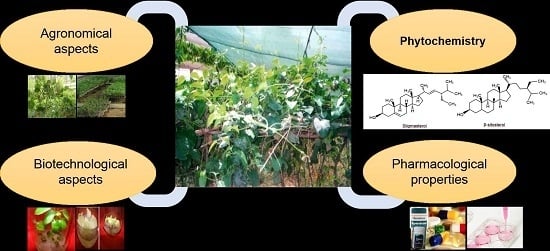
Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): Botanical, Agronomical, Phytochemical, Pharmacological, and Biotechnological Aspects
Abstract
:1. Introduction
2. Botanical Aspects of L. reticulata
2.1. Taxonomy
| Kingdom | Viridiplantae |
| Phylum | Streptophyta |
| Class | Magnoliopsida |
| Order | Gentianales |
| Family | Apocynaceae |
| Sub-family | Asclepiadoideae |
| Genus | Leptadenia |
| Species | Leptadenia reticulata (Retz.) Wight & Arn. |
2.2. Origin and Distribution
2.3. Morphology
3. Agronomical Aspects of L. reticulata
3.1. Climate and Soil
3.2. Propagation Technique
3.3. Spacing of Propagules
3.4. Preparation of Land and Fertilizer
3.5. Irrigation
3.6. Control of Weeds
3.7. Crop Protection
3.8. Intercropping
3.9. Harvesting
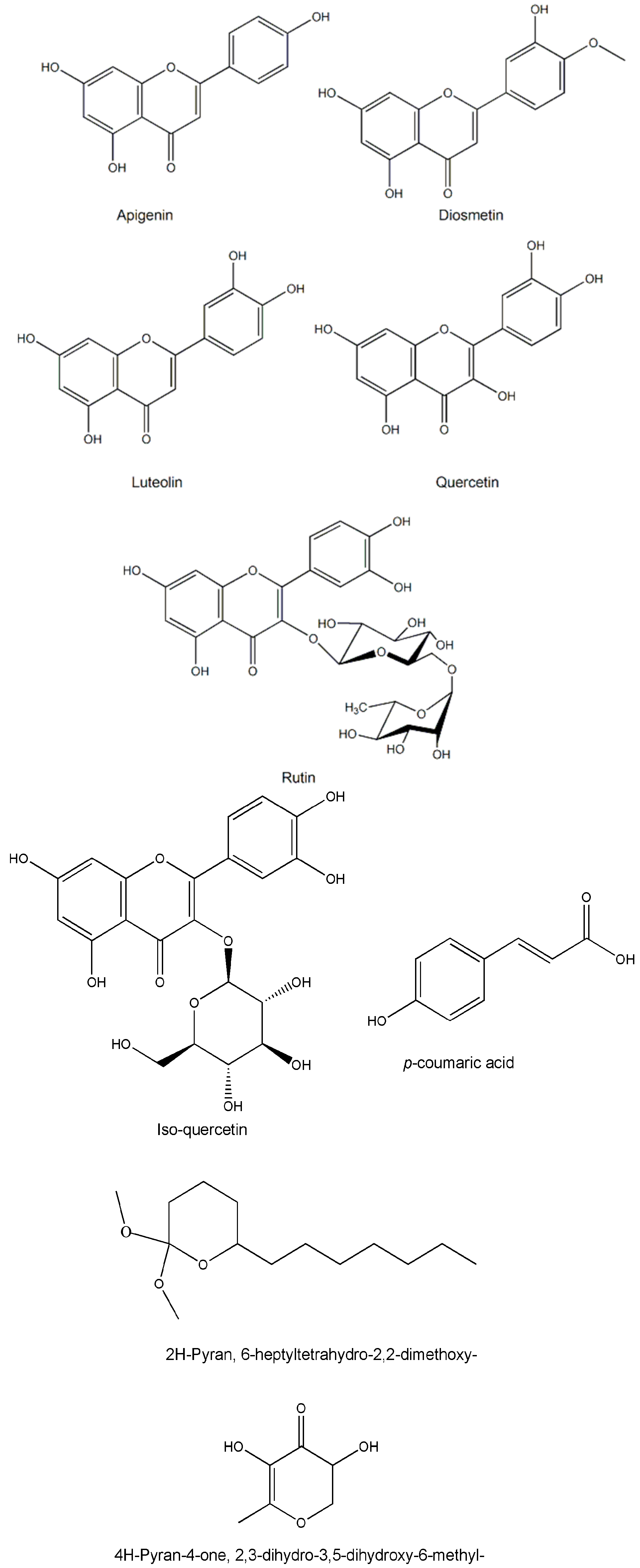
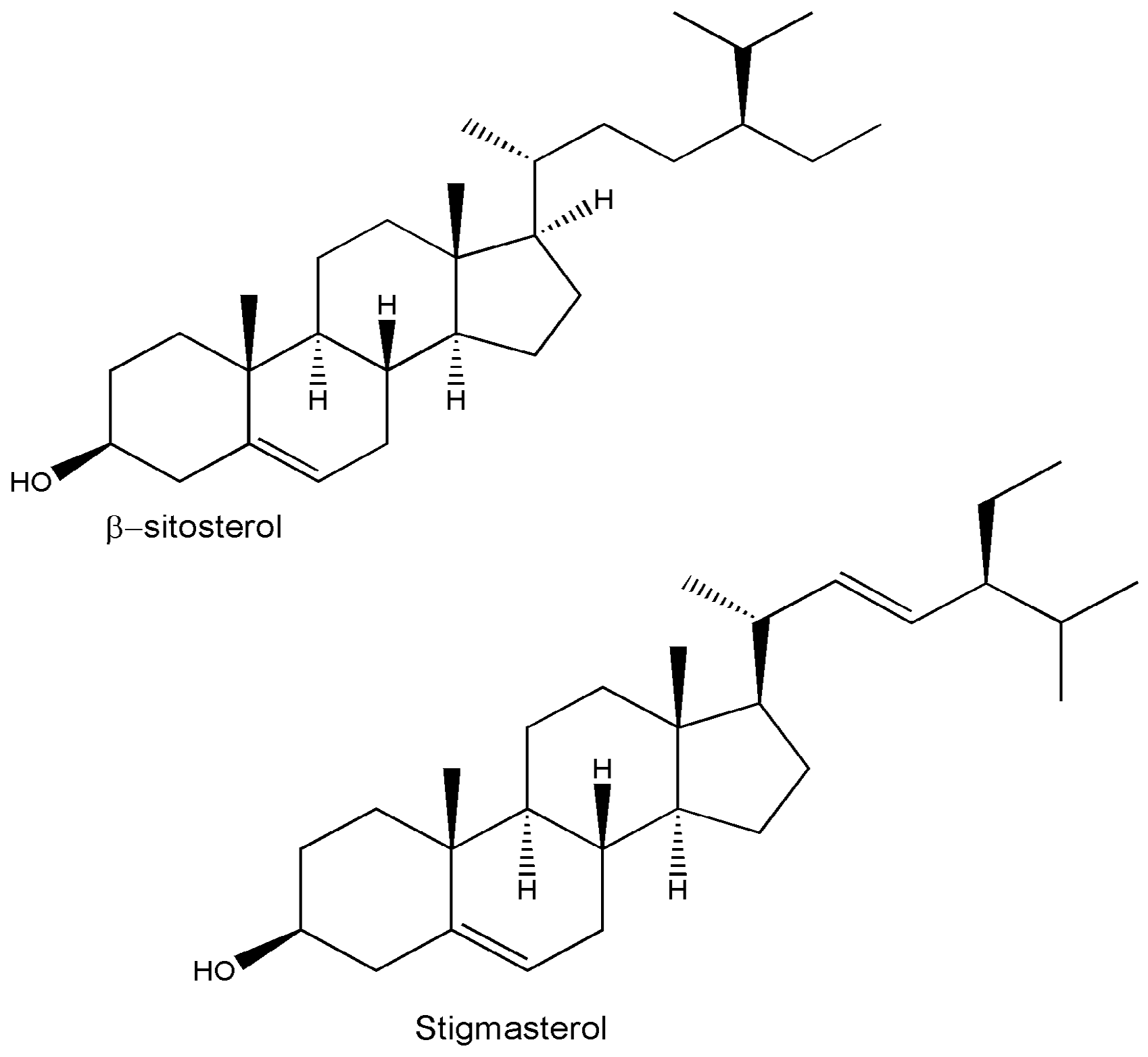
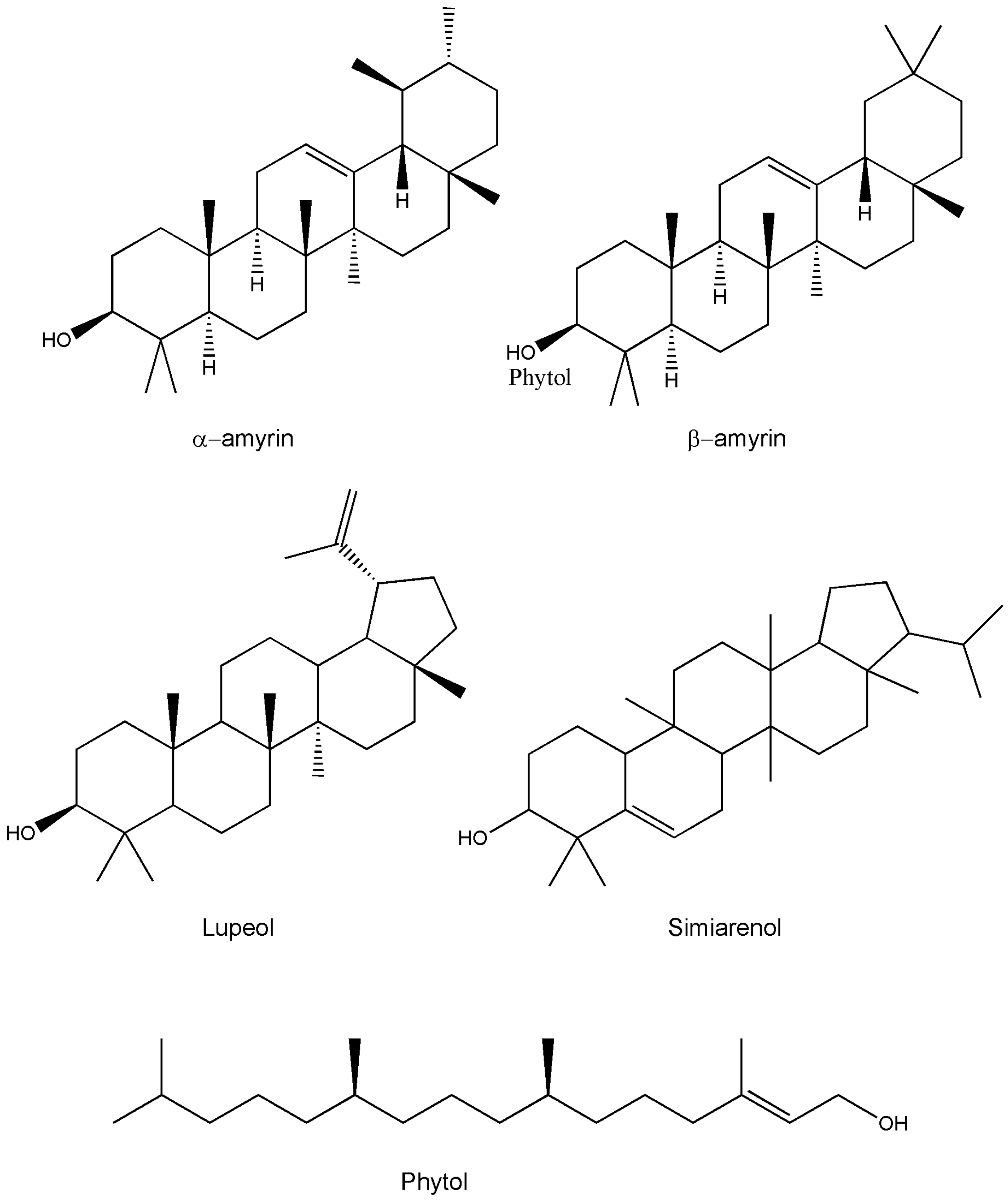
4. Phytochemistry
5. Pharmacological Properties of L. reticulata
5.1. Antiabortifacient Effect
5.2. Antianaphylactic Activity
5.3. Antidepressant Effect
5.4. Antiepileptic Potential
5.5. Anti-Implantation Activity
5.6. Antimicrobial Activity
5.7. Antitumor and In Vitro Cytotoxic Activity
5.8. Antioxidant Activity
5.9. Antipyretic, Analgesic, and Anti-Inflammatory Activity
5.10. Antiulcer Activity
5.11. Anxiolytic Activity
5.12. Diuretic Activity
5.13. Galactagogue Property
5.14. Hepatoprotective Activity
5.15. Immunomodulatory Activity
5.16. Treatment of Oligospermia
5.17. Other Studies
6. Biotechnological Aspects of L. reticulata
Plant Tissue Culture, Plant Regeneration and Conservation Studies
7. Current Demand
8. Adulteration and Authentication of L. reticulata
9. Conclusions and Future Scope
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| AMF | Arbuscular mycorrhizal fungi |
| BA | Benzyladenine |
| CAT | Catalase |
| DAL | Dalton’s ascites lymphoma |
| DPPH | Diphenylpicrylhydrazyl |
| EI-MS | Electron ionization mass spectrum |
| FYM | Farm yard manure |
| FAB | Fast atom bombardment |
| GA3 | Gibberellic acid |
| GC-MS | Gas chromatography-mass spectroscopy |
| HPLC | High pressure liquid chromatography |
| HPTLC | High performance thin layer chromatography |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| IR | Infrared |
| Kn | Kinetin |
| MIC | Minimum inhibitory concentration |
| NAA | α-naphthaleneacetic acid |
| NMR | Nuclear magnetic resonance |
| NPK | Nitrogen, Phosphorus, Potassium |
| SOD | Superoxide dismutase |
| TLC | Thin layer chromatography |
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, Phytochemical, Pharmacological and Nutritional Significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef] [PubMed]
- Kumara Swamy, M.; Pokharen, N.; Dahal, S.; Anuradha, M. Phytochemical and antimicrobial studies of leaf extract of Euphorbia nerifolia. J. Med. Plants Res. 2011, 5, 5785–5788. [Google Scholar]
- Kumara, S.M.; Sudipta, K.M.; Lokesh, P.; Neeki, A.; Rashmi, W.; Bhaumik, H.; Darshil, H.; Vijay, R.; Kashyap, S.S.N. Phytochemical screening and in vitro antimicrobial activity of Bougainvillea spectabilis flower extracts. Int. J. Phytomed. 2012, 4, 375–379. [Google Scholar]
- Swamy, M.K.; Sinniah, U.R.; Akhtar, M.S. In vitro pharmacological activities and GC-MS analysis of different solvent extracts of Lantana camara leaves collected from tropical region of Malaysia. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: An aromatic medicinal plant of industrial importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef] [PubMed]
- Chermahini, S.H.; Majid, F.A.A.; Sarmid, M.R. Cosmeceutical value of herbal extracts as natural ingredients and novel technologies in anti-aging. J. Med. Plants Res. 2011, 5, 3074–3077. [Google Scholar]
- Joshi, L.S.; Pawar, H.A. Herbal Cosmetics and Cosmeceuticals: An Overview. Nat. Prod. Chem. Res. 2015, 3, 170. [Google Scholar] [CrossRef]
- Sivarajan, V.V.; Balachandran, I. Ayurvedic Drugs and Their Plant Sources; Oxford IBH Co. Pvt. Ltd.: Delhi, India, 1994; pp. 195–200. [Google Scholar]
- Anjaria, J.V.; Gupta, I. Studies on lactogenic property of Leptadenia reticulata and leptaden tablet in goats, sheep, cows and buffaloes. Indian Vet. J. 1967, 44, 967–974. [Google Scholar] [PubMed]
