Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms
Abstract
:1. Introduction
2. Plant-Derived Alkaloids: A Fountain of Bioactive Agents
3. Emerging Cytotoxic Alkaloids: Apoptotic Strategies
3.1. DNA Damaging Alkaloids: A Useful Damage
3.2. Apoptotic Alkaloids: Caspase Activators
3.3. Anti-Proliferative Alkaloids: Cell Growth Inhibitors
3.3.1. Cell-Cycle Arrest
3.3.2. Alteration of the MAPK Pathway
3.3.3. Suppression of the NF-κB Pathway
3.4. Other Deadly Mechanisms: An Infinite Diversity
3.4.1. Formation of G-Quadruplexes
3.4.2. HER2 Targeting
3.4.3. Inhibition of the p-Glycoprotein ABCB1
4. Most Researched Alkaloids: A Comprehensive Molecular Machinery
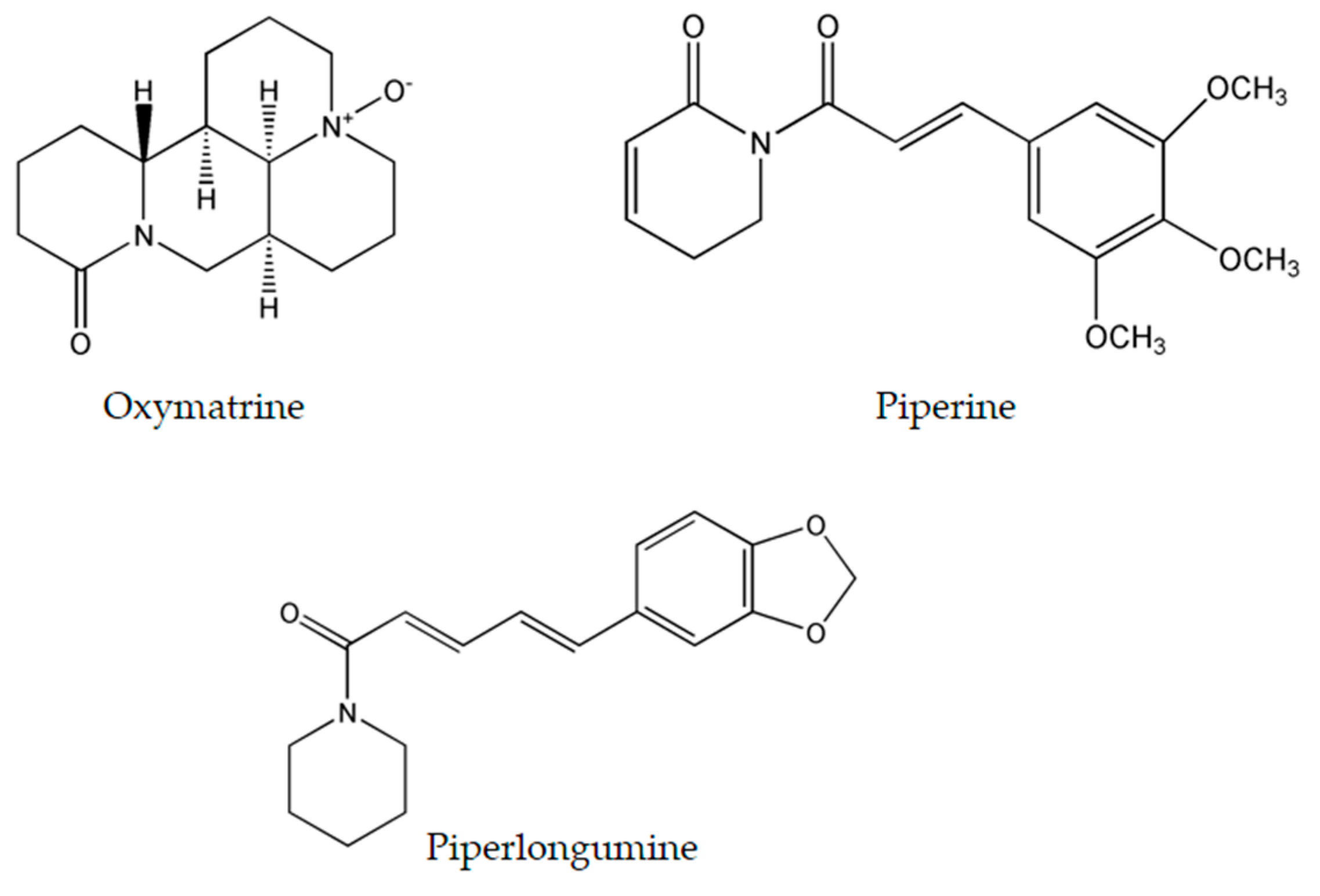
4.1. Oxymatrine
4.2. Piperine
4.3. Piperlongumine
5. Selectivity against Cancer Cells: The Trail to Non-Toxic Anticancer Agents
6. Future Perspectives
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Cavallo, F.; de Giovanni, C.; Nanni, P.; Forni, G.; Lollini, P.L. The immune hallmarks of cancer. Cancer Immunol. Immunother. 2011, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat. Rev. Drug Discov. 2008, 7, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J. Drug resistance and the microenvironment: Nature and nurture. Drug Resist. Updates 2003, 6, 169–172. [Google Scholar] [CrossRef]
- Halberstein, R.A. Medicinal Plants: Historical and Cross-Cultural Usage Patterns. Ann. Epidemiol. 2005, 15, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Dhanjal, J.K. Medicinal plants, a gold mine of anticancer compounds. Am. Int. J. Res. Formal Appl. Nat. Sci. 2015, 9, 14–23. [Google Scholar]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R. Impurity profiling of active pharmaceutical ingredients and finished drug products. Int. J. Drug Res. Technol. 