β-Defensins in the Fight against Helicobacter pylori
Abstract
:1. Introduction
2. HP Virulence Factors
2.1. Antimicrobial Peptides
2.2. Defensins: The Classification
2.3. Structure and Function: Human α-Defensins
2.4. Human β-Defensins
2.5. Killing Mechanism: Bacteria
3. β-Defensins and Hp Infection
3.1. Hp-Induced Gastritis
3.2. Peptic Ulcer
3.3. Gastric Cancer
4. Antimicrobial Activity of β-Defensins against Hp
5. Synthetic Amps Analogs
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suerbaum, S.; Michetti, P. Helicobacter Hp infection. N. Engl. J. Med. 2002, 15, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Peek, R.M., Jr.; Crabtree, J.E. Helicobacter pylori infection and gastric neoplasia. J. Pathol. 2006, 2, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, G.; Moccia, F.; Marra, T.; Buonomo, M.; Pascotto, B.; Pezzullo, A.; Napolitano, V.; Schettino, P.; Avellino, M.; Conzo, G.; et al. Does Helicobacter pylori infection have influence on outcome of laparoscopic sleeve gastrectomy for morbid obesity? Int. J. Surg. 2014, 12 (Suppl. S1), S68–S71. [Google Scholar] [CrossRef] [PubMed]
- Pounder, R.E.; Ng, D. The prevalence of Helicobacter pylori infection in different countries. Aliment. Pharmacol. Ther. 1995, 9 (Suppl. S2), 33–39. [Google Scholar]
- Bajaj-Elliott, M.; Fedeli, P.; Smith, G.V.; Domizio, P.; Maher, L.; Ali, R.S.; Quinn, A.G.; Farthing, M.J.G. Modulation of host antimicrobial peptide (β-defensins 1 and 2) expression during gastritis. Gut 2002, 53, 356–361. [Google Scholar] [CrossRef]
- Umit, H.; Tezel, A.; Bukavaz, S.; Unsal, G.; Otkun, M.; Soylu, A.R.; Tucer, D.; Otkun, M.; Bilgi, S. The relationship between virulence factors of Helicobacter pylori and severity of gastritis in infected patients. Dig. Dis. Sci. 2009, 54, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.R.; Coyle, A.J.; et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Fujioka, T.; Kodama, K.; Nishizono, A.; Nasu, M. Virulence-associated genes as markers of strain diversity in Helicobacter pylori infection. J. Gastroenterol. Hepatol. 1997, 12, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.L.; Fagundes e Souza, E.K.; Leite, K.R.; Bastos, E.L.; Vicentini, L.R.; Silva, L.C. cagA vacA alelles and babA2 genotypes of Helicobacter pylori associated with gastric disease in Brazilian adult patients. Diagn. Microbiol. Infect. Dis. 2005, 51, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.C.; Cover, T.L.; Papini, E.; Telford, J.L. Vacuolating Cytotoxin. In Helicobacter Pylory: Physiology and Genetics; Mobley, H.L.T., Mendz, G.L., Hazell, S.L., Eds.; ASM Press: Washington, DC, USA, 2001; Chapter 9. [Google Scholar]
- Rhead, J.L.; Letley, D.P.; Mohammadi, M.; Hussein, N.; Mohagheghi, M.A.; Eshagh Hosseini, M.; Atherton, J.C. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 2007, 3, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Amedei, A.; Cappon, A.; Codolo, G.; Cabrelle, A.; Polenghi, A.; Benagiano, M.; Tasca, E.; Azzurri, A.; D’Elios, M.M.; Del Prete, G.; et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J. Clin. Investig. 2006, 116, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Basak, C.; Pathak, S.K.; Bhattacharyya, A.; Pathak, S.; Basu, J.; Kundu, M. The secreted peptidyl prolyl cis, trans-isomerase HP0175 of Helicobacter pylori induces apoptosis of gastric epithelial cells in a TLR4- and apoptosis signal-regulating kinase 1-dependent manner. J. Immunol. 