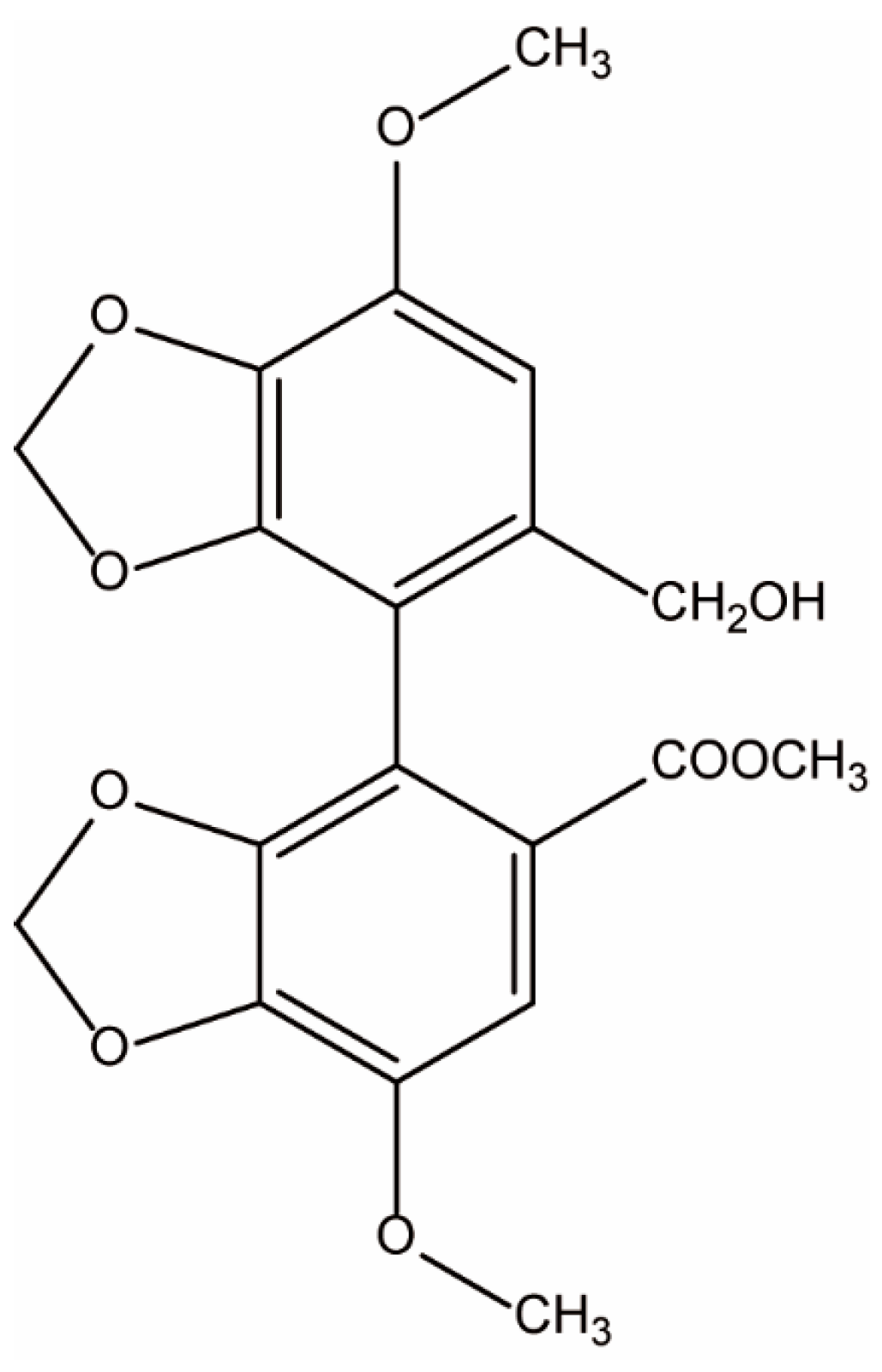
Protective Effect of Bicyclol on Anti-Tuberculosis Drug Induced Liver Injury in Rats
Abstract
:1. Introduction
2. Results
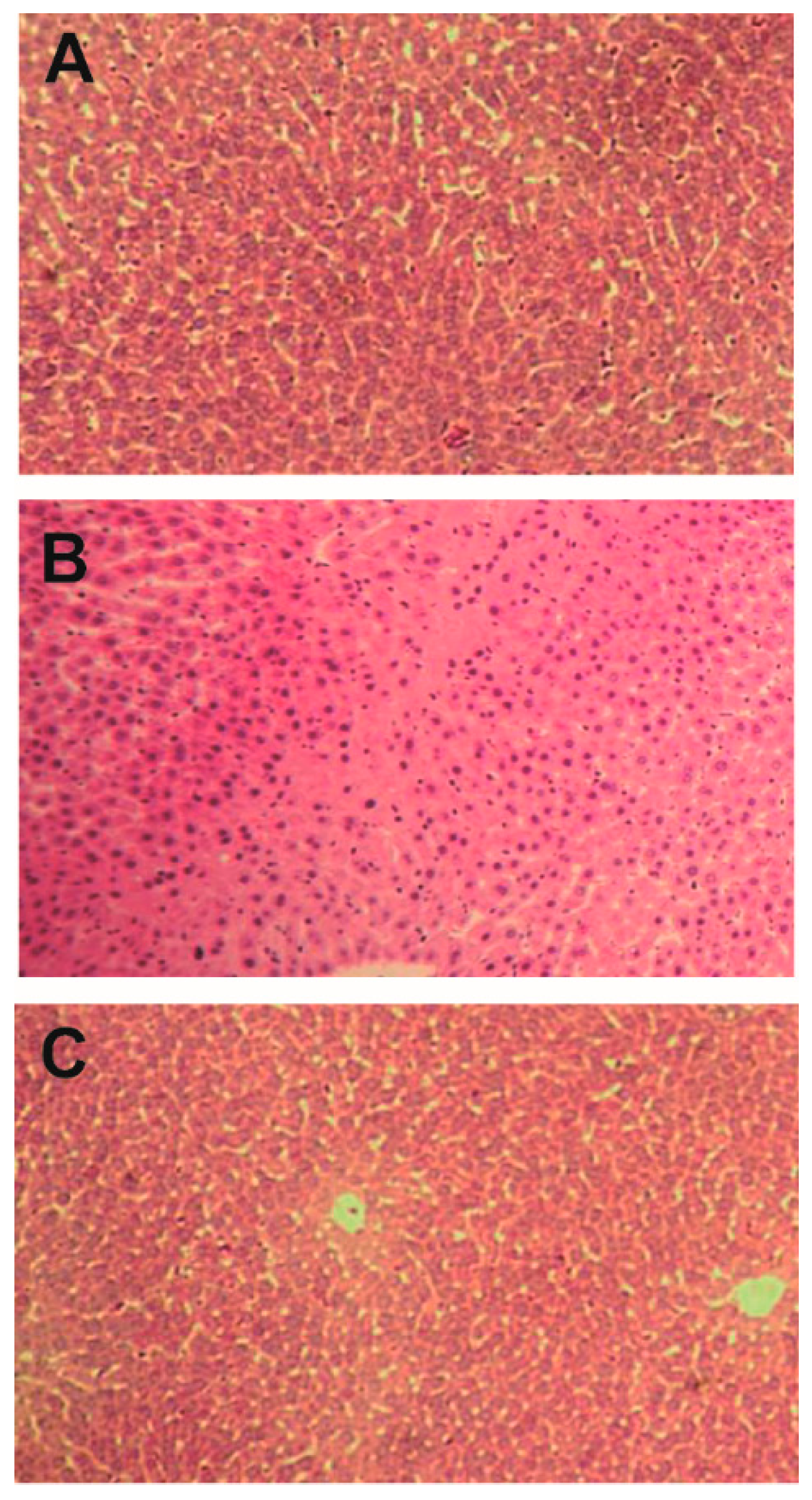
2.1. Effect of Bicyclol on Liver Injury Induced by Anti-TB Drug
2.2. Effect of Bicyclol on Oxidative Stress Induced by Anti-TB Drug
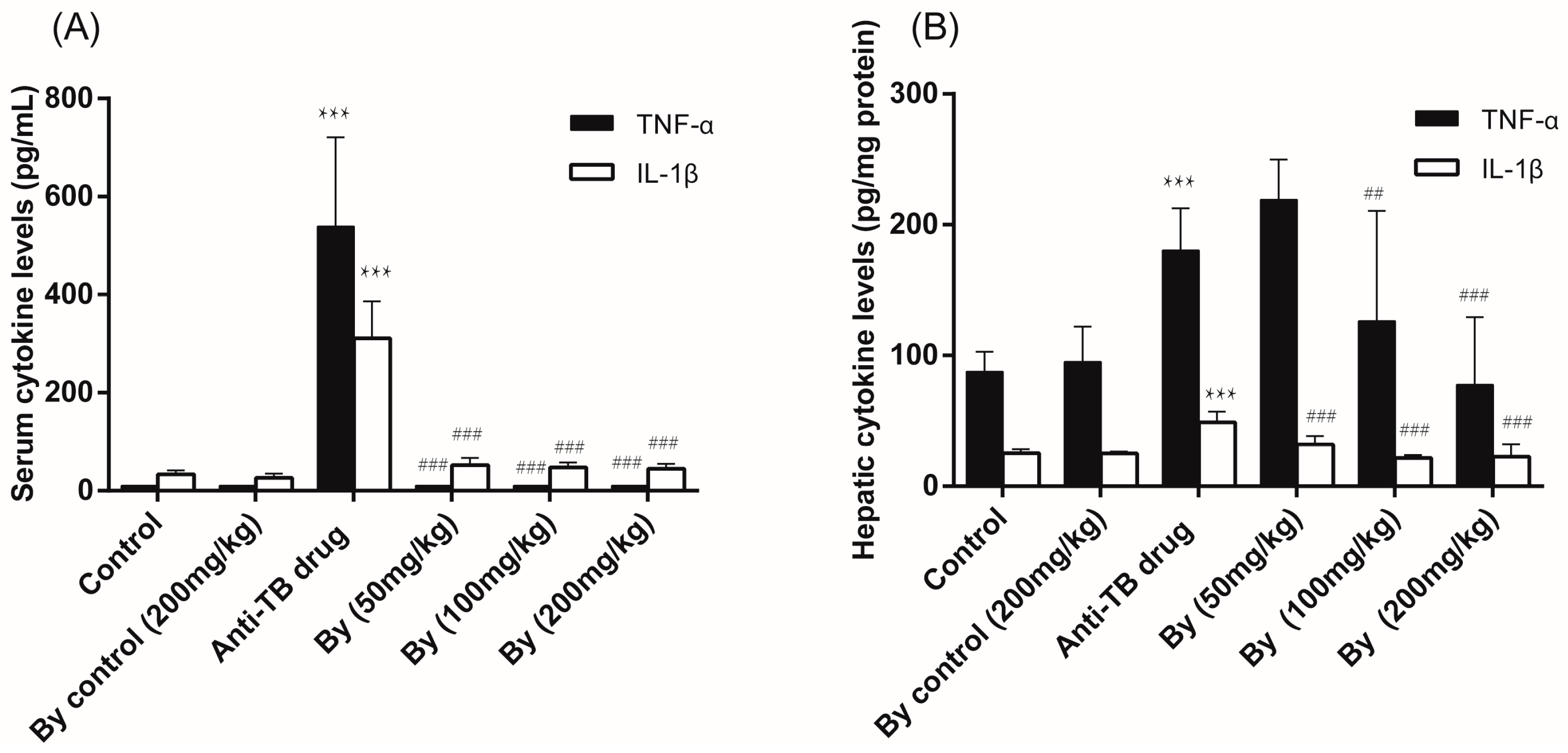
2.3. Effect of Bicyclol on the Levels of Serum and Hepatic TNF-α and IL-1β in Anti-TB Drug-Intoxicated Rats
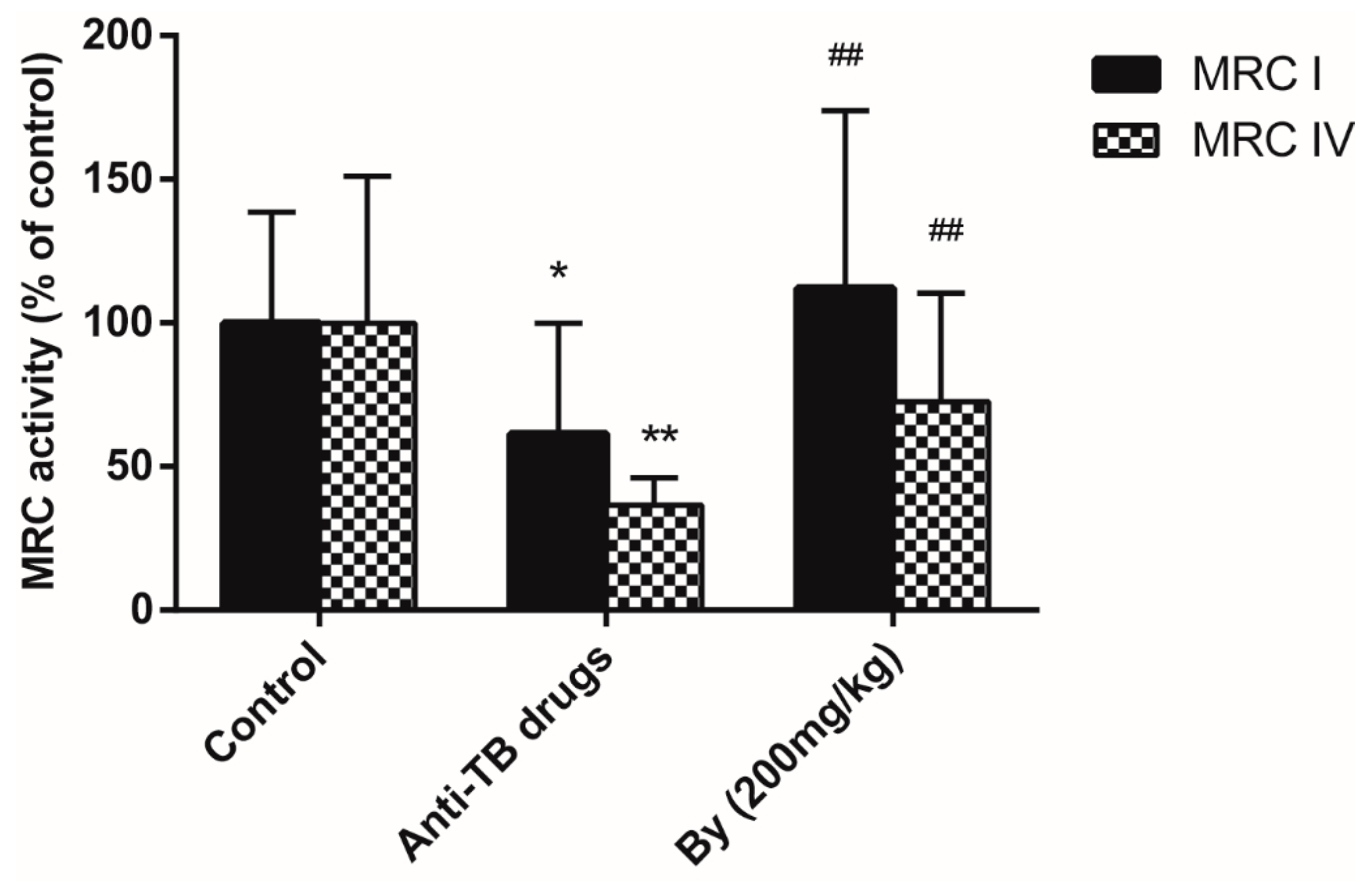
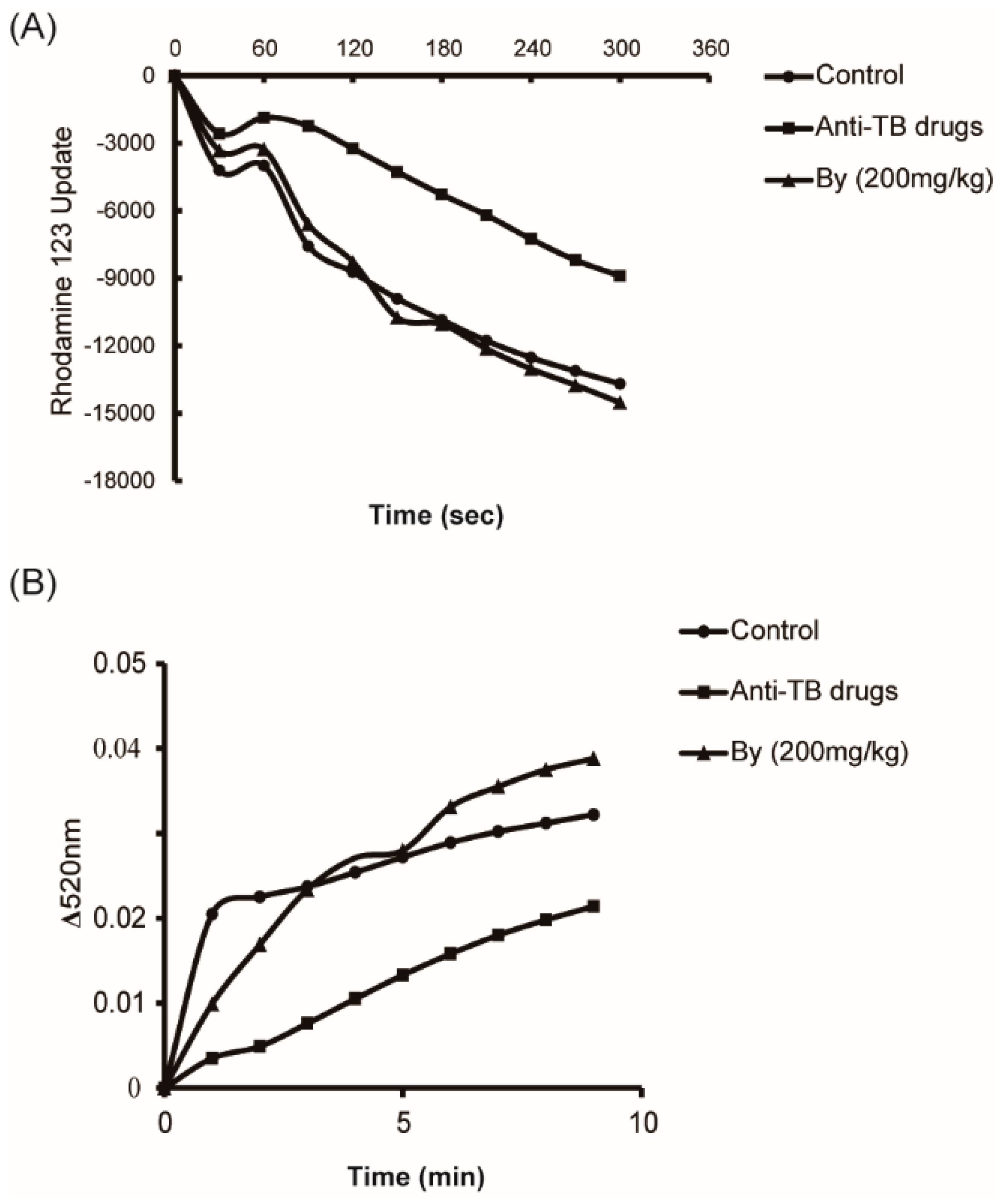
2.4. Effects of Bicyclol on MRC Activity and MPT in Anti-TB Drug-Intoxicated Rats
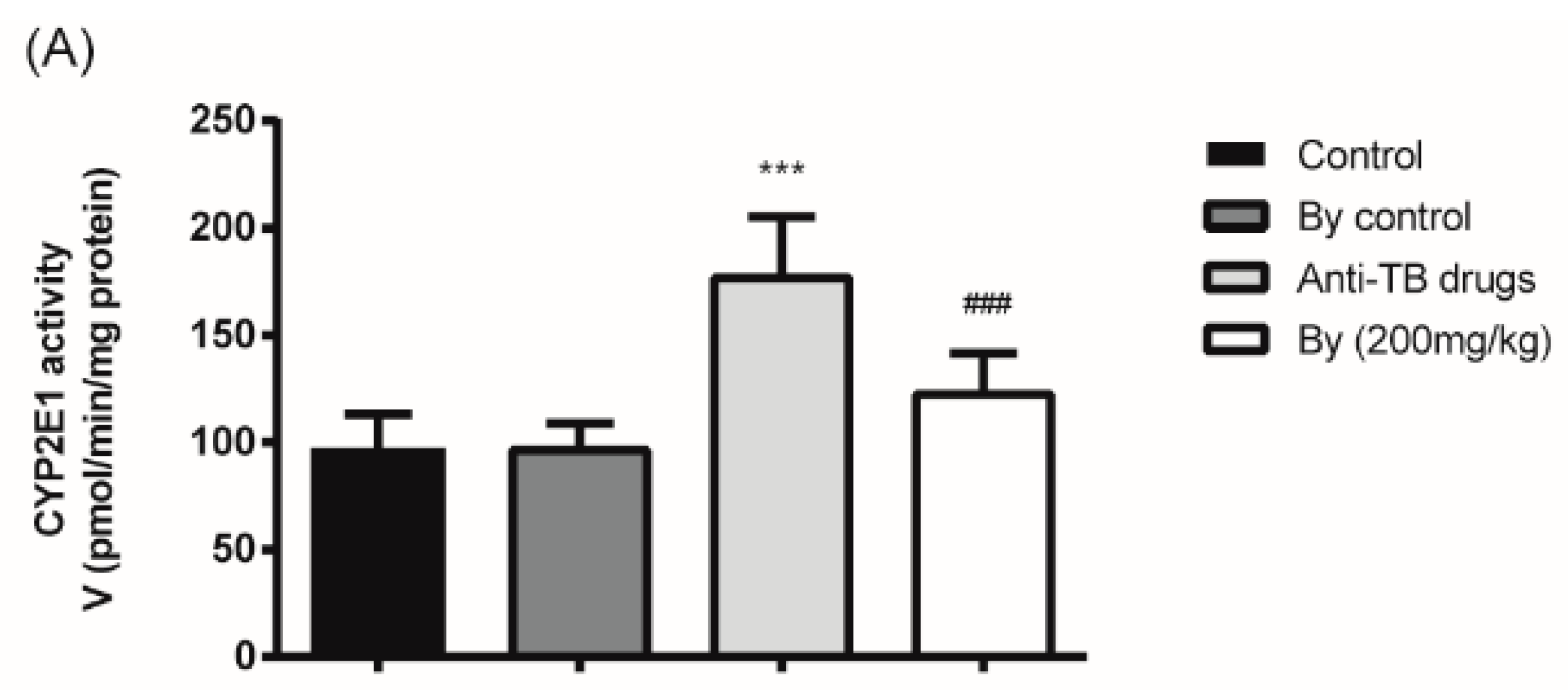
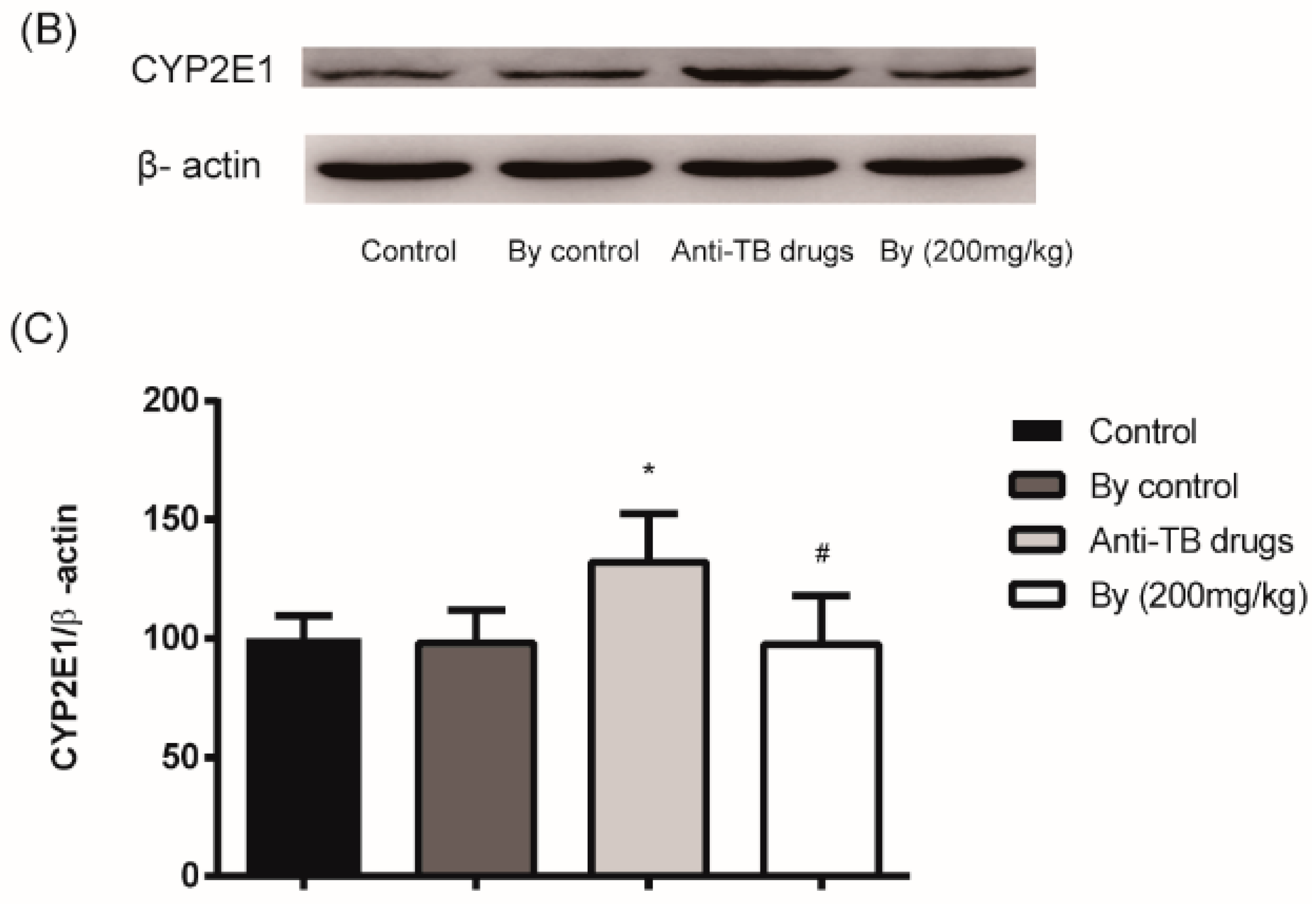
2.5. Effect of Bicyclol on the Activity and Protein Expression of Hepatic Microsomal CYP2E1 in Anti-TB Drug-Intoxicated Rats
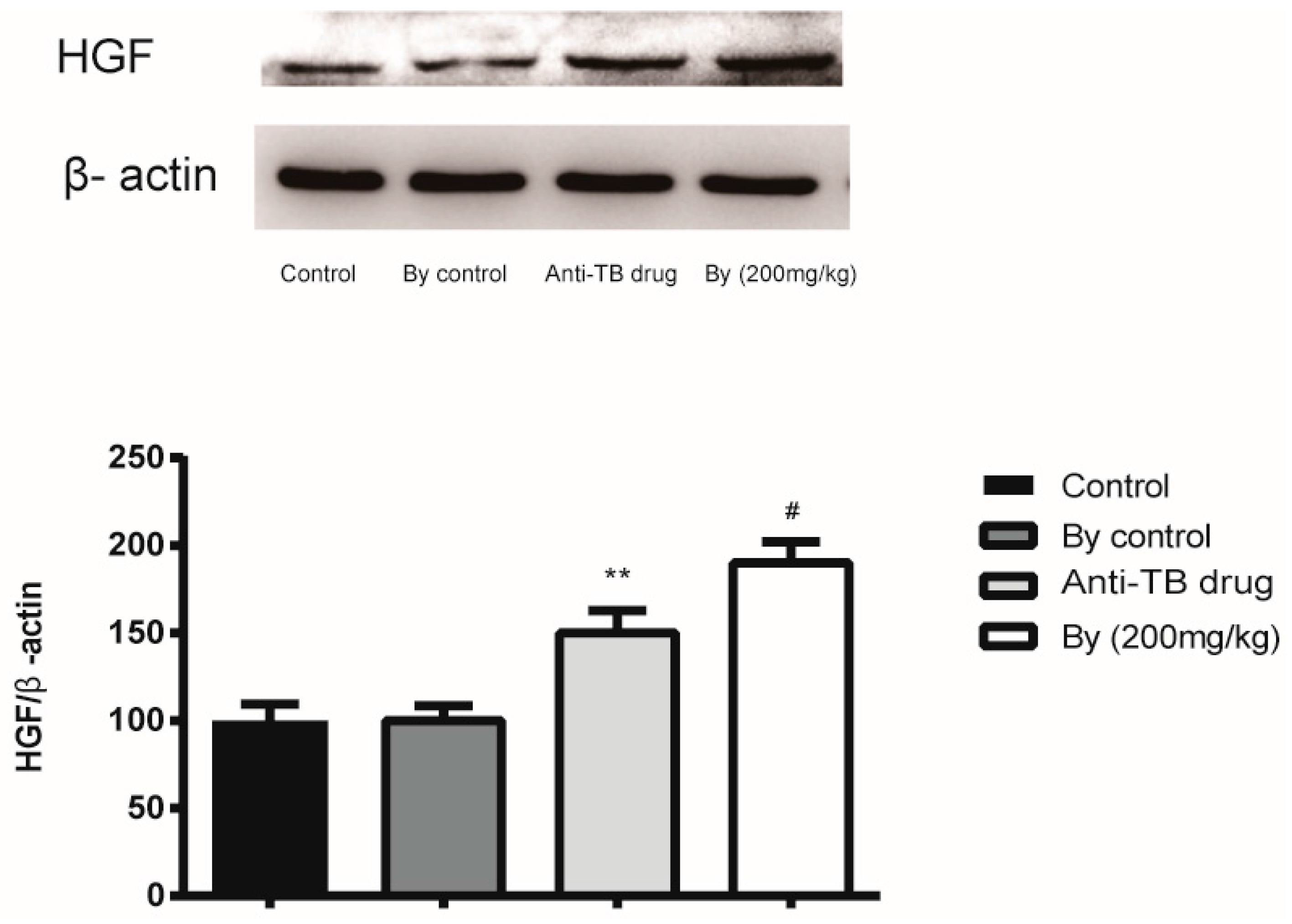
2.6. Effect of Bicyclol on the Protein Expression of HGF in Anti-TB Drug-Intoxicated Rats
2.7. Pharmacokinetic Studies
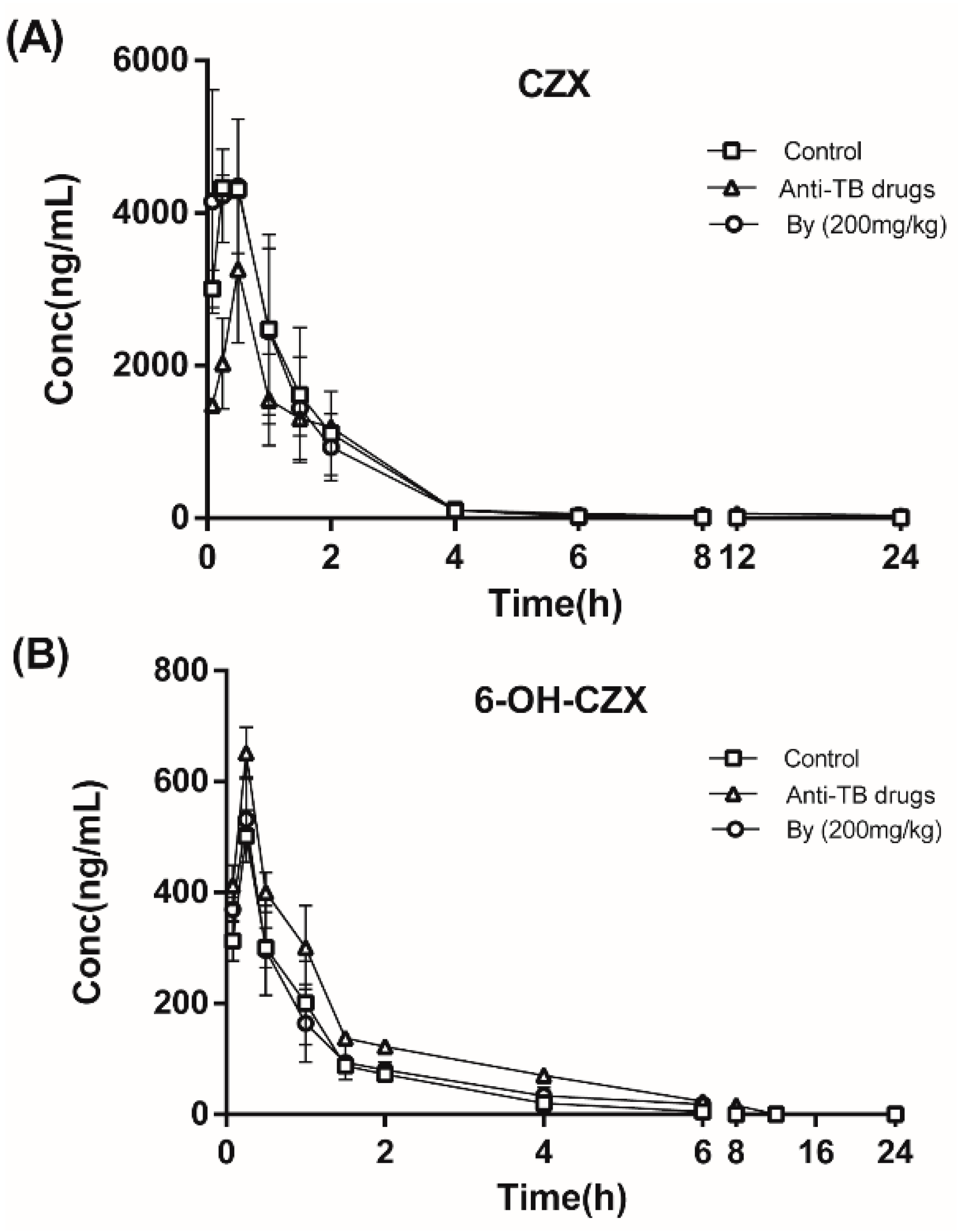
2.7.1. Effects of Bicyclol on the Pharmacokinetics of CZX and 6-OH-CZX in Anti-TB Drug-Intoxicated Rats
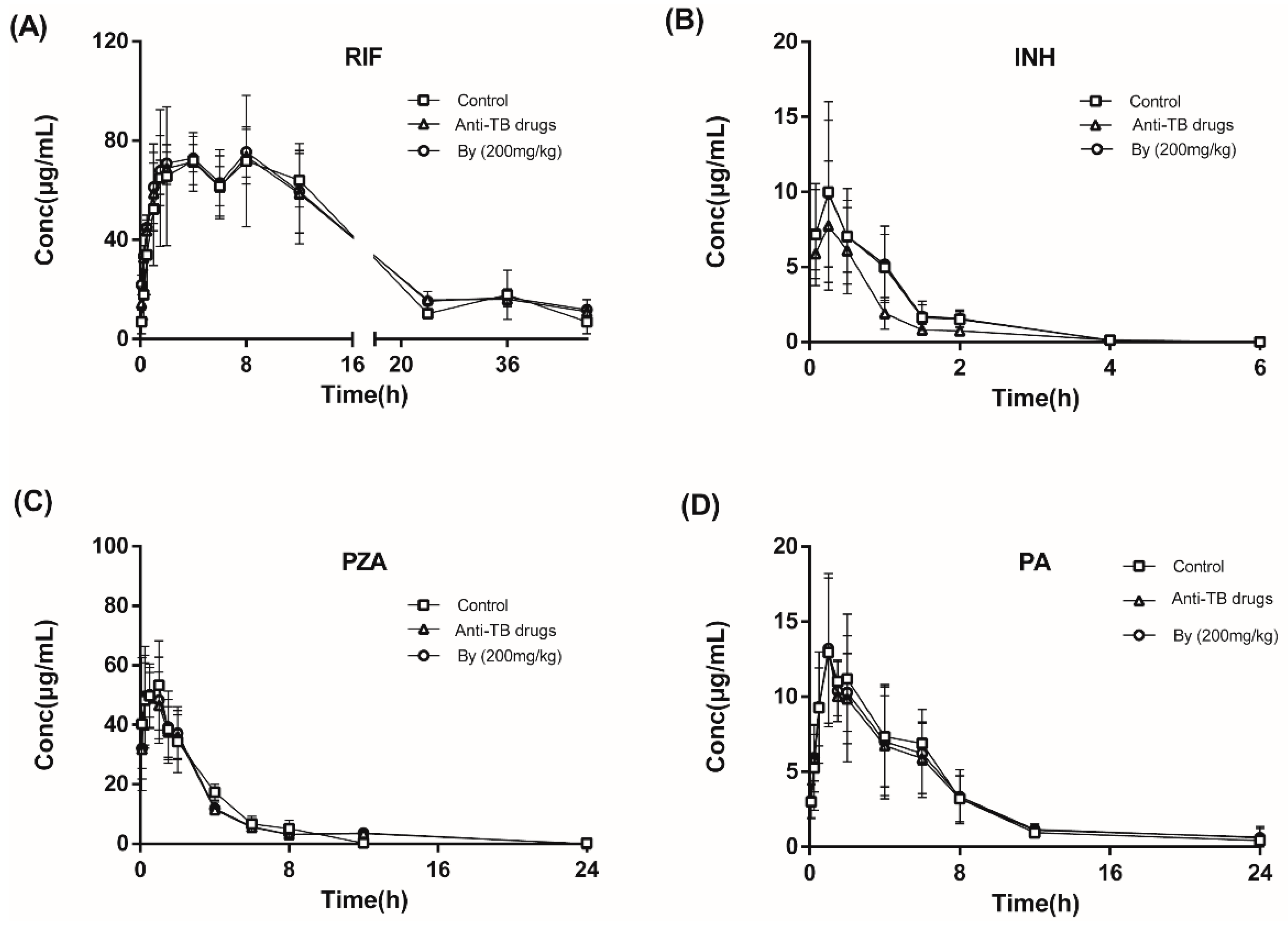
2.7.2. Effects of Bicyclol on the Pharmacokinetics of INH, RIF, PZA, and PA in Anti-TB Drug-Intoxicated Rats
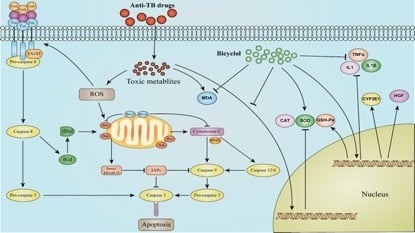
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Treatment of Animals
4.3. Biochemical Assays
4.4. Liver Histopathological Study
4.5. Serum and Hepatic TNF-α and IL-1β Assays
4.6. Preparation of Hepatic Subcellular Fractions
4.7. Mitochondrial Permeability Transition (MPT) Assay
4.8. Measurement of Enzyme Activities
4.9. Western Blotting Analysis
4.10. Pharmacokinetic Studies