- Krishna, P.V.G.; Venkata, R.E.; Venkata, R.D. Crystalline principles from the leaves and twigs of Leptadenia reticulata. Planta Med. 1975, 27, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.S.; Lakshman, A.J. On the constituents of Leptadenia reticulata Wight & Arn. occurrence of simiarenol. Indian J. Chem. 1977, 5, 180. [Google Scholar]
- Sastry, B.S.; Vijayalaxmi, T.; Venkata, R.D.; Venkata, R.E. Chemical constituents of stem bark of Leptadenia reticulata. Indian Drug 1985, 22, 611–612. [Google Scholar]
- Rawat, G.S. Special Habitats and Threatened Plants of India; ENVIS Bulletin: Wildlife and Protected Areas; Wildlife Institute of India: Dehradun, India, 2008; Volume 11, p. 239. [Google Scholar]
- Mishra, M.; Kotwal, P.C.; Prasad, C. Harvesting of medicinal plants in the forest of central India and its impact on quality of raw materials, A case of Nagpur district, India. Ecoprint 2009, 16, 35–42. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. Patchouli (Pogostemon cablin Benth.): Botany, agrotechnology and biotechnological aspects. Ind. Crop. Prod. 2016, 87, 161–176. [Google Scholar] [CrossRef]
- Sathyanarayana, N.; Rajesha, R.; Vikas, P.B.; Bharath, T.N. Somatic embryogenesis and plant regeneration from stem explant of Leptadenia reticulata (Retz.). Indian J. Biotechnol. 2008, 7, 250–254. [Google Scholar]
- Kasera, P.K.; Shukla, J.K. Bio–medicinal properties and cultivation of Leptadaenia reticulata (Jivanti)—An endangered plant of the Thar desert, India. Curr. Sci. 2003, 84, 877–879. [Google Scholar]
- Khare, C.P. Indian Medicinal Plant: An Illustrated Dictionary; Springer: Heidelberg, Germany, 2007; p. 480. [Google Scholar]
- Mammen, D.; Daniel, M.; Sane, R.T. Pharmacognostic and Phytochemical studies on Leptadenia reticulata (retz.) Wight & Arn. and Ichnocarpus frutescens R. Br. for identification of distinguishing biomarkers. Pharmacognosy 2011, 2, 7–12. [Google Scholar]
- Mallikarjuna, B.; Usha, N.R.; Gayathri, D.; Rama, G. In vitro antioxidant and free radical scavenging potential of Holostemma adakodien K. Schum., an important rare medicinal plant. Int. J. Pharm. Sci. Res. 2011, 2, 2413–2418. [Google Scholar]
- Grubben, G.J.H. Plant Resources of Tropical Africa: Vegetables Backhuys; PROTA Foundation: Wageningen, The Netherlands, 2004; Volume 2, pp. 367–368. [Google Scholar]
- Godara, P.; Rao, D.V.; Dulara, B.; Barwar, N. Multidimensional approach of endangered ayurvedic plant Leptadenia reticulata: A review. Int. J. Appl. Sci. Eng. Res. 2015, 4, 531–543. [Google Scholar]
- Schmelzer, G.H.; Gurub-Fakim, A.; Arroo, R.R.J. Plant Resources of Tropical Africa 11. Medicinal Plants 2; PROTA Foundation: Wageningen, The Netherlands, 2013; pp. 158–159. [Google Scholar]
- Bawra, B.; Dixit, M.; Chauhan, N.S.; Dixit, V.K.; Saraf, D.K. Leptadenia reticulata a Rasayana Herbs: A Review. Asian J. Plant Sci. 2010, 9, 314–319. [Google Scholar] [CrossRef]
- Mammen, D.; Daniel, M.; Sane, R.T. Seasonal and geographical varifations in chemical constituents of Leptadenia reticulata. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 111–116. [Google Scholar]
- Panwar, J.; Tarafdar, J.C. Distribution of three endangered medicinal plant species and their colonization with arbuscular mycorrhizal fungi. J. Arid Environ. 2006, 65, 337–350. [Google Scholar] [CrossRef]
- Anonymous. Agro Techniques of Selected Medicinal Plants; National Medicinal Plants Board, Department of AYUSH, Ministry of Health and Family Welfare Government of India: New Delhi, India, 2008; Volume 1, pp. 111–114.
- Kalidass, C.; Mohan, V.R.; Sivalingam, R. Pharmacognostical studies on a multipurpose medicinal plant Leptadenia reticulata (retz.) Wight & Arn. Pharmacologyonline 2011, 3, 713–718. [Google Scholar]
- Nagarajaiah, C.; Shivanna, J. Influence of nitrogen, phosphorus and potassium on growth and yield of Jeevanthi (Leptadenia reticulata Wight and Arn.). My For. 2007, 43, 417–422. [Google Scholar]
- Panwar, J.; Vyas, A. AM fungi: A biological approach towards conservation of endangered plants in Thar desert India. Curr. Sci. 2002, 82, 576–578. [Google Scholar]
- Swamy, M.K.; Mohanty, S.K.; Sinniah, U.R.; Maniyam, A. Evaluation of patchouli (Pogostemon cablin Benth.) cultivars for growth, yield and quality parameters. J. Essent. Oil Bear. Plants 2015, 18, 826–832. [Google Scholar] [CrossRef]
- Verma, S.C.I.; Agarwal, S.L. Studies on Leptadenia reticulata (part II). Preliminary chemical investigations. Indian J. Med. Res. 1962, 50, 439–445. [Google Scholar] [PubMed]