2005, 174, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- Alvi, A.; Ansari, S.A.; Ehtesham, N.Z.; Rizwan, M.; Devi, S.; Sechi, L.A.; Qureshi, I.A.; Hasnain, S.E.; Ahmed, N. Concurrent proinflammatory and apoptotic activity of a Helicobacter pylori protein (HP986) points to its role in chronic persistence. PLoS ONE 2011, 6, e22530. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, J.; Guan, H.; Cai, W.; Bai, X.; Fang, X.; Hu, X.; Wang, Y.; Wang, H.; Zheng, Z.; et al. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts. PLoS ONE 2014, 9, e98228. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Schauber, J.; Stange, E.F. Defensins and cathelicidins in gastrointestinal infections. Curr. Opin. Gastroenterol. 2007, 23, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bajaj-Elliott, M.; Turner, M. Innate immunity in health and disease. Mol. Immunol. 2005, 8, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Josenhans, C. Helicobacter pylori and the innate immune system. Int. J. Med. Microbiol. 2005, 295, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Menendez, A.; Brett Finlay, B. Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 2007, 19, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The human β-defensin-3, anantibacterial peptide with multiple biological functions. Biochim. Biophys. Acta 2006, 9, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Lichtenstein, A.K.; Ganz, T. Defensins: Antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 1993, 11, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Cobo, E.R.; Chadee, K. Antimicrobial human β-defensins in the colon and their role in infectious and non-infectious diseases. Pathogens 2013, 1, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.N.; Kleshchenko, Y.Y.; Nde, P.N.; Simmons, K.J.; Lima, M.F.; Villalta, F. Human defensin α-1 causes Trypanosoma cruzi membrane pore formation and induces DNA fragmentation, which leads to trypanosome destruction. Infect Immun. 2007, 10, 4780–4791. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Biragyn, A.; Hoover, D.M.; Lubkowski, J.; Oppenheim, J.J. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2004, 22, 181–215. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. Lond. B Biol. Sci. 1922, 93, 306–317. [Google Scholar] [CrossRef]
- Ridley, F. Lysozyme: An antibacterial body present in great concentration in tears, and its relation to infection of the human eye. Proc. R. Soc. Med. 1928, 21, 1495–1506. [Google Scholar] [PubMed]
- Bals, R. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 2000, 1, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- The Antimicrobial Peptide Database. Available online: http://aps.unmc.edu/AP/main.php/ (accessed on 3 March 2017).
- Cederlund, A.; Gudmundsson, G.H.; Agerberth, B. Antimicrobial peptides important in innate immunity. FEBS J. 2011, 20, 3942–3951. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I. Primate defensins. Nat. Rev. Microbiol. 2004, 9, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins in the urinary tract and other tissues. J. Infect. Dis. 2001, 183, S41–S42. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.; Wu, M. Defensins in innate immunity. Cell Tissue Res. 2011, 1, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E. Theta-defensins: Cyclic antimicrobial peptides produced by binary ligation of truncated α-defensins. Curr. Protein Pept. Sci. 2004, 5, 65–71. [Google Scholar] [CrossRef]