4.10.1. Pharmacokinetics of CZX and 6-OH-CZX in Rats
4.10.2. Pharmacokinetics of INH, RIF, PZA and PA in Rats
4.10.3. LC–MS/MS Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glickman, S.W.; Rasiel, E.B.; Hamilton, C.D.; Kubataev, A.; Schulman, K.A. Medicine. A portfolio model of drug development for tuberculosis. Science 2006, 311, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shalimar; Bhatia, V.; Khanal, S.; Sreenivas, V.; Gupta, S.D.; Panda, S.K.; Acharya, S.K. Antituberculosis therapy-induced acute liver failure: Magnitude, profile, prognosis, and predictors of outcome. Hepatology 2010, 51, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Santra, A.; Bhattacharjee, K.; Ghatak, S.; Saha, D.R.; Dhali, G.K. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J. Hepatol. 2006, 45, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Lu, S.R.; Lin, Y.; Yang, Q.L.; Yu, B. Oxidative stress potentiated by diallylsulfide, a selective CYP2E1 inhibitor, in isoniazid toxic effect on rat primary hepatocytes. Toxicol. Lett. 2008, 183, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Tafazoli, S.; Mashregi, M.; O’Brien, P.J. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol. Appl. Pharmacol. 2008, 229, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sarich, T.C.; Adams, S.P.; Petricca, G.; Wright, J.M. Inhibition of isoniazid-induced hepatotoxicity in rabbits by pretreatment with an amidase inhibitor. J. Pharmacol. Exp. Ther. 1999, 289, 695–702. [Google Scholar] [PubMed]