2012, 2, 231–238. [Google Scholar]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant Defense Against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Vermaak, I.; Viljoen, A. From arrow poison to herbal medicine—The ethnobotanical, phytochemical and pharmacological significance of Cissampelos (Menispermaceae). J. Ethnopharmacol. 2014, 155, 1011–1128. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Zenk, M.H.; Juenger, M. Evolution and current status of the phytochemistry of nitrogenous compounds. Phytochemistry 2007, 68, 2757–2772. [Google Scholar] [CrossRef] [PubMed]
- Aniszewski, T. Alkaloids-Secrets of Life, 1st ed.; Elsevier: Oxford, UK, 2007; pp. 1–352. [Google Scholar]
- Mohan, K.; Jeyachandran, R. Alkaloids as anticancer agents. Ann. Phytomed. 2012, 1, 46–53. [Google Scholar]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Qu, X.X.; Wang, D.; Chen, X.X.; Tian, X.C.; Gao, F.; Zhou, X.L. Recent advances in design, synthesis and bioactivity of paclitaxel-mimics. Fitoterapia 2016, 110, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Said, R. Pharmacokinetic evaluation of vincristine for the treatment of lymphoid malignancies. Expert Opin. Drug Metab. Toxicol. 2014, 10, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef] [PubMed]
- Sobarzo-Sánchez, E. (Ed.) Alkaloids: Biosynthesis, Biological Roles and Health Benefits; Nova Sciences Publishers: Hauppauge, NY, USA, 2015.
