Synthesis of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles and Its Distribution and Dissipation in Cucumber Plants
Abstract
:1. Introduction
2. Results and Discussions
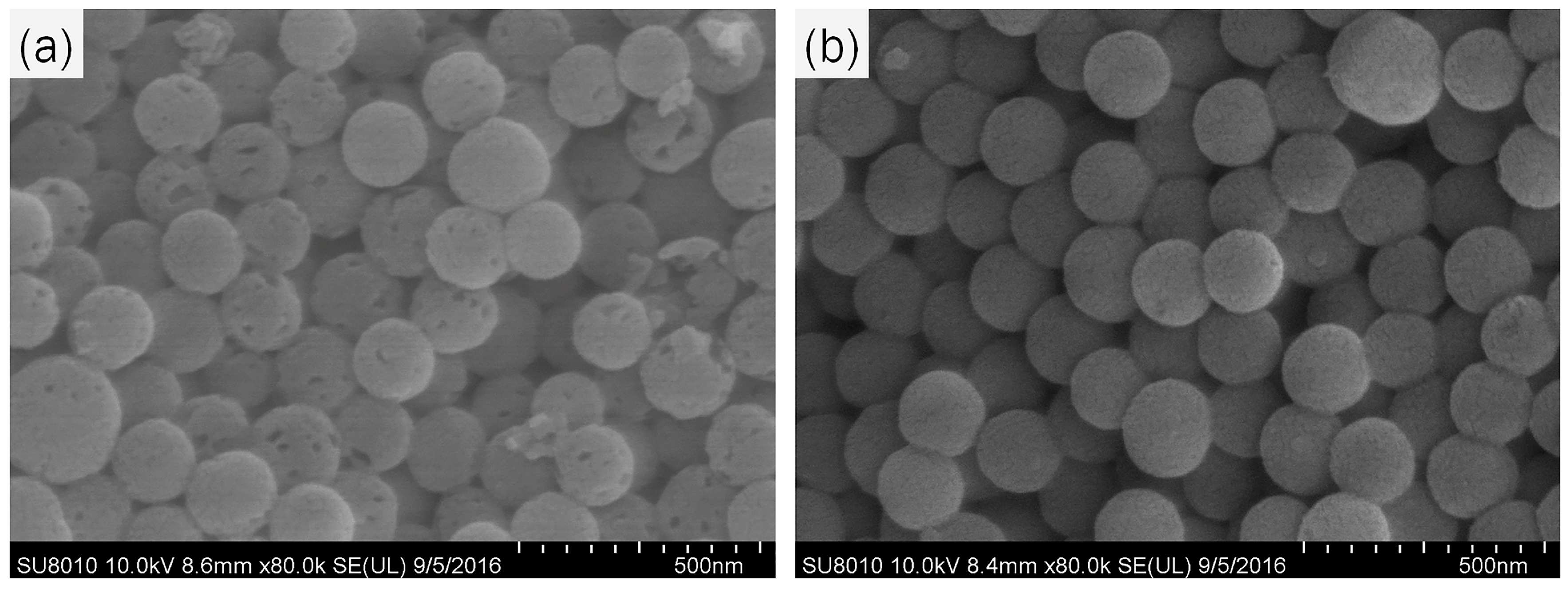
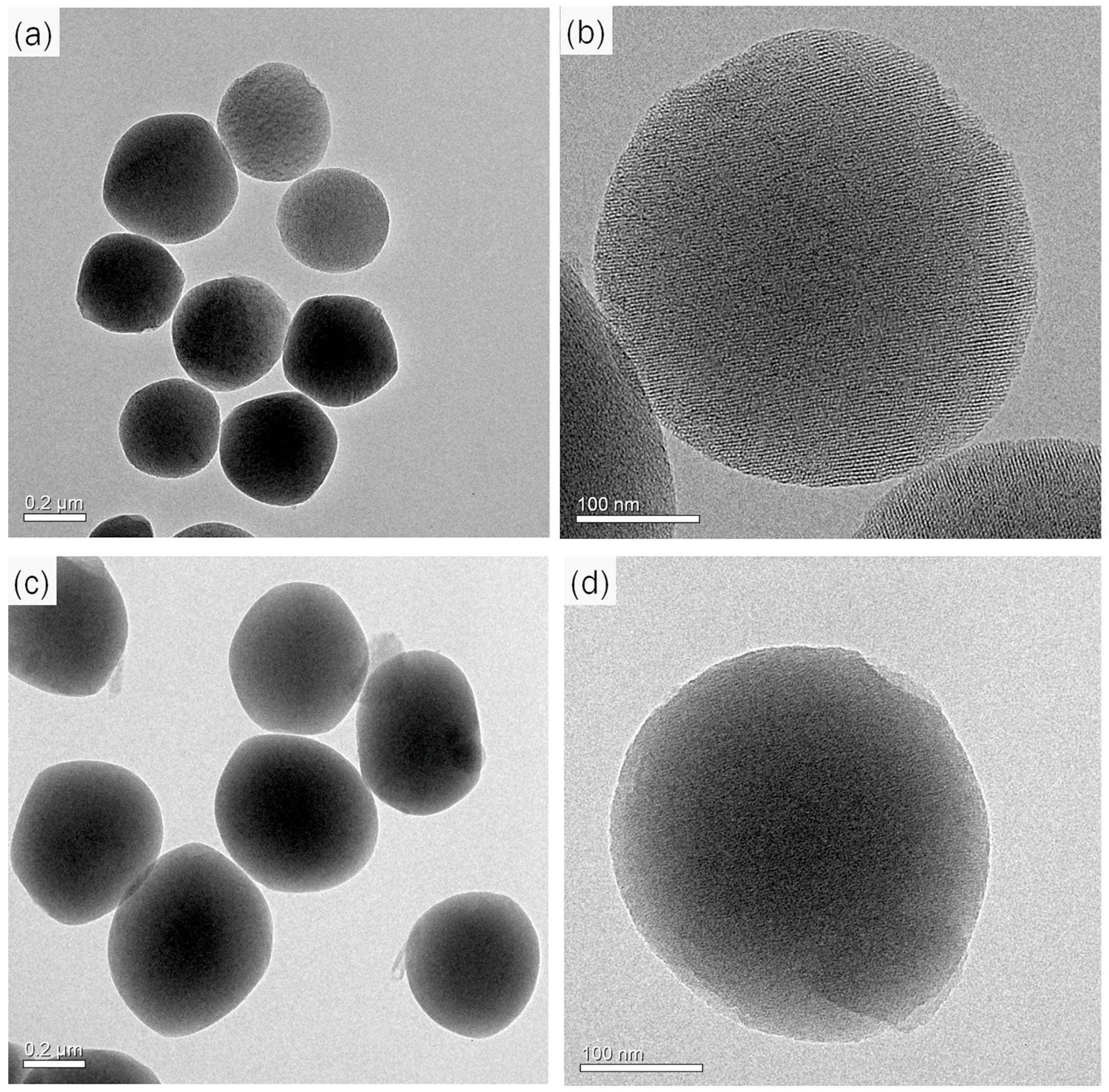
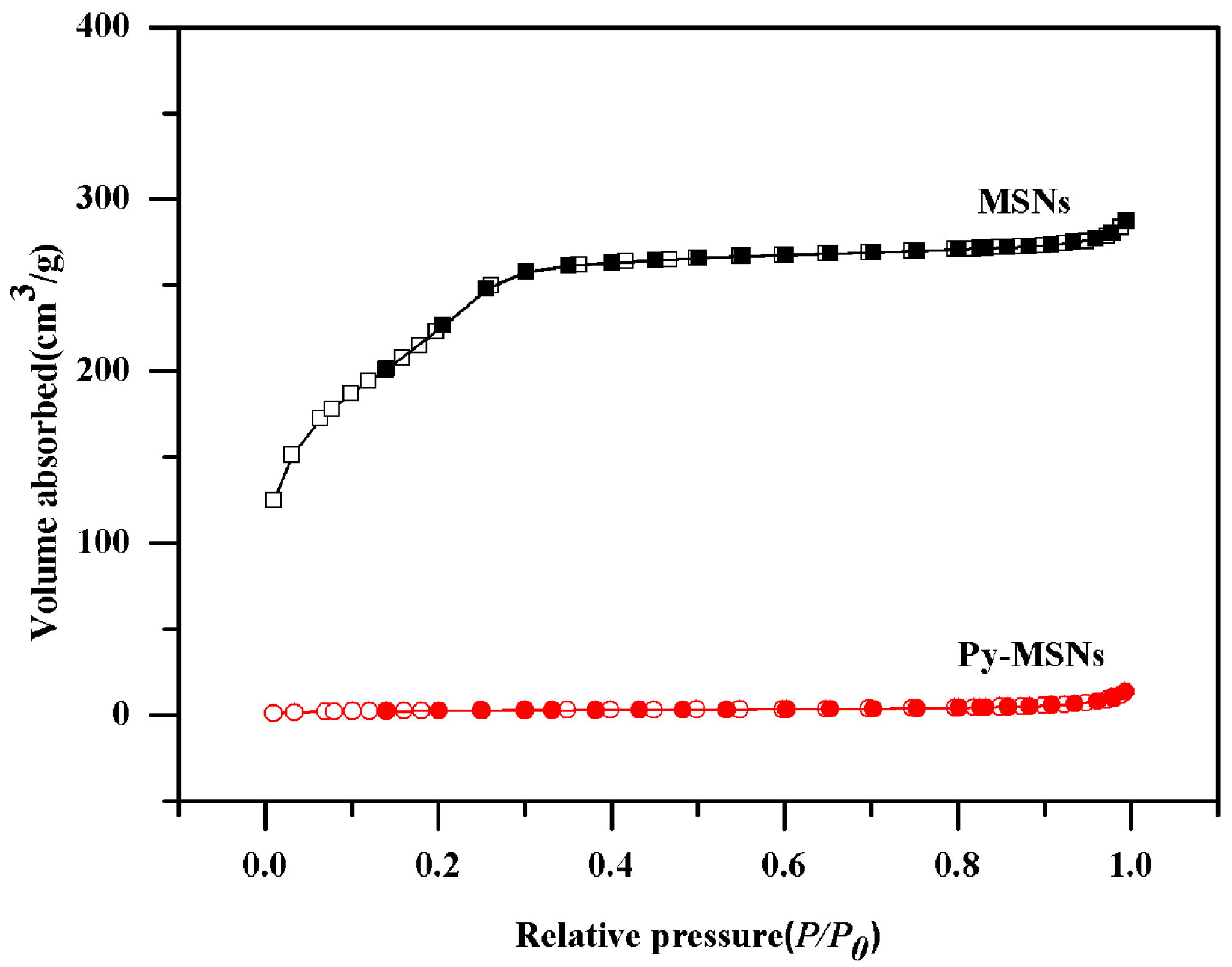
2.1. Preparation and Characterization of MSNs and Py-MSNs
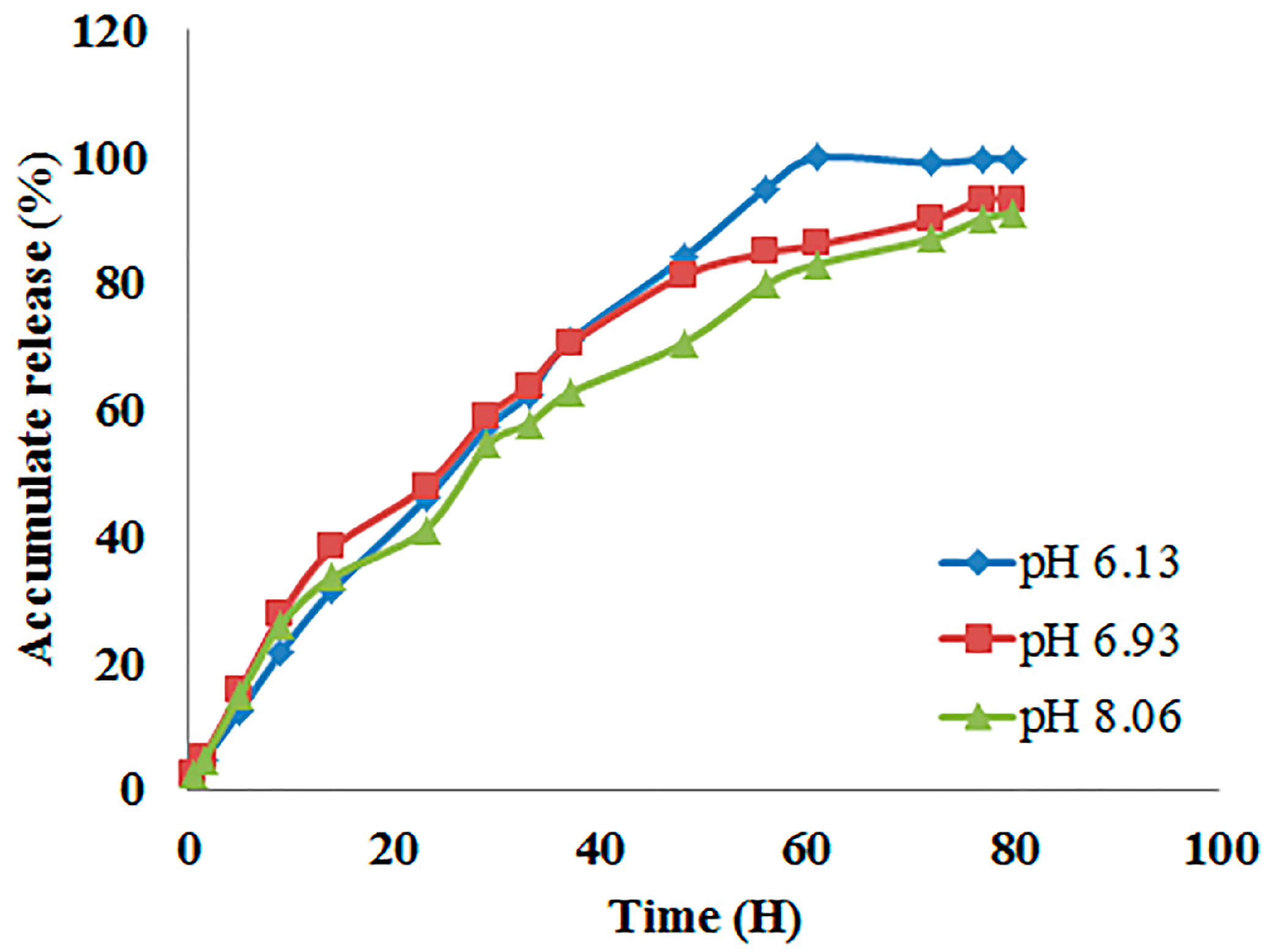
2.2. In Vitro Release of Pymethanil
2.3. Analytical Method Validation
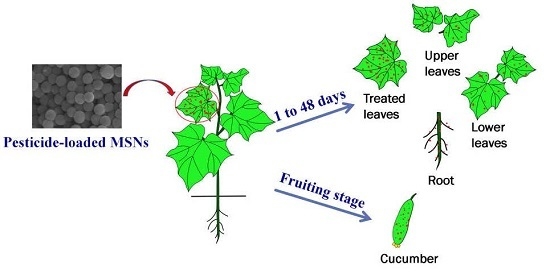
