New Marine Sterols from a Gorgonian Pinnigorgia sp.
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.4. Generation of Superoxide Anions and Release of Elastase by Human Neutrophils
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Chang, Y.-C.; Kuo, L.-M.; Su, J.-H.; Hwang, T.-L.; Kuo, Y.-H.; Lin, C.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Pinnigorgiols A–C, 9,11-secosterols with a rare ring arrangement from a gorgonian coral Pinnigorgia sp. Tetrahedron 2016, 72, 999–1004. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kuo, L.-M.; Hwang, T.-L.; Yeh, J.; Wen, Z.-H.; Fang, L.-S.; Wu, Y.-C.; Lin, C.-S.; Sheu, J.-H.; Sung, P.-J. Pinnisterols A–C, new 9,11-secosterols from a gorgonian Pinnigorgia sp. Mar. Drugs 2016, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Cheng, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Sung, P.-J. New anti-inflammatory sterols from a gorgonian Pinnigorgia sp. Bioorg. Med. Chem. Lett. 2016, 26, 3060–3063. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chen, N.-F.; Hwang, T.-L.; Tseng, C.-C.; Wu, T.-Y.; Peng, B.-R.; Wen, Z.-H.; Fang, L.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. New marine sterols from an algal-bearing gorgonian coral Pinnigorgia sp. Steroids 2016, 115, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Hwang, T.-L.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. New anti-inflammatory 9,11-secosterols with a rare tricyclo[5,2,1,1]decane ring from a Formosan gorgonian Pinnigorgia sp. Mar. Drugs 2016, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Hwang, T.-L.; Kuo, L.-M.; Sung, P.