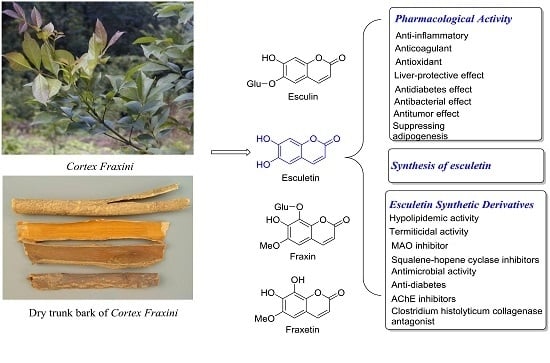
Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review
Abstract
:1. Introduction
2. Pharmacological Effects of Esculetin
2.1. Anti-Inflammatory Effects
2.2. Anticoagulant
2.3. Antioxidant
2.4. Liver-Protective Effects
2.5. Antidiabetic Effects
2.6. Antibacterial Effects
2.7. Antitumour Effects
2.8. Suppressed Adipogenesis
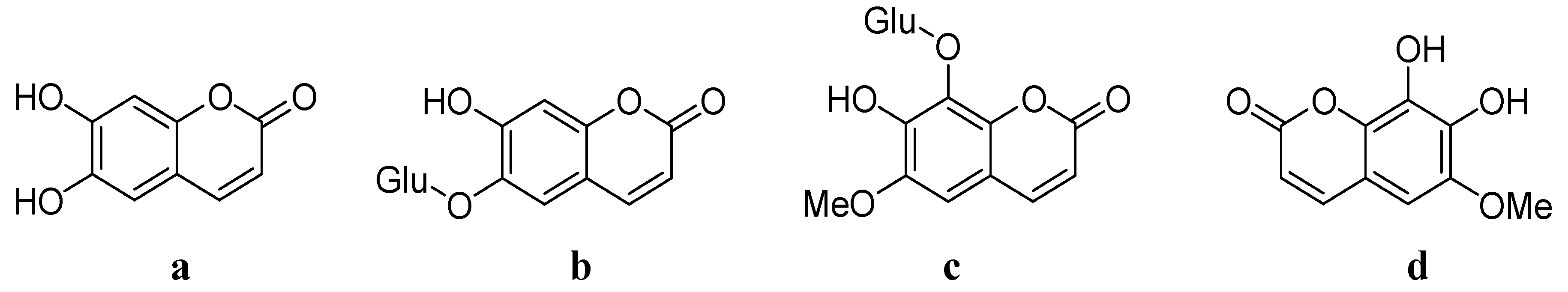
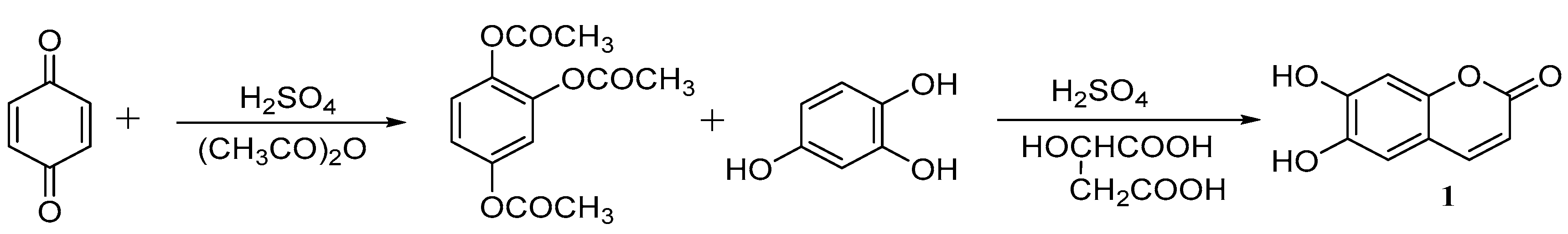
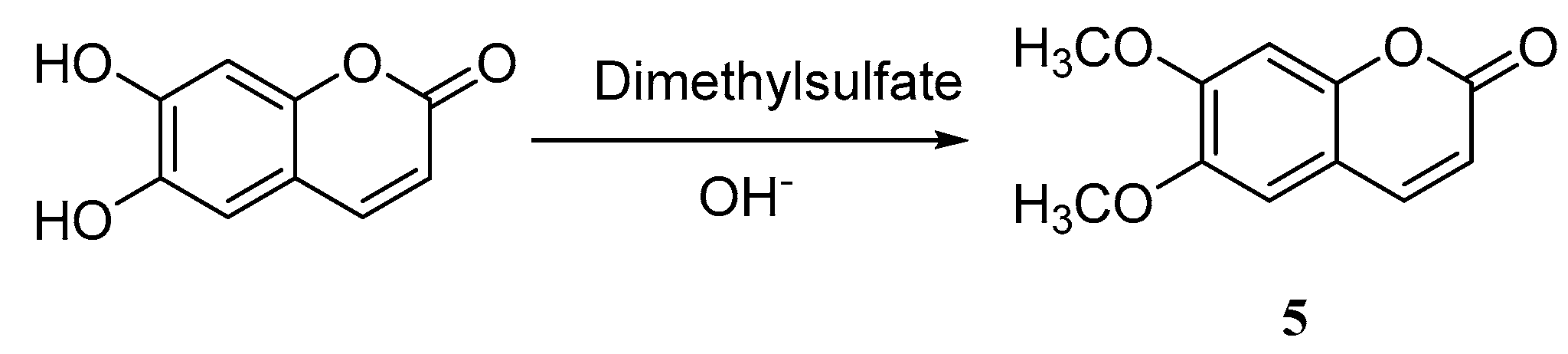
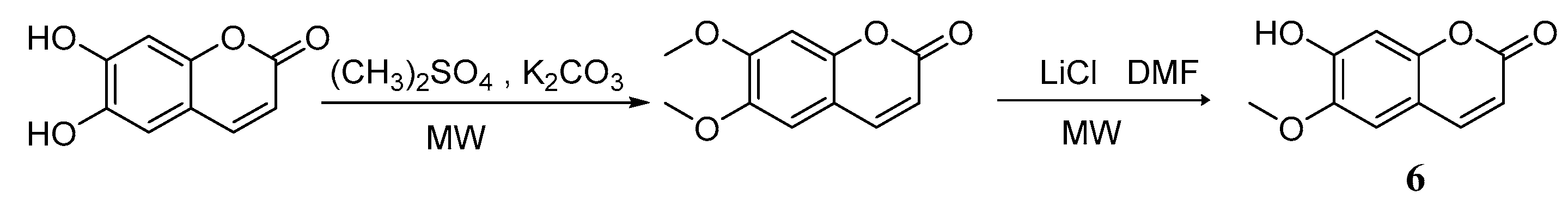
3. Synthesis of Esculetin
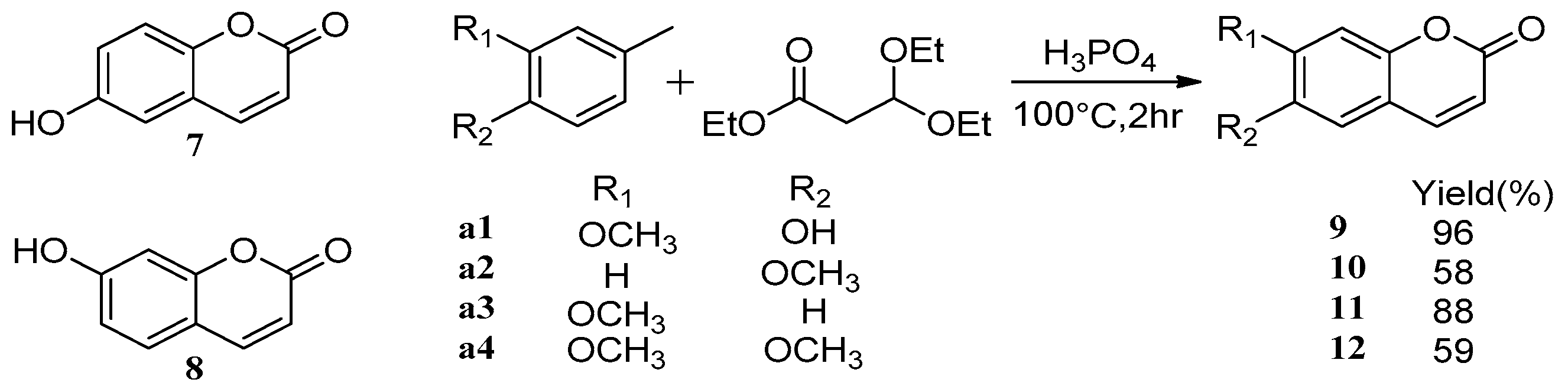
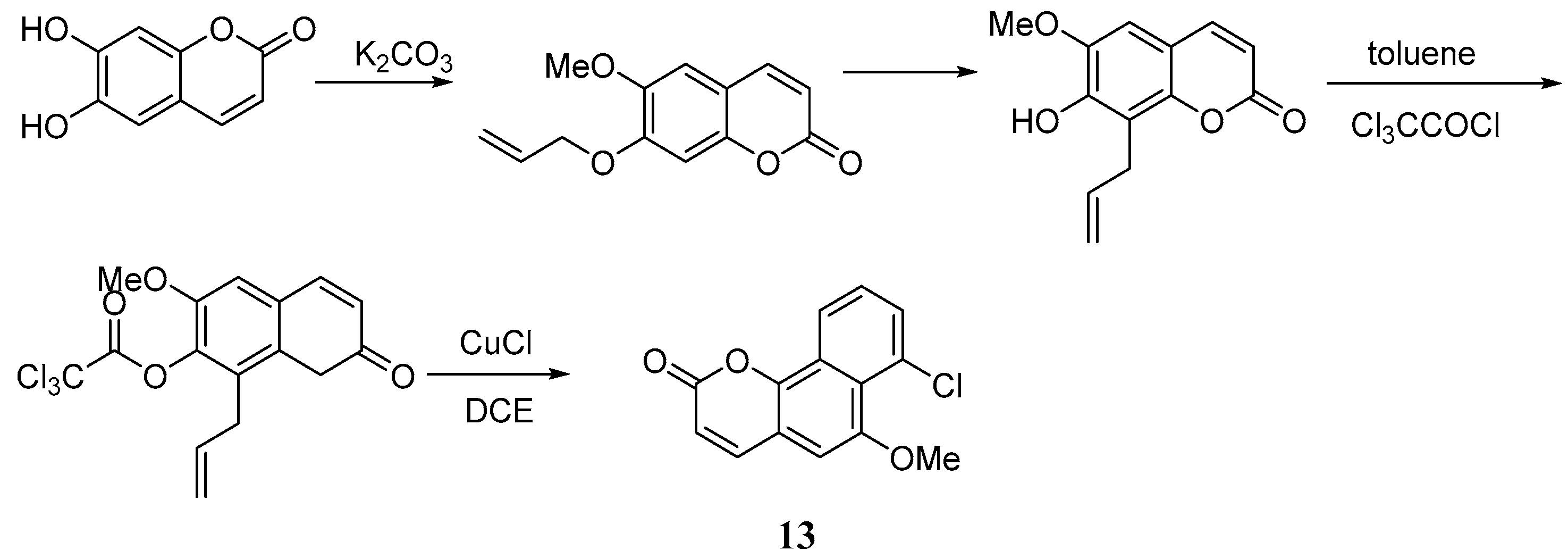
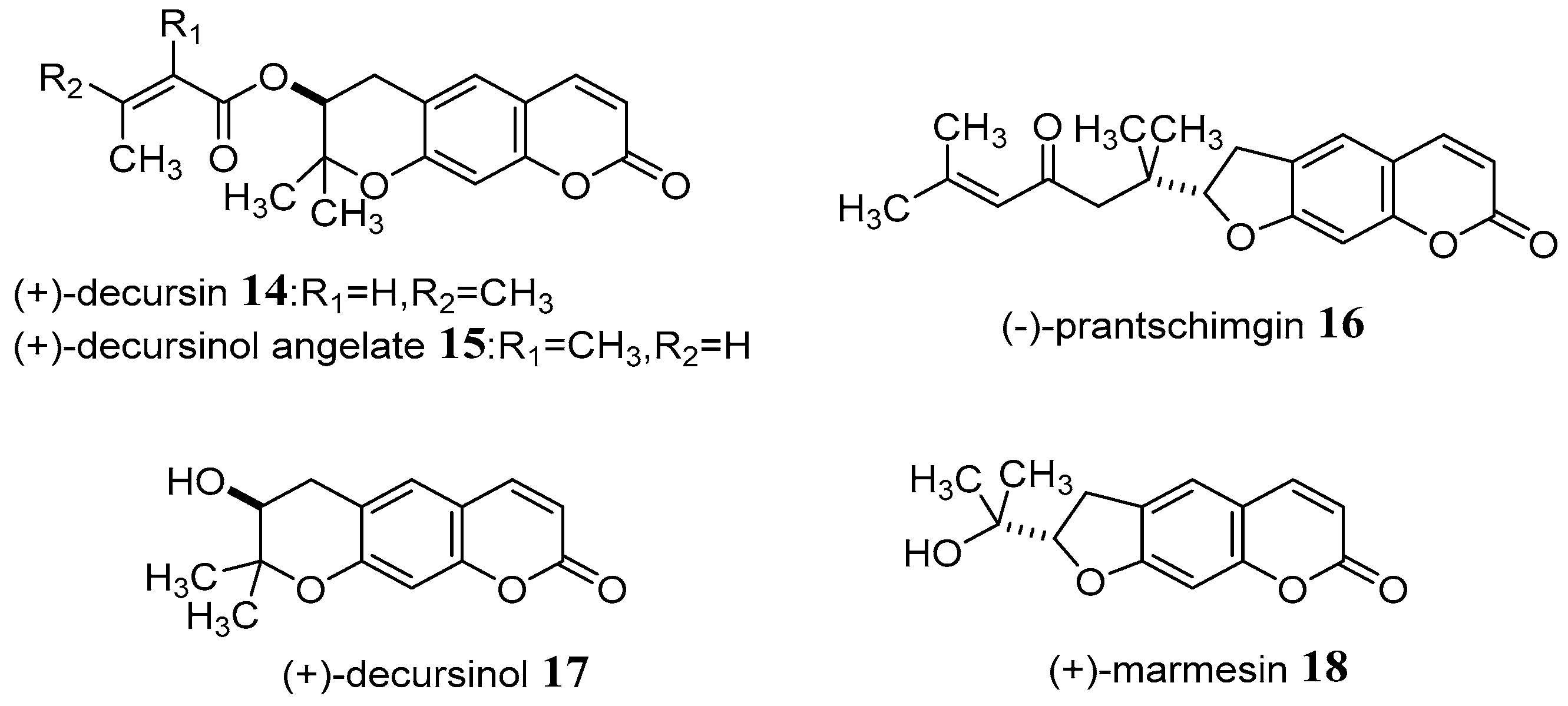
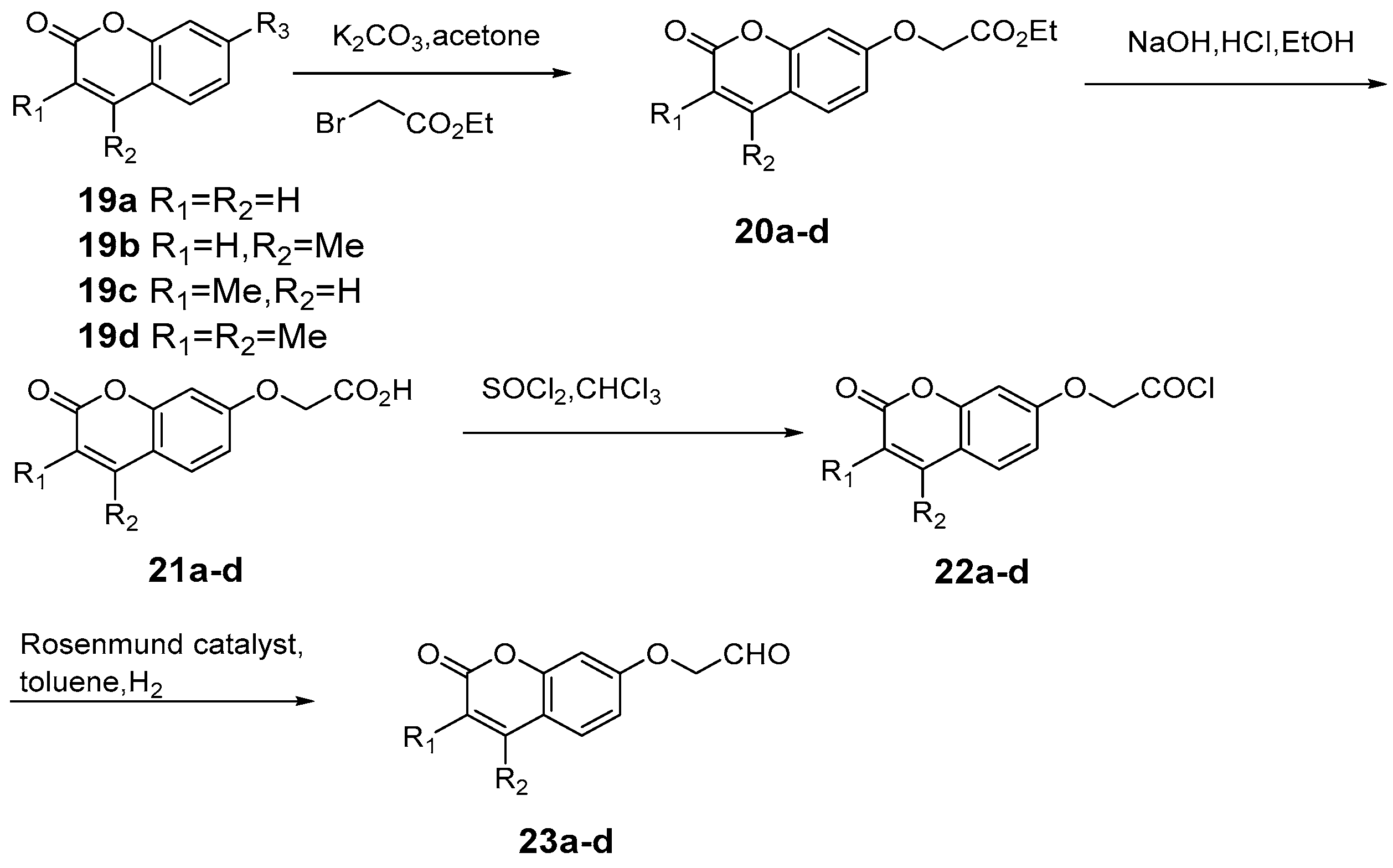
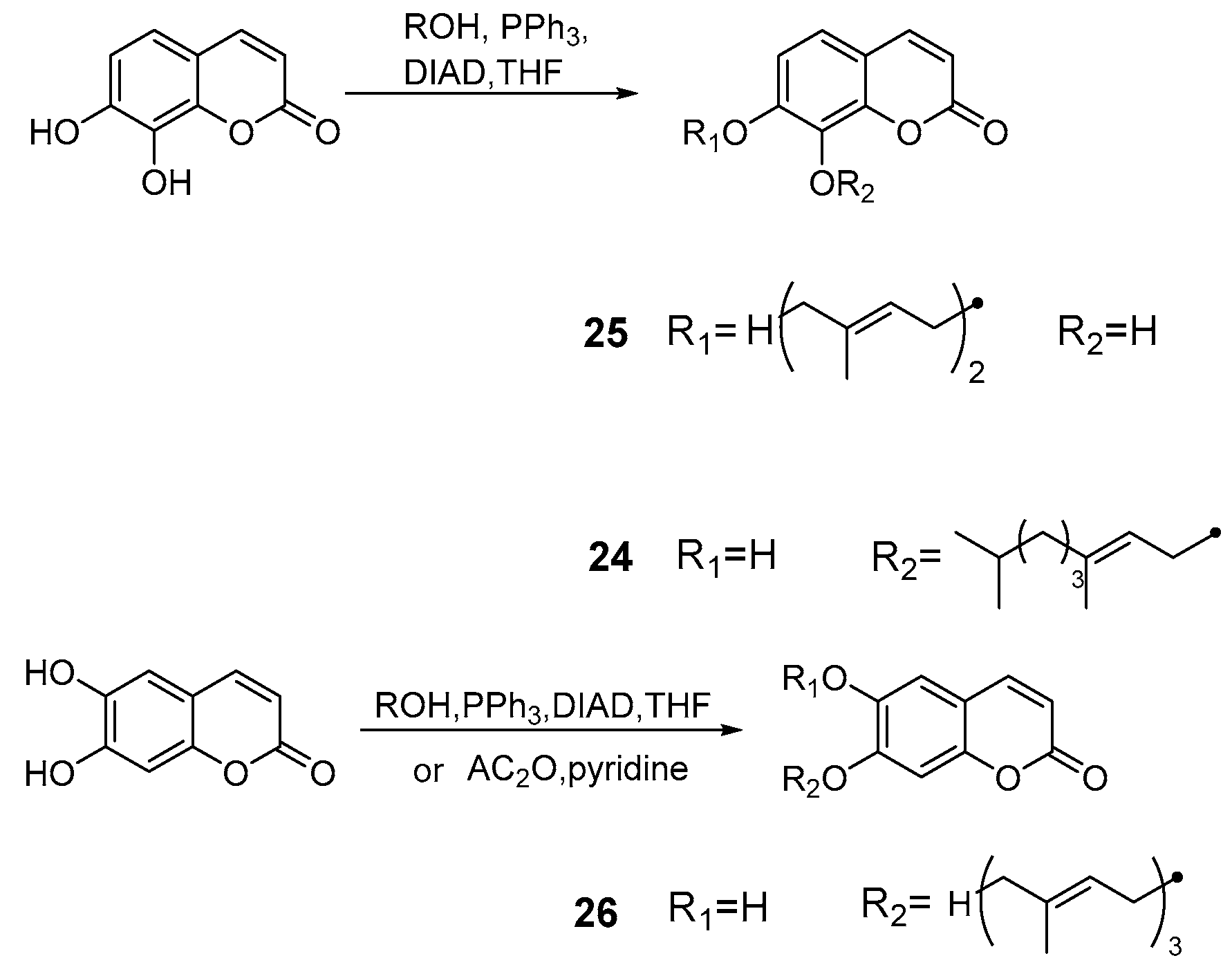
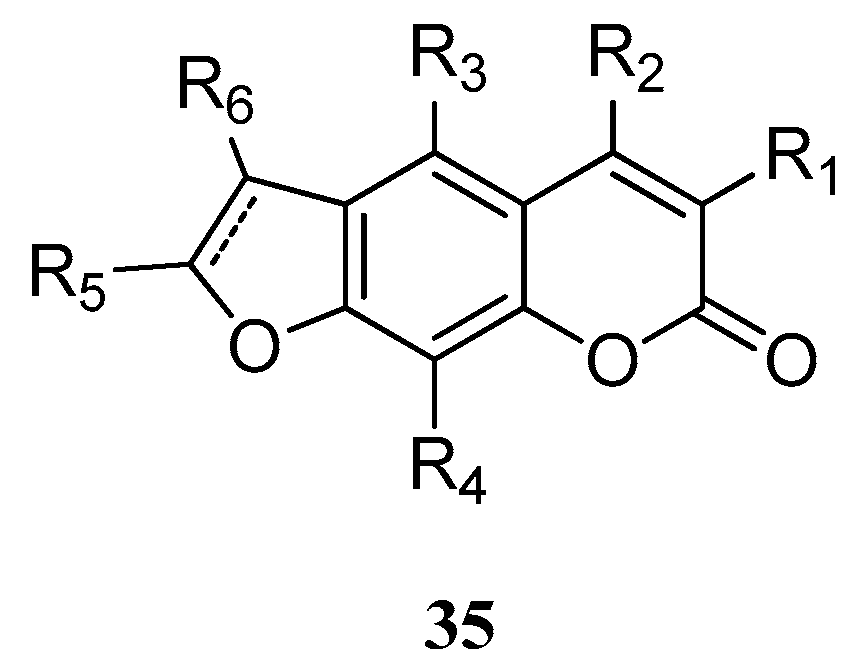
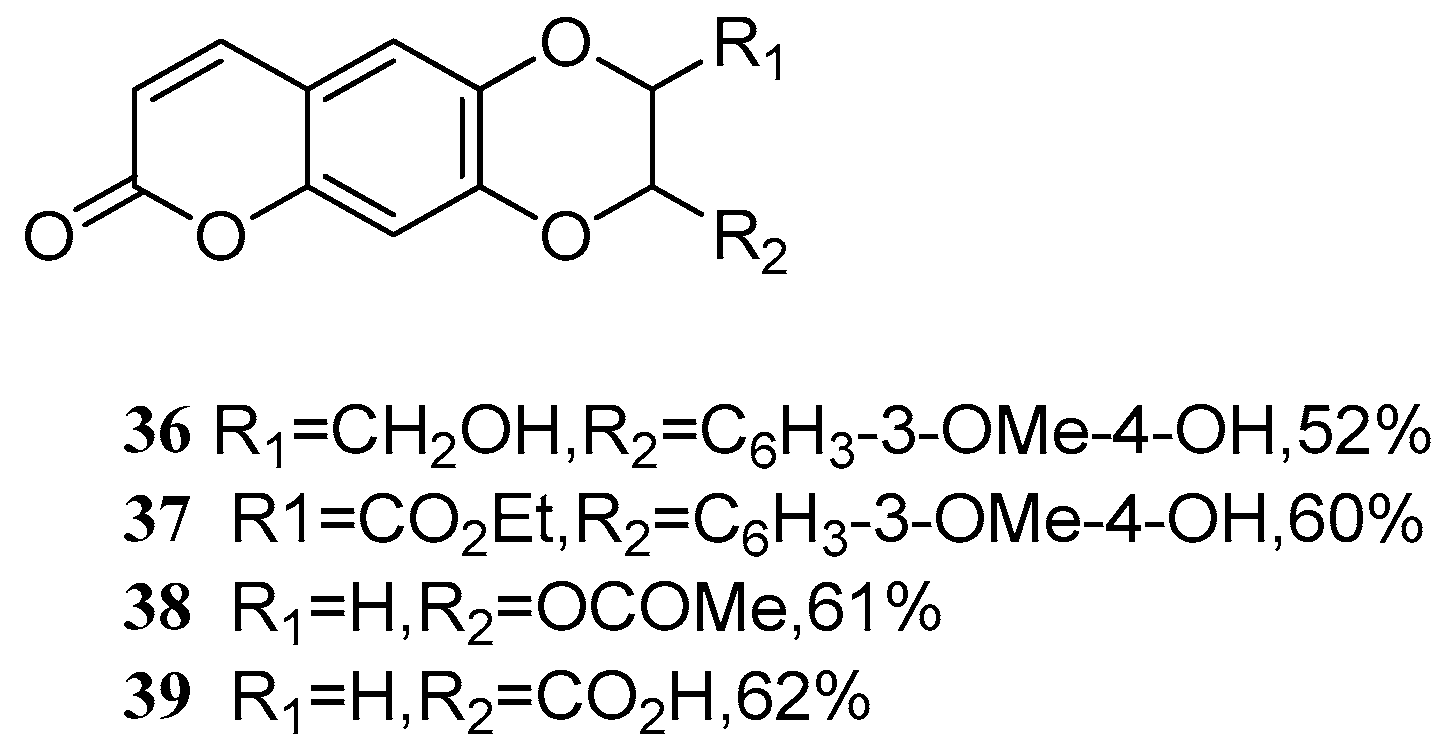
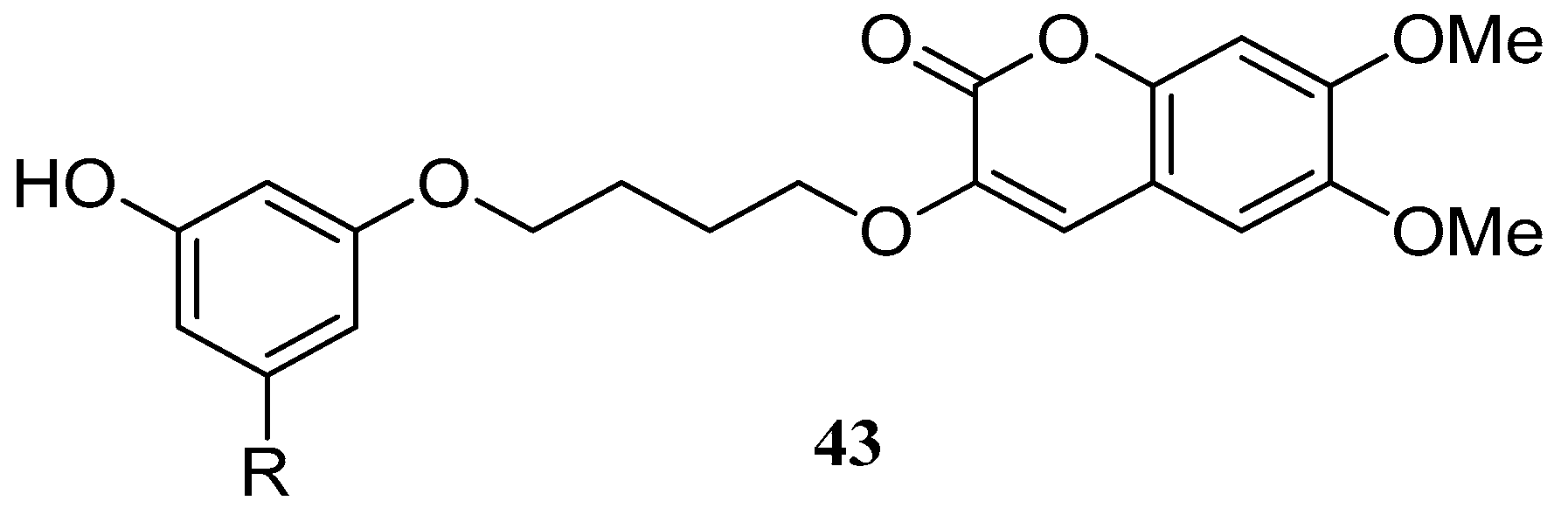
4. Synthesis and Pharmacological Evaluation of Esculetin Derivatives
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weng, Y.C.; Liu, J.W.; Cui, C.; Zhao, Q.C. Isolation and identification of the constituents from Fraxini Cortex and their in vitro antibacterial activity. Chin. J. Med. Chem. 2014, 1, 40–47. [Google Scholar]
- Liu, S.Q.; He, L.; Peng, H.; Liu, J. Effect of ash bark on matrix metalloproteinase 1, nitricoxide and prostaglandin E2 in rabbits with experimental osteoarthritis. Chin. J. Clin. Rehabil. 2005, 9, 150–153. [Google Scholar]
- Przemyslaw, R.; Emilia, G.; Slawomir, M.; Bujalskazadrozny, M. Antinociceptive properties of esculetin in non-inflammatory and inflammatory models of pain in rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 213–219. [Google Scholar]