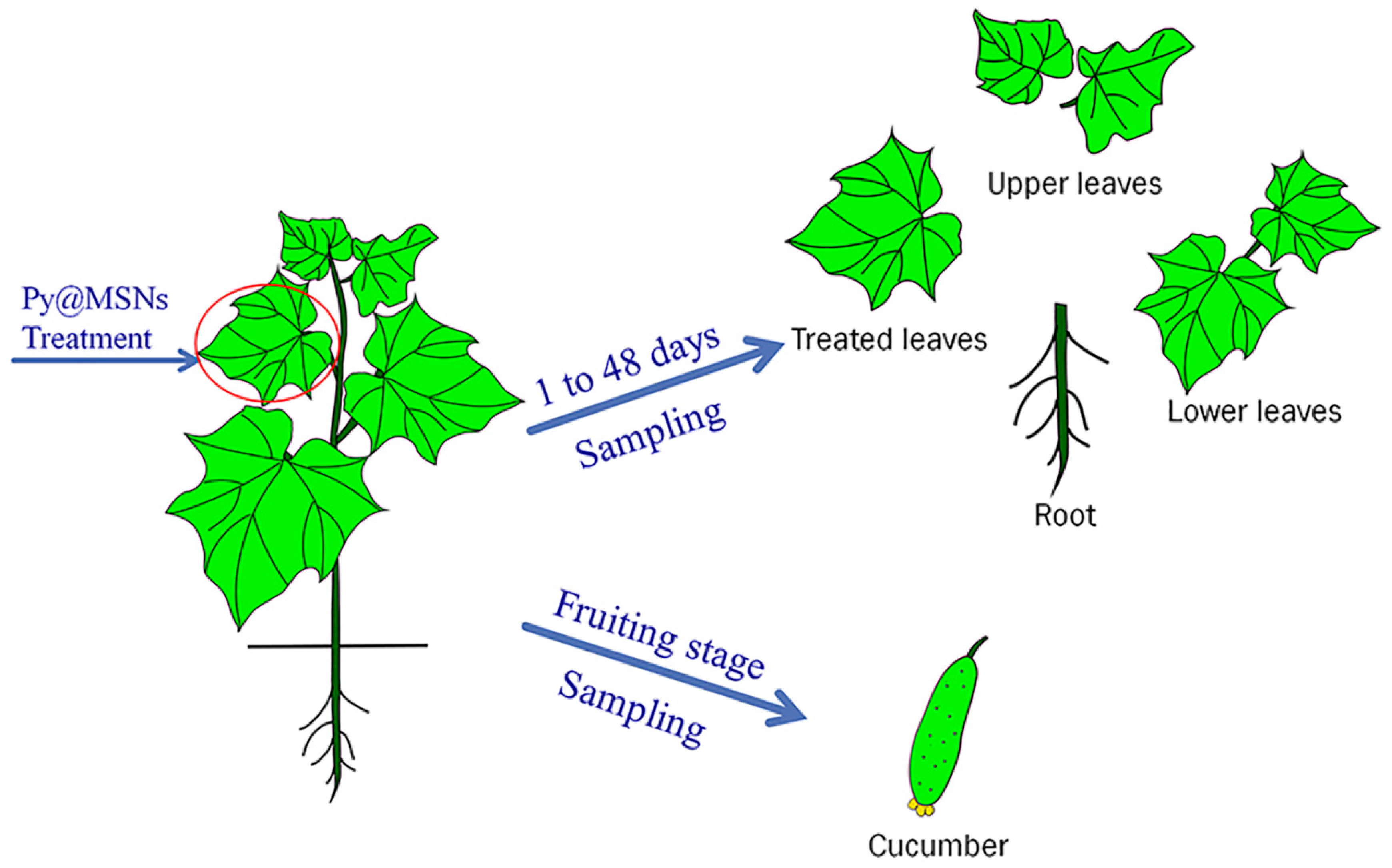
2.4. Distribution of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles in Cucumber Plant
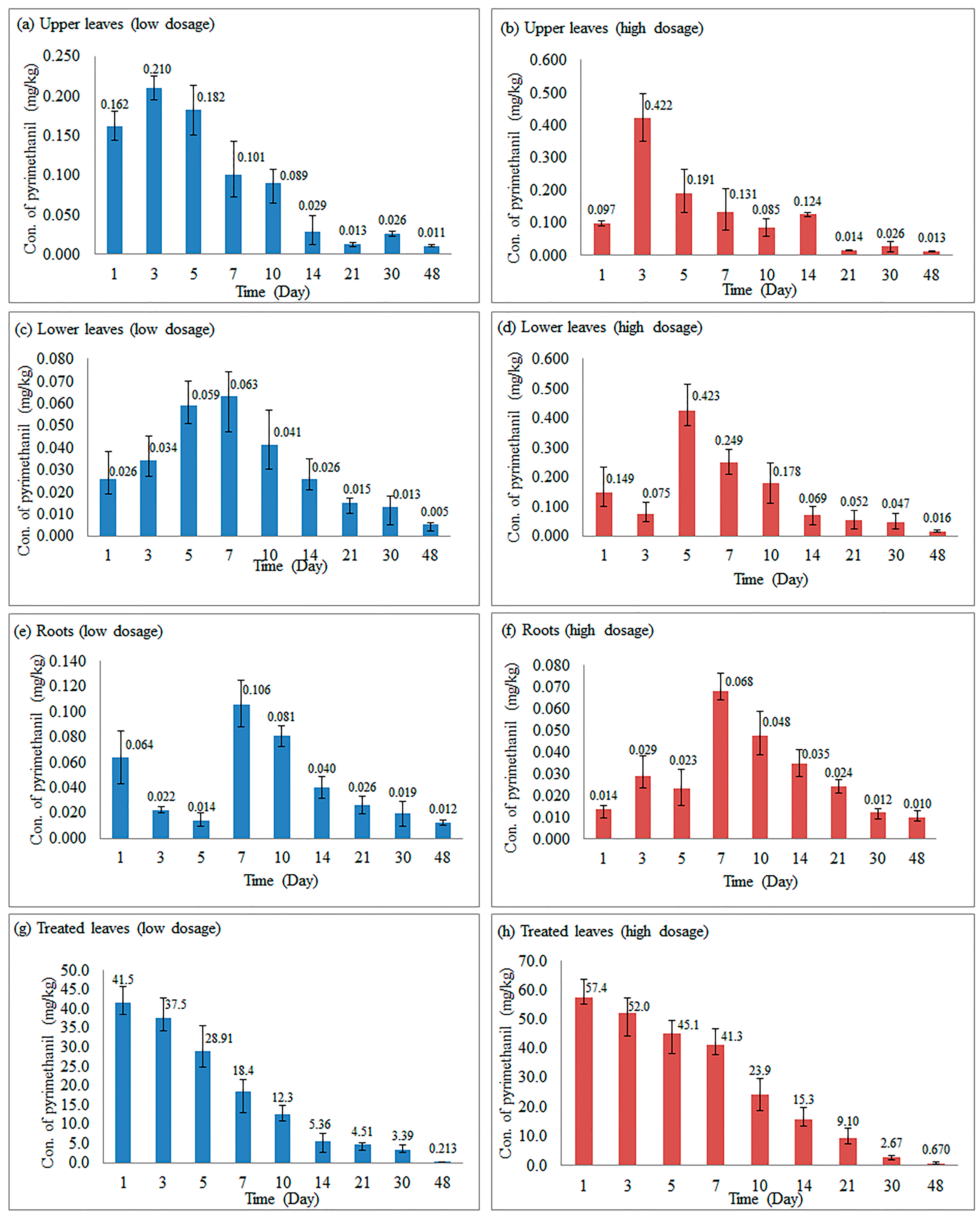
2.5. Dissipation of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles in Different Parts of Cucumber Plants
2.6. Final Residues of Pyrimethanil in Cucumber
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Mesoporous Silica Nanoparticles
3.3. Loading of Pyrimethanil into Mesoporous Silica Nanoparticles
3.4. Pyrimethanil Release