- Patil, A.; Hughes, A.L.; Zhang, G. Rapid evolution and diversification of mammalian α-defensins as revealed by comparative analysis of rodent and primate genes. Physiol. Genom. 2004, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, R.N. α-Defensins in the gastrointestinal tract. Mol. Immunol. 2003, 7, 463–467. [Google Scholar] [CrossRef]
- Schneider, J.J.; Unholzer, A.; Schaller, M.; Schäfer-Korting, M.; Korting, H.C. Human defensins. J. Mol. Med. 2005, 8, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Pazgier, M.; Hoover, D.M.; Yang, D.; Lu, W.; Lubkowski, J. Human β-defensins. Cell. Mol. Life Sci. 2006, 11, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Quiñones-Mateu, M.E.; Lederman, M.M. Role of human β-defensins in HIV infection. Adv. Dent. Res. 2006, 1, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Batoni, G.; Esin, S.; Florio, W.; Bottai, D.; Favilli, F.; Campa, M. In vitro bactericidal activity of human β-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob. Agents Chemother. 2006, 50, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 4, 553–560. [Google Scholar] [CrossRef]
- Bensch, K.W.; Raida, M.; Mägert, H.J.; Schulz-Knappe, P.; Forssmann, W.G. hBD-1: A novel β-defensin from human plasma. FEBS Lett. 1995, 2, 331–335. [Google Scholar] [CrossRef]
- Valore, E.V.; Park, C.H.; Quayle, A.J.; Wiles, K.R.; McCray, P.B., Jr.; Ganz, T. Human β-defensin-1: An antimicrobial peptide of urogenital tissues. J. Clin. Investig. 1998, 8, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.M.; Chertov, O.; Lubkowski, J. The structure of human β-defensin-1: New insights into structural properties of β-defensins. J. Biol. Chem. 2001, 42, 39021–39026. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Fleming, D.C.; Critchley, H.O.; Kelly, R.W. Regulation of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Mol. Hum. Reprod. 2002, 4, 341–349. [Google Scholar] [CrossRef]
- Seo, S.J.; Ahn, S.W.; Hong, C.K.; Ro, B.I. Expressions of β-defensins in human keratinocyte cell lines. J. Dermatol. Sci. 2001, 3, 183–191. [Google Scholar] [CrossRef]
- Wehkamp, J.; Fellermann, K.; Herrlinger, K.R.; Baxmann, S.; Schmidt, K.; Schwind, B.; Duchrow, M.; Wohlschläger, C.; Feller, A.C.; Stange, E.F. Human β-defensin 2 but not β-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2002, 14, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human β defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 8, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, S.; Ashitani, J.; Ishimoto, H.; Date, Y.; Mukae, H.; Chino, N.; Nakazato, M. Isolation of human β-defensin-4 in lung tissue and its increase in lower respiratory tract infection. Respir. Res. 2005, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Fleming, D.C.; Critchley, H.O.; Kelly, R.W. Differential expression of the natural antimicrobials, β-defensins 3 and 4, in human endometrium. J. Reprod. Immunol. 2003, 1, 1–16. [Google Scholar] [CrossRef]
- García, J.R.; Jaumann, F.; Schulz, S.; Krause, A.; Rodríguez-Jiménez, J.; Forssmann, U.; Adermann, K.; Klüver, E.; Vogelmeier, C.; Becker, D.; et al. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001, 306, 257–264. [Google Scholar] [CrossRef]
