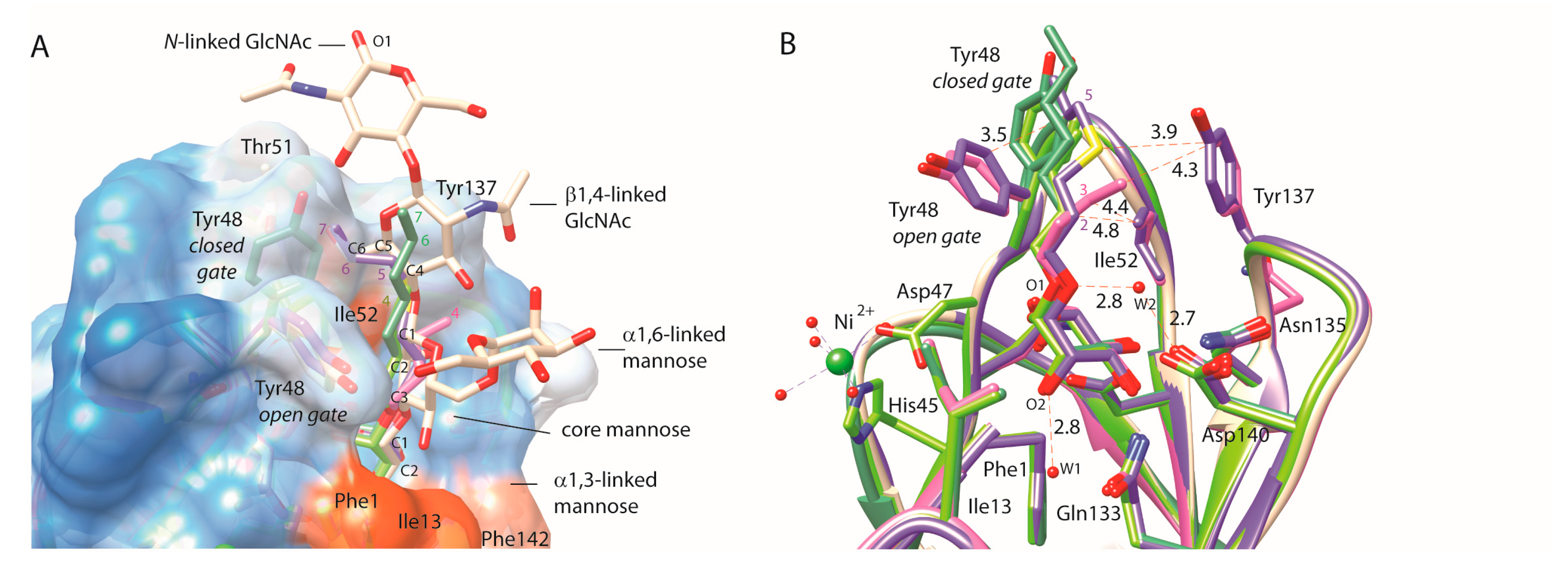
2.1. Alkane Aglycons, the Nature and Dynamics of Their Interactions
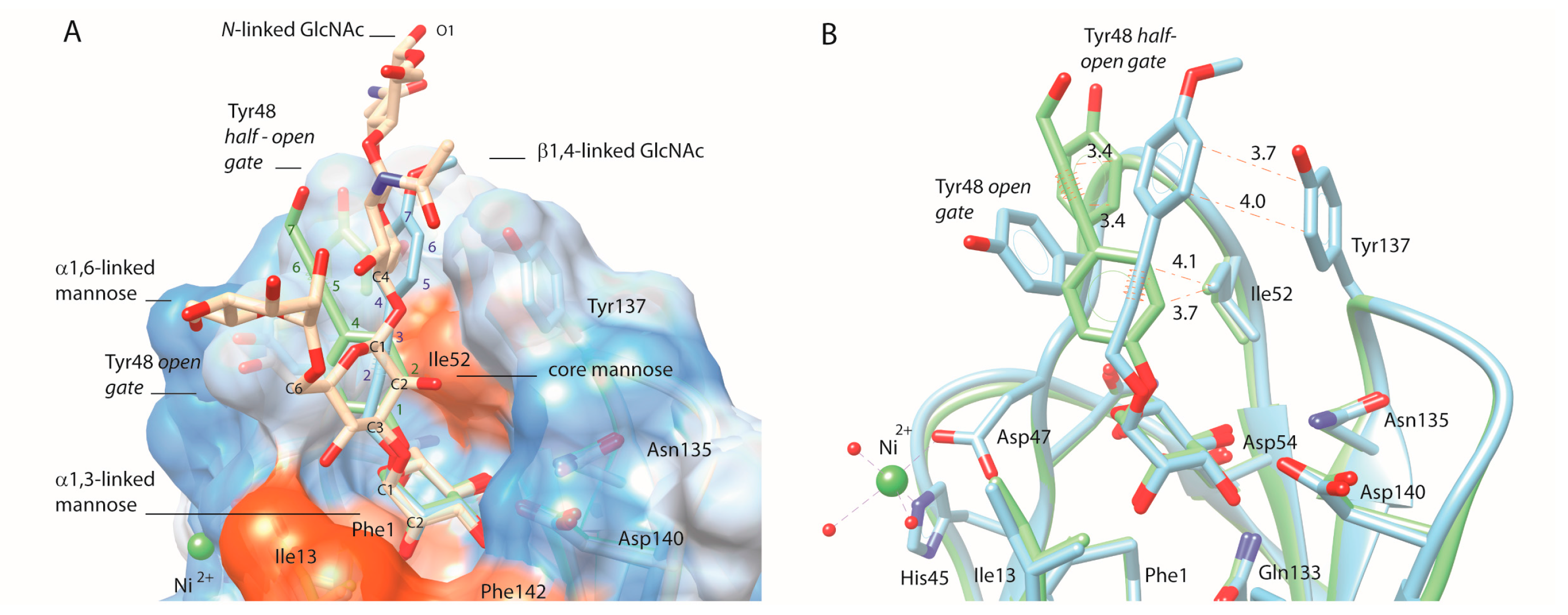
2.2. An Alkene, But Not an Alkyne, Linker Increases Affinity by Interaction with Ile52
2.3. Sulfonamide and Amide Spacers: Worse Fit and Bulky Compensation
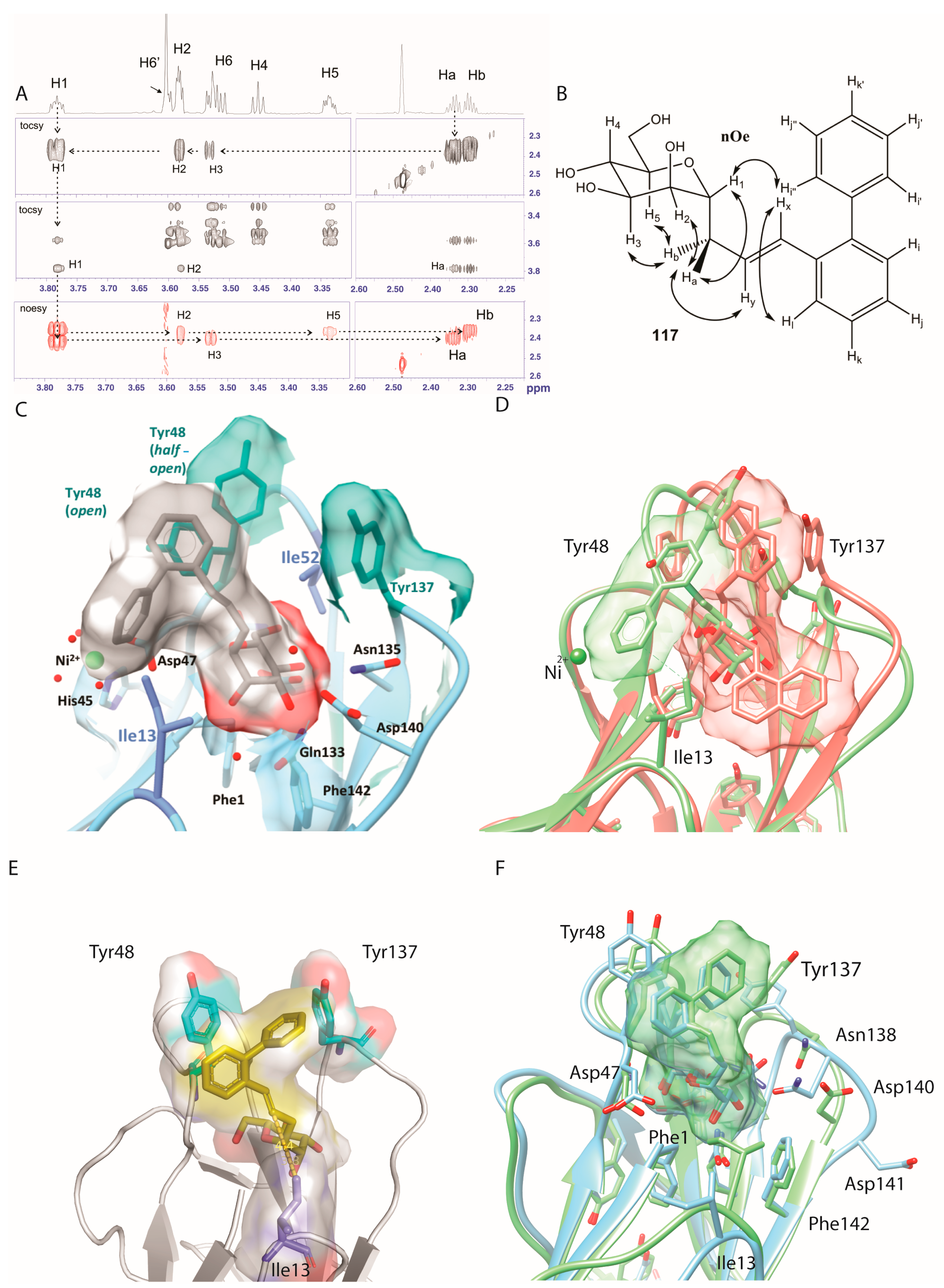
2.4. C-Mannopyranosides versus O-Mannopyranosides
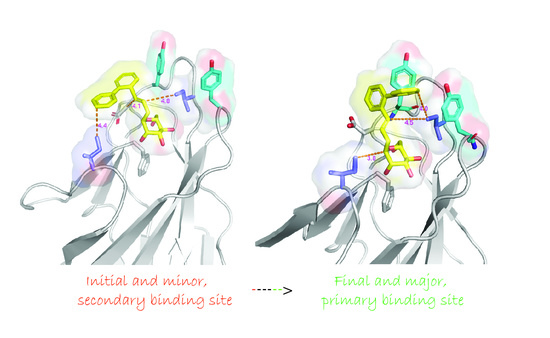
2.5. Ortho-Biphenyl C-Mannopyranoside 117
3. Discussion
4. Materials and Methods
4.1. Solution Affinity of Ligands for the FimH Adhesin Using SPR
4.1.1. Steady State Affinity Measurement
4.1.2. Competition Method
4.2. Haemagglutination Inhibition Assay
4.3. Induced Fit Docking
4.4. Molecular Dynamics Simulations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kau, A.L.; Hunstad, D.A.; Hultgren, S.J. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr. Opin. Microbiol. 2005, 8, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Barnich, N.; Sivignon, A.; Darcha, C.; Chan, C.H.; Stanners, C.P.; Darfeuille-Michaud, A. Crohn’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human ceacam. J. Exp. Med. 2009, 206, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Firon, N.; Ofek, I.; Sharon, N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae and Salmonella typhimurium. Carbohydr. Res. 1983, 120, 235–249. [Google Scholar] [CrossRef]
- Bouckaert, J.; Berglund, J.; Schembri, M.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.S.; Pinkner, J.; Slattegard, R.; Zavialov, A.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Wellens, A.; Garofalo, C.; Nguyen, H.; van Gerven, N.; Slattegard, R.; Hernalsteens, J.P.; Wyns, L.; Oscarson, S.; de Greve, H.; Hultgren, S.; et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the Fimh-oligomannose-3 complex. PLoS ONE 2008, 3, e2040. [Google Scholar] [CrossRef]
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Luthi, C.; Scharenberg, M.; Bezencon, J.; et al. Fimh antagonists for the oral treatment of urinary tract infections: From design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 2010, 53, 8627–8641. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Pinkner, J.S.; Ford, B.; Obermann, R.; Nolan, W.; Wildman, S.A.; Hobbs, D.; Ellenberger, T.; Cusumano, C.K.; Hultgren, S.J.; et al. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J. Med. Chem. 2010, 53, 4779–4792. [Google Scholar] [CrossRef] [PubMed]