-J. Pinnisterols D−J, new 11-acetoxy-9,11-secosterols with a 1,4-quinone moiety from Formosan gorgonian coral Pinnigorgia sp. (Gorgoniidae). Mar. Drugs 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Rueda, A.; Zubía, E.; Ortega, M.J.; Carballo, J.L.; Salvá, J. New metabolites from the sponge Spongia agaricina. J. Nat. Prod. 1998, 61, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.C.; McInnes, A.G.; Shimizu, S.; Smith, D.G.; Walter, J.A.; Idler, D.; Khalil, W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonances spectroscopy. Can. J. Chem. 1978, 56, 1898–1903. [Google Scholar]
- Aiello, A.; Fattorusso, E.; Menna, M.; Carnuccio, R.; Iuvone, T. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the lagoon of Venice. Steroids 1995, 60, 666–673, The compound that was reported to possess the same structure as that of 1 was listed as compound 7 in this article. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y. Isolation and structure elucidation of lobophytosterol, depresosterol and three other closely related sterols. Tetrahedron 1981, 37, 2397–2403. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chang, Y.-C.; Chen, W.-F.; Hwang, T.-L.; Fang, L.-S.; Wen, Z.-H.; Chen, Y.-H.; Wu, Y.-C.; Sung, P.-J. Pubinernoid A and apo-9′-fucoxanthinone, secondary metabolites from a gorgonian coral Pinnigorgia sp. Nat. Prod. Commun. 2016, 11, 707–708. [Google Scholar] [PubMed]
- Huang, S.-X.; Yang, J.; Xiao, W.-L.; Zhu, Y.-L.; Li, R.-T.; Li, L.-M.; Pu, J.-X.; Li, X.; Li, S.-H.; Sun, H.-D. Three novel terpenoids from Schisandra pubescens var. pubinervis. Helv. Chim. Acta 2006, 89, 1169–1175. [Google Scholar] [CrossRef]
- Hodges, R.; Porte, A.L. The structure of loliolide, a terpene from Lolium perenne. Tetrahedron 1964, 20, 1463–1467. [Google Scholar] [CrossRef]