- Kaneko, T.; Tahara, S.; Takabayashi, F. Inhibitory effect of natural coumarin compounds, esculetin and esculin, on oxidative DNA damage and formation of aberrant crypt foci and tumors induced by 1,2-dimethylhydrazine in rat colons. Biol. Pharm. Bull. 2007, 30, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Marinova, E.M.; Yanishlieva, N.V.; Kostova, I.N. Antioxidative action of the ethanolic extract and some hydroxycoumarins of Fraxinus ornus bark. Food Chem. 1994, 51, 125–132. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zou, L.B.; Lin, S.; Shi, J.G.; Zhu, H.B. Anti-apoptotic effect of esculin on dopamine-induced cytotoxicity in the human neuroblastoma SH-SY5Y cell line. Neuropharmacology 2007, 53, 724. [Google Scholar] [CrossRef] [PubMed]
- Shabanov, P.D.; Vislobokov, A.I. Neuronoprotective action of cortexin andcortagen. Rev. Clin. Pharmacol. Drug Ther. 2013, 11, 17. [Google Scholar] [CrossRef]
- Pan, Y.M. Antioxidant activity of ethanolic extract of Cortex fraxini and use in peanut oil. Food Chem. 2007, 103, 913–918. [Google Scholar] [CrossRef]
- Issa, Y.M.; El-Hawary, W.F.; Moustafa, M.E.; Refaat, M. ChemInform abstract: Spectroscopic studies on some new azo dyes derived from 4-methylesculetin and their biological activity. J. Indian Chem. Soc. 1997, 74, 777–780. [Google Scholar] [CrossRef]
- Duan, H.Q.; Zhang, Y.D.; Fan, K.; Suo, Z.W.; Hu, G.; Mu, X. Anti-inflammatory mechanism of esculetin. Chin. J. Vet. Med. 2007, 43, 45–46. [Google Scholar]
- Liu, S.Q.; He, L.; Peng, H. Effect of esculetin on osteoarthritis in rabbit. Med. J. Wuhan Univ. 2004, 9, 567–570. [Google Scholar]
- Wang, Z.Q.; Xia, Y. Protective effect of esculetin on acute myocardial ischemia reperfusion injury in rats. J. Chengdu Med. Coll. 2011, 6, 49–51. [Google Scholar]