- Pal, A.; Sharma, P.P.; Pandya, T.N.; Acharya, R.; Patel, B.R.; Shukla, V.J.; Rabishankar, B. Phytochemical evaluation of aqueous extract of Jivanti (L. reticulata). Ayu 2012, 33, 557–560. [Google Scholar] [PubMed]
- Hewageegana, S.P.; Arawwawala, M.; Dhammaratana, I.; Ariyawansa, H.; Tisser, A. Proximate analysis and standardization of leaves: Leptadenia reticulata (Retz) Wight and Arn (Jeevanti). World J. Pharm. Res. 2014, 3, 1603–1612. [Google Scholar]
- Nema, A.K.; Agarwal, A.; Kashaw, V. Screening of hepatoprotective potential of Leptadenia reticulata stems against paracetamol–induced hepatotoxicty in rats. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 666–671. [Google Scholar]
- Mammen, D.; Daniel, M.; Sane, R.T. Phytochemical Investigation of Water Soluble Phytoconstituents of Leptadenia reticulata (Retz.) Wight & Arn. Int. J. Res. Pharm. Sci. 2010, 1, 486–489. [Google Scholar]
- Sudipta, K.M.; Balasubramanya, S.; Anuradha, M. Growth studies and phytochemical analysis of Leptadenia reticulata cell suspension cultures. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 34–37. [Google Scholar]
- Anjaria, J.V.; Mankad, B.N.; Gulati, O.D. Isolation of stigmasterol and tocopherols from Leptadenia reticulata by shortcut method. Indian J. Pharm. 1974, 36, 373. [Google Scholar]
- Mallick, S.S.; Dighe, V.V. Detection and Estimation of alpha-Amyrin, beta-Sitosterol, Lupeol, and n-Triacontane in Two Medicinal Plants by High Performance Thin Layer Chromatography. Adv. Chem. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Geetha, N.P.; Mahesh, M.; Bettadaiah, B.K.; Kini, R.K.; Prakash, H.S. HPLC Method for Determination of p-coumaric acid from the Medicinal Herb Leptadinia reticulata. Int. J. Phytomed. 2011, 3, 319. [Google Scholar]
- Mohanty, S.K.; Swamy, M.K.; Middha, S.K.; Prakash, L.; Subbanarashiman, B.; Maniyam, A. Analgesic, anti-inflammatory, anti-lipoxygenase activity and characterization of three bioactive compounds in the most active fraction of Leptadenia reticulata (Retz.) Wight & Arn.—A valuable medicinal plant. Iran. J. Pharm. Res. 2015, 14, 933–942. [Google Scholar] [PubMed]
- Srivastav, S.; Deepak, D.; Khare, A. Three novel pregnane glycosides from Leptadenia reticulata Wight & Arn. Tetrahedron 1994, 50, 789–798. [Google Scholar]
- Hamrapurkar, P.; Karishma, P. HPTLC Determination of Stigmasterol and Tocopherol Acetate in Leptadenia reticulata and in its formulation. J. Planar Chromatogr. 2007, 20, 183–187. [Google Scholar] [CrossRef]
- Prashanth, K.V.; Ravishankara, M.N.; Padh, H.; Rajani, M. High-performance thin-layer chromatographic method for estimation of rutin in medicinal plants. J. Planar Chromatogr. 2003, 16, 386–389. [Google Scholar] [CrossRef]
- Rajanna, L.N.; Seetharam, Y.N.; Devendra, N.K. Isolation and characterization of lupeol from roots of Leptadenia reticulata and its antimicrobial activity. Pharmacologyonline 2009, 3, 489–494. [Google Scholar]
- Rajeswari, J.; Rani, S. GC-MS analysis of whole plant of Leptadenia reticulata. Int. J. PharmTech Res. 2014, 6, 2043–2050. [Google Scholar]
- Mangeshikar, S.N. Use of herbal drugs in habitual abortions. Abid 1958, 55, 487. [Google Scholar]
- Sharma, S.C. A Possible Mechanism of Leptaden action by inhibiting prostaglandin F2a synthesis. Ind. J. Med. Res. 1976, 64, 97–600. [Google Scholar]
- Philips, F.S. Clinicnl trial with Leptaden for recurrent and threatened abortions and premature labour. Curr. Med. Pract. 1977, 21, 317–320. [Google Scholar]
- Naik, M.G. Preliminary observations on the use of an Indian Indigenous drug in certain uterine haemorrhages. Indian Pract. 1957, 10, 1. [Google Scholar]
- Padmalatha, K.; Venkataraman, B.V.; Roopa, R. Antianaphylactic effect of DLH-3041 (polyherbal formulation) on rat mesenteric mast cell degranulation. Indian J. Pharmacol. 2002, 34, 119–122. [Google Scholar]
- Hakim, R.A. A preliminary report on the use of Malkanguni with other indigenous drugs in the treatment of depression. Indian J. Psychiatry 1964, 6, 142–146. [Google Scholar]
- Pushpa, K.B.; Mahipal, R.R.; Manasa, V.B.; Mohan, B.T.; Ranganayakulu, D. Antiepileptic activity and neuropharmacological screening of methanolic extract of Leptadenia reticulata against different experimental models. J. Adv. Drug Res. 2010, 1, 1–8. [Google Scholar]