- Xia, J.; Chen, J.; Zhang, Z.; Song, P.; Tang, W.; Kokudo, N. A map describing the association between effective components of traditional Chinese medicine and signaling pathways in cancer cells in vitro and in vivo. Drug Discov. Ther. 2014, 8, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Majid, N.A.; Hashim, N.M.; Rahman, M.A.; Hassan, Z.; Ali, H.M. Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression. Drug Des. Dev. Ther. 2015, 9, 1437–1448. [Google Scholar]
- Li, L.; Xu, Y.; Wang, B. Liriodenine induces the apoptosis of human laryngocarcinoma cells via the upregulation of p53 expression. Oncol. Lett. 2014, 15, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- DeBono, A.; Capuano, B.; Scammells, P.J. Progress Toward the Development of Noscapine and Derivatives as Anticancer Agents. J. Med. Chem. 2015, 58, 5699–5727. [Google Scholar] [CrossRef] [PubMed]
- Sajadian, S.; Vatankhah, M.; Majdzadeh, M.; Kouhsari, S.M.; Ghahremani, M.H.; Ostad, S.N. Cell cycle arrest and apoptogenic properties of opium alkaloids noscapine and papaverine on breast cancer stem cells. Toxicol. Mech. Methods 2015, 25, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Gooderham, N.J. Mechanisms of induction of cell cycle arrest and cell death by cryptolepine in human lung adenocarcinoma A549 cells. Toxicol. Sci. 2006, 91, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.A.; Sowa, Y.; Murata, H.; Takagi, K.; Nakanishi, R.; Aoki, S.; Yoshikawa, M.; Kobayashi, M.; Sakabe, T.; Kubo, T.; et al. The plant alkaloid cryptolepine induces p21WAF1/CIP1 and cell cycle arrest in a human osteosarcoma cell line. Int. J. Oncol. 2007, 31, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Laryea, D.; Isaksson, A.; Wright, C.W.; Larsson, R.; Nygren, P. Characterization of the cytotoxic activity of the indoloquinoline alkaloid cryptolepine in human tumour cell lines and primary cultures of tumour cells from patients. Investig. New Drugs 2009, 27, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Waziri, P.M.; Abdullah, R.; Yeap, S.K.; Omar, A.R.; Kassim, N.K.; Malami, I.; How, C.W.; Etti, I.C.; Abu, M.L. Clausenidin induces caspase-dependent apoptosis in colon cancer. BMC Complement. Altern. Med. 2016, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Schelz, Z.; Ocsovszki, I.; Bózsity, N.; Hohmann, J.; Zupko, I. Antiproliferative Effects of Various Furanoacridones Isolated from Ruta graveolens on Human Breast Cancer Cell Lines. Anticancer Res. 2016, 36, 2751–2758. [Google Scholar] [PubMed]
- Uche, F.I.; Drijfhout, F.P.; McCullagh, J.; Richardson, A.; Wen, L.W. Cytotoxicity Effects and Apoptosis Induction by Bisbenzylisoquinoline Alkaloids from Triclisia subcordata. Phytother. Res. 2016, 30, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Li, C.Y.; Jiang, M.M.; Li, D.; Wen, P.; Song, X.; Chen, J.D.; Guo, L.X.; Hu, X.P.; Li, G.Q.; et al. Induction of apoptosis in human leukemia cells through an intrinsic pathway by cathachunine, a unique alkaloid isolated from Catharanthus roseus. Phytomedicine 2016, 23, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, P.; Zhang, M.; Ma, F.; Su, L. Brucine inhibits the proliferation of human lung cancer cell line PC-9 via arresting cell cycle. Zhongguo Fei Ai Za Zhi 2014, 17, 444–450. [Google Scholar]