- Kim, Y.; Park, Y.; Namkoong, S.; Lee, J. Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes. Food Funct. 2014, 5, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wang, L.; Wu, H. Bio-function summary of marine oligosaccharides. Int. J. Biol. 2010, 3, 74–86. [Google Scholar] [CrossRef]
- Dai, R.; Zheng, Q. L.; Xu, Q.S.; Zhu, W. Inhibitory effects of esculetin on proliferation of rat vascular smooth muscle cells in vitro and the underlying mechanism. Acta. Med. Univ. Sci. Technol. Huazhong 2009, 38, 239–242. [Google Scholar]
- Pan, S.L.; Huang, Y.W.; Guh, J.H.; Chang, Y.L.; Peng, C.Y.; Teng, C.M. Esculetin inhibits Ras-mediated cell proliferation and attenuates vascular restenosis following angioplasty in rats. Biochem. Pharmacol. 2003, 65, 1897–1905. [Google Scholar] [CrossRef]
- He, C.; Huang, Y.; Li, B.S.; Zhou, J.J.; Hu, L.; Guo, X.M. Effect of the esculetin on the cholesterol transport protein ABCA1 and ABCG1 in smooth muscle-derived foam cells. Guangdong Med. J. 2012, 33, 3368–3371. [Google Scholar]
- Wang, C.; Pei, A.; Chen, J.; Yu, H.; Sun, M.L.; Liu, C.F.; Xu, X. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J. Neurochem. 2012, 121, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Tsai, S.H.; Chen, C.S.; Chang, Y.C.; Lee, C.M.; Lai, Z.Y.; Lin, C.M. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem. Pharmacol. 2008, 75, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Lee, S.Y.; Lee, H.J.; Sim, G.S.; Kim, J.H.; Kim, J.H.; Cho, Y.H.; Lee, D.H.; Pyo, H.B.; Choe, T.B. Anti-oxidative and photo-protective effects of coumarins isolated from Fraxinus chinensis. Arch. Pharm. Res. 2007, 30, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tahara, S.; Takabayasi, F. Suppression of lipid hydroperoxide-induced oxidative damage to cellular DNA by esculetin. Biol. Pharm. Bull. 2003, 26, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Liang, M. Isolation and activity of antioxidant ingredients in Cortex fraxini. Sci. Technol. Food Ind. 2006, 27, 64–66. [Google Scholar]