- Yang, D.; Chertov, O.; Bykovskaia, S.N.; Chen, Q.; Buffo, M.J.; Shogan, J.; Anderson, M.; Schröder, J.M.; Wang, J.M.; Howard, O.M.; et al. β-Defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999, 5439, 525–528. [Google Scholar] [CrossRef]
- Colavita, I.; Nigro, E.; Sarnataro, D.; Scudiero, O.; Granata, V.; Daniele, A.; Zagari, A.; Pessi, A.; Salvatore, F. Membrane protein 4F2/CD98 is a cell surface receptor involved in the internalization and trafficking of human β-Defensin 3 in epithelial cells. Chem. Biol. 2015, 2, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A. Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J. Clin. Investig. 1991, 1, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C.; Selsted, M.E.; White, S.H. Interactions between human defensins and lipid bilayers: Evidence for formation of multimeric pores. Protein Sci. 1994, 3, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Fujii, G.; Selsted, M.E.; Eisenberg, D. Defensins promote fusion and lysis of negatively charged membranes. Protein Sci. 1993, 8, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Sahl, H.G.; Pag, U.; Bonness, S.; Wagner, S.; Antcheva, N.; Tossi, A. Mammalian defensins: Structures and mechanism of antibiotic activity. J. Leukoc. Biol. 2005, 4, 466–745. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 9, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Sass, V.; Schneider, T.; Wilmes, M.; Körner, C.; Tossi, A.; Novikova, N.; Shamova, O.; Sahl, H.G. Human β-defensin 3 inhibits cell wall biosynthesis in Staphylococci. Infect. Immun. 2010, 6, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Mukae, H.; Ishimoto, H.; Date, Y.; Nishi, Y.; Inoue, K.; Wada, A.; Hirayama, T.; Nakazato, M.; Kohno, S. Elevated concentrations of α-defensins in gastric juice of patients with Helicobacter pylori infection. Am. J. Gastroenterol. 2004, 99, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Kapas, S. Adrenomedullin expression by gastric epithelial cells in response to infection. Clin. Diagn. Lab. Immunol. 2003, 4, 546–551. [Google Scholar] [CrossRef]
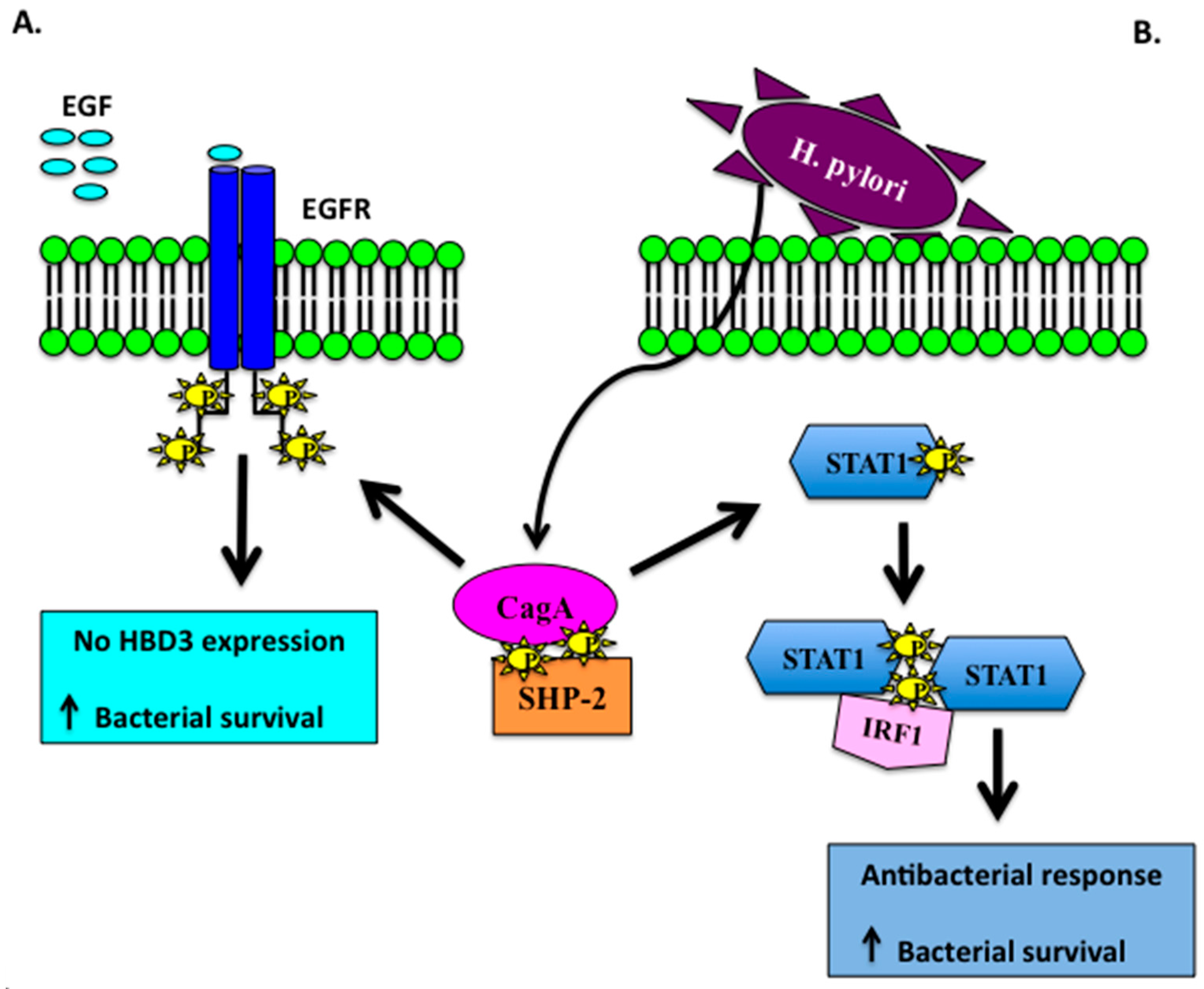
- Boughan, P.K.; Argent, R.H.; Body-Malapel, M.; Park, J.H.; Ewings, K.E.; Bowie, A.G.; Ong, S.J.; Cook, S.J.; Sorensen, O.E.; Manzo, B.A.; et al. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: Critical regulators of β-defensins during Helicobacter pylori infection. J. Biol. Chem. 2006, 17, 11637–11648. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Smith, K.; Letley, D.P.; Cook, K.W.; Memon, A.A.; Ingram, R.J.; Staples, E.; Backert, S.; Zaitoun, A.M.; Atherton, J.C.; et al. Helicobacter pylori downregulates expression of human β-defensin 1 in the gastric mucosa in a type IV secretion-dependent fashion. Cell. Microbiol. 2013, 12, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.M.; Neumann, H.M.; Brand, S.; Schrader, H.; Schmidt, W.E.; Schmitz, F. Expression of β-defensin 4 is increased in human gastritis. Eur. J. Clin. Investig. 2009, 2, 126–238. [Google Scholar] [CrossRef] [PubMed]
- Peek, R.M., Jr.; Miller, G.G.; Tham, K.T.; Perez-Perez, G.I.; Zhao, X.; Atherton, J.C.; Blaser, M.J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 1995, 6, 760–770. [Google Scholar]