- Schwardt, O.; Rabbani, S.; Hartmann, M.; Abgottspon, D.; Wittwer, M.; Kleeb, S.; Zalewski, A.; Smiesko, M.; Cutting, B.; Ernst, B. Design, synthesis and biological evaluation of mannosyl triazoles as FimH antagonists. Bioorg. Med. Chem. 2011, 19, 6454–6473. [Google Scholar] [CrossRef] [PubMed]
- Wellens, A.; Lahmann, M.; Touaibia, M.; Vaucher, J.; Oscarson, S.; Roy, R.; Remaut, H.; Bouckaert, J. The tyrosine gate as a potential entropic lever in the receptor-binding site of the bacterial adhesin FimH. Biochemistry 2012, 51, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Abgottspon, D.; Kleeb, S.; Rabbani, S.; Scharenberg, M.; Wittwer, M.; Haug, M.; Schwardt, O.; Ernst, B. Antiadhesion therapy for urinary tract infections-a balanced pk/pd profile proved to be key for success. J. Med. Chem. 2012, 55, 4700–4713. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Pinkner, J.S.; Ford, B.; Chorell, E.; Crowley, J.M.; Cusumano, C.K.; Campbell, S.; Henderson, J.P.; Hultgren, S.J.; Janetka, J.W. Lead optimization studies on FimH antagonists: Discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J. Med. Chem. 2012, 55, 3945–3959. [Google Scholar] [CrossRef] [PubMed]
- Brument, S.; Sivignon, A.; Dumych, T.I.; Moreau, N.; Roos, G.; Guerardel, Y.; Chalopin, T.; Deniaud, D.; Bilyy, R.O.; Darfeuille-Michaud, A.; et al. Thiazolylaminomannosides as potent anti-adhesives of type 1 piliated Escherichia coli isolated from Crohn’s disease patients. J. Med. Chem. 2013, 56, 5395–5406. [Google Scholar] [CrossRef] [PubMed]
- Roos, G.; Wellens, A.; Touaibia, M.; Yamakawa, N.; Geerlings, P.; Roy, R.; Wyns, L.; Bouckaert, J. Validation of reactivity descriptors to assess the aromatic stacking within the tyrosine gate of FimH. ACS Med. Chem. Lett. 2013, 4, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Vanwetswinkel, S.; Volkov, A.N.; Sterckx, Y.G.; Garcia-Pino, A.; Buts, L.; Vranken, W.F.; Bouckaert, J.; Roy, R.; Wyns, L.; van Nuland, N.A. Study of the structural and dynamic effects in the FimH adhesin upon alpha-d-heptyl mannose binding. J. Med. Chem. 2014, 57, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S.G.; Roos, G.; Bouckaert, J. Discovery and application of FimH antagonists. Top. Med. Chem. 2014, 12, 123–168. [Google Scholar]