- Gilani, A.H.; Janbaz, K.H.; Shah, B.H. Esculetin prevents liver damage induced by paracetamol and CCl4. Pharmacol. Res. 1998, 37, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Wang, C.J.; Tsai, Y.Y.; Liu, C.L.; Hwang, J.M.; Tseng, T.H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch. Toxic. 2000, 74, 467–472. [Google Scholar] [CrossRef]
- Hassan, H.A.; Yousef, M.I. Ameliorating effect of chicory (Cichorium intybus L.)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2010, 48, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Ham, J.R.; Lee, M.K. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem. Biol. Interact. 2016, 260, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chou, F.P.; Tseng, T.H.; Hsieh, M.H.; Lin, M.C.; Wang, C.J. Hibiscus protocatechuic acid or esculetin can inhibit oxidative ldl induced by either copper ion or nitric oxide donor. J. Agric. Food Chem. 2002, 50, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, D.; Ashokkumar, N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 2013, 95, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, D.; Ashokkumar, N. Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats. J. Funct. Foods 2012, 4, 776–783. [Google Scholar] [CrossRef]
- Duncan, S.H.; Leitch, E.C.; Stanley, K.N.; Richardson, A.J.; Laven, R.A.; Flint, H.J.; Stewart, C.S. Effects of esculin and esculetin on the survival of Escherichia coli O157 in human faecal slurries, continuous-flow simulations of the rumen and colon and in