- Isoe, S.; Hyeon, S.B.; Katsumura, S.; Sakan, T. Photo-oxygenation of carotenoids. II. The absolute configuration of loliolide and dihydroactinidiolide. Tetrahedron Lett. 1972, 13, 2517–2520. [Google Scholar] [CrossRef]
- Pettit, G.R.; Herald, C.L.; Ode, R.H.; Brown, P.; Gust, D.J.; Michel, C. The isolation of loliolide from an Indian Ocean opisthobranch mollusc. J. Nat. Prod. 1980, 43, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.N.; Murphy, P.T.; Lidgard, R.O.; Warren, R.G.; Wells, R.J. C18 terpenoid metabolites of the brown alga Cystophora moniliformis. Aust. J. Chem. 1982, 35, 171–182. [Google Scholar] [CrossRef]
- Valdes, L.J., III. Loliolide from Salvia divinorum. J. Nat. Prod. 1986, 49, 171. [Google Scholar] [CrossRef]
- Mori, K.; Khlebnikov, V. Synthesis of (+)-dihydroactinidiolide, (+)- and (–)-actinidiolide, (+)- and (–)-loliolide as well as (+)- and (–)-epiloliolide. Liebigs Ann. Chem. 1993, 77–82. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chen, B.-Y.; Chen, Y.-H.; Chiang, M.Y.; Lin, M.-R. Loliolide: Occurrence of a carotenoid metabolite in the octocoral Briareum excavatum (Briareidae). Biochem. Syst. Ecol. 2010, 38, 116–118. [Google Scholar] [CrossRef]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed.; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 218–219. [Google Scholar]
- Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; Hwang, T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1 and 2 are not available from the authors.
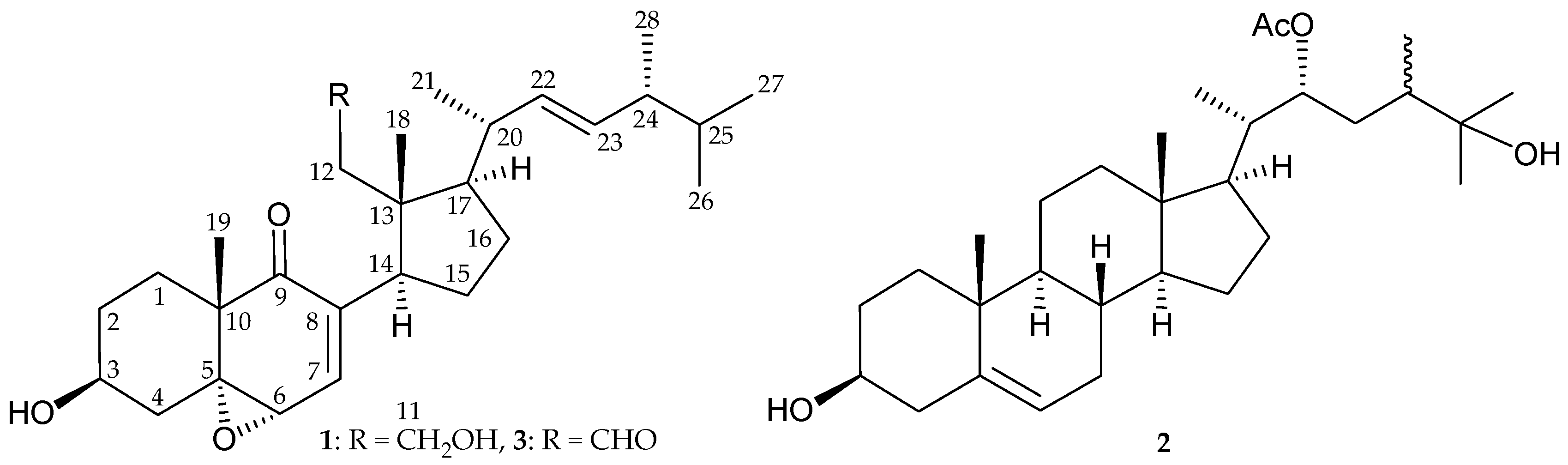
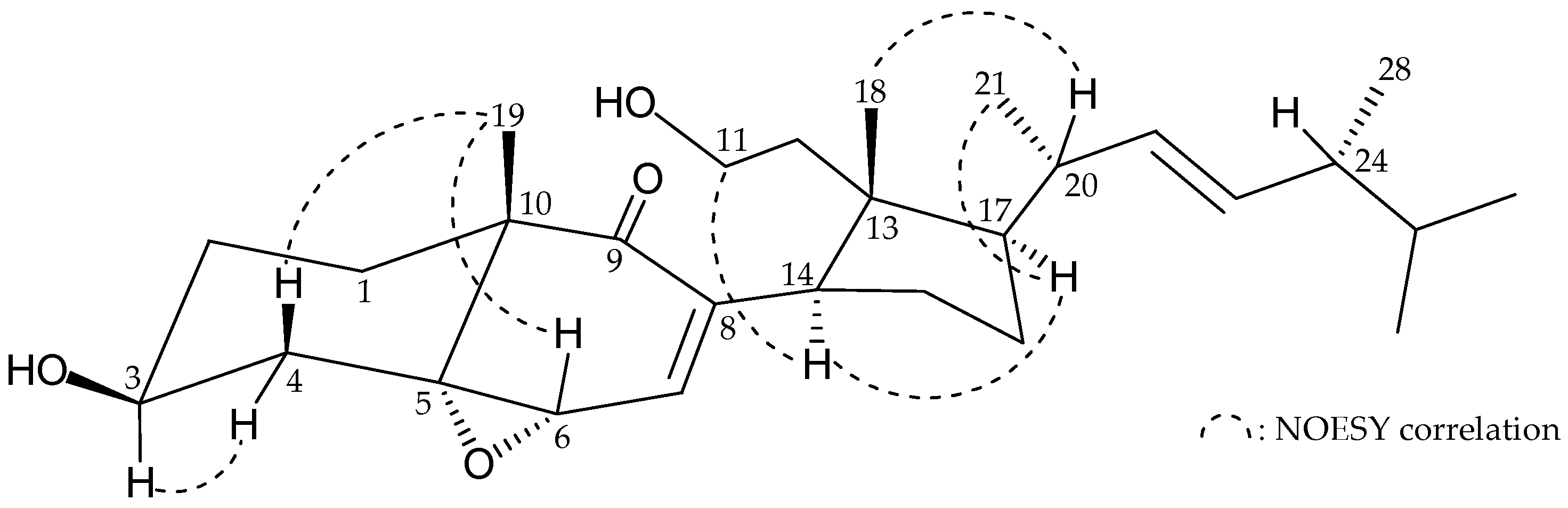