- Chalopin, T.; Brissonnet, Y.; Sivignon, A.; Deniaud, D.; Cremet, L.; Barnich, N.; Bouckaert, J.; Gouin, S.G. Inhibition profiles of mono- and polyvalent FimH antagonists against 10 different Escherichia coli strains. Org. Biomol. Chem. 2015, 13, 11369–11375. [Google Scholar] [CrossRef] [PubMed]
- Sivignon, A.; Yan, X.; Alvarez Dorta, D.; Bonnet, R.; Bouckaert, J.; Fleury, E.; Bernard, J.; Gouin, S.G.; Darfeuille-Michaud, A.; Barnich, N. Development of heptylmannoside-based glycoconjugate antiadhesive compounds against adherent-invasive Escherichia coli bacteria associated with Crohn’s disease. mBio 2015, 6, e01298-15. [Google Scholar] [CrossRef] [PubMed]
- Mydock-McGrane, L.; Cusumano, Z.; Han, Z.; Binkley, J.; Kostakioti, M.; Hannan, T.; Pinkner, J.S.; Klein, R.; Kalas, V.; Crowley, J.; et al. Antivirulence C-mannosides as antibiotic-sparing, oral therapeutics for urinary tract infections. J. Med. Chem. 2016, 59, 9390–9408. [Google Scholar] [CrossRef] [PubMed]
- Mydock-McGrane, L.K.; Cusumano, Z.T.; Janetka, J.W. Mannose-derived FimH antagonists: A promising anti-virulence therapeutic strategy for urinary tract infections and Crohn’s disease. Expert Opin. Ther. Pat. 2016, 26, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, C.; Han, Z.; Kalas, V.; Klein, R.; Pinkner, J.S.; Ford, B.; Binkley, J.; Cusumano, C.K.; Cusumano, Z.; Mydock-McGrane, L.; et al. Antivirulence isoquinolone mannosides: Optimization of the biaryl aglycone for FimH lectin binding affinity and efficacy in the treatment of chronic UTI. ChemMedChem 2016, 11, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, T.; Alvarez Dorta, D.; Sivignon, A.; Caudan, M.; Dumych, T.I.; Bilyy, R.O.; Deniaud, D.; Barnich, N.; Bouckaert, J.; Gouin, S.G. Second generation of thiazolylmannosides, FimH antagonists for E. coli-induced Crohn’s disease. Org. Biomol. Chem. 2016, 14, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Dorta, D.; Sivignon, A.; Chalopin, T.; Dumych, T.I.; Roos, G.; Bilyy, R.O.; Deniaud, D.; Krammer, E.M.; de Ruyck, J.; Lensink, M.F.; et al. The antiadhesive strategy in crohn’s disease: Orally active mannosides to decolonize pathogenic Escherichia coli from the gut. Chembiochem 2016, 17, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Kleeb, S.; Jiang, X.; Frei, P.; Sigl, A.; Bezencon, J.; Bamberger, K.; Schwardt, O.; Ernst, B. Fimh antagonists: Phosphate prodrugs improve oral bioavailability. J. Med. Chem. 2016, 59, 3163–3182. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Krammer, E.-M.; Roos, G.; Zalewski, A.; Preston, R.; Eid, S.; Zihlmann, P.; Prevost, M.; Lensink, M.F.; Thompson, A.; et al. Mutation of Tyr137 of the universal Escherichia coli fimbrial adhesin FimH relaxes the tyrosine gate prior to mannose binding. IUCrJ 2017, 4, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar]
- Dreux, N.; Denizot, J.; Martinez-Medina, M.; Mellmann, A.; Billig, M.; Kisiela, D.; Chattopadhyay, S.; Sokurenko, E.; Neut, C.; Gower-Rousseau, C.; et al. Point mutations in FimH adhesin of Crohn’s disease-associated adherent-invasive Escherichia coli enhance intestinal inflammatory response. PLoS Pathog. 2013, 9, e1003141. [Google Scholar] [CrossRef] [PubMed]
- Asensio, J.L.; Arda, A.; Canada, F.J.; Jimenez-Barbero, J. Carbohydrate-aromatic interactions 11. Acc. Chem. Res. 2013, 46, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Fiege, B.; Rabbani, S.; Preston, R.C.; Jakob, R.P.; Zihlmann, P.; Schwardt, O.; Jiang, X.; Maier, T.; Ernst, B. The tyrosine gate of the bacterial lectin FimH: A conformational analysis by NMR spectroscopy and X-ray crystallography. Chembiochem 2015, 16, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Dorta, D.; Chalopin, T.; Sivignon, A.; De Ruyck, J.; Dumych, T.; Bilyy, R.; Deniaud, D.; Barnich, N.; Bouckaert, J.; Gouin, S.G. Physiochemical tuning of potent E. coli antiadhesives by microencapsulation and methylene homologation. ChemMedChem 2017, 12, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Ladeveze, S.; Cioci, G.; Roblin, P.; Mourey, L.; Tranier, S.; Potocki-Veronese, G. Structural bases for N-glycan processing by mannoside phosphorylase. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, C.; Bednarski, M.D. Modern methods in carbohydrate synthesis. In Synthesis of C-Glycosides: Stable Mimics of O-Glycosidic Linkages; Harwood Academic Publishers: Reading, UK, 1996; pp. 316–351. [Google Scholar]
