Metabolomics Characterization of Two Apocynaceae Plants, Catharanthus roseus and Vinca minor, Using GC-MS and LC-MS Methods in Combination
Abstract
:1. Introduction
2. Results
2.1. Leaf Morphology Characteristics
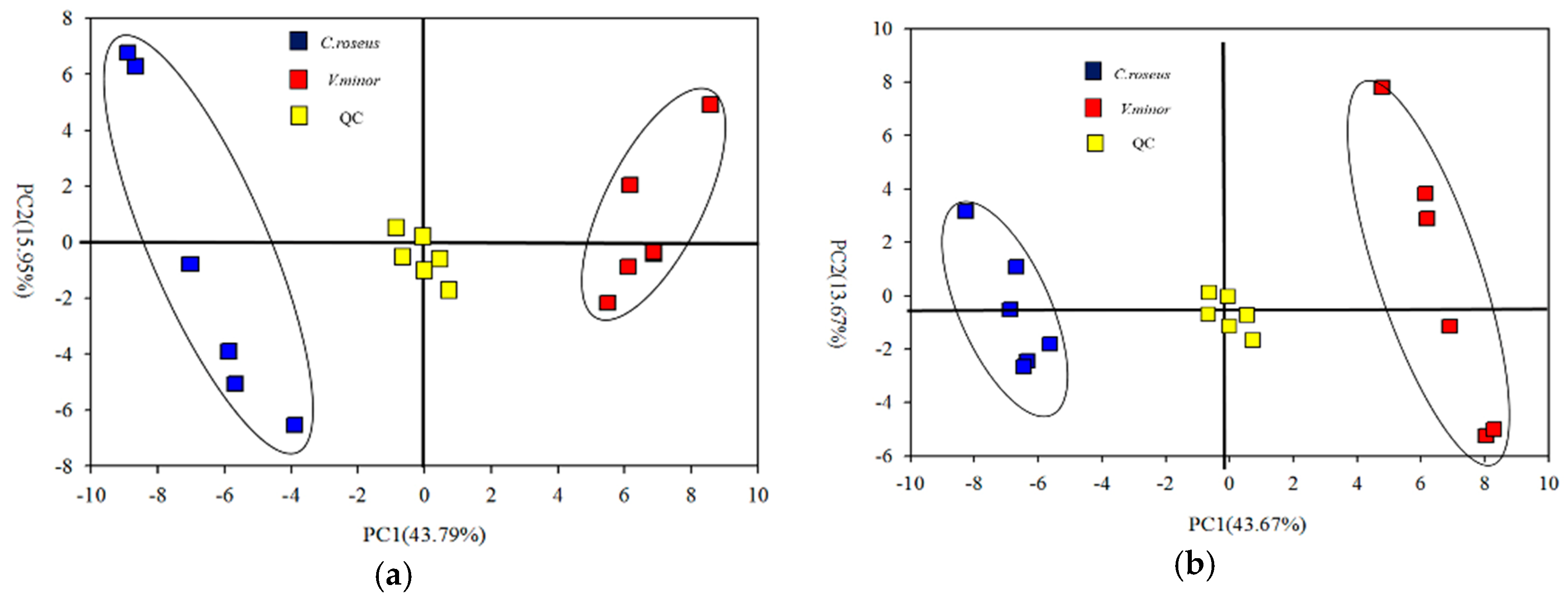
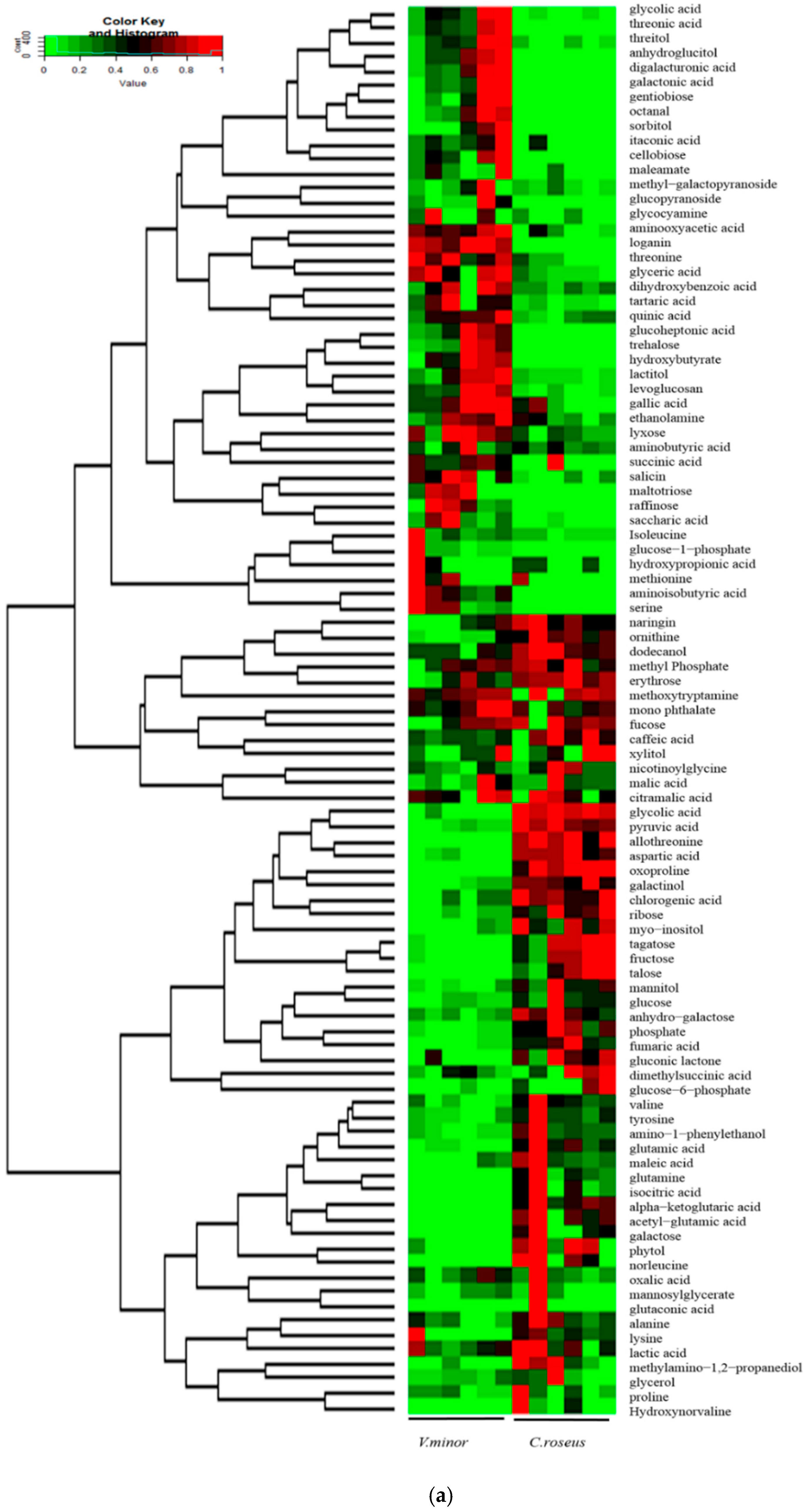
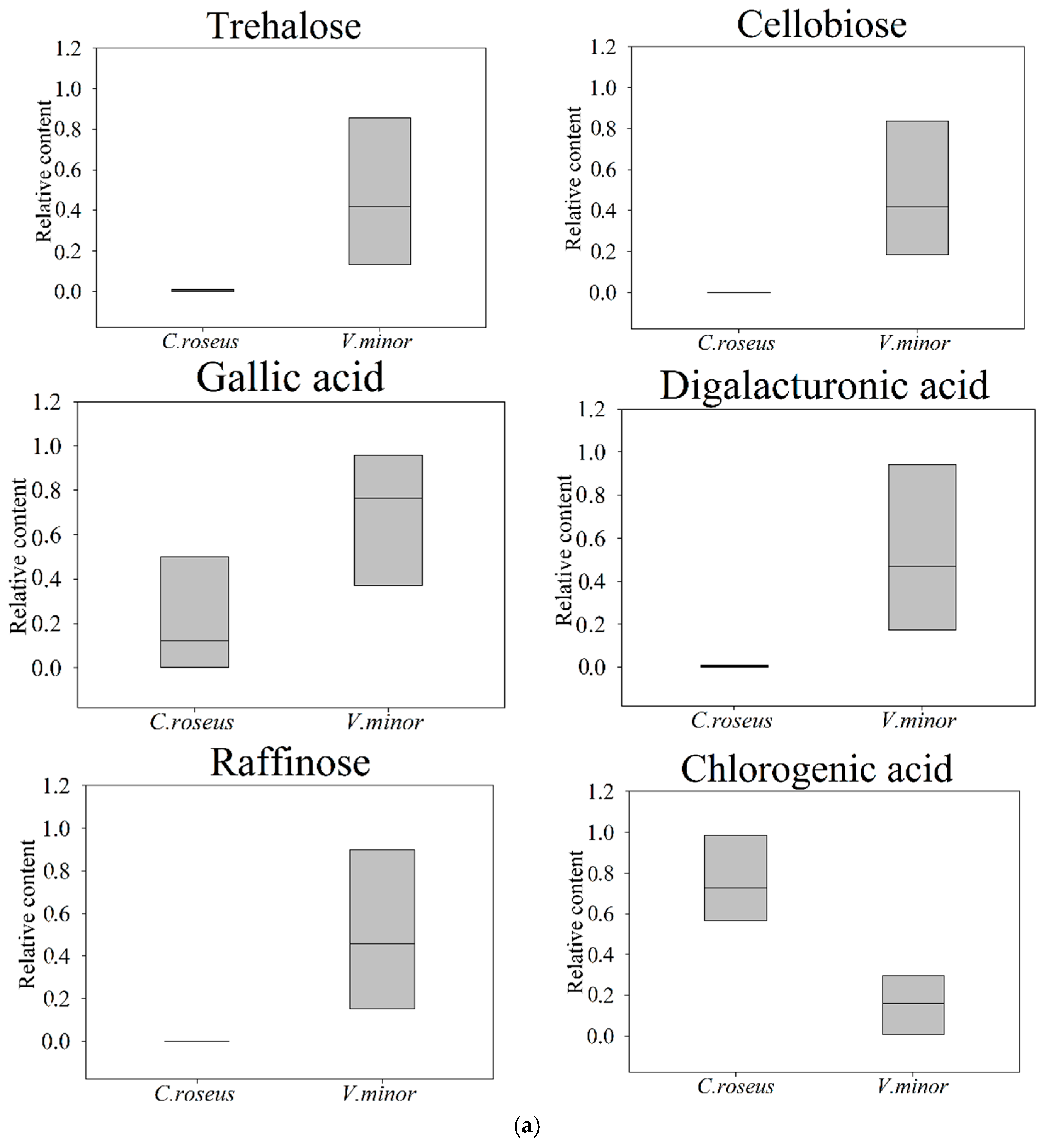
2.2. Primary Metabolic Profiling
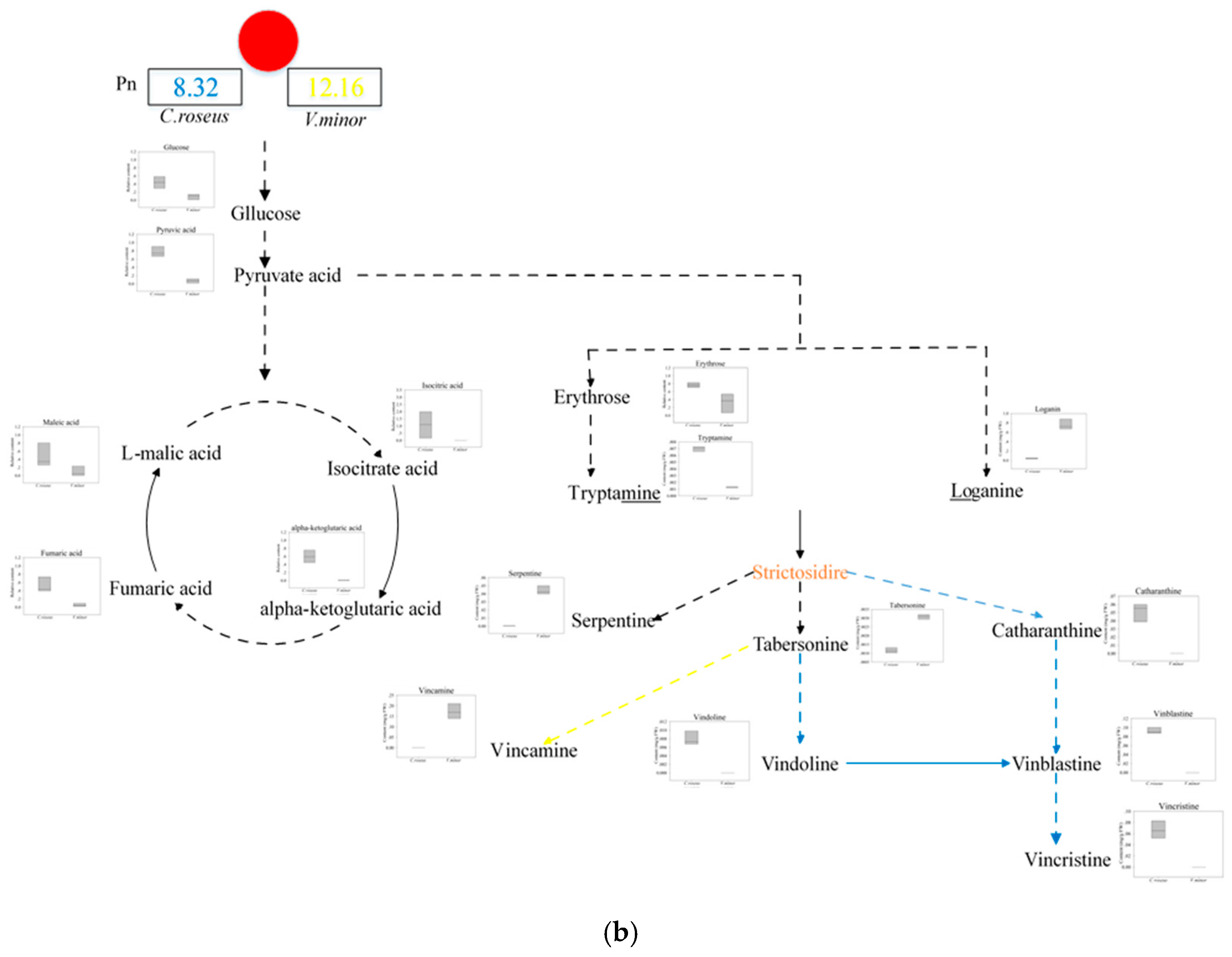
2.3. The Content of TIAs
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Leaf Morphology and Physiological Parameters
4.3. GC-MS Analysis
4.4. LC-MS Analysis
4.5. Statistical Analysis
Supplementary materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verma, P.; Sharma, A.; Khan, S.A.; Shanker, K.; Mathur, A.K. Over-expression of Catharanthus roseus tryptophan decarboxylase and strictosidine synthase in rol gene integrated transgenic cell suspensions of Vinca minor. Protoplasma 2015, 252, 373–381. [Google Scholar] [CrossRef]
- Verma, P.; Mathur, A.K.; Shanker, K. Enhanced vincamine production in selected tryptophan-overproducing shoots of Vinca minor. PCTOC 2012, 111, 239–245. [Google Scholar] [CrossRef]
- Belal, T.S.; Barary, M.H.; Sabry, S.M.; Elsayed, M.; Ibrahim, A.L. Kinetic Spectrophotometric Analysis of Naftidrofuryl Oxalate and Vincamine in Pharmaceutical Preparations Using Alkaline Potassium Permanganate. J. Food Drug Anal. 2009, 17, 415–423. [Google Scholar]
- Debast, S.; Nunes-Nesi, A.; Hajirezaei, M.R.; Hofmann, J.; Sonnewald, U.; Fernie, A.R.; Börnke, F. Altering Trehalose-6-Phosphate Content in Transgenic Potato Tubers Affects Tuber Growth and Alters Responsiveness to Hormones during Sprouting. Plant Physiol. 2011, 156, 1754–1771. [Google Scholar] [CrossRef]
- Molchan, O.; Romashko, S.; Yurin, V. l-tryptophan decarboxylase activity and tryptamine accumulation in callus cultures of Vinca minor L. PCTOC 2012, 108, 535–539. [Google Scholar] [CrossRef]
- Verma, P.; Khan, S.A.; Mathur, A.K.; Shanker, K.; Lal, R.K. Regulation of vincamine biosynthesis and associated growth promoting effects through abiotic elicitation, cyclooxygenase inhibition, and precursor feeding of bioreactor grown Vinca minor hairy roots. Appl. Biochem. Biotechnol. 2014, 173, 663–672. [Google Scholar] [CrossRef]