calves. Br. J. Nutr. 2004, 91, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Jang, J.Y.; Shim, J.H.; Myung, P.K.; Chae, J.I. Esculetin, a coumarin derivative, exhibits anti-proliferative and pro-apoptotic activity in G361 human malignant melanoma. Cancer Prev. 2015, 20, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Anand, J.R.; Rijhwani, H.; Malapati, K.; Kumar, P.; Saikia, K.; Lakhar, M. Anticancer activity of esculetin via-modulation of Bcl-2 and NF-κB expression in benzo[a]pyrene induced lung carcinogenesis in mice. Biomed. Prev. Nutr. 2013, 3, 107–112. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.L.; He, X.R.; Qiu, G.F.; Hu, X.M. Aesculetin-induced apoptosis through a ROS-mediated mitochondrial dysfunction pathway in human cervical cancer cells. J. Asian Nat. Prod. Res. 2010, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Della-Fera, M.A.; Baile, C.A. Esculetin induces mitochondria-mediated apoptosis in 3T3-L1 adipocytes. Apoptosis 2006, 11, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J. Esculetin, a coumarin derivative, suppresses adipogenesis through modulation of the AMPK pathway in 3T3-L1 adipocytes. J. Funct. Foods 2015, 12, 509–515. [Google Scholar] [CrossRef]
- Cao, W.Q.; Xue, J.F.; Shi, C.M.; Ding, C.F.; Zhu, X.G.; Liu, F.; Zhou, X.J. Synthesis of 6,7-Dihydroxy Coumarin. Fine Chem. Intermed. 2013, 43, 39–41. [Google Scholar]
- Zhang, T. Synthesis of 6,7-Dihydroxyeoumarin. Master’s Thesis, Nanjing University Science Technology, Nanjing, China, 2007. [Google Scholar]
- Yang, X.J.; Gao, H.H. Study on the synthesis of esculetin undermicrowave irradiation. Appl. Chem. Ind. 2011, 40, 627–629. [Google Scholar]