- Pang, L.; Kleeb, S.; Lemme, K.; Rabbani, S.; Scharenberg, M.; Zalewski, A.; Schadler, F.; Schwardt, O.; Ernst, B. Fimh antagonists: Structure-activity and structure-property relationships for biphenyl α-d-mannopyranosides. ChemMedChem 2012, 7, 1404–1422. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, J.; Mackenzie, J.; de Paz, J.L.; Chipwaza, B.; Choudhury, D.; Zavialov, A.; Mannerstedt, K.; Anderson, J.; Pierard, D.; Wyns, L.; et al. The affinity of the Fimh fimbrial adhesin is receptor-driven and quasi-independent of Escherichia coli pathotypes. Mol. Microbiol. 2006, 61, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.F.; Taganna, J.; Bouckaert, J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli Fimh-towards novel bacterial anti-adhesives. Chem. Commun.(Camb.) 2011, 47, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, M.; Schwardt, O.; Rabbani, S.; Ernst, B. Target selectivity of FimH antagonists. J. Med. Chem. 2012, 55, 9810–9816. [Google Scholar] [CrossRef] [PubMed]
- Le Trong, I.; Aprikian, P.; Kidd, B.A.; Forero-Shelton, M.; Tchesnokova, V.; Rajagopal, P.; Rodriguez, V.; Interlandi, G.; Klevit, R.; Vogel, V.; et al. Structural basis for mechanical force regulation of the adhesin FimH via finger trap-like beta sheet twisting. Cell 2010, 141, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Le Trong, I.; Aprikian, P.; Kidd, B.A.; Thomas, W.E.; Sokurenko, E.V.; Stenkamp, R.E. Donor strand exchange and conformational changes during E. coli fimbrial formation. J. Struct. Biol. 2010, 172, 380–388. [Google Scholar] [CrossRef] [PubMed]
- De Ruyck, J.; Lensink, M.F.; Bouckaert, J. Structures of C-mannosylated anti-adhesives bound to the type 1 fimbrial FimH adhesin. IUCrJ 2016, 3, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Rabbani, S.; Gobec, M.; Raščan, I.M.; Podlipnik, C.; Ernst, B.; Anderluh, M. Branched α-d-mannopyranosides: A new class of potent FimH antagonists. MedChemComm 2014, 5, 1247–1253. [Google Scholar] [CrossRef]