- Verma, P.; Mathur, A.K.; Srivastava, A.; Mathur, A. Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloid pathway in Catharanthus roseus: A literature update. Protoplasma 2012, 249, 255–268. [Google Scholar] [CrossRef]
- Van, D.H.R.; Jacobs, D.I.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar]
- Suttipanta, N.; Pattanaik, S.; Kulshrestha, M.; Patr, B.; Singh, S.K.; Yuan, L. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011, 157, 2081–2093. [Google Scholar] [CrossRef]
- Afrin, S.; Huang, J.J.; Luo, Z.Y. JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 2015, 60, 1062–1072. [Google Scholar] [CrossRef]
- Nunes, C.; Primavesi, L.F.; Patel, M.K.; Martinez-Barajas, E.; Powers, S.J.; Sagar, R.; Fevereiro, P.S.; Davis, B.G.; Paul, M.J. Inhibition of SnRK1 by metabolites: Tissue-dependent effects and cooperative inhibition by glucose 1-phosphate in combination with trehalose 6-phosphate. Plant Physiol. Biochem. 2012, 63, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Baenagonzález, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Signaling Role of Sucrose Metabolism in Development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef]
- Vandesteene, L.; Dijck, P.V. Expansive evolution of the trehalose-6-phosphate phosphatase gene family in Arabidopsis. Plant Physiol. 2012, 160, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Ecker, J.R. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef]
- Schluepmann, H.; Paul, M. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6849–6854. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, A.; Tiessen, A.; Schluepmann, H.; Paul, M.; Ulrich, S.; Geigenberger, P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA 2005, 102, 11118–11123. [Google Scholar] [CrossRef]
- Saito, M.; Yoshida, M. Expression analysis of the gene family associated with raffinose accumulation in rice seedlings under cold stress. J. Plant Physiol. 2011, 168, 2268–2271. [Google Scholar] [CrossRef]