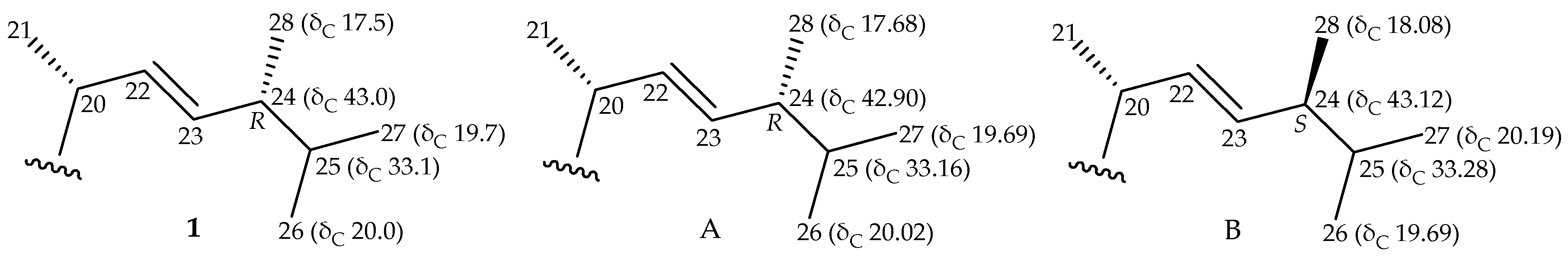
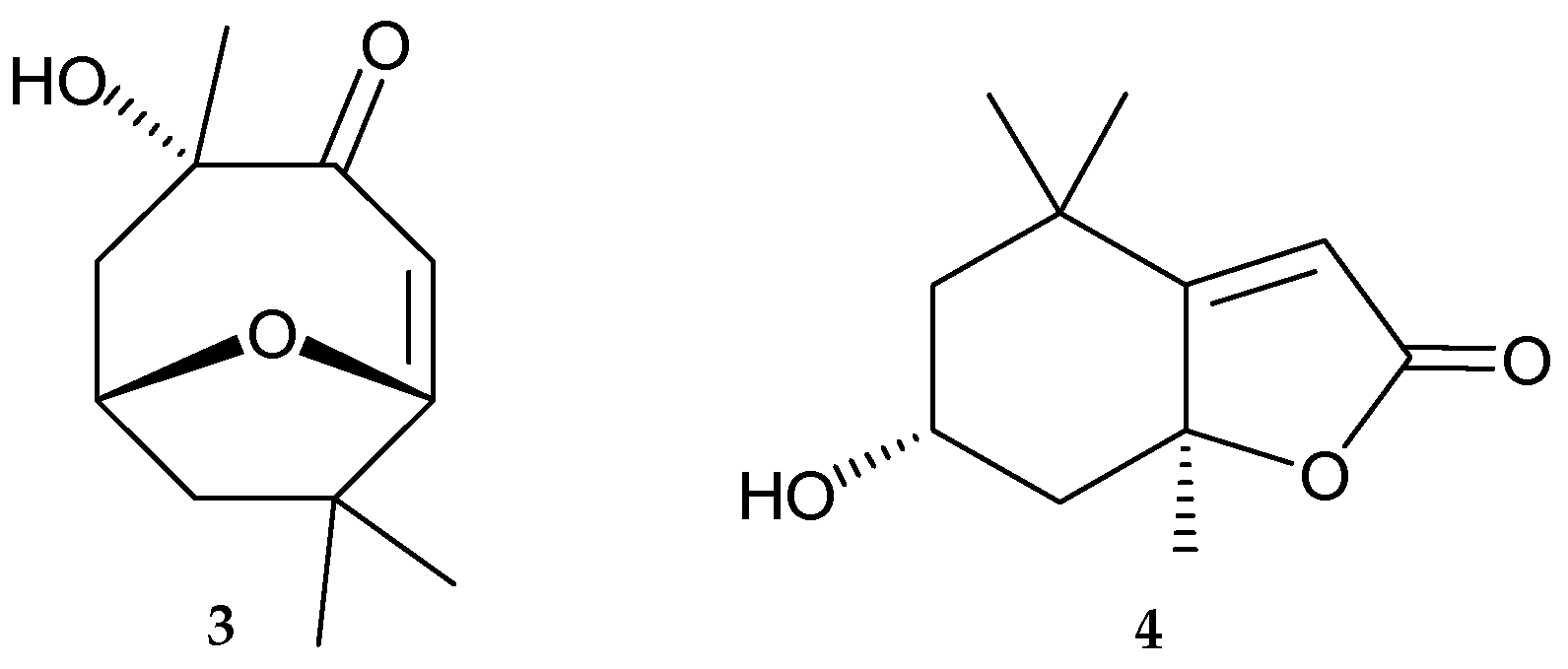
| Position | 1 | 3 | ||||
|---|---|---|---|---|---|---|
| δH (J in Hz) a | δCb | 1H-1H | HMBC | δH (J in Hz)c | δCc | |
| 1a/b | 2.09 m; 1.72 m | 27.8, CH2 | H2-2 | C-5 | 27.8, CH2 | |
| 2a/b | 2.09 m; 1.65 m | 30.5, CH2 | H2-1, H-3 | C-10 | 2.09 m; 1.68 m | 30.5, CH2 |
| 3 | 3.98 m | 68.3, CH | H2-2, H2-4 | n. o. d | 3.98 m | 68.3, CH |
| 4α | 1.57 m | 37.4, CH2 | H-3, H-4β | C-2, -3, -5 | 1.56 m | 37.5, CH2 |
| β | 2.18 dd (12.8, 11.6) | H-3, H-4α | C-3 | 2.18 m | ||
| 5 | 63.2, C | 63.5, C | ||||
| 6 | 3.39 d (4.8) | 53.5, CH | H-7 | C-7, -8 | 3.40 d (4.6) | 53.5, CH |
| 7 | 6.81 d (4.8) | 139.3, CH | H-6 | C-5, -6, -9, -14 | 6.84 dd (4.6, 1.0) | 139.7, CH |
| 8 | 141.4, C | 140.5, C | ||||
| 9 | 201.9, C | 200.6, C | ||||
| 10 | 45.6, C | 45.4, C | ||||
| 11a | 3.81 ddd (10.4, 10.4, 6.0) | 59.1, CH2 | H-11b, H2-12 | n. o. | 9.88 dd (3.8, 1.7) | 203.4, CH |
| b | 3.68 ddd (10.4, 8.8, 6.0) | H-11a, H2-12 | n. o. | |||
| 12a | 1.61 m | 40.4, CH2 | H2-11, H-12b | n. o. | 2.27 dd (15.9, 3.8) | 50.8, CH2 |
| b | 1.12 m | H2-11, H-12a | C-11, -13, -17 | 2.00 dd (15.9, 1.7) | ||
| 13 | 46.1, C | 46.3, C | ||||
| 14 | 3.37 dd (10.8, 8.0) | 43.8, CH | H2-15 | n. o. | 3.51 dd (10.3, 9.2) | 45.0, CH |
| 15a/b | 1.69–1.56 m | 26.9, CH2 | H-14, H2-16 | C-13, -14 | 1.78 m; 1.71 m | 26.7, CH2 |
| 16a/b | 1.69 m; 1.44 m | 25.4, CH2 | H2-15, H-17 | n. o. | 25.8, CH2 | |
| 17 | 1.74 m | 49.6, CH | H2-16, H-20 | n. o. | 51.9, CH | |
| 18 | 0.68 s | 17.8, CH3 | C-12, -13, -14, -17 | 0.76 s | 17.1, CH3 | |
| 19 | 1.25 s | 21.4, CH3 | C-1, -5, -9, -10 | 1.21 s | 20.0, CH3 | |