- Vuilleumier, N.; Rossier, M.F.; Chiappe, A.; Degoumois, F.; Dayer, P.; Mermillod, B.; Nicod, L.; Desmeules, J.; Hochstrasser, D. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur. J. Clin. Pharmacol. 2006, 62, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Peng, R.; Chen, J.; Liu, Y.; Dong, G. Effects of rifampin on CYP2E1-dependent hepatotoxicity of isoniazid in rats. Pharmacol. Res. 2009, 59, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Peng, R.X.; Yang, J.; Kong, R.; Liu, J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol. Sin. 2004, 25, 699–704. [Google Scholar] [PubMed]
- Nicod, L.; Viollon, C.; Regnier, A.; Jacqueson, A.; Richert, L. Rifampicin and isoniazid increase acetaminophen and isoniazid cytotoxicity in human HepG2 hepatoma cells. Hum. Exp. Toxicol. 1997, 16, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tasduq, S.A.; Kaiser, P.; Sharma, S.C.; Johri, R.K. Potentiation of isoniazid-induced liver toxicity by rifampicin in a combinational therapy of antitubercular drugs (rifampicin, isoniazid and pyrazinamide) in Wistar rats: A toxicity profile study. Hepatol. Res. 2007, 37, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Peng, R. Does CYP2E1 play a major role in the aggravation of isoniazid toxicity by rifampicin in human hepatocytes? Br. J. Pharmacol. 2009, 157, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Pitre, D.; Facino, R.M.; Carini, M.; Carlo, A. In vitro biotransformation of pyrazinamide by rat liver: Identification of a new metabolite. Pharmacol. Res. Commun. 1981, 13, 351–362. [Google Scholar] [CrossRef]
- Shih, T.Y.; Pai, C.Y.; Yang, P.; Chang, W.L.; Wang, N.C.; Hu, O.Y. A novel mechanism underlies the hepatotoxicity of pyrazinamide. Antimicrob. Agents Chemother. 