- Er: the accumulative release (%) of pyrimethanil from the nanoparticles;
- Ve: the volume of the release medium taken in a time interval (Ve = 0.8 mL);
- Ci: the pyrimethanil concentration in release medium;
- i: release time
- V0: the volume of release medium (250 mL);
- n: the sample number;
- Mp: the total amount of pesticide enwrapped in the particles.
3.5. Greenhouse Study
3.6. Sample Preparation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MSNs | mesoporous silica nanoparticles |
| Py-MSNs | pyrimethanil-loaded MSNs |
| CTAB | cetyltrimethylammonium bromide |
| TEOS | tetraethyl orthosilicate |
| PSA | primary secondary amine |
| GCB | graphitized carbon black |
| SEM | scanning electron microscopy |
| DAD | diode array detector |
| HPLC-MS/MS | high performance liquid chromatography tandem mass spectrometry |
| QuEChERS | quick, easy, cheap, effective, rugged and safe |
| RSDs | relative standard deviations |
| LOQs | limits of quantification |
| MRL | maximum residue limit |
References
- Shi, J.; Wang, J.; Li, R.; Li, D.; Xu, F.; Sun, Q.; Zhao, B.; Mao, A.J.; Guo, Y.D. Expression patterns of genes encoding plasma membrane aquaporins during fruit development in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2015, 96, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Carr, K.M.; Grumet, R. Transcriptome analyses of early cucumber fruit growth identifies distinct gene modules associated with phases of development. BMC Genom. 2012, 13, 518. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization Corporate Statistical Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; Available online: http://faostat3.fao.org/download/Q/QC/E (accessed on 15 May 2017).
- Berdugo, C.A.; Zito, R.; Paulus, S.; Mahlein, A.K. Fusion of sensor data for the detection and differentiation of plant diseases in cucumber. Plant Pathol. 2015, 63, 1344–1356. [Google Scholar] [CrossRef]
- All 48 Fruits and Vegetables with Pesticide Residue Data; The Environmental Working Group: Washington, DC, USA, 2017; Available online: https://www.ewg.org/foodnews/list.php (accessed on 15 May 2017).