- Eris, D.; Preston, R.C.; Scharenberg, M.; Hulliger, F.; Abgottspon, D.; Pang, L.; Jiang, X.; Schwardt, O.; Ernst, B. The conformational variability of FimH: Which conformation represents the therapeutic target? Chembiochem 2016, 17, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, L.M.; Hernaiz, M.J.; Martin-Pastor, M.; Skrydstrup, T.; Jimenez-Barbero, J. Conformation of glycomimetics in the free and protein-bound state: Structural and binding features of the C-glycosyl analogue of the core trisaccharide α-d-man-(1 --> 3)-[α-d-man-(1 --> 6)]-d-man. J. Am. Chem. Soc. 2002, 124, 14940–14951. [Google Scholar] [CrossRef] [PubMed]
- Kisiela, D.I.; Avagyan, H.; Friend, D.; Jalan, A.; Gupta, S.; Interlandi, G.; Liu, Y.; Tchesnokova, V.; Rodriguez, V.B.; Sumida, J.P. Inhibition and reversal of microbial attachment by an antibody with parasteric activity against the fimh adhesin of uropathogenic E. coli. PLoS Pathog. 2015, 11, e1004857. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Zagorodko, O.; Cogez, V.; Dumych, T.; Chalopin, T.; Alvarez Dorta, D.; Sivignon, A.; Barnich, N.; Harduin-Lepers, A.; Larroulet, I.; et al. Differentiation of Crohn’s disease-associated isolates from other pathogenic Escherichia coli by fimbrial adhesion under shear force. Biology (Basel) 2016, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J. Optimization of parameters for semiempirical methods vi: More modifications to the nddo approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Storer, J.W.; Giesen, D.J.; Cramer, C.J.; Truhlar, D.G. Class IV charge models: A new semiempirical approach in quantum chemistry. J. Comput. Aided Mol. Des. 1995, 9, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Vergoten, G.; Mazur, I.; Lagant, P.; Michalski, J.C.; Zanetta, J.P. The spasiba force field as an essential tool for studying the structure and dynamics of saccharides. Biochimie 2003, 85, 65–73. [Google Scholar] [CrossRef]
- Lagant, P.; Nolde, D.; Stote, R.; Vergoten, G.; Karplus, M. Increasing normal modes analysis accuracy: The SPASIBA spectroscopic force field introduced into the CHARMM program. J. Phys. Chem. A 2004, 108, 4019–4029. [Google Scholar] [CrossRef]