- Gilmour, S.J.; Thomashow, M.F. Overexpression of the Arabidopsis CBF3 Transcriptional Activator Mimics Multiple Biochemical Changes Associated with Cold Acclimation. Plant Physiol. 2000, 124, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.R.; Jouve, L.; Hausman, J.F.; Hoffmann, L.; Stewart, J.M. Alteration of oxidative and carbohydrate metabolism under abiotic stress in two rice (Oryza sativa L.) genotypes contrasting in chilling tolerance. J. Plant Physiol. 2007, 164, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef]
- Lou, Z.X.; Wang, H.X.; Lv, W.P.; Ma, C.Y.; Wang, Z.P.; Chen, S.W. Assessment of antibacterial activity of fractions from burdock leaf against food-related bacteria. Food Control 2010, 21, 1272–1278. [Google Scholar] [CrossRef]
- Ferreira, A.A.; Leal, A.S.; Ferraz, V.P.; Machado, Y.; Takahashi, J.A. Effects of gamma radiation on the chemistry and antibacterial activity of artichoke leaves. Bol. Latinoam. Caribe Plant Med. Aromat. 2010, 9, 232–237. [Google Scholar]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Zhang, L.; Zhu, F.H.; He, P.L.; Tang, W.; et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Ncube, E.N.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Chlorogenic Acids Biosynthesis in Centella asiatica Cells Is not Stimulated by Salicylic Acid Manipulation. Appl. Biochem. Biotechnol. 2016, 179, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Lee, S.; Kim, H.Y.; Lee, C.H. MS-Based Metabolite Profiling of Aboveground and Root Components of Zingiber mioga and Officinale. Molecules 2015, 20, 16170–16185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nägele, T.; Doerfler, H.; Fragner, L.; Chaturvedi, P.; Nukarinen, E.; Bellaire, A.; Huber, W.; Weiszmann, J.; Engelmeier, D.; et al. System level analysis of cacao seed ripening reveals a sequential interplay of primary and secondary metabolism leading to polyphenol accumulation and preparation of stress resistance. Plant J. Cell Mol. Biol. 2016, 87, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. Cell Mol. Biol. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.-M.; Stittet, M.; et al. The sucrose-trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J. Exp. Bot. 2014, 65, 1051–1068. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, S.; Ozdemir, F.; Bor, M. Contribution of trehalose biosynthetic pathway to drought stress tolerance of Capparis ovata Desf. Plant Biol. 2015, 17, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Pellny, T.K.; Ghannoum, O.; Conroy, J.P.; Schluepmann, H.; Smeekens, S.; Andralojc, J.; Krause, K.P.; Goddijn, O.; Paul, M.J. Genetic modification of photosynthesis with E. coli genes for trehalose synthesis. Plant Biotechnol. J. 2004, 2, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E. Gene families and evolution of trehalose metabolism in plants. Funct. Plant Biol. 2007, 34, 550–563. [Google Scholar] [CrossRef]
- Houtte, H.V.; Lópezgalvis, L.; Vandesteene, L.; Beeckman, T.; Dijck, P.V. Redundant and non-redundant roles of the trehalose-6-phosphate phosphatases in leaf growth, root hair specification and energy-responses in Arabidopsis. Plant Signal. Behav. 2013, 8, e23209. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.C.; Hejazi, M.; Fettke, J.; Steup, M.; Feil, R.; Krause, U.; Arrivault, S.; Vosloh, D.; Figueroa, C.M.; Ivakov, A.; et al. Feedback inhibition of starch degradation in Arabidopsis leaves mediated by trehalose 6-phosphate. Plant Physiol. 2013, 163, 1142–1163. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Flis, A.; Ivakov, A.A.; Apelt, F.; Krohn, N.; Encke, B.; Abel, C.; Feil, R.; Lunn, J.E.; Stitt, M. Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol. Plant 2014, 7, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.K.C.; Lee, S.J. Straying off the highway: Trafficking of secreted plant proteins and complexity in the plant cell wall proteome. Plant Physiol. 2010, 153, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Kohorn, B.D.; Kohorn, S.L. The cell wall-associated kinases, WAKs, as pectin receptors. Front. Plant Sci. 2012, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Getachew, G.; Pittroff, W.; Putnam, D.H.; Dandekar, A.; Goyal, S.; Depeters, E.J. The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Anim. Feed Sci. Technol. 2008, 140, 444–461. [Google Scholar] [CrossRef]