- Rani, S.; Manavalan, R.; Kilimozhi, D.; Balamurugan, K. Preliminary study on anti–implantation activity of Leptadenia reticulata in female rats. Int. J. PharmTech Res. 2009, 1, 1403–1405. [Google Scholar]
- Vaghasiya, Y.; Chanda, S.V. Screening of methanol and acetone extracts of fourteen Indian medicinal plants for antimicrobial activity. Turk. J. Biol. 2007, 31, 243–248. [Google Scholar]
- Natarajan, V.; Dhas, A.S.A.G. Phytochemical Composition and in vitro Antimicrobial, Antioxidant Activities of Ethanolic Extract of Leptadenia reticulata [W & A] Leaves. Middle East J. Sci. Res. 2014, 21, 1698–1705. [Google Scholar]
- Kalidass, C.; Glory, M.; Francis, B.; Manickam, V.S. Antibacterial Activity of Leptadenia reticulata (Retz.) Wight & Arn. (Asclepidaceae). Anc. Life Sci. 2009, 28, 10–12. [Google Scholar]
- Swamy, M.K.; Sudipta, K.M.; Jayanta, K.; Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. 2015, 5, 73–81. [Google Scholar] [CrossRef]
- Mishra, M.K.; Tiwari, P.; Dash, D.K.; Jadon, R.S.; Ghosh, G.; Barik, B.B. Antifungal activity of Leptadenia reticulata Wight & Arn. aerial parts. Int. J. Phytomed. 2010, 2, 172–176. [Google Scholar]
- Suresh, K.P. Anti-fungal activity of Leptadenia reticulata in rat animal model in vivo. J. Basic Appl. Biol. 2008, 2, 9–13. [Google Scholar]
- Sathiyanarayanan, L.; Sinnathambi, A.; Chidambaranathan, A. Anticarcinogenic activity of Leptadenia reticulata against Daltons ascitic lymphoma. Iran. J. Pharmacol. Ther. 2007, 6, 133–135. [Google Scholar]
- Mohanty, S.K.; Mallappa, K.S.; Godavarthi, A.; Subbanarasiman, B.; Maniyam, A. Evaluation of antioxidant, in vitro cytotoxicity of micropropagated and naturally grown plants of Leptadenia reticulata (Retz.) Wight & Arn.—An endangered medicinal plant. Asian Pac. J. Trop. Med. 2014, 7, S267–S271. [Google Scholar]
- Wakade, A.S.; Juvekar, A.R.; Hole, R.C.; Nachankar, R.S.; Kulkarni, M.P. Antioxidant and cardioprotective effect of Leptadenia reticulata against adriamycin–induced myocardial oxidative damage in rat experiments. Planta Med. 2007, 73, 443. [Google Scholar] [CrossRef]
- Pravansha, S.; Thippeswamy, B.S.; Veerapur, V.P. Immunomodulatory and antioxidant effect of Leptadenia reticulata leaf extract in rodents: Possible modulation of cell and humoral immune response. Immunopharm. Immunot. 2012, 34, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Sudipta, K.M.; Kumara Swamy, M.; Balasubramanya, S.; Anuradha, M. Assessment of genetic fidelity, antioxidant enzyme activity and proline content of micropropagated and field grown plants of Leptadenia reticulata (Wight & Arn.)—An endangered medicinal plant. Plant Cell Biotechnol. Mol. Biol. 2014, 15, 127–135. [Google Scholar]
- Sneha, B.; Ganga, R.M.; Divya, N. Evaluation of Antipyretic and Anti–Inflammatory Activity of Aqueous Extract of Leptadenia reticulata in Animal Models. J. Nat. Remedies 2016. [Google Scholar] [CrossRef]
- Bodhanapu, S.; Sreedhar, S.; Rupeshkumar, M.; Tamizhmani, P.P.; Satya, K.B.; Mohamed, N.K. Antiulcer activity of aqueous extract of Leptadenia reticulata leaves. Pharmacologyonline 2011, 2, 1190–1196. [Google Scholar]
- Rajpurohit, B.; Gilhotra, U.K.; Verma, A.K.; Genwa, C. Evalution of anxiolytic activity of Leptadenia reticulata plant. Int. J. Pharm. Sci. Res. 2016, 7, 5099. [Google Scholar]
- Mohanraj, S.; Santhoshkumar, C.; Chandran, A. Diuretic activity of whole plant extractof Leptadenia reticulata. Res. J. Pharmacol. Pharmacodyn. 2012, 4, 84–86. [Google Scholar]
- Patel, N.V. A Suggestion to Gynaecologists. Antiseptic 1947, 44, 377–380. [Google Scholar] [PubMed]
- Trivedi, S.B. Can lactation be stimulated. Indian Pract. 1956, 9, 219. [Google Scholar]
- Moulvi, M.V. Lactogenic properties of Leptaden. Indian Vet. J. 1963, 40, 657. [Google Scholar]
- Narasimhamurthy, G. A preliminary note on the study of lactogenic properties of Lepaden. Indian Vet. J. 1969, 46, 510. [Google Scholar] [PubMed]
- Achari, K.; Sinha, R. Treatment of recurrent abortin (A clinical study of 62 cases with Leptaden). Patna J. Med. 1966, 30, 1–3. [Google Scholar]
- Patel, M.C. Leptaden in idiopathic habitual abortions. Curr. Med. Pract. 1965, 9, 764. [Google Scholar]
- Dash, S.K.; Owens, M.J.; Voelker, H.H. Effects of Feeding Leptaden to Dairy Cows. J. Dairy Sci. 1972, 55, 102–106. [Google Scholar] [CrossRef]