- Zheng, L.; Wang, X.; Luo, W.; Zhan, Y.; Zhang, Y. Brucine, an effective natural compound derived from nux-vomica, induces G1 phase arrest and apoptosis in LoVo cells. Food Chem. Toxicol. 2013, 58, 332–339. [Google Scholar] [CrossRef]
- Shu, G.; Mi, X.; Cai, J.; Zhang, X.; Yin, W.; Yang, X.; Li, Y.; Chen, L.; Deng, X. Brucine, an alkaloid from seeds of Strychnos nux-vomica Linn., represses hepatocellular carcinoma cell migration and metastasis: The role of hypoxia inducible factor 1 pathway. Toxicol. Lett. 2013, 222, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Looi, C.Y.; Paydar, M.; Cheah, F.K.; Leong, K.H.; Wong, W.F.; Mustafa, M.R.; Litaudon, M.; Awang, K. Subditine, a new monoterpenoid indole alkaloid from bark of Nauclea subdita (Korth.) Steud. Induces apoptosis in human prostate cancer cells. PLoS ONE 2014, 9, e87286. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.K.; Xu, M.Y.; Xu, G.S.; Zhang, Y.L.; Xu, Z.X. In Vitro and in Vivo Antitumor Activity of Scutebarbatine A on Human Lung Carcinoma A549 Cell Lines. Molecules 2014, 19, 8740–8751. [Google Scholar] [CrossRef] [PubMed]
- Safia; Kamil, M.; Jadiya, P.; Sheikh, S.; Haque, E.; Nazir, A.; Lakshmi, V.; Mir, S.S. The chromone alkaloid, Rohitukine, affords anti-cancer activity via modulating apoptosis pathways in A549 cell line and yeast mitogen activated protein kinase (MAPK) pathway. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, T.A.; Borralho, P.M.; Dewanjee, S.; Mulhovo, S.; Rodrigues, C.M.P.; Ferreira, M.J.U. Monoterpene bisindole alkaloids, from the African medicinal plant Tabernaemontana elegans, induce apoptosis in HCT116 human colon carcinoma cells. J. Ethnopharmacol. 2013, 149, 463–470. [Google Scholar] [CrossRef]
- Lou, C.; Yokoyama, S.; Saiki, I.; Hayakawa, Y. Selective anticancer activity of hirsutine against HER2positive breast cancer cells by inducing DNA damage. Oncol. Rep. 2015, 33, 2072–2076. [Google Scholar] [PubMed]
- Lou, C.; Takahashi, K.; Irimura, T.; Saiki, I.; Hayakawa, Y. Identification of Hirsutine as an anti-metastatic phytochemical by targeting NF-κB activation. Int. J. Oncol. 2014, 45, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Patima, A.; Chen, Y.; Zeng, F.; He, W.; Luo, L.; Jie, Y.; Zhu, Y.; Zhang, L.; Lei, J.; Xie, X.; Zhang, H. Cytotoxic effects of beta-carboline alkaloids on human gastric cancer SGC-7901 cells. Int. J. Clin. Exp. Med. 2015, 8, 12977–12982. [Google Scholar] [PubMed]
- Wang, K.B.; Li, D.H.; Hu, P.; Wang, W.J.; Lin, C.; Wang, J.; Lin, B.; Bai, J.; Pei, Y.H.; Jing, Y.K.; et al. A Series of β-Carboline Alkaloids from the Seeds of Peganum harmala Show G-Quadruplex Interactions. Org. Lett. 2016, 18, 3398–3401. [Google Scholar] [CrossRef] [PubMed]
- Zupkó, I.; Réthy, B.; Hohmann, J.; Molnár, J.; Ocsovszki, I.; Falkay, G. Antitumor activity of alkaloids derived from amaryllidaceae species. In Vivo (Brooklyn) 2009, 23, 41–48. [Google Scholar]