- Vogel, J. Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 2008, 11, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Hématy, K.; Höfte, H. Growth control and cell wall signaling in plants. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.L.; Meng, F.Z.; Wang, H.Y.; Wei, Y.X.; Li, R.F.; Wang, Z.Y.; Hu, L.P.; Wang, S.-H.; Zhang, Z.-X. Molecular cloning, characteristics and low temperature response of raffinose synthase gene in Cucumis sativus L. J. Plant Physiol. 2012, 169, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Obendorf, R.L.; Sensenig, E.M.; Wu, J.; Ohashi, M.; O’Sullivan, T.E.; Kosina, S.M.; Schnebly, S.R. Soluble carbohydrates in mature soybean seed after feeding d-chiro-inositol, myo-inositol, or d-pinitol to stem-leaf-pod explants of low-raffinose, low-stachyose lines. Plant Sci. 2008, 175, 650–655. [Google Scholar] [CrossRef]
- Knaupp, M.; Mishra, K.B.; Nedbal, L.; Heyer, A.G. Evidence for a role of raffinose in stabilizing photosystem II during freeze-thaw cycles. Planta 2011, 234, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Carbo, M.; Aroca, R.; Gonzàlez-Meler, M.A.; Irigoyen, J.J.; Sánchez-Dı́az, M. The Electron Partitioning between the Cytochrome and Alternative Respiratory Pathways during Chilling Recovery in Two Cultivars of Maize Differing in Chilling Sensitivity. Plant Physiol. 2000, 122, 199–204. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are provided by the authors. |

| Indicator/Parameter | C. roseus | V. minor |
|---|---|---|
| LL (cm) | 5.08 ± 0.37 | 2.36 ± 0.25 |
| LW (cm) | 2.50 ± 0.21 | 1.41 ± 0.19 |
| LA (cm2) | 8.42 ± 0.86 | 2.67 ± 0.41 |
| FW (g) | 0.15 ± 0.010 | 0.07 ± 0.0087 |
| LT (cm) | 0.115 ± 0.012 | 0.19 ± 0.018 |
| RWC (%) | 88.67 ± 7.32 | 51.43 ± 3.81 |
| LMA (g/cm2) | 0.018 ± 0.0021 | 0.027 ± 0.0013 |
| Pn (μmol CO2 m−2 s−1) | 8.03 ± 0.23 | 11.67 ± 0.50 |
| Gs (mol H2O m−2 s−1) | 0.042 ± 0.0002 | 0.038 ± 0.0008 |
| Tr (mmol H2O m−2 s−1) | 1.56 ± 0.0164 | 1.49 ± 0.016 |
| Fv/Fm | 0.815 ± 0.0083 | 0.841 ± 0.0084 |
| Y (II) | 0.711 ± 0.0199 | 0.761 ± 0.0112 |
| Chl (mg/g) | 11.23 ± 2.54 | 7.93 ± 1.65 |
| Chla/chlb | 4.17 ± 0.55 | 3.34 ± 0.36 |
| Temperature (°C) | 20–33 | 15–25 |
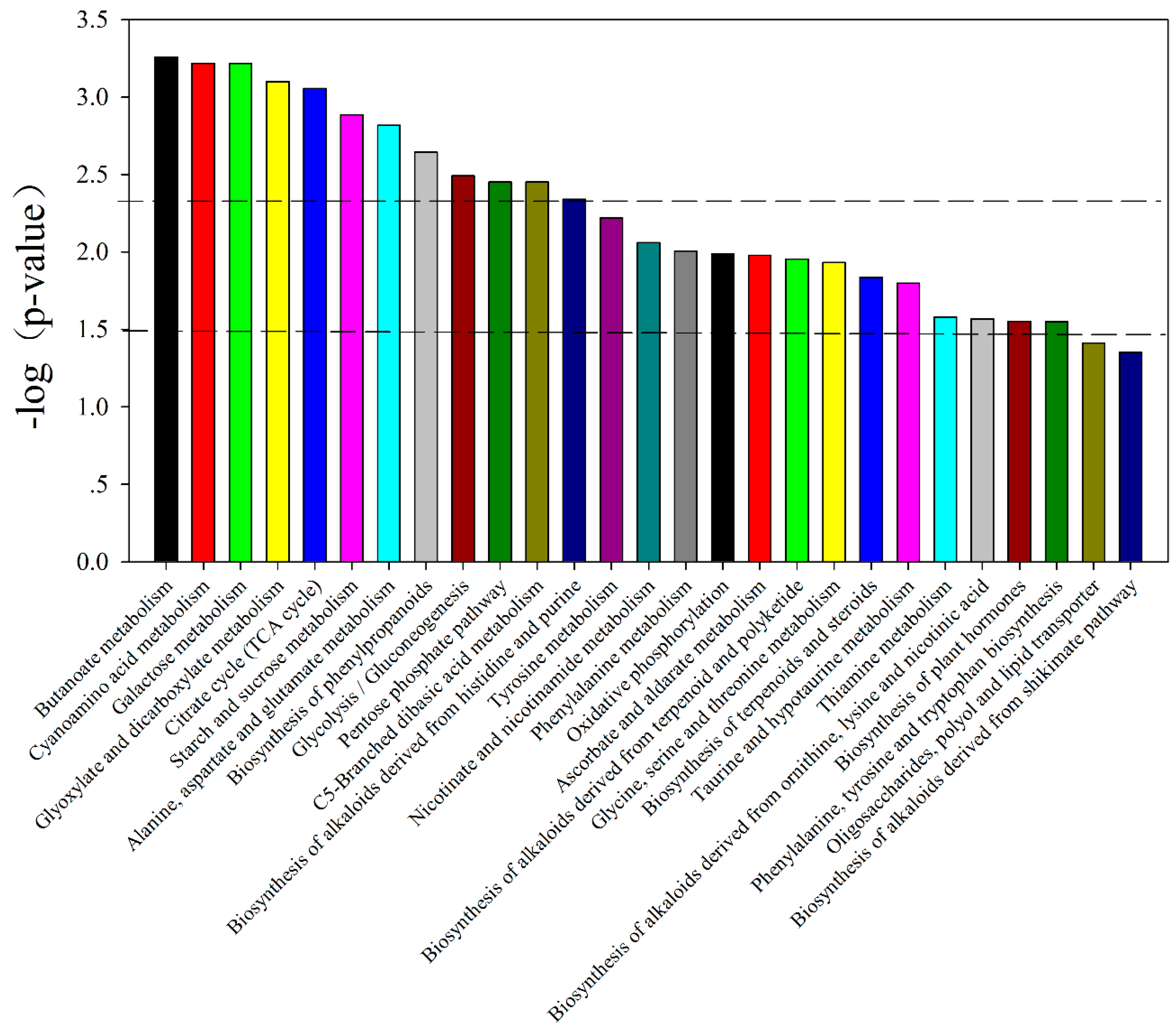
| KEGG (p-Value) | Different Metabolites | Vip | Change Fold (V. minor/C. roseus) | p-Value |
|---|---|---|---|---|
| Galactose metabolism (4.74 × 10−4) | Raffinose # | 1.06 | —— | ** —— |
| Myo-inositol ## | 1.24 | 0.13 | ** 0.005 | |
| Galactinol # | 1.42 | 0.19 | ** 9.7 × 10−6 | |