- Anjaria, J.V.; Varia, M.R.; Janakiraman, K.; Gulati, O.D. Studies on Leptadenia reticulata: Lactogenic effect on rats. Indian J. Exp. Biol. 1975, 13, 448–449. [Google Scholar] [PubMed]
- Baig, M.I.; Bhagwat, V.G. Study the efficacy of Galactin Vet Bolus on milk yield in dairy cows. Vet. World 2009, 2, 140–142. [Google Scholar]
- Girishkumar, V.; Sreepriya, M.S.; Praveenkumar, S.; Bali, G.; Jagadeesh, M.S. Modulating effect of Leptadenia reticulata (Retz) Wight & Arn against chromate (VI)-induced immunosuppression and oxidative stress on mouse splenic lymphocytes and bone marrow derived macrophages. J. Ethnopharmacol. 2010, 131, 505–508. [Google Scholar] [PubMed]
- Madan, S.; Madaan, T.R. Speman in oligospermia. Probe 1985, 24, 115–117. [Google Scholar]
- Agarwal, S.L.; Deshmankar, B.S.; Verma, S.C.L.; Saxena, S.P. Studies on Leptadenia reticulata. Part I: Pharmacological action of aqueous extract. Indian J. Med. Res. 1960, 48, 457–464. [Google Scholar]
- Satyavati, G.V.; Gupta, A.K.; Tandon, N; Seth, S.D. Medicinal Plant of India; Indian Council of Medical Research: New Delhi, India, 1987; Volume 2, pp. 289–299. [Google Scholar]
- Marya, S.K.S.; Garg, P.; Gupta, A.K.; Sharma, V.K. Role of speman in benign prostatic hyperplasia. Surg. J. North India 1995, 11, 126–131. [Google Scholar]
- Prakash, L.; Middha, S.K.; Mohanty, S.K.; Swamy, M.K. Micropropagation and validation of genetic and biochemical fidelity among regenerants of Nothapodytes nimmoniana (Graham) Mabb. employing ISSR markers and HPLC. 3 Biotech 2016, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Balasubramanya, S.; Anuradha, M. In vitro multiplication of Pogostemon cablin Benth. through direct regeneration. Afr. J. Biotechnol. 2010, 9, 2069–2075. [Google Scholar]
- Swamy, M.K.; Balasubramanya, S.; Anuradha, M. Germplasm conservation of patchouli (Pogostemon cablin Benth.) by encapsulation of in vitro derived nodal segments. Int. J. Biodivers. Conserv. 2009, 1, 224–230. [Google Scholar]
- Sudipta, K.M.; KumaraSwamy, M.; Balasubramanya, S.; Anuradha, M. Cost effective approach for in vitro propagation of (Leptadenia reticulata Wight & Arn.)—A threatened plant of medicinal importance. J. Phytol. 2011, 3, 72–79. [Google Scholar]
- Kaushik, P.S.; Swamy, M.K.; Balasubramanya, S.; Anuradha, M. Rapid plant regeneration, analysis of genetic fidelity and camptothecin content of micropropagated plants of Ophiorrhiza mungos Linn.—A potent anticancer plant. J. Crop Sci. Biotechnol. 2015, 18, 1–8. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plant Res. 2009, 3, 1222–1239. [Google Scholar]
- Swamy, M.K.; Mohanty, S.K.; Anuradha, M. The effect of plant growth regulators and natural supplements on in vitro propagation of Pogostemon cablin Benth. J. Crop Sci. Biotechnol. 2014, 17, 71–78. [Google Scholar] [CrossRef]
- Arya, V.; Shekhawat, N.S.; Singh, R.P. Micropropagation of Leptadenia reticulate—A medicinal plant. In Vitro Cell. Dev. Biol. Plant 2003, 39, 180–185. [Google Scholar] [CrossRef]
- Hariharan, M.; Sebastian, D.P.; Benjamin, S.; Prashy, P. Somatic embryogenesis in Leptadenia reticulata Wight & Arn. A medicinal plant. Phytomorphology 2002, 52, 155–160. [Google Scholar]
- Martin, K.P. Benzyladenine induced somatic embryogenesis and plant regeneration of Leptadenia reticulata. Biol. Plant. 2004, 48, 285–288. [Google Scholar] [CrossRef]
- Shekhawat, N.S.; Kackar, A.; Rathore, M.S.; Singh, M.; Dagla, H.R.; Arya, V. Establishment and economic evaluation of micropropagated Jeewanti Leptadenia reticulata (Wight & Arn.) plants in field. Nat. Prod. Radiance 2006, 5, 311–314. [Google Scholar]
- Parabia, M.F.; Gami, B.; Kothari, L.I.; Mohan, J.S.S.; Parabia, H.M. Effect of plant growth regulators on in vitro morphogenesis of L. reticulata from nodal explants. Curr. Sci. 2007, 92, 1290–1293. [Google Scholar]
- Rathore, M.S.; Shakhawat, N.S. Ex vivo implication of phytohormones on various in vitro responses in Leptadenia reticulata (Retz.) Weight &Arn.—An endengered plant. Environ. Exp. Bot. 2013, 86, 86–93. [Google Scholar]
- Ayurvedherbs.com. Available online: http://ayurvedherbs.com/leptadenia-reticulata-(jivanti).html (accessed on 8 March 2017).
- Jivanti (Holostemma Adakodien) Herb—View Latest Price...—IndiaMART. Available online: https://www.indiamart.com/proddetail/jivanti-holostemma-adakodien-herb-13210353788.html (accessed on 8 March 2017).