| 20 | 2.15 m | 38.8, CH | H-17, H3-21, H-22 | n. o. | 2.18 m | 43.0, CH |
| 21 | 1.03 d (6.8) | 21.4, CH3 | H-20 | C-17, -20, -22 | 1.00 d (6.8) | 19.7, CH3 |
| 22 | 5.24 dd (15.2, 6.8) | 134.4, CH | H-20, H-23 | C-20, -24 | 5.20 dd (17.6, 7.4) | 133.4, CH |
| 23 | 5.21 dd (15.2, 6.4) | 133.0, CH | H-22, H-24 | C-20, -24 | 5.24 dd (17.6, 7.4) | 134.0, CH |
| 24 | 1.86 m | 43.0, CH | H-23, H-25, H3-28 | C-22, -23, -25 | 1.87 m | 38.8, CH |
| 25 | 1.47 m | 33.1, CH | H-24, H3-26, H3-27 | C-23, -24, -28 | 1.47 m | 33.2, CH |
| 26 | 0.83 d (7.2) | 20.0, CH3 | H-25 | C-24, -25, -27 | 0.82 d (6.8) | 21.9, CH3 |
| 27 | 0.82 d (6.8) | 19.7, CH3 | H-25 | C-24, -25, -26 | 0.83 d (6.8) | 21.1, CH3 |
| 28 | 0.91 d (6.8) | 17.5, CH3 | H-24 | C-23, -24, -25 | 0.91 d (7.0) | 17.8, CH3 |
| Position | δH (J in Hz) | δC, Multiple | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1a/b | 1.84 m; 1.06 m | 37.2, CH2 | H2-2 | C-2, -5 |
| 2a/b | 1.84 m; 1.51 m | 31.6, CH2 | H2-1, H-3 | n. o. a |
| 3 | 3.52 m | 71.8, CH | H2-2, H2-4 | n. o. |
| 4a/b | 2.30 m; 2.24 m | 42.3, CH2 | H-3 | C-2, -3, -5, -6, -10 |
| 5 | 140.7, C | |||
| 6 | 5.35 d (5.2) | 121.6, CH | H2-7 | C-4, -7, -8, -10 |
| 7a/b | 1.97 m; 1.53 m | 31.8, CH2 | H-6, H-8 | C-6, -14 |
| 8 | 1.46 m | 31.9, CH | H2-7, H-9, H-14 | C-14 |
| 9 | 0.92 m | 50.1, CH | H-8, H2-11 | C-7, -8 |
| 10 | 36.5, C | |||
| 11a/b | 1.50–1.40 m | 21.1, CH2 | H-9, H2-12 | C-9 |
| 12a/b | 1.97 m; 1.17 m | 39.7, CH2 | H2-11 | n. o. |
| 13 | 42.7, C | |||
| 14 | 0.98 m | 56.3, CH | H-8, H2-15 | n. o. |
| 15a/b | 1.56 m; 1.07 m | 24.3, CH2 | H-14, H2-16 | C-14 |
| 16a/b | 1.81 m; 1.53 m | 27.2, CH2 | H2-15, H-17 | n. o. |