- Zhang, S.F.; Ma, J.H.; Chen, S.R.; Li, H.Y.; Xin, J.F. Improved synthesis technics of 6,7-dimethoxy coumarin. J. Hebei Univ. Sci. Technol. 2007, 28, 24–25. [Google Scholar]
- Zhao, L.J. Synthesis and research of 6,7-dimethoxycoumarin. Ph.D. Thesis, Nanjing University of Science and Technology, Nanjing, China, 9 June 2012. [Google Scholar]
- Oshima, N.; Narukawa, Y.; Takeda, T.; Kiuchi, F. Collagenase inhibitors from Viola yedoensis. J. Nat. Med. 2013, 67, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Yang, X.M. Extraction and Preparation of Natural Pharmaceutical Ingredients. Ph.D. Thesis, Beijing Medical University, Beijing, China, 7 July 2006. [Google Scholar]
- Demyttenaere, J.; Vervisch, S.; Debenedetti, S.; Coussio, J.; Maes, D.; Kimpe, N.D. Synthesis of virgatol (Ia) and virgatenol (Ib), two naturally occurring coumarins from Pterocaulon virgatum (L.) DC, and 7-(2,3-Epoxy-3-methylbutoxy)-6-methoxycoumarin (Ic), Isolated from Conyza obscura DC. Synth 2004, 11, 1844–1848. [Google Scholar] [CrossRef]
- Fang, Z.; He, G.L.; He, L. Synthesis of scopoletin with assisted microwave irradiation. West China J. Pharm. Sci. 2007, 22, 302–303. [Google Scholar]
- Adfa, M.; Yoshimura, T.; Komura, K.; Koketsu, M. Antitermite activities of coumarin derivatives and scopoletin from Protium javanicum Burm. f. J. Chem. Ecol. 2010, 36, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.A.; Lujan, C.; Hutchings, M.G.; Peter, Q. Application of the BHQ benzannulation reaction to the synthesis of benzo-fused coumarins. Tetrahedron Lett. 2009, 50, 3617–3620. [Google Scholar] [CrossRef]
- Nemoto, T.; Ohshima, T.; Shibasaki, M. Enantioselective total syntheses of (+)-decursin and related natural compounds using catalytic asymmetric epoxidation of an enon. ChemInform 2003, 59, 6889–6897. [Google Scholar]