- Agro-Techniques of Selected Medicinal Plants. Available online: http://www.fdcm.nic.in/PDF/Agro-techniques%20of%20selected%20medicinal%20plants%20Vol%20-%20I%20Part%20-%20II.pdf (accessed on 8 March 2017).
- Raval, M.A.; Mishra, S.H. Parameters for differentiation of Leptadenia reticulata from substitutes. J. Herbs Spices Med. Plants 2010, 16, 147–159. [Google Scholar] [CrossRef]
- Girija, T.P.; Shree, A.R. Comparative histological, histochemical and phytochemical studies of the raw drug jivanti from different raw drug markets of Kerala. Int. J. Pharm. Sci. Res. 2016, 7, 2122. [Google Scholar]


| Language | Vernacular Names (Language) |
|---|---|
| Hindi | Dori |
| Bengali | Bhadjivai |
| English | Jiwanti or Jeevanti |
| Gujarati | Methidodi, Dodi saka/Dodi Saag, Dori |
| Marathi | Haranvel, Hiranvel |
| Kannada | Hiriyahalle |
| Sanskrit | Madhusrava, Jivniya, Jivapushpa or Jivani |
| Tamil | Palaikkodi |
| Telugu | Kalasa |
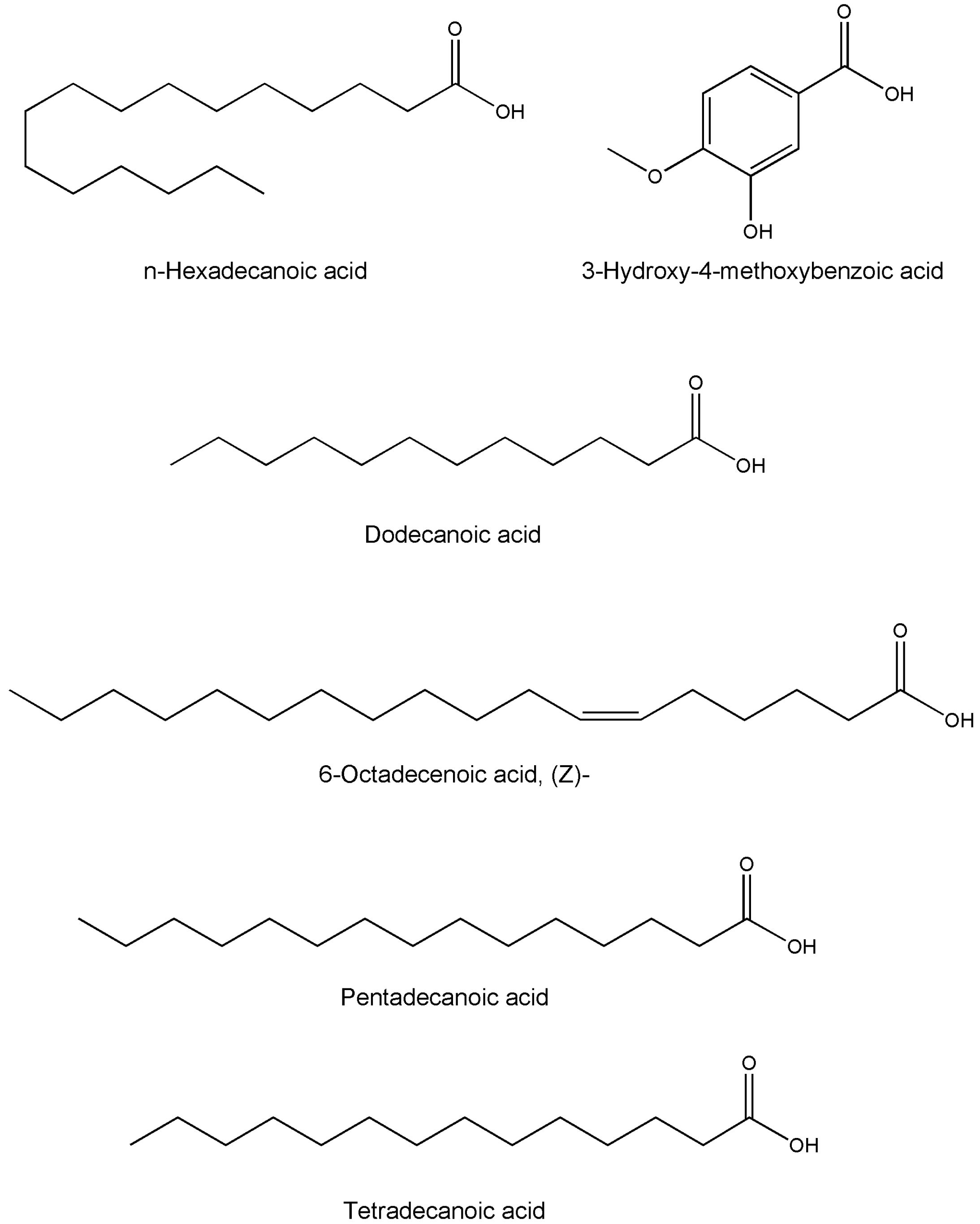
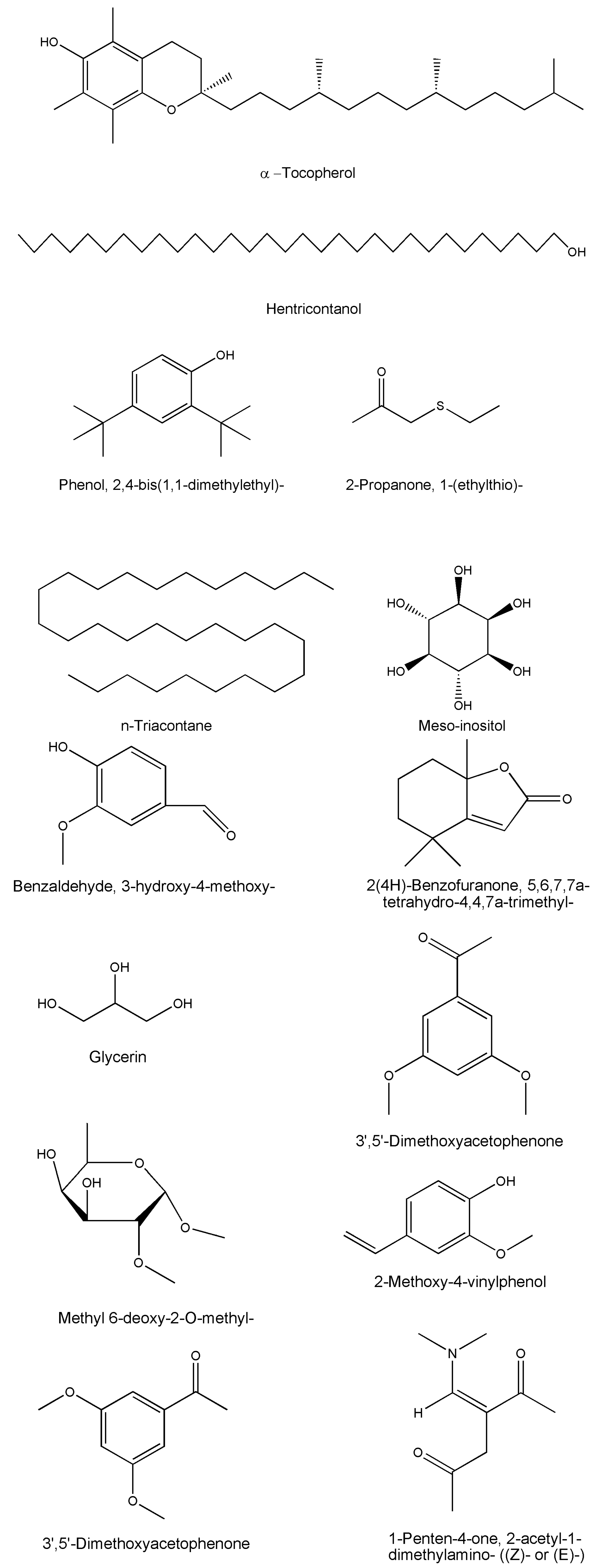
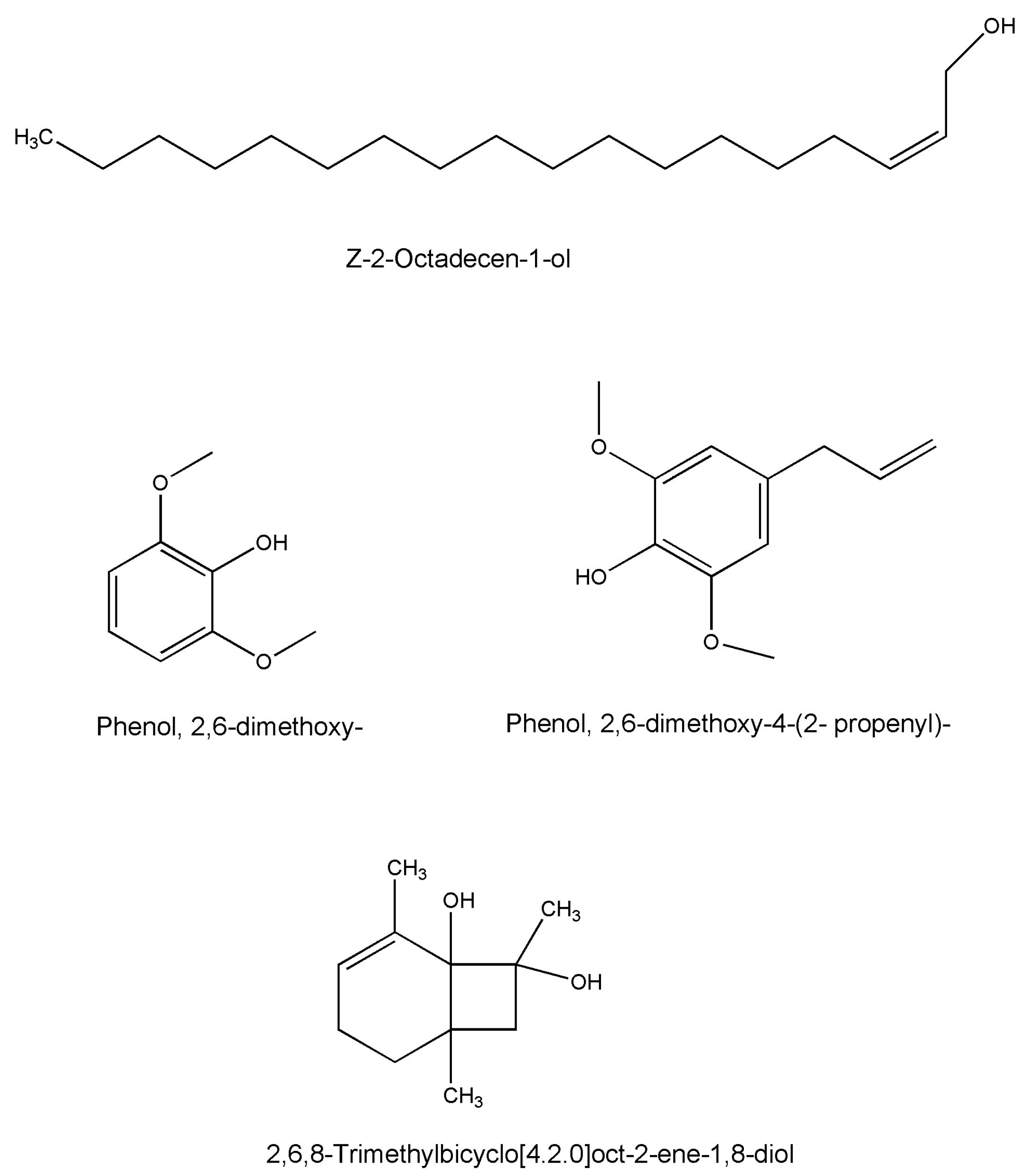
| Compound Name | Formula | Plant Part | Method | References |
|---|---|---|---|---|
| Flavonoids | ||||
| Apigenin | C48H80O17 | Stem bark | - | [14,15] |
| Diosmetin | C16H12O6 | Whole plant/leaves/twigs | Chromatography/HPTLC/NMR | [14,15] |
| Luteolin | C16H12O6 | Whole plant/leaves/twigs | Chromatography/HPTLC/NMR | [14,15] |
| Rutin | C27H30O16 | Leaves | HPLC/HPLC | [27,43,46,47] |
| Quercetin | C15H10O7 | Whole plant | TLC/HPTLC | [36,44] |
| Iso-quercetin | C21H20O12 | Whole plant | TLC/HPTLC | [12,29] |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | Whole plant | GC-MS | [49] |
| 2H-Pyran, 6-heptyltetrahydro-2,2-dimethoxy- | C14H28O3 | Whole plant | GC-MS | [49] |
| Phenolic compound | ||||
| p-coumaric acid | C9H8O3 | Whole plant/leaves | HPLC | [29,40,43,44] |
| Terpenes/Terpenoids | ||||
| α-amyrin | C30H50O | Whole plant/leaves/twigs | Chromatography/HPTLC/IR | [13,15,28,47] |
| β-amyrin | C30H50O | Whole plant/leaves/twigs | Chromatography/HPTLC/IR | [11,25,27,44] |
| Lupeol | C30H50O | Whole plant/roots | HPTLC/NMR | [42,48] |
| Simiarenol | C30H50O | Whole plant | - | [14] |
| Phytol | C20H40 | Whole plant | GC-MS | [49] |
| Esters | ||||
| Benzene carboxylic acid | C7H6O2 | Whole plant | GC-MS | [49] |
| Ethyl hydrogen succinate | C6H10O4 | Whole plant | GC-MS | [49] |
| Hexadecanoic acid, ethyl ester | C18H36O2 | Whole plant | GC-MS | [49] |
| 4-Oxazolecarboxylic acid, 4,5-dihydro-2-phenyl-, 1-methylethyl ester | C13H15NO3 | Whole plant | GC-MS | [49] |
| 9,12-Octadecadienoic acid, ethyl Ester | C20H36O2 | Whole plant | GC-MS | [49] |
| 9-Octadecenoic acid, ethylester | C20H38O2 | Whole plant | GC-MS | [49] |
| Fatty acids | ||||
| Dodecanoic acid | C12H24O2 | Whole plant | GC-MS | [49] |
| n-Hexadecanoic acid | C16H32O2 | Whole plant | GC-MS | [49] |
| 3-Hydroxy-4-methoxybenzoic acid | C8H8O4 | Whole plant | GC-MS | [49] |
| 6-Octadecenoic acid, (Z)- | C18H34O2 | Whole plant | GC-MS | [49] |
| Pentadecanoic acid | C15H30O2 | Whole plant | GC-MS | [49] |
| Tetradecanoic acid | C14H28O2 | Whole plant | GC-MS | [49] |
| Pregnane glycosides | ||||
| Deniculatin | C34H56O11 | Aerial parts | NMR/FAB/EI-MS | [45] |