- Shih, Y.W.; Shieh, J.M.; Wu, P.F.; Lee, Y.C.; Chen, Y.Z. Alpha-tomatine inactivates PI3K/Akt and ERK signaling pathways in human lung adenocarcinoma A549 cells: Effect on metastasis. Food Chem. Toxicol. 2009, 47, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Wong, P.F.; Cheah, S.C. Alpha-Tomatine Induces Apoptosis and Inhibits Nuclear Factor-Kappa B Activation on Human Prostatic Adenocarcinoma PC-3 Cells. PLoS ONE 2011, 6, e18915. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2010, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, L.E. Cell Cycle Regulation and Hepatocarcinogenesis. Cancer Biol. Ther. 2016, 3, 1200–1207. [Google Scholar] [CrossRef]
- Dickson, M.A.; Schwartz, G.K. Development of cell-cycle inhibitors for cancer therapy. Curr. Oncol. 2009, 16, 36–43. [Google Scholar] [PubMed]
- Fruman, D.A.; Rommel, C. PI3K and Cancer: Lessons, Challenges and Opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Liu, J.; Wang, Y.; Zhang, B. Novel therapeutic strategies for patients with triple-negative breast cancer. Onco Targets Ther. 2016, 9, 6519–6528. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Joo, S.H. Downregulation of Reactive Oxygen Species in Apoptosis. J Cancer Prev. 2016, 21, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.A.; Witteveen, P.E.; Mergui-Roelvink, M.; Smith, D.A.; Lewis, L.D.; Mendelson, D.S.; Bang, Y.J.; Chung, H.C.; Dar, M.M.; Huitema, A.D.; et al. Pharmacodynamics and pharmacokinetics of oral topotecan in patients with advanced solid tumours and impaired renal function. Br. J. Clin. Pharmacol. 2014, 80, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Musa, F.; Blank, S.; Muggia, F. Drug Evaluation A pharmacokinetic evaluation of topotecan as a cervical cancer therapy. Expert Opin. Drug Metab. Toxicol. 2013, 9, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Nicum, S.J.; O’Brien, M.E.R. Topotecan for the treatment of small-cell lung cancer. Expert Rev. Anticancer Ther. 2007, 7, 795–801. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.R.; Eckardt, J.; Ramlau, R. Recent advances with topotecan in the treatment of lung cancer. Oncologist 2007, 12, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Mcilwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a0086562016. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, E.A.; Zamaraev, A.V.; Kopeina, G.S.; Zhivotovsky, B.; Lavrik, I.N. Role of the nucleus in apoptosis: Signaling and execution. Cell. Mol. Life Sci. 2015, 72, 4593–4612. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Hashemi, M.; Ande, S.R.; Yeganeh, B.; Xiao, W.; Eshraghi, M.; Bus, C.J.; Kadkhoda, K.; Wiechec, E.; Halayko, J.; et al. Apoptosis and cancer: Mutations within caspase genes. J. Med. Genet. 2009, 46, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Debatin, K.M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004, 53, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.A.; Yellen, P.; Xu, L.; Saqcena, M. Regulation of G1 Cell Cycle Progression: Distinguishing the Restriction Point from a Nutrient-Sensing Cell Growth Checkpoint(s). Genes Cancer 2010, 1, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.; Della Monica, R.; Grieco, D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016, 35, 153. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.; Roberts, T.G. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell. 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Holmsten, K.; Dohn, L.; Jensen, N.V.; Shah, C.H.; Jäderling, F.; Pappot, H.; Ullén, A. Vinflunine treatment in patients with metastatic urothelial cancer: A Nordic retrospective multicenter analysis. Oncol. Lett. 2016, 12, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Gourmelon, C.; Bourien, H.; Augereau, P.; Patsouris, A.; Frenel, J.-S.; Campone, M. Vinflunine for the treatment of breast cancer. Expert Opin. Pharmacother. 2016, 17, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Dongol, R.; Hewett, Y.; Shah, B.K.; Joseph, S.; Medical, R. Vincristine-induced blindness: A case report and review of literature. Anticancer Res. 2014, 6734, 6731–6733. [Google Scholar]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Weinberg, R.A. The Biology of Cancer, 2nd ed.; Garland Science: New York, NY, USA, 2013. [Google Scholar]
- Sundaram, V. RTK/Ras/MAPK signaling. WormBook 2006, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.B.A.; Alok, C. Nuclear factor-kappa B and cancer: Its role in prevention and therapy. Biochem. Pharmacol. 2002, 64, 883–888. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed. Res. Int. 2015, 2015, 413076. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, D.Y.; Okamoto, K. Structural insights into G-quadruplexes: Towards new anticancer drugs. Future Med. Chem. 2010, 2, 619–646. [Google Scholar]
- Neidle, S. Quadruplex Nucleic Acids as Novel Therapeutic Targets. J. Med. Chem. 2016, 59, 5987–6011. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A.; Hochhauser, D.; Boone, J.J.; Bhosle, J.; Tilby, M.J. Involvement of the HER2 pathway in repair of DNA damage produced by chemotherapeutic agents. Mol. Cancer Ther. 2009, 8, 3015–3023. [Google Scholar]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary metabolites inhibiting ABC transporters and reversing resistance of cancer cells and fungi to cytotoxic and antimicrobial agents. Frontiers in Microbiology. Front. Microbiol. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Li, D.; Zhang, L. Oxymatrine mediates Bax and Bcl-2 expression in human breast cancer MCF-7 cells. Pharmazie 2016, 71, 154–157. [Google Scholar] [PubMed]
- Li, J.; Jiang, K.; Zhao, F. Oxymatrine suppresses proliferation and facilitates apoptosis of human ovarian cancer cells through upregulating microRNA-29b and downregulating matrix metalloproteinase-2 expression. Mol. Med. Rep. 2015, 12, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, C.; Huang, W.; Guo, Y.; Xia, P.; Sun, X.; Pan, X. Oxymatrine inhibits the proliferation of prostate cancer cells in vitro and in vivo. Mol. Med. Rep. 2015, 11, 4129–4134. [Google Scholar]
- Huang, J.; Liang, L. Oxymatrine inhibits epithelial-mesenchymal transition through regulation of NF-κB signaling in colorectal cancer cells. Oncol. Rep. 2016, 36, 1333–1338. [Google Scholar]
- Zhu, Y.; Wang, B.; Han, Q. Oxymatrine inhibited cell proliferation by inducing apoptosis in human lung cancer A549 cells. Biomed. Mater. Eng. 2015, 26, S165–S172. [Google Scholar]