- Chimichi, S.; Boccalini, M.; Cosimelli, B. A new convenient route to 2-oxoethoxycoumarins: Key intermediates in the synthesis of natural products. Tetrahedron 2002, 58, 4851–4858. [Google Scholar] [CrossRef]
- Cravotto, G.; Chimichi, S.; Robaldoa, B.; Boccalinib, M. Monoalkylation of dihydroxycoumarins via Mitsunobu dehydroalkylation under high intensity ultrasound. The synthesis of ferujol. Tetrahedron Lett. 2003, 44, 8383–8386. [Google Scholar] [CrossRef]
- Singh, M.M.; Gupta, D.N.; Wadhwa, V.; Jain, G.K.; Khanna, N.M.; Kamboj, V.P. Contraceptive efficacy and hormonal profile of ferujol: A new coumarin from Ferula jaeschkeana. Planta Med. 1985, 3, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Hang, C.T.; Doong, S.L.; Tsai, I.L.; Chen, I.S. Coumarins and anti-HBV constituents from Zanthoxylum schinifolium. Phytochemistry 1997, 45, 1419–1422. [Google Scholar]
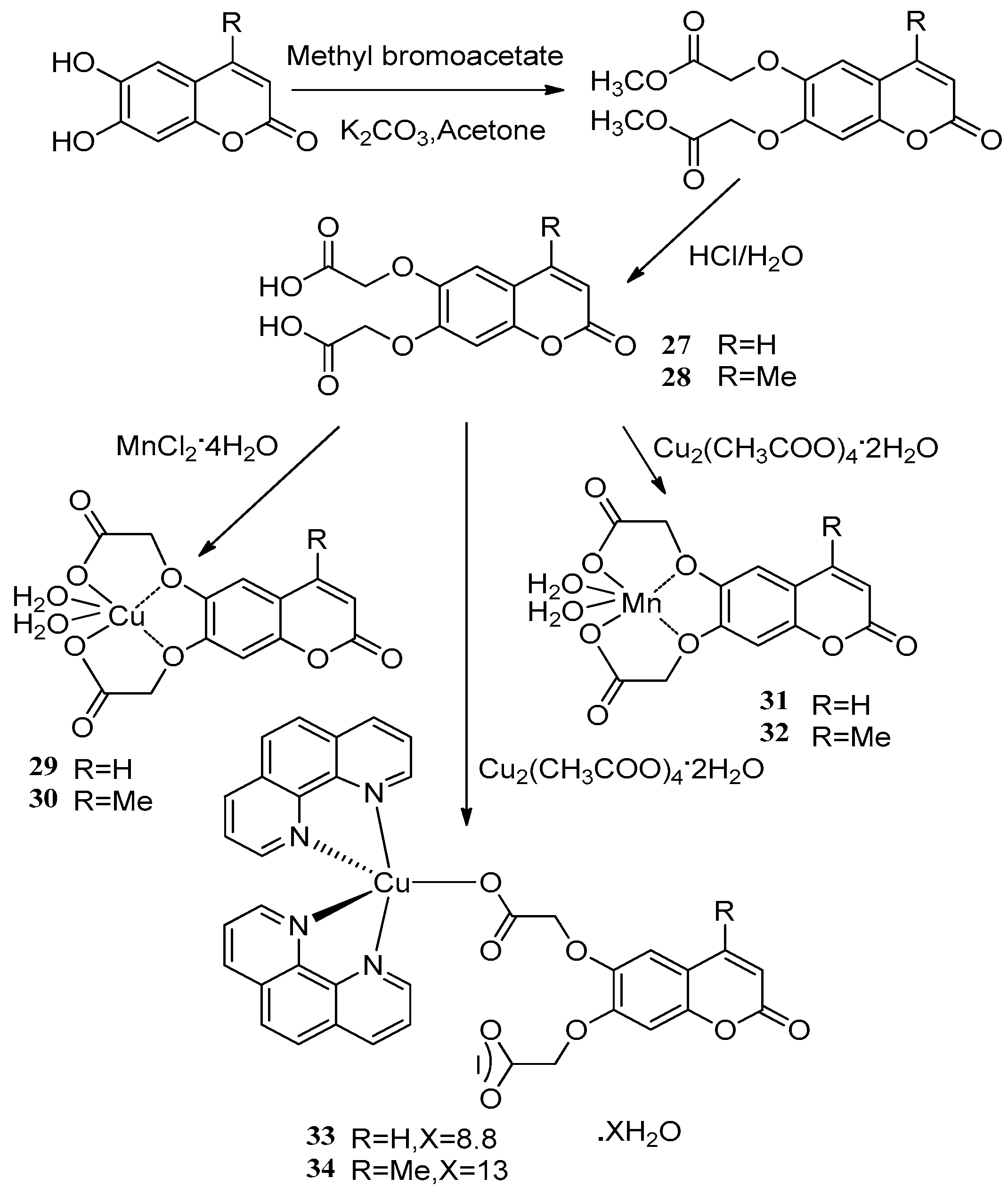
- Creaven, B.S.; Egan, D.A.; Karcz, D.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterisation and antimicrobial activity of copper(II)and manganese(II) complexes of coumarin-6,7-dioxyacetic acid (cdoaH2) and 4-methylcoumarin-6,7-dioxyacetic acid(4-Mecdoa H2): X-ray crystal structures of [Cu(cdoa)(phen)2] 8.8H2O and [Cu(4-Mecdoa)(phen)2]13H2O (phen = 1,10-phenanthroline). J. Inorg. Biochem. 2007, 101, 1108–1119. [Google Scholar] [PubMed]
- Hu, L.H.; Shen, X.; Jiang, H.L.; Zhang, Y.; Ma, L. Preparation of Furanocoumarin Derivatives for Treatment of Diabetes Mellitus. Ph.D. Thesis, Lanzhou Jiaotong University, Lanzhou, China, 24 June 2007. [Google Scholar]
- Bhardwaj, S.; Mishra, A.K.; Kaushik, N.K. Synthesis of some novel coumarinolignans newer catalyst for phenolic oxidative coupling. Trends Appl. Sci. Res. 2006, 1, 115–122. [Google Scholar]