| Fructose # | 1.28 | 0.03 | ** 0.004 | |
| Glucose # | 1.10 | 0.18 | * 0.020 | |
| TCA cycle (7.31 × 10−4) | Fumaric acid ### | 1.28 | 0.09 | ** 0.004 |
| alpha-ketoglutaric acid ### | 1.38 | 0.02 | ** 0.0009 | |
| Pyruvic acid ### | 1.47 | 0.09 | ** 3.3 × 10−7 | |
| Starch and sucrose metabolism (1.02 × 10−3) | Trehalose # | 1.06 | 77.64 | * 0.029 |
| Cellobiose # | 1.09 | —— | ** —— | |
| Fructose | ||||
| Glucose | ||||
| Oligosaccharides, polyol and lipid transporter (3.45 × 10−2) | Raffinose | |||
| Mannitol ## | 1.25 | 0.13 | ** 0.004 | |
| Trehalose | ||||
| Cellobiose | ||||
| Biosynthesis of phenylpropanoids (1.70 × 10−3) | alpha-ketoglutaric acid | |||
| Salicin # | 1.10 | 5.26 | * 0.018 | |
| Gallic acid ### | 1.01 | 3.02 | * 0.019 | |
| Pyruvic acid | ||||
| Fumaric acid | ||||
| Biosynthesis of terpenoids and steroids (1.17 × 10−2) | Fumaric acid | |||
| Pyruvic acid | ||||
| alpha-ketoglutaric acid | ||||
| Loganin # | 1.48 | 18.19 | ** 3.4 × 10−5 | |
| Glyoxylate and dicarboxylate metabolism (6.24 × 10−4) | alpha-ketoglutaric acid | |||
| Pyruvic acid | ||||
| Glycolic acid ### | 1.49 | 0.05 | ** 1.2 × 10−8 | |
| Cyanoamino acid metabolism (4.74 × 10−4) | Tartaric acid ### | 1.08 | 6.19 | * 0.023 |
| Glutamic acid #### | 1.30 | 0.08 | ** 0.003 | |
| 4-Hydroxymandelonitrile | 1.26 | 4.87 | ** 0.004 | |
| Serine #### | 1.12 | 52.77 | * 0.022 | |
| Tyrosine #### | 1.14 | 0.18 | * 0.012 | |
| Butanoate metabolism (3.63 × 10−2) | Pyruvic acid | |||
| α-Ketoglutaric acid | ||||
| Fumaric acid | ||||
| Maleic acid ### | 1.02 | 0.21 | * 0.017 | |
| Biosynthesis of alkaloids derived from shikimate pathway (9.31 × 10−3) | Tyrosine | |||
| Fumaric acid | ||||
| Pyruvic acid | ||||
| α-Ketoglutaric acid | ||||
| Biosynthesis of alkaloids derived from terpenoid and polyketide (9.31 × 10−3) | α-Ketoglutaric acid | |||
| Fumaric acid | ||||
| Pyruvic acid | ||||
| Pentose phosphate pathway (2.95 × 10−3) | Glucose | |||
| Pyruvic acid | ||||
| Gluconic lactone # | 1.11 | 0.16 | ** 0.0076 | |
| Glycolysis/Gluconeogenesis (2.69 × 10−3) | Salicin # | |||
| Glucose | ||||
| Pyruvic acid | ||||
| C5-Branched dibasic acid metabolism (2.95 × 10−3) | Itaconic acid ### | 1.00 | 5.45 | * 0.02 |