- Stephenson, G.R. Pesticide use and world food production: Risks and benefits. In Environmental Fate and Effects of Pesticides; Coats, J.R., Yamanoto, H., Eds.; American Chemical Society: Washington, DC, USA, 2003; pp. 261–270. [Google Scholar]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-Guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Abhilash, P.C.; Fraceto, L.F. Nanotechnology Applied to Bio-Encapsulation of Pesticides. J. Nanosci. Nanotechnol. 2016, 16, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Ghosh, J.; Sil, P.C. 2-New pesticides: A cutting-edge view of contributions from nanotechnology for the development of sustainable agricultural pest control A2-Grumezescu, Alexandru Mihai. In New Pesticides and Soil Sensors; Grumezescu, A., Ed.; Academic Press: London, UK, 2017; pp. 47–79. [Google Scholar]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, S.; Ganguli, A.K.; Shanmugam, V. Anti-drift nano-stickers made of graphene oxide for targeted pesticide delivery and crop pest control. Carbon 2017, 115, 781–790. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous silica nanoparticles for stimuliresponsive controlled drug delivery: Advances, challenges, and outlook. Int. J. Nanomed. 2017, 12, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ortigosa, S.; Peterson, D.J.; Valenstein, J.S.; Lin, V.S.Y.; Trewyn, B.G.; Lyznik, L.A.; Wang, K. Mesoporous silica nanoparticle-mediated intracellular cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 2014, 164, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.P.; Kuang, L.Y.; Huang, C.A.; Jane, W.N.; Hung, Y.; Hsing, Y.C.; Mou, C.Y. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J. Mater. Chem. B 2013, 1, 5279–5287. [Google Scholar] [CrossRef]
- Chang, J.H.; Thai, P.H.; Chen, W.; Chiou, S.H.; Mou, C.Y. Dual delivery of siRNA and plasmid DNA using mesoporous silica nanoparticles to differentiate induced pluripotent stem cells into dopaminergic neurons. J. Mater. Chem. B 2017, 5, 3012–3023. [Google Scholar] [CrossRef]
- Kankala, R.K.; Kuthati, Y.; Liu, C.L.; Mou, C.Y.; Lee, C.H. Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ROS. RSC Adv. 2015, 5, 86072–86081. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Li, J.; Yan, M.; Wang, C. Surface modification of MSNs with β-CD and use as a drug delivery system. Mater. Technol. 2016, 31, 1–5. [Google Scholar] [CrossRef]
- Tsai, C.H.; Viveroescoto, J.L.; Slowing, I.I.; Fang, I.J.; Trewyn, B.G.; Lin, V.S. Surfactant-assisted controlled release of hydrophobic drugs using anionic surfactant templated mesoporous silica nanoparticles. Biomaterials 2011, 32, 6234–6244. [Google Scholar] [CrossRef] [PubMed]
- Malfanti, A.; Miletto, I.; Bottinelli, E.; Zonari, D.; Blandino, G.; Berlier, G.; Arpicco, S. Delivery of Gemcitabine Prodrugs Employing Mesoporous Silica Nanoparticles. Molecules 2016, 21, 522. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Wu, L.C.; Lu, S.Y.; Chang, L.L.; Teng, I.; Yang, C.M.; Ho, J.A. Biofunctionalized Phospholipid-Capped Mesoporous Silica Nanoshuttles for Targeted Drug Delivery: Improved Water Suspensibility and Decreased Nonspecific Protein Binding. ACS Nano 2010, 4, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Qing, M.L.G. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Sun, C.; Zhao, X.; Cui, B. Construction and evaluation of controlled-release delivery system of Abamectin using porous silica nanoparticles as carriers. Nanoscale Res. Lett. 2014, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Wanyika, H.; Gatebe, E.; Kioni, P.; Tang, Z.; Gao, Y. Mesoporous silica nanoparticles carrier for urea: Potential applications in agrochemical delivery systems. J. Nanosci. Nanotechnol. 2012, 12, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.X.; Cahill, D.M. Mesoporous silica nanoparticles as a biomolecule delivery vehicle in plants. J. Nanopart. Res. 2013, 15, 1676. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Cao, C.; Zhang, J.; Li, F.; Huang, Q. Quaternized Chitosan-Capped Mesoporous Silica Nanoparticles as Nanocarriers for Controlled Pesticide Release. Nanomaterials 2016, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Liu, J.; Hu, Q.; Kennedy, M.; Peters, B.; Lu, G.Q.; Qiao, S.Z. Adsorption and release of biocides with mesoporous silica nanoparticles. Nanoscale 2012, 4, 970–975. [Google Scholar] [CrossRef] [PubMed]