| 17 | 1.15 m | 53.1, CH | H2-16, H-20 | C-12, -20 |
| 18 | 0.67 s | 11.9, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.00 s | 19.4, CH3 | C-1, -5, -9 | |
| 20 | 1.75 m | 39.8, CH | H-17, H3-21, H-22 | n. o. |
| 21 | 0.93 d (7.2) | 13.0, CH3 | H-20 | C-17, -20, -22 |
| 22 | 5.02 ddd (10.8, 2.8, 2.4) | 78.3, CH | H-20, H2-23 | n. o. |
| 23a/b | 1.84 m; 1.15 m | 29.3, CH2 | H-22, H-24 | C-20, -22 |
| 24 | 1.43 m | 43.1, CH | H2-23, H3-28 | n. o. |
| 25 | 73.6, C | |||
| 26 | 1.13 s | 25.0, CH3 | C-24, -25, -27 | |
| 27 | 1.20 s | 28.4, CH3 | C-24, -25, -26 | |
| 28 | 0.91 d (7.2) | 16.9, CH3 | H-24 | C-23, -24, -25 |
| 22-OAc | 171.0, C | |||
| 2.04, s | 21.6, CH3 | Acetate carbonyl |
| Compound | Superoxide Anions | Elastase Release |
|---|---|---|
| IC50 (μM) a | IC50 (μM) | |
| 1 | 8.65 ± 0.19 | 5.86 ± 0.95 |
| 2 | > 10 | > 10 |
| LY294002 b | 1.06 ± 0.06 | 3.85 ± 1.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Hwang, T.-L.; Chao, C.-H.; Sung, P.-J. New Marine Sterols from a Gorgonian Pinnigorgia sp. Molecules 2017, 22, 393. https://doi.org/10.3390/molecules22030393
Chang Y-C, Hwang T-L, Chao C-H, Sung P-J. New Marine Sterols from a Gorgonian Pinnigorgia sp. Molecules. 2017; 22(3):393. https://doi.org/10.3390/molecules22030393
Chicago/Turabian StyleChang, Yu-Chia, Tsong-Long Hwang, Chih-Hua Chao, and Ping-Jyun Sung. 2017. "New Marine Sterols from a Gorgonian Pinnigorgia sp." Molecules 22, no. 3: 393. https://doi.org/10.3390/molecules22030393
APA StyleChang, Y.-C., Hwang, T.-L., Chao, C.-H., & Sung, P.-J. (2017). New Marine Sterols from a Gorgonian Pinnigorgia sp. Molecules, 22(3), 393. https://doi.org/10.3390/molecules22030393