- Guo, B.; Zhang, T.; Su, J.; Wang, K.; Li, X. Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Cancer Chemother. Pharmacol. 2015, 75, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Su, B.S.; Chang, L.H.; Gao, Q.; Chen, K.L.; An, P.; Huang, C.; Yang, J.; Li, Z.F. Oxymatrine induces apoptosis in human cervical cancer cells through guanine nucleotide depletion. Anticancer Drugs 2014, 25, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Luo, J.; Lai, F.; Wang, Z.; Tong, H.; Lu, D.; Bu, H.; Zhang, R.; Lin, S. Antiangiogenic effects of oxymatrine on pancreatic cancer by inhibition of the NF-κB-mediated VEGF signaling pathway. Oncol. Rep. 2013, 30, 589–595. [Google Scholar] [PubMed]
- Liu, Y.; Bi, T.; Dai, W.; Wang, G.; Qian, L.; Gao, Q.; Shen, G. Effects of Oxymatrine on the Proliferation and Apoptosis of Human Hepatoma Carcinoma Cells. Technol. Cancer Res. Treat. 2016, 15, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.J.; Jin, B.; Chen, X.W.; Xie, J.; Xu, H.M.; Dong, P. Oxymatrine downregulates HPV16E7 expression and inhibits cell proliferation in laryngeal squamous cell carcinoma Hep-2 cells in vitro. Biomed. Res. Int. 2015, 2015, 150390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S.; Chen, J.; Ren, P.; Hu, Y.; Cao, Z.; Sun, H.; Ding, Y. Oxymatrine induces mitochondria dependent apoptosis in human osteosarcoma MNNG/HOS cells through inhibition of PI3K/Akt pathway. Tumour Biol. 2014, 35, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.W.; Qiu, M.K.; Qi, X.Q.; Dai, Y.X.; Wang, S.Q.; Quan, Z.W.; Liu, Y.B.; Ou, J.M. Oxymatrine suppresses proliferation and induces apoptosis of hemangioma cells through inhibition of HIF-1a signaling. Int. J. Immunopathol. Pharmacol. 2015, 28, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Zeng, J.; Gao, Y.; Li, F.; Li, W.; Zhou, H.; Yang, Y.; Wu, R.; Chen, Y.; Liu, J. Oxymatrine inhibits the proliferation of CaSki cells via downregulating HPV16E7 expression. Oncol. Rep. 2016, 36, 291–298. [Google Scholar] [PubMed]
- Bairagya, H.R.; Mukhopadhyay, B.P.; Bera, A.K. Role of salt bridge dynamics in inter domain recognition of human IMPDH isoforms: An insight to inhibitor topology for isoform-II. J. Biomol. Struct. Dyn. 2011, 29, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Piao, B.; Zhang, Y.; Hua, B.; Hou, W.; Xu, W.; Qi, X.; Zhu, X.; Pei, Y.; Lin, H. Oxymatrine diminishes the side population and inhibits the expression of β-catenin in MCF-7 breast cancer cells. Med. Oncol. 2011, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 2015, 54, 1070–1085. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, X.; Li, H.; Li, B.; Sun, L.; Xie, T.; Zhu, T.; Zhou, H.; Ye, Z. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 2015, 24, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.Y.; Zeng, L.H.; Pan, H.; Xu, L.H.; Wang, Y.; Liu, K.P.; He, X.H. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. 2013, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Liao, H.; Li, L.; Lin, Y. Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumour Biol. 2014, 35, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Historical Spice as a Future Drug: Therapeutic Potential of Piperlongumine. Curr. Pharm. Des. 2016, 22, 4151–4159. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Zhang, B.; Deng, C.; Cao, Y.; Zhou, F.; Wu, L.; Chen, M.; Shen, S.; Xu, G.; Zhang, S.; et al. Piperlongumine induces gastric cancer cell apoptosis and G2/M cell cycle arrest both in vitro and in vivo. Tumour Biol. 2016, 37, 10793–10804. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Song, J.; Kim, S.H.; Parikh, A.K.; Mo, X.; Palanichamy, K.; Kaur, B.; Yu, J.; Yoon, S.O.; Nakano, I.; et al. Piperlongumine treatment inactivates peroxiredoxin 4, exacerbates endoplasmic reticulum stress, and preferentially kills high-grade glioma cells. Neuro Oncol. 2014, 16, 135–164. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, U.; Eckols, T.K.; Kolosov, M.; Kasembeli, M.M.; Adam, A.; Torres, D.; Zhang, X.; Dobrolecki, L.; Wei, W.; Lewis, M.T.; et al. Drug-repositioning screening identified piperlongumine as a direct STAT3 inhibitor with potent activity against breast cancer. Oncogene 2015, 34, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Shen, Y.; Xu, X.; Yao, Y.; Fu, C.; Yan, Z.; Wu, Q.; Cao, J.; Sang, W.; Zeng, L.; et al. Piperlongumine selectively suppresses ABC-DLBCL through inhibition of NF-κB p65 subunit nuclear import. Biochem. Biophys. Res. Commun. 2015, 462, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Golovine, K.; Makhov, P.; Naito, S.; Raiyani, H.; Tomaszewski, J.; Mehrazin, R.; Tulin, A.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine and its analogs down-regulate expression of c-Met in renal cell carcinoma. Cancer Biol. Ther. 2015, 16, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, S.; Golovine, K.V.; Makhov, P.B.; Uzzo, R.G.; Kutikov, A.; Kolenko, V.M. Piperlongumine inhibits NF-κB activity and attenuates aggressive growth characteristics of prostate cancer cells. Prostate 2014, 74, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, H.; Kibble, K.; Zeng, H.; Moyer, M.P.; Reindl, K.M. Activation of ERK signaling and induction of colon cancer cell death by piperlongumine. Toxicol. Vitr. 2013, 27, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Xia, Y.; Ji, J.; Chen, W.; Zhang, J.; Chen, X.; Rajamanickam, V.; Chen, G.; Wang, Z.; Chen, L.; et al. Piperlongumine as a direct TrxR1 inhibitor with suppressive activity against gastric cancer. Cancer Lett. 2015, 375, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Park, J.; Kim, J.W.; Kwon, M.; Lee, B.H. Piperlongumine selectively kills cancer cells and increases cisplatin antitumor activity in head and neck cancer. Oncotarget 2014, 5, 9227–9238. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, Y.; Xu, G.; Yang, F.; Guan, Z.; Wang, M.; Fang, Y. Oxymatrine extracted from Sophora flavescens inhibited cell growth and induced apoptosis in human osteosarcoma MG-63 cells in vitro. Cell Biochem. Biophys. 2014, 70, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Bonavida, B.E.; Ng, C.P.; Jazirehi, A.; Schiller, G.A.; Mizutani, Y.O. Selectivity of TRAIL-mediated apoptosis of cancer cells and synergy with drugs: The trail to non-toxic cancer therapeutics (review). Int. J. Oncol. 1999, 15, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [PubMed]
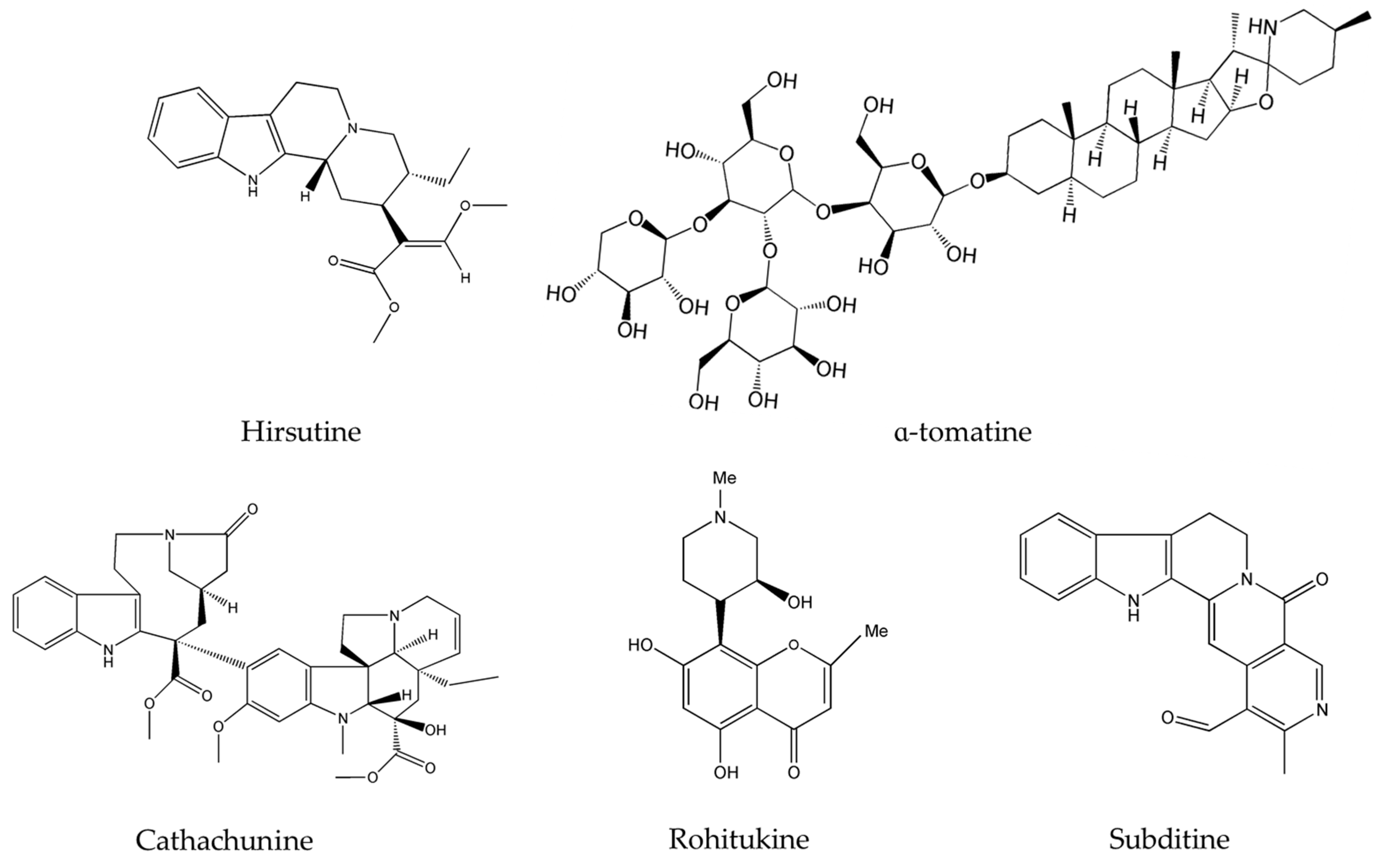