- Bas, D.C.; Rogers, D.M.; Jensen, J.H. Very fast prediction and rationalization of pKa values for protein-ligand complexes. Proteins 2008, 73, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. Charmm general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.Y.D.; Pedersen, L. Particle mesh ewald:An n*log(n) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Tuckerman, M.B.; Berne, B.J.; Martyna, G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992, 97, 1990. [Google Scholar] [CrossRef]
- Andersen, H.C. Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 1983, 52, 24–34. [Google Scholar] [CrossRef]
- Pronk, S.; Pall, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. Gromacs 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| R | Cpd | Kd SPR (nM) Kd ITC (nM) HAI (µM) | R | Cpd | Kd SPR (nM) Kd ITC (nM) HAI (µM) | Cpd | Kd SPR (nM) Kd ITC (nM) HAI (µM) |
|---|---|---|---|---|---|---|---|
| H | 4 | 151 [4] 156 ± 45 [9] 100 |  | 35 | 16 ± 2.4 -- -- | 106 | 25 ± 6.3 -- -- |
 | 5a | 21± 0.2 [4] -- -- |  | 36 | 22 ± 2.7 -- -- | 107 | 22 ± 2.8 -- -- |
 | 5 | 4.3 ± 0.3 [4] 7.3 ± 1.8 [9] 6.25 |  | 37 | 14 ± 5.0 59.5 ± 4.7 [9] 25 | 108 | 36 ± 9.0 -- -- |
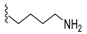 | 5b | 18 ± 0.4 Ss 17 ± 0.4 -- |  | 38 | 14 ± 7.6 -- -- | 109 | 78 ± 5.6 -- -- |
 | 39 | 11 ± 6.1 -- -- | 110 | 32 ± 4.1 -- -- |

| R | Cpd | Kd SPR (nM) Kd ITC (nM) HAI (µM) | Cpd | Kd SPR (nM) Kd ITC nM) HAI (µM) | Cpd | Kd SPR (nM) Kd ITC (nM) HAI (µM) |
|---|---|---|---|---|---|---|
 | 22 | 53 ± 7.5 -- -- | 26 | 59 ± 8.5 -- -- | 50 | 168 ± 15 -- -- |
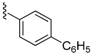 | 23 | 3.0 ± 1.1 -- 25 | 27 | 10 ± 0.2 1.5 ± 0.3 25 | 51 | 405 ± 24 61 ± 9.6 100 |
 | 24 | 4.3 ± 0.8 -- -- | 28 | 13 ± 1.7 -- -- | 52 | 2250 ± 291 -- -- |
 | 25 | 5.0 ± 1.4 71 ± 23 50 | 29 | 22 ± 3.0 -- 25 | 53 | 120 ± 18 -- 50 |

| Cpd | R | Kd SPR (nM) | Kd ITC (nM) | HAI (µM) |
|---|---|---|---|---|
| 41 | H | 490 ± 55 | -- | -- |
| 54 |  | 133 ± 31 | -- | -- |
| 55 |  | 83 ± 5.6 | 105 ± 34 | 50 |
| 56 |  | 53 ± 2.3 | 104.6 ± 21.9 | 50 |