- Cook, K.W.; Letley, D.P.; Ingram, R.J.; Staples, E.; Skjoldmose, H.; Atherton, J.C. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut 2014, 10, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Ding, J.L. Ubiquitination by SAG regulates macrophage survival/death and immune response during infection. Cell. Death. Differ. 2014, 9, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Kurokawa, K.; Nakamura, K.; Lee, B.L.; Sekimizu, K.; Kubagawa, H.; Hiramatsu, K.; Yagita, H.; Okumura, K.; Takai, T.; et al. Inhibitory receptor paired Ig-like receptor B is exploited by Staphylococcus aureus for virulence. J. Immunol. 2012, 12, 5903–5911. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; McAleer, J.P.; Zheng, M.; Pociask, D.A.; Kaplan, M.H.; Qin, S.; Reinhart, T.A.; Kolls, J.K. Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia. J. Exp. Med. 2013, 3, 551–561. [Google Scholar] [CrossRef]
- Rolán, H.G.; Durand, E.A.; Mecsas, J. Identifying Yersinia YopH-targeted signal transduction pathways that impair neutrophil responses during in vivo murine infection. Cell Host Microbe 2013, 3, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 2, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Daëron, M. Immunoreceptor tyrosine-based inhibition motifs. Immunol. Today 1997, 6, 286–291. [Google Scholar] [CrossRef]
- Higashi, H.; Tsutsumi, R.; Muto, S.; Sugiyama, T.; Azuma, T.; Asaka, M.; Hatakeyama, M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 2002, 295, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Wex, T.; Kuester, D.; Meyer, T.; Malfertheiner, P. Differential expression of human β defensin 2 and 3 in gastric mucosa of Helicobacter pylori-infected individuals. Helicobacter 2013, 18, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Mori, N.; Oishi, K.; Hojo, H.; Nakahara, Y.; Hamanaka, Y.; Nagashima, M.; Sekine, I.; Ogushi, K.; Niidome, T.; et al. Induction of human β-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem. Biophys. Res. Commun. 1999, 263, 770–774. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, D.A.; Cole, S.P.; Martin-Porter, E.; Housley, M.P.; Liu, L.; Ganz, T.; Kagnoff, M.F. Regulation of human β-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect. Immun. 2000, 9, 5412–5415. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Nakashima, M.; Wada, A.; Ito, M.; Kurazono, H.; Hojo, H.; Nakahara, Y.; Kohno, S.; Hirayama, T.; Sekine, I. Expression of human β-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: Antibacterial effect of hBD-2 against Helicobacter pylori. Gut 2001, 4, 481–487. [Google Scholar] [CrossRef]
- Allison, C.C.; Kufer, T.A.; Kremmer, E.; Kaparakis, M.; Ferrero, R.L. Helicobacter pylori induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J. Immunol. 2009, 183, 8099–8109. [Google Scholar]
- Grubman, A.; Kaparakis, M.; Viala, J.; Allison, C.; Badea, L.; Karrar, A.; Boneca, I.G.; Le Bourhis, L.; Reeve, S.; Smith, I.A.; et al. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell. Microbiol. 2010, 12, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Asano, N.; Kitani, A.; Fuss, I.J.; Chiba, T.; Strober, W. NOD1-Mediated Mucosal Host Defense against Helicobacter pylori. Int. J. Inflam. 2010, 2010, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Argent, R.H.; Atherton, J.C. The inflammatory and immune response to Helicobacter pylori infection. Best Pract. Res. Clin. Gastroenterol. 2007, 2, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.A.; Jaramillo, E.; Suzuki, G.; Lagergren, P.; Nesi, G. Antralization of the gastric mucosa of the incisura angularis and its gastrin expression. Int. J. Clin. Exp. Pathol. 2009, 2, 65–70. [Google Scholar] [PubMed]