| Leptaculatin | C40H66O16 | Aerial parts | NMR/FAB/EI-MS | [14,15] |
| Reticulin | C34H56O11 | Aerial parts | NMR/FAB/EI-MS | [15,16,29] |
| Steroids | ||||
| β-sitosterol | C29H50O | Whole plant/leaves/twigs | TLC/HPTLC | [14,15,44,46] |
| Stigmasterol | C29H48O | Whole plant/leaves/twigs/roots | Chromatography/HPTLC/IR | [12,14,15,25,29,44,46] |
| Others (Carbohydrates, carboxylic acid, ethers, alcohols, aldehydes etc.) | ||||
| Benzaldehyde, 3-hydroxy-4-methoxy- | C8H8O3 | Whole plant | GC-MS | [49] |
| 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- | C11H16O2 | Whole plant | GC-MS | [50] |
| Glycerin | C3H8O3 | Whole plant | GC-MS | [49] |
| 3′,5′-Dimethoxyacetophenone | C10H12O3 | Whole plant | GC-MS | [49] |
| Hentricontanol | C31H64O | Leaves/twigs | Chromatography/HPTLC/NMR | [14,15] |
| Methyl 6-deoxy-2-O-methyl- | C8H16O5 | Whole plant | GC-MS | [49] |
| 2-Methoxy-4-vinylphenol | C9H10O2 | Whole plant | GC-MS | [49] |
| Meso-inositol | C6H12O6 | Whole plant | - | [12] |
| Z-2-Octadecen-1-ol | C18H36O | Whole plant | GC-MS | [49] |
| 1-Penten-4-one, 2-acetyl-1-dimethylamino- ((Z)- or (E)-) | C9H15NO2 | Whole plant | GC-MS | [49] |
| Phenol, 2,6-dimethoxy- | C8H10O3 | Whole plant | GC-MS | [49] |
| Phenol, 2,6-dimethoxy-4-(2-propenyl)- | C11H14O3 | Whole plant | GC-MS | [49] |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | C14H22O | Whole plant | GC-MS | [49] |
| 2-Propanone, 1-(ethylthio)- | C5H10O | Whole plant | GC-MS | [49] |
| α-tocopherol | C29H50O2 | Roots/stems/leaves | HPTLC | [12,13,25,44,46] |
| 2,6,8-Trimethylbicyclo[4.2.0]oct-2-ene-1,8-diol | C11H18O2 | Whole plant | GC-MS | [49] |
| n-triacontane | C30H62 | Whole plant | HPTLC | [45] |
| Pharmacological Activity or Therapeutic Use | Part of the Plant Used or Extract | Animal Used | References |
|---|---|---|---|
| Antiabortifacient effect | Plant powder and Leptaden tablet | Human, Guinea pig | [50,51,52,53] |
| Anti-anaphylactic activity | The herbal formulation (DLH-3041) | Normal rats | [54] |
| Antidepressant effect | A polyherbal formulation (Malkanguni) | Human | [55] |
| Anti-epileptic potential | Leaf | - | [56] |
| Anti-implantation activity | Leaf | Albino rats | [57] |
| Antimicrobial activity | Whole plant, stem, leaf and root | - | [58,59,60,61,62,63] |
| Antitumor/in vitro cytotoxic activity | Whole plant, leaf, in vitro plants, callus | Swiss albino mice | [64,65] |
| Antioxidant activity | Leaf, in vitro plants, callus, whole plant | Normal rats | [66,67,68] |
| Antipyretic, analgesic, and anti-inflammatory activity | Whole plant | Albino rats | [44,69] |
| Antiulcer activity | Leaf | Normal rats | [70,71] |
| Anxiolytic activity | Leaf | Wister albino rats | [72] |
| Cardioprotective activity | Leaf | Normal rats | [66] |
| Diuretic activity | Whole plants | Normal rats | [72] |
| Galactogogue property/Lactogenic effect/improving milk quality | Whole plant and Leptaden (Herbal formulation) | Human, cows, normal rat | [12,73,74,75,76,77,78,79,80,81] |
| Hepatoprotective activity | Stem | Swiss albino mice | [38] |
| Immunomodulatory activity | Whole plant, leaf | Swiss albino mice | [67,82] |
| Rejuvenating properties | Whole plant | Humans | [83,84,85,86] |