2013, 57, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Clavijo-Cornejo, D.; Enriquez-Cortina, C.; Lopez-Reyes, A.; Dominguez-Perez, M.; Nuno, N.; Dominguez-Meraz, M.; Bucio, L.; Souza, V.; Factor, V.M.; Thorgeirsson, S.S.; et al. Biphasic regulation of the NADPH oxidase by HGF/c-Met signaling pathway in primary mouse hepatocytes. Biochimie 2013, 95, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Quiroz, L.E.; Factor, V.M.; Kaposi-Novak, P.; Coulouarn, C.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte-specific c-Met deletion disrupts redox homeostasis and sensitizes to Fas-mediated apoptosis. J. Biol. Chem. 2008, 283, 14581–14589. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Arzate, A.; Luna, A.; Bucio, L.; Licona, C.; Clemens, D.L.; Souza, V.; Hernandez, E.; Kershenobich, D.; Gutierrez-Ruiz, M.C.; Gomez-Quiroz, L.E. Hepatocyte growth factor protects hepatocytes against oxidative injury induced by ethanol metabolism. Free Radic. Biol. Med. 2009, 47, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.T.; Li, Y.; Wei, H.L.; Zhang, H.; Xu, J.Y.; Yu, L.H. Mechanism of protective action of bicyclol against CCl-induced liver injury in mice. Liver Int. 2005, 25, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, H.; Li, Y. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. Eur. J. Pharmacol. 2008, 586, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Wang, B.L.; Zhao, J.; Yao, X.M.; Gu, Y.; Li, Y. Protective effect of bicyclol on tetracycline-induced fatty liver in mice. Toxicology 2009, 261, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.J. Clinical study on bicyclol tablets in preventing liver damage caused by anti-tuberculosis drugs. Chin. J. Prim. Med. Pharm. 2013, 20, 16–17. [Google Scholar]
- Bhadauria, S.; Singh, G.; Sinha, N.; Srivastava, S. Isoniazid induces oxidative stress, mitochondrial dysfunction and apoptosis in Hep G2 cells. Cell. Mol. Biol. (Noisy-Le-Grand) 2007, 53, 102–114. [Google Scholar]