- Nakajima, S.; Nishiyama, Y.; Yamaoka, M.; Yasuoka, T.; Cho, E. Changes in the prevalence of Helicobacter Hp infection and gastrointestinal diseases in the past 17 years. J. Gastroenterol. Hepatol. 2010, 25, S99–S110. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Choi, I.J.; Kim, C.G.; Kook, M.C.; Lee, J.Y.; Kim, B.C.; Ryu, K.H.; Nam, S.Y.; Kim, Y.W. Risk factors associated with gastric cancer in patients with a duodenal ulcer. Helicobacter 2010, 6, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Schmidt, K.; Herrlinger, K.R.; Baxmann, S.; Behling, S.; Wohlschläger, C.; Feller, A.C.; Stange, E.F.; Fellermann, K.; et al. Defensin pattern in chronic gastritis: HBD-2 is differentially expressed with respect to Helicobacter Hp status. J. Clin. Pathol. 2003, 55, 352–357. [Google Scholar] [CrossRef]
- Kocsis, A.K.; Kiss, Z.F.; Tiszlavicz, L.; Tiszlavicz, Z.; Mándi, Y. Potential role of human β-defensin 1 in Helicobacter pylori-induced gastritis. Scand. J. Gastroenterol. 2009, 44, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, K.; Yagihashi, A.; Tsuji, N.; Uehara, N.; Furuya, D.; Kobayashi, D.; Watanabe, N. Human β-defensin-3 induction in Hp-infected gastric mucosal tissues. World J. Gastroenterol. 2006, 12, 5793–5797. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Günther, G.; Bullwinkel, J.; Lange, C.; Heine, H. Increased expression of β-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS ONE 2011, 6, e21898. [Google Scholar] [CrossRef] [PubMed]
- Laube, D.M.; Yim, S.; Ryan, L.K.; Diamond, G. Antimicrobial peptides in the airway. Curr. Top. Microbiol. Immunol. 2006, 306, 153–182. [Google Scholar] [PubMed]
- Kocsis, A.K.; Lakatos, P.L.; Somogyvári, F.; Fuszek, P.; Papp, J.; Fischer, S. Association of β-defensin 1 single nucleotide polymorphisms with Crohn’s disease. Scand. J. Gastroenterol. 2008, 43, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Wang, T.Y.; Wang, W.; Sun, H.Z. Association between Genetic Polymorphisms in DEFB1 and Susceptibility to Digestive Diseases. Med. Sci. Monit. 2015, 21, 2240–2250. [Google Scholar] [PubMed]
- Li, P.; Lv, H.; Yang, H.; Qian, J.M. IRF5, but not TLR4, DEFB1, or VDR, is associated with the risk of ulcerative colitis in a Han Chinese population. Scand. J. Gastroenterol. 2013, 48, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Yang, J.J.; Oh, S.; Cho, L.Y.; Ma, S.H.; Shin, A.; Ko, K.-P.; Park, T.; Yoo, K.-Y.; Kang, D. Innate immunity and non-Hodgkin’s lymphoma (NHL) related genes in a nested case-control study for gastric cancer risk. PLoS ONE 2012, 7, e45274. [Google Scholar] [CrossRef] [PubMed]
- Sheh, A.; Fox, J.G. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes 2013, 4, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.S.; Faccenda, E.; Angerson, W.J.; Balsitis, M.; Kelly, R.W. Gastric epithelial anti-microbial peptides-histological correlation and influence of anatomical site and peptic ulcer disease. Dig. Liver Dis. 2005, 37, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, A.; Arnold, M.; Ferlay, J.; Goodman, K.J.; Forman, D.; Soerjomataram, I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 2015, 64, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Helicobacter pylori and gastric carcinogenesis. Am. J. Surg. Pathol. 1995, 19, S37–S43. [Google Scholar]