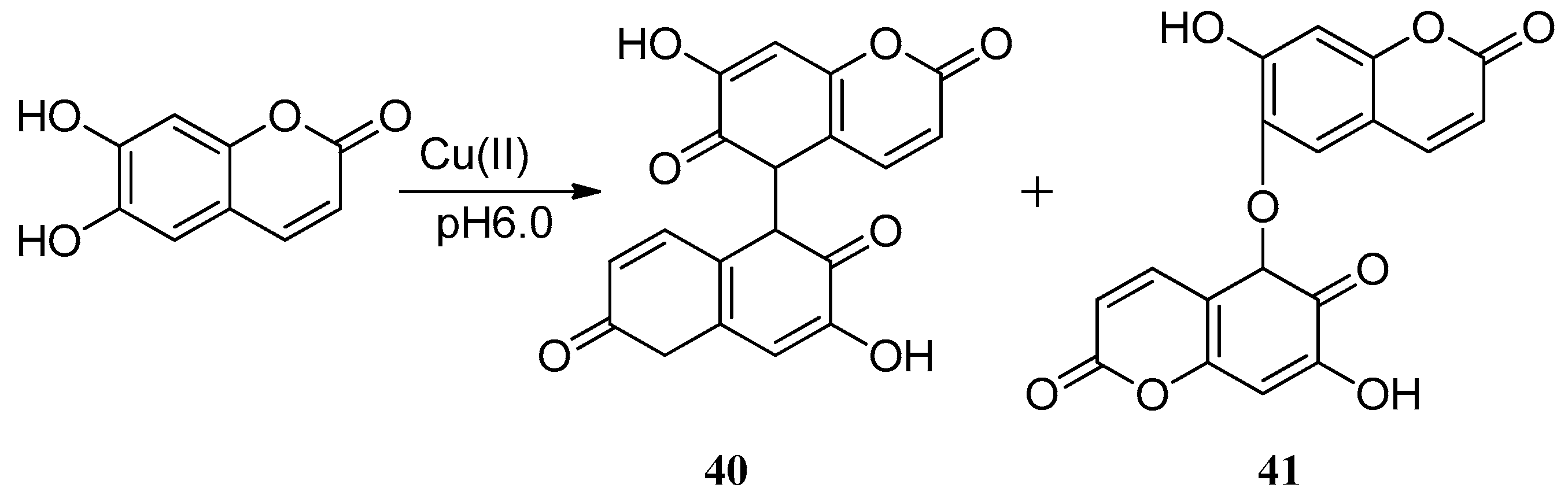
- Castaldi, P.; Garau, G.; Palma, A.; Deiana, S. Formation of biopolymers owing to the oxidation of esculetine by Cu(II)ions in a Ca-polygalacturonate network. J. Inorg. Biochem. 2012, 108, 30–35. [Google Scholar] [CrossRef] [PubMed]
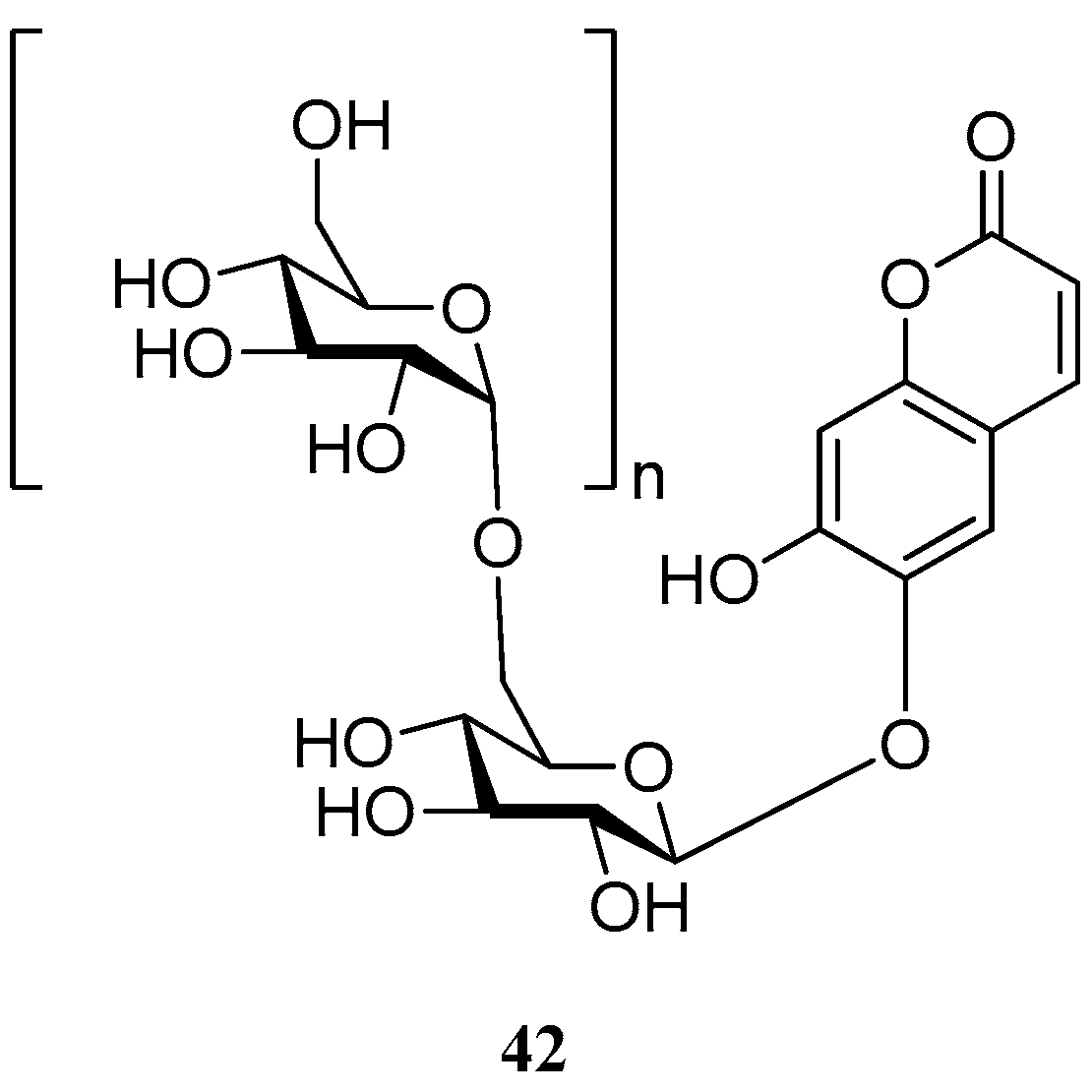
- Auriol, D.; Aurelie, G.; Fabrice, L.; Renaud, N. Hydrosoluble [6-O-α-d-Glcp-(1]n-6-O-β-d-Glcp-)1-phenolic Derivatives with Dermocosmetic, Nutritional and Therapeutic Applications, and Compositions Containing Said Water Soluble Compounds. EP2379576 A1, 26 October 2011. [Google Scholar]
- Pisani, L.; Catto, M.; Giangreco, I.; Leonetti, F.; Nicolotti, O.; Stefanachi, A.; Cellamare, S.; Carotti, A. Design, synthesis, and biological evaluation of coumarin derivatives tethered to an edrophonium-like fragment as highly potent and selective dual binding site acetylcholinesterase inhibitors. ChemMedChem 2010, 5, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
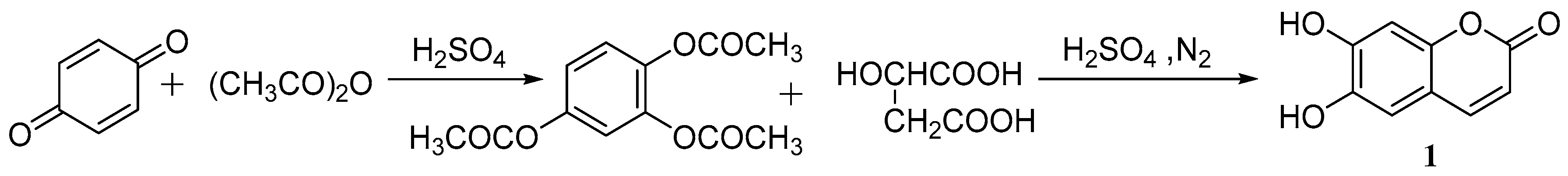
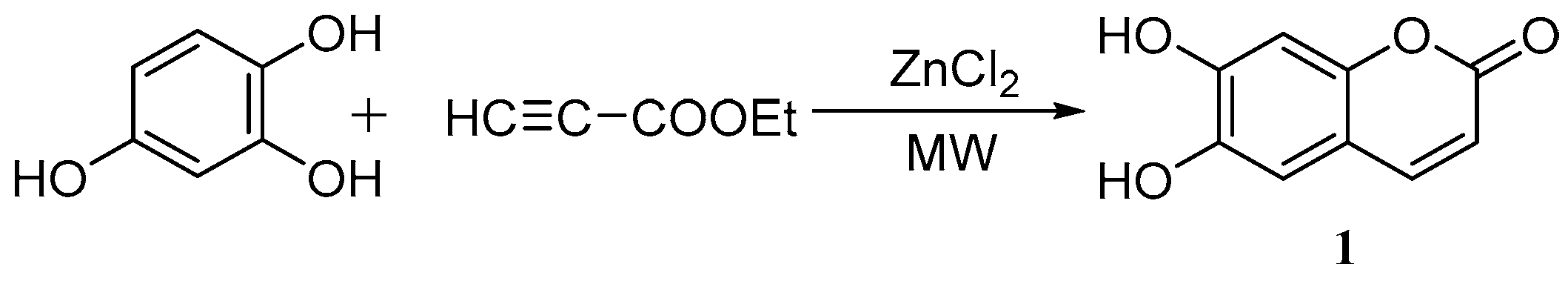

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules 2017, 22, 387. https://doi.org/10.3390/molecules22030387
Liang C, Ju W, Pei S, Tang Y, Xiao Y. Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules. 2017; 22(3):387. https://doi.org/10.3390/molecules22030387
Chicago/Turabian StyleLiang, Chengyuan, Weihui Ju, Shaomeng Pei, Yonghong Tang, and Yadong Xiao. 2017. "Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review" Molecules 22, no. 3: 387. https://doi.org/10.3390/molecules22030387
APA StyleLiang, C., Ju, W., Pei, S., Tang, Y., & Xiao, Y. (2017). Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules, 22(3), 387. https://doi.org/10.3390/molecules22030387