| Treatment of oligospermia | The Speman tablet (Himalaya Drug Co., Bengaluru) | Human | [83] |
| Treatment of asthma, bronchitis and throat trouble | Whole plant | Human | [11] |
| Treatment of Skin infection against ringworms and wounds | Whole plant | Human | [85] |
| Treatment of oligospermia | Speman (Herbal formulation) | Human | [86] |
| Treatment of benign prostatic hyperplasia, Prostatitis | Speman (Herbal formulation) | Human | [86] |
| Vasodilator, Hypotensive effect | Stem | Dog | [84] |
| Name of the Product | Company | Uses |
|---|---|---|
| Confido (Speman forte) | Himalaya Drug House, Bengaluru, India | Useful in oligospermia |
| Speman | Himalaya Drug House, Bengaluru, India | Helps in Spermatogenesis |
| Galactin Vet (bolus) | Himalaya Drug House, Bengaluru, India | Stimulate activity of alveolar tissue, stimulate lactogenesis, improve fat percentage |
| Speman forte vet | Himalaya Drug House, Bengaluru, India | Spermatogenic and increases libido |
| Speman vet | Himalaya Drug House, Bengaluru, India | Promotes spermatogenesis |
| HimalayaTM Chyavanprasha | Himalaya Drug House, Bengaluru, India | Useful in debilitating disorders like cough, cold, infection. Boost immunity of the body |
| Calshakti | Intas Pharmaceuticals Ltd., Ahmedabad, India | Feed suppliments (Animal health) |
| Safe herbs | VASU Healthcare Pvt. Ltd., Hyderabad, India | Improves lactation (Women care) |
| Jivanti Powder/capsule | Evaidyaji Wellness Pvt. Ltd., Jaipur, India | Used in allergic response, constipation, cardiac and bleeding disorders, possess diuretic property |
| Enviro Care | Satveda, India/Herbs Forever, Los Angeles, CA, USA | Used as antioxidants, immunobuilder, rejuvenating and vitalizing tonic |
| Antisept | Zydus Cadila Healthcare Ltd., Ahmedabad, India | Possess antiseptic and antibacterial properties |
| Praas™ (Chyawanprash) | Komal Herbals, Inc., Sewickley, PA, USA | Enhance general health, increase mental and physical energy, increase resistance to disease |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, S.K.; Swamy, M.K.; Sinniah, U.R.; Anuradha, M. Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): Botanical, Agronomical, Phytochemical, Pharmacological, and Biotechnological Aspects. Molecules 2017, 22, 1019. https://doi.org/10.3390/molecules22061019
Mohanty SK, Swamy MK, Sinniah UR, Anuradha M. Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): Botanical, Agronomical, Phytochemical, Pharmacological, and Biotechnological Aspects. Molecules. 2017; 22(6):1019. https://doi.org/10.3390/molecules22061019
Chicago/Turabian StyleMohanty, Sudipta Kumar, Mallappa Kumara Swamy, Uma Rani Sinniah, and Maniyam Anuradha. 2017. "Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): Botanical, Agronomical, Phytochemical, Pharmacological, and Biotechnological Aspects" Molecules 22, no. 6: 1019. https://doi.org/10.3390/molecules22061019
APA StyleMohanty, S. K., Swamy, M. K., Sinniah, U. R., & Anuradha, M. (2017). Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): Botanical, Agronomical, Phytochemical, Pharmacological, and Biotechnological Aspects. Molecules, 22(6), 1019. https://doi.org/10.3390/molecules22061019