- Uehara, N.; Yagihashi, A.; Kondoh, K.; Tsuji, N.; Fujita, T.; Hamada, H.; Watanabe, N. Human β-defensin-2 induction in Helicobacter Hp-infected gastric mucosal tissues: Antimicrobial effect of overexpression. J. Med. Microbiol. 2003, 52 Pt 1, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Nuding, S.; Gersemann, M.; Hosaka, Y.; Konietzny, S.; Schaefer, C.; Beisner, J.; Schroeder, B.O.; Ostaff, M.J.; Saigenji, K.; Ott, G.; et al. Gastric antimicrobial peptides fail to eradicate Helicobacter pylori infection due to selective induction and resistance. PLoS ONE 2013, 9, e73867. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.W.; Giles, D.K.; Wolf, L.N.; Ecobichon, C.; Boneca, I.G.; Trent, M.S. Helicobacter pylori versus the host: Remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog. 2011, 7, e1002454. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, D.H. Antibacterial and neutralizing effect of human β-defensins on Enterococcus faecalis and Enterococcus faecalis lipoteichoic acid. J. Endod. 2012, 3, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lim, K.B.; Poduje, C.M.; Daniel, M.; Gunn, J.S.; Hackett, M.; Miller, S.I. Lipid acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 1998, 2, 189–198. [Google Scholar] [CrossRef]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Bokarewa, M.; Foster, T.; Mitchell, J.; Higgins, J.; Tarkowski, A. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J. Immunol. 2004, 2, 1169–1176. [Google Scholar] [CrossRef]
- Schmidtchen, A.; Frick, I.M.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 2002, 1, 157–168. [Google Scholar] [CrossRef]
- Basile, A.; Senatore, F.; Gargano, R.; Sorbo, S.; Del Pezzo, M.; Lavitola, A.; Ritieni, A.; Bruno, M.; Spatuzzi, D.; Rigano, D.; et al. Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J. Ethnopharmacol. 2006, 107, 240–248. [Google Scholar] [CrossRef]
- Radzishevsky, I.S.; Kovachi, T.; Porat, Y.; Ziserman, L.; Zaknoon, F.; Danino, D.; Mor, A. Structure-activity relationships of antibacterial acyl-lysine oligomers. Chem. Biol. 2008, 15, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Makobongo, M.O.; Gancz, H.; Carpenter, B.M.; McDaniel, D.P.; Merrell, D.S. The oligo-acyllysyl antimicrobial peptide C12K-2β12exhibits a dual mechanism of action and demonstrates strong in vivo efficacy against Helicobacter pylori. Antimicrob. Agents Chemother. 2012, 56, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Makobongo, M.O.; Kovachi, T.; Gancz, H.; Mor, A.; Merrell, D.S. In vitro antibacterial activity of acyl-lysyl oligomers against Helicobacter pylori. Antimicrob. Agents Chemother. 2009, 10, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Galdiero, S.; Cantisani, M.; Di Noto, R.; Vitiello, M.; Galdiero, M.; Naclerio, G.; Cassiman, J.J.; Pedone, C.; Castaldo, G.; et al. Novel synthetic, salt-resistant analogs of human β-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob. Agents Chemother. 2010, 54, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Galdiero, S.; Nigro, E.; Del Vecchio, L.; Di Noto, R.; Cantisani, M.; Colavita, I.; Galdiero, M.; Cassiman, J.J.; Daniele, A.; et al. Chimeric β-defensin analogs, including the novel 3NI analog, display salt-resistant antimicrobial activity and lack toxicity in human epithelial cell lines. Antimicrob. Agents Chemother. 2013, 4, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Nigro, E.; Cantisani, M.; Colavita, I.; Leone, M.; Mercurio, F.A.; Galdiero, M.; Pessi, A.; Daniele, A.; Salvatore, F.; et al. Design and activity of a cyclic mini-β-defensin analog: A novel antimicrobial tool. Int. J. Nanomed. 2015, 10, 6523–6539. [Google Scholar]