| 57 |  | 816 ± 105 | -- | -- |
| 61 | H | 36.9 ± 3.0 | 18.3 ± 5.9 | 12.5 |
| 62 | Me | nf * | 59.5 ± 2.3 | 25 |

| Cpd | R | Kd (nM) | Cpd | R | Kd (nM) | Cpd | R | Kd (nM) | Cpd | R | Kd (nM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 70 | 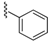 | 146 ± 26 | 73 |  | 122 ± 11 | 91 | 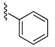 | 372 ± 26 | 94 | 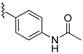 | 227 ± 54 |
| 71 |  | 408 ± 52 | 74 | 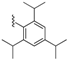 | 37 ± 5 | 92 |  | 397 ± 83 | 95 |  | 328 ± 63 |
| 72 |  | 168 ± 64 | 75 | 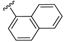 | 148 ± 18 | 93 |  | 105 ± 44 | 96 | 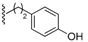 | 970 ± 382 |

| R | Cpd | Kd (nM) | Cpd | Kd (nM) |
|---|---|---|---|---|
 | 115 | 182 ± 25 | 119 | 49 ± 5.3 |
 | 116 | 17 ± 3.5 | 120 | nf |
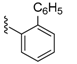 | 117 | 6.9 ± 5.7 | 121 | 123 ± 21 |
 | 118 | nf | 122 | nf |
| H | 78 | 118 ± 20 | 123 | 266 ± 26 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touaibia, M.; Krammer, E.-M.; Shiao, T.C.; Yamakawa, N.; Wang, Q.; Glinschert, A.; Papadopoulos, A.; Mousavifar, L.; Maes, E.; Oscarson, S.; et al. Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules 2017, 22, 1101. https://doi.org/10.3390/molecules22071101
Touaibia M, Krammer E-M, Shiao TC, Yamakawa N, Wang Q, Glinschert A, Papadopoulos A, Mousavifar L, Maes E, Oscarson S, et al. Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules. 2017; 22(7):1101. https://doi.org/10.3390/molecules22071101
Chicago/Turabian StyleTouaibia, Mohamed, Eva-Maria Krammer, Tze C. Shiao, Nao Yamakawa, Qingan Wang, Anja Glinschert, Alex Papadopoulos, Leila Mousavifar, Emmanuel Maes, Stefan Oscarson, and et al. 2017. "Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin" Molecules 22, no. 7: 1101. https://doi.org/10.3390/molecules22071101
APA StyleTouaibia, M., Krammer, E.-M., Shiao, T. C., Yamakawa, N., Wang, Q., Glinschert, A., Papadopoulos, A., Mousavifar, L., Maes, E., Oscarson, S., Vergoten, G., Lensink, M. F., Roy, R., & Bouckaert, J. (2017). Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules, 22(7), 1101. https://doi.org/10.3390/molecules22071101