- European Commission Directorate General Health and Consumer Protection. Guidance Document on Quality Control Procedures for Pesticide Residues Analyses; EU Laboratories for Residues of Pesticides: Fellbach, Germany, 2006; SANCO/10232/2006. [Google Scholar]
- Malhat, F.M.; Loutfy, N.M.; Thabet, W. Dissipation profile and human risk assessment of pyrimethanil residues in cucumbers and strawberries. J. Health Pollut. 2014, 4, 36–41. [Google Scholar] [CrossRef]
- Liu, C.; Lu, D.; Wang, Y.; Wan, K.; Huang, J.; Wang, F. Pyrimethanil residue and dissipation in tomatoes and soil under field conditions. Environ. Monit. Assess. 2013, 185, 9397–9402. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Luo, Z.; Pang, W.; Fan, Y.; Chen, X.; Cui, F. Dilute Solution Routes to Various Controllable Morphologies of MCM-41 Silica with a Basic Medium. Chem. Mater. 2001, 13, 258–263. [Google Scholar] [CrossRef]
- Fowler, C.E.; Khushalani, D.; Lebeau, B.; Mann, S. Nanoscale Materials with Mesostructured Interiors. Adv. Mater. 2001, 13, 649–652. [Google Scholar] [CrossRef]
- Nooney, R.I.; Thirunavukkarasu, D.; Chen, Y.; Josephs, R.; Ostafin, A.E. Synthesis of Nanoscale Mesoporous Silica Spheres with Controlled Particle Size. Chem. Mater. 2002, 14, 4721–4728. [Google Scholar] [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Spataru, C.I.; Ianchis, R.; Petcu, C.; Nistor, C.L; Purcar, V.; Trica, B.; Nitu, S.G.; Somoghi, R.; Alexandrescu, E.; Oancea, F.; et al. Synthesis of Non-Toxic Silica Particles Stabilized by Molecular Complex Oleic-Acid/Sodium Oleate. Int. J. Mol. Sci. 2016, 17, 1936. [Google Scholar] [CrossRef] [PubMed]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning, “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [PubMed]
- Anastassiades, M.; Maštovská, K.; Lehotay, S.J. Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J. Chromatogr. A 2003, 1015, 163–184. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Sample | Fortified Level (mg/kg) | Average Recoveries (%) | RSD (%) | LOQ (mg/kg) | Linear Equation | R2 |
|---|---|---|---|---|---|---|
| Leaf | 0.01 | 78 | 8 | 0.004 | y = 3,964,048x + 9802 | 0.993 |
| 0.1 | 82 | 9 | ||||
| 1 | 85 | 6 | ||||
| Root | 0.01 | 87 | 5 | 0.001 | y = 5,007,122x + 3234 | 0.998 |
| 0.1 | 94 | 7 | ||||
| 1 | 99 | 8 | ||||
| Cucumber | 0.001 | 74 | 10 | 0.001 | y = 4,817,122x + 1329 | 0.996 |
| 0.01 | 90 | 7 | ||||
| 0.1 | 87 | 8 |
| Part | Day | Dosage (mg/mL) | Regression Equation | Determination Coefficient (R2) | Half-life (Days) |
|---|---|---|---|---|---|
| Upper leaves | 3–48 | 0.5 | y = 0.1243e−0.064(x − 3) | 0.7277 | 13.8 |
| 2 | y = 0.1934e−0.073(x − 3) | 0.7484 | 12.5 | ||
| Lower leaves | 5–48 | 0.5 | y = 0.0540e−0.059(x − 5) | 0.9484 | 16.7 |
| 2 | y = 0.2417e−0.068(x − 5) | 0.8671 | 15.2 | ||
| Root | 7–48 | 0.5 | y = 0.0757e−0.051(x − 7) | 0.8703 | 20.6 |
| 2 | y = 0.052e−0.047(x − 7) | 0.8893 | 21.7 | ||
| Treated leaves | 1–48 | 0.5 | y = 42.407e−0.106x | 0.9582 | 6.5 |
| 2 | y = 68.458e−0.099x | 0.9916 | 7.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Synthesis of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles and Its Distribution and Dissipation in Cucumber Plants. Molecules 2017, 22, 817. https://doi.org/10.3390/molecules22050817
Zhao P, Cao L, Ma D, Zhou Z, Huang Q, Pan C. Synthesis of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles and Its Distribution and Dissipation in Cucumber Plants. Molecules. 2017; 22(5):817. https://doi.org/10.3390/molecules22050817
Chicago/Turabian StyleZhao, Pengyue, Lidong Cao, Dukang Ma, Zhaolu Zhou, Qiliang Huang, and Canping Pan. 2017. "Synthesis of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles and Its Distribution and Dissipation in Cucumber Plants" Molecules 22, no. 5: 817. https://doi.org/10.3390/molecules22050817
APA StyleZhao, P., Cao, L., Ma, D., Zhou, Z., Huang, Q., & Pan, C. (2017). Synthesis of Pyrimethanil-Loaded Mesoporous Silica Nanoparticles and Its Distribution and Dissipation in Cucumber Plants. Molecules, 22(5), 817. https://doi.org/10.3390/molecules22050817