- Nigro, E.; Colavita, I.; Sarnataro, D.; Scudiero, O.; Zambrano, G.; Granata, V.; Daniele, A.; Carotenuto, A.; Galdiero, S.; Folliero, V.; et al. An ancestral host defence peptide within human β-defensin 3 recapitulates the antibacterial and antiviral activity of the full-length molecule. Sci. Rep. 2015, 5, 18450. [Google Scholar] [CrossRef] [PubMed]
- Rotem, S.; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta 2009, 8, 1582–9152. [Google Scholar] [CrossRef] [PubMed]
- Zaknoon, F.; Sarig, H.; Rotem, S.; Livne, L.; Ivankin, A.; Gidalevitz, D.; Mor, A. Antibacterial properties and mode of action of a short acyl-lysyl oligomer. Antimicrob. Agents Chemother. 2009, 53, 3422–3429. [Google Scholar] [CrossRef]
- Rotem, S.; Raz, N.; Kashi, Y.; Mor, A. Bacterial capture by peptide-mimetic oligoacyllysine surfaces. Appl. Environ. Microbiol. 2010, 10, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, K.; Namiot, A.; Fein, D.E.; Wen, Q.; Namiot, Z.; Savage, P.B.; Diamond, S.; Janmey, P.A.; Bucki, R. Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiol. 2009, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Jiang, A.M.; Ma, Z.Y.; Li, X.B.; Xiong, Y.Y.; Dou, J.F.; Wang, J.F. The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-Helicobacter pylori activity in vitro and in vivo. Molecules 2015, 20, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- Cassone, M.; Otvos, L., Jr. Synergy among antibacterial peptides and betweenpeptides and small-molecule antibiotics. Expert Rev. Anti-Infec. Ther. 2010, 6, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Nuding, S.; Frasch, T.; Schaller, M.; Stange, E.F.; Zabel, L.T. Synergistic effects of antimicrobial peptides and antibiotics against Clostridium difficile. Antimicrob. Agents Chemother. 2014, 58, 5719–5725. [Google Scholar] [CrossRef] [PubMed]
- Narayana, J.L.; Huang, H.N.; Wu, C.J.; Chen, J.Y. Efficacy of the antimicrobial peptide TP4 against Helicobacter pylori infection: In vitro membrane perturbation via micellization and in vivo suppression of host immune responses in a mouse model. Oncotarget 2015, 15, 2936–2954. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pero, R.; Coretti, L.; Nigro, E.; Lembo, F.; Laneri, S.; Lombardo, B.; Daniele, A.; Scudiero, O. β-Defensins in the Fight against Helicobacter pylori. Molecules 2017, 22, 424. https://doi.org/10.3390/molecules22030424
Pero R, Coretti L, Nigro E, Lembo F, Laneri S, Lombardo B, Daniele A, Scudiero O. β-Defensins in the Fight against Helicobacter pylori. Molecules. 2017; 22(3):424. https://doi.org/10.3390/molecules22030424
Chicago/Turabian StylePero, Raffaela, Lorena Coretti, Ersilia Nigro, Francesca Lembo, Sonia Laneri, Barbara Lombardo, Aurora Daniele, and Olga Scudiero. 2017. "β-Defensins in the Fight against Helicobacter pylori" Molecules 22, no. 3: 424. https://doi.org/10.3390/molecules22030424
APA StylePero, R., Coretti, L., Nigro, E., Lembo, F., Laneri, S., Lombardo, B., Daniele, A., & Scudiero, O. (2017). β-Defensins in the Fight against Helicobacter pylori. Molecules, 22(3), 424. https://doi.org/10.3390/molecules22030424