- Yu, Y.N.; Chen, H.; Li, Y. Effect of bicyclol on cisplatin-induced hepatotoxicity in the hepatocarcinoma 22 tumour-bearing mice. Basic Clin. Pharmacol. Toxicol. 2009, 104, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Mansouri, A.; Fromenty, B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G193–G199. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Shedlofsky, S.; Barve, S.; Hill, D.B. Cytokines and alcoholic liver disease. Alcohol Health Res. World 1997, 21, 317–320. [Google Scholar] [PubMed]
- Kono, H.; Arteel, G.E.; Rusyn, I.; Sies, H.; Thurman, R.G. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic. Biol. Med. 2001, 30, 403–411. [Google Scholar] [CrossRef]
- Kono, H.; Rusyn, I.; Uesugi, T.; Yamashina, S.; Connor, H.D.; Dikalova, A.; Mason, R.P.; Thurman, R.G. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, 1005–1012. [Google Scholar]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 1), 188–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.N.; Chen, H.; Li, Y. Protect effect of bicyclol on cisplatin-induced nephrotoxicity in mice. Arch. Toxicol. 2009, 83, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhao, J.; Yao, X.M.; Li, Y. Effects of bicyclol on immunological liver fibrosis in rats. J. Asian Nat. Prod. Res. 2010, 12, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.A.; Brennan, B.; Chenery, R.; Houston, J.B. In vivo disposition of caffeine predicted from hepatic microsomal and hepatocyte data. Drug Metab. Dispos. 1995, 23, 349–353. [Google Scholar] [PubMed]
- Omura, T.; Sato, R. Carbon monoxide-binding pigment of liver microsomes, I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [PubMed]