| α-Ketoglutaric acid | ||||
| Pyruvic acid | ||||
| Ascorbate and aldarate metabolism (8.78 × 10−3) | α-Ketoglutaric acid | |||
| Pyruvic acid | ||||
| Myo-inositol | ||||
| Biosynthesis of alkaloids derived from histidine and purine (3.82 × 10−3) | α-Ketoglutaric acid | |||
| Pyruvic acid | ||||
| Fumaric acid | ||||
| Biosynthesis of plant hormones (2.38 × 10−2) | α-Ketoglutaric acid | |||
| Pyruvic acid | ||||
| Fumaric acid | ||||
| Phenylalanine metabolism(8.27 × 10−3) | Tyrosine | |||
| Pyruvic acid | ||||
| Fumaric acid | ||||
| Nicotinate and nicotinamide metabolism (7.31 × 10−3) | Pyruvic acid | |||
| Fumaric acid | ||||
| Maleic acid ### | ||||
| Tyrosine metabolism(3.18 × 10−2) | Pyruvic acid | |||
| Fumaric acid | ||||
| Tyrosine | ||||
| Glycine, serine and threonine metabolism (9.85 × 10−3) | Pyruvic acid | |||
| L-Allothreonine #### | 1.46 | 0.01 | ** 7.9 × 10−5 | |
| Serine #### | 1.12 | 52.77 | * 0.022 | |
| Biosynthesis of alkaloids derived from ornithine, lysine and nicotinic acid (2.29 × 10−2) | Pyruvic acid | |||
| Fumaric acid | ||||
| α-Ketoglutaric acid | ||||
| Alanine, aspartate and glutamate metabolism (1.26 × 10−3) | α-Ketoglutaric acid | |||
| Pyruvic acid | ||||
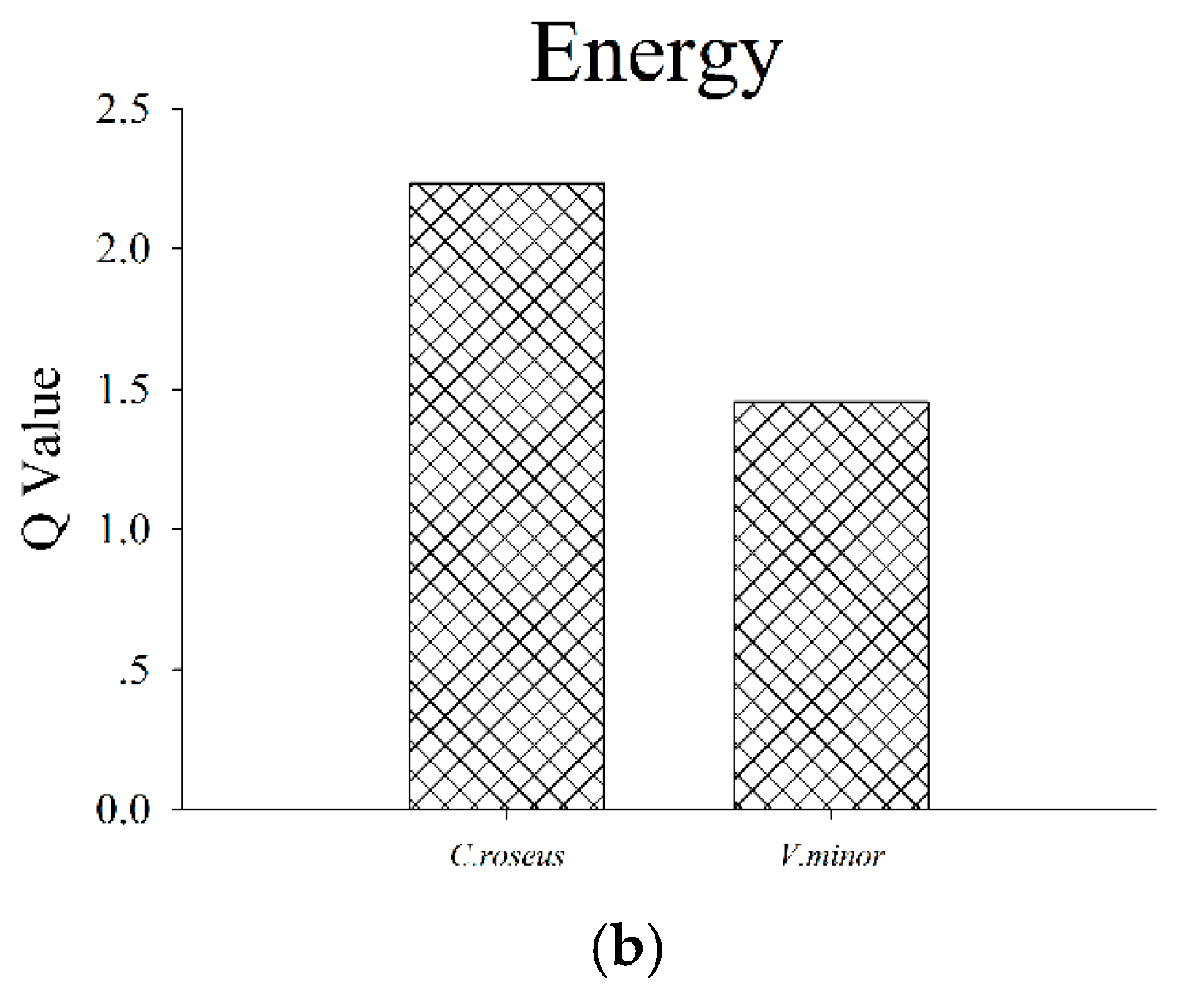
| Oxidative phosphorylation(9.13 × 10−3) | Phosphate | 1.33 | 0.07 | ** 0.002 |
| Fumaric acid | ||||
| Thiamine metabolism(2.34 × 10−2) | Pyruvic acid | |||
| Tyrosine | ||||
| Phenylalanine, tyrosine and tryptophan biosynthesis (2.51 × 10−2) | Quinic acid ### | 1.17 | 3.28 | ** 0.004 |
| Tyrosine | ||||
| Taurine and hypotaurine metabolism (1.41 × 10−2) | 2-Aminoethanethiol | 1.14 | 0.19 | * 0.010 |
| Pyruvic acid | ||||
| Others | Tagatose # | 1.30 | 0.05 | ** 0.003 |
| Levoglucosan # | 1.23 | 11.66 | ** 0.006 | |
| Erythrose # | 1.13 | 0.44 | ** 0.006 | |
| Lyxose # | 1.13 | 3.02 | ** 0.005 | |
| Ribose # | 1.34 | 0.13 | ** 0.0002 | |
| d-Talose# | 1.19 | 0.03 | * 0.011 | |
| 3,6-Anhydro-d-galactose # | 1.29 | 0.24 | ** 0.0005 | |
| Naringin # | 1.08 | 0.35 | ** 0.0098 | |
| Glucoheptonic acid ### | 1.20 | 130715.28 | ** 0.0096 | |
| Gluconic acid ### | 1.17 | 15.46 | * 0.013 | |