- Zamzami, N.; Kroemer, G. Methods to measure membrane potential and permeability transition in the mitochondria during apoptosis. Methods Mol. Biol. 2004, 282, 103–115. [Google Scholar] [PubMed]
- Hunter, F.E., Jr.; Gebicki, J.M.; Hoffsten, P.E.; Weinstein, J.; Scott, A. Swelling and lysis of rat liver mitochondria induced by ferrous ions. J. Biol. Chem. 1963, 238, 828–835. [Google Scholar] [PubMed]
- Kharasch, E.D.; Thummel, K.E.; Mhyre, J.; Lillibridge, J.H. Single-dose disulfiram inhibition of chlorzoxazone metabolism: A clinical probe for P450 2E1. Clin. Pharmacol. Ther. 1993, 53, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Jackson-Lewis, V.; Muthane, U.; Jiang, H.; Ferreira, M.; Naini, A.B.; Fahn, S. Chronic levodopa administration alters cerebral mitochondrial respiratory chain activity. Ann. Neurol. 1993, 34, 715–723. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Groups | Liver GSH (mg/g Tissue) | Liver MDA (nmol/mg Tissue) | Liver SOD (U/mg Tissue) | Liver CAT (U/mg Tissue) | Liver GSH-Px (U/mg Tissue) |
|---|---|---|---|---|---|
| Normal control | 12.77 ± 6.71 | 3.96 ± 1.13 | 627.72 ± 320.72 | 48.19 ± 17.33 | 256.32 ± 43.45 |
| By control | 14.54 ± 12.16 | 3.97 ± 1.01 | 766.85 ± 945.57 | 42.52 ± 13.38 | 269.18 ± 48.94 |
| Anti-TB drug | 4.14 ± 1.28 *** | 9.03 ± 2.71 *** | 301.82 ± 67.17 ** | 22.43 ± 11.96 *** | 182.26 ± 18.28 * |
| By (50 mg/kg) | 7.84 ± 4.94 | 4.35 ± 0.85 ### | 493.61 ± 364.85 | 29.30 ± 7.59 | 260.21 ± 44.85 |
| By (100 mg/kg) | 6.53 ± 2.27 # | 4.07 ± 0.69 ### | 471.62 ± 215.07 | 31.09 ± 10.41 ## | 280.08 ± 68.25 |
| By (200 mg/kg) | 8.01 ± 2.40 ## | 3.81 ± 0.84 ### | 487.15 ± 152.62 ## | 40.61 ± 9.80 ### | 427.77 ± 53.28 ## |
| Parameters | Unit | CZX | 6-OH-CZX | ||||
|---|---|---|---|---|---|---|---|
| Normal Control | Anti-TB Drug | By (200 mg/kg) | Normal Control | Anti-TB Drug | By (200 mg/kg) | ||
| t1/2z | h | 2.37 ± 0.83 | 2.81 ± 0.63 | 2.24 ± 0.19 | - | - | - |
| Tmax | h | 0.42 ± 0.14 | 0.50 ± 0.00 | 0.48 ± 0.21 | 0.25 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 |
| Cmax | μg/L | 4402.13 ± 108.37 | 3263.51 ± 965.98 | 4395.34 ± 537.32 | 501.44 ± 46.93 | 651.44 ± 46.93 | 521.72 ± 76.42 |
| AUC(0−t) | μg/L*h | 6765.56 ± 1715.18 | 5180.76 ± 542.58 | 6670.83 ± 1299.81 | 551.7 ± 84.89 | 954.45 ± 68.02 | 534.81 ± 109.62 |
| Vz/F | L/kg | 10.57 ± 4.57 | 9.67 ± 1.60 | 10.51 ± 18.85 | - | - | - |
| CLz/F | L/h/kg | 3.06 ± 0.70 | 2.41 ± 0.16 | 3.04 ± 0.56 | - | - | - |
| MRT(0−t) | h | 1.58 ± 0.12 | 1.48 ± 1.13 | 1.82 ± 0.79 | 1.35 ± 0.41 | 2.19 ± 0.04 | 1.97 ± 0.48 |
| Parameters | t1/2z | Tmax | Cmax | AUC(0−t) | Vz | CLz | MRT(0−t) | |
|---|---|---|---|---|---|---|---|---|
| Unit | h | h | μg/mL | μg/mL·h | L/kg | L/h/kg | h | |
| RIF | Normal control | 6.97 ± 5.11 | 4.75 ± 4.02 | 205.72 ± 220.27 | 1394.39 ± 836.99 | 2.18 ± 0.41 | 0.14 ± 0.05 | 11.02 ± 8.34 |
| Anti-TB drug | 15.31 ± 1.86 | 4.5 ± 3.28 | 79.40 ± 10.38 | 1999.83 ± 246.72 | 3.26 ± 0.85 | 0.13 ± 0.01 | 21.94 ± 2.42 | |
| By (200 mg/kg) | 17.29 ± 5.17 | 4.5 ± 3.28 | 76.21 ± 2.27 | 1901.43 ± 140.29 | 3.16 ± 0.63 | 0.13 ± 0.02 | 23.88 ± 5.23 | |
| INH | Normal control | 0.60 ± 0.08 | 0.50 ± 0.43 | 10.90 ± 4.91 | 11.02 ± 2.78 | 4.19 ± 1.78 | 4.76 ± 1.32 | 0.95 ± 0.19 |
| Anti-TB drug | 0.56 ± 0.21 | 0.19 ± 0.10 | 8.25 ± 3.60 | 7.75 ± 1.74 | 21.82 ± 29.56 | 6.68 ± 1.59 | 1.90 ± 1.92 | |