| Chlorogenic Acid ### | 1.35 | 0.21 | ** 0.0002 | |
| Threonic acid ### | 1.16 | 21.18 | * 0.014 | |
| d-Glyceric acid ### | 1.15 | 4.95 | * 0.012 | |
| Galactonic acid ### | 1.02 | 19.60 | * 0.040 | |
| Aminooxyacetic acid ### | 1.29 | 4.30 | ** 0.0004 | |
| Digalacturonic acid ### | 1.11 | 101.91 | * 0.023 | |
| 3-Aminoisobutyric acid ### | 1.29 | 32.74 | ** 0.005 | |
| Dodecanol ## | 1.16 | 0.46 | ** 0.004 | |
| Lactitol ## | 1.13 | 6.12 | * 0.016 | |
| Threitol ## | 1.07 | 7.92 | * 0.025 | |
| 3-Methylamino-1,2-propanediol ## | 1.04 | 0.06 | * 0.029 | |
| 1,5-Anhydroglucitol ## | 1.21 | 198.25 | ** 0.009 | |
| Phytol ## | 1.13 | 0.06 | * 0.015 | |
| 2-Amino-1-phenylethanol ## | 1.10 | 0.16 | * 0.018 | |
| Threonine #### | 1.31 | 5.26 | ** 0.0004 | |
| Aspartic acid #### | 1.44 | 0.11 | ** 8.6 × 10−5 | |
| Ornithine #### | 1.25 | 0.23 | ** 0.001 | |
| Valine #### | 1.19 | 0.18 | ** 0.003 | |
| 2-Kydroxypyridine | 1.41 | 0.11 | ** 1.4 × 10−5 | |
| Octanal | 1.05 | —— | ** —— | |
| 2-aminoethanethiol | 1.14 | 0.19 | * 0.010 | |
| Gluconic lactone | 1.11 | 0.16 | ** 0.0076 | |
| Glutamine | 1.08 | 0.01 | * 0.024 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Lu, X.; Guo, X.; Guo, Q.; Li, D. Metabolomics Characterization of Two Apocynaceae Plants, Catharanthus roseus and Vinca minor, Using GC-MS and LC-MS Methods in Combination. Molecules 2017, 22, 997. https://doi.org/10.3390/molecules22060997
Chen Q, Lu X, Guo X, Guo Q, Li D. Metabolomics Characterization of Two Apocynaceae Plants, Catharanthus roseus and Vinca minor, Using GC-MS and LC-MS Methods in Combination. Molecules. 2017; 22(6):997. https://doi.org/10.3390/molecules22060997
Chicago/Turabian StyleChen, Qi, Xueyan Lu, Xiaorui Guo, Qingxi Guo, and Dewen Li. 2017. "Metabolomics Characterization of Two Apocynaceae Plants, Catharanthus roseus and Vinca minor, Using GC-MS and LC-MS Methods in Combination" Molecules 22, no. 6: 997. https://doi.org/10.3390/molecules22060997
APA StyleChen, Q., Lu, X., Guo, X., Guo, Q., & Li, D. (2017). Metabolomics Characterization of Two Apocynaceae Plants, Catharanthus roseus and Vinca minor, Using GC-MS and LC-MS Methods in Combination. Molecules, 22(6), 997. https://doi.org/10.3390/molecules22060997