| By (200 mg/kg) | 0.60 ± 0.08 | 0.50 ± 0.43 | 10.89 ± 3.53 | 11.22 ± 2.09 | 4.00 ± 1.42 | 4.57 ± 0.95 | 0.95 ± 0.19 | |
| PZA | Normal control | 2.93 ± 0.49 | 0.53 ± 0.46 | 54.10 ± 14.29 | 192.02 ± 40.57 | 2.31 ± 0.79 | 0.54 ± 0.11 | 3.23 ± 0.35 |
| Anti-TB drug | 2.28 ± 0.16 | 0.50 ± 0.43 | 57.07 ± 12.57 | 188.24 ± 34.24 | 1.80 ± 0.37 | 0.54 ± 0.09 | 3.70 ± 0.35 | |
| By (200 mg/kg) | 2.28 ± 0.16 | 0.50 ± 0.43 | 58.21 ± 7.28 | 194.97 ± 33.3 | 1.76 ± 0.40 | 0.53 ± 0.09 | 3.70 ± 0.35 | |
| PA | Normal control | 4.78 ± 0.69 | 1.33 ± 0.58 | 14.80 ± 1.80 | 82.28 ± 16.1 | 8.61 ± 2.20 | 1.25 ± 0.26 | 6.15 ± 0.92 |
| Anti-TB drug | 5.03 ± 1.72 | 1.17 ± 0.29 | 13.54 ± 3.94 | 82.40 ± 22.1 | 9.04 ± 2.74 | 1.29 ± 0.41 | 7.42 ± 2.74 | |
| By (200 mg/kg) | 5.03 ± 1.72 | 1.17 ± 0.29 | 13.88 ± 3.21 | 84.66 ± 15.97 | 8.89 ± 2.89 | 1.23 ± 0.28 | 7.40 ± 2.74 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhao, M.; Mi, J.; Chen, H.; Sheng, L.; Li, Y. Protective Effect of Bicyclol on Anti-Tuberculosis Drug Induced Liver Injury in Rats. Molecules 2017, 22, 524. https://doi.org/10.3390/molecules22040524
Liu X, Zhao M, Mi J, Chen H, Sheng L, Li Y. Protective Effect of Bicyclol on Anti-Tuberculosis Drug Induced Liver Injury in Rats. Molecules. 2017; 22(4):524. https://doi.org/10.3390/molecules22040524
Chicago/Turabian StyleLiu, Xin, Manman Zhao, Jiaqi Mi, Hui Chen, Li Sheng, and Yan Li. 2017. "Protective Effect of Bicyclol on Anti-Tuberculosis Drug Induced Liver Injury in Rats" Molecules 22, no. 4: 524. https://doi.org/10.3390/molecules22040524
APA StyleLiu, X., Zhao, M., Mi, J., Chen, H., Sheng, L., & Li, Y. (2017). Protective Effect of Bicyclol on Anti-Tuberculosis Drug Induced Liver Injury in Rats. Molecules, 22(4), 524. https://doi.org/10.3390/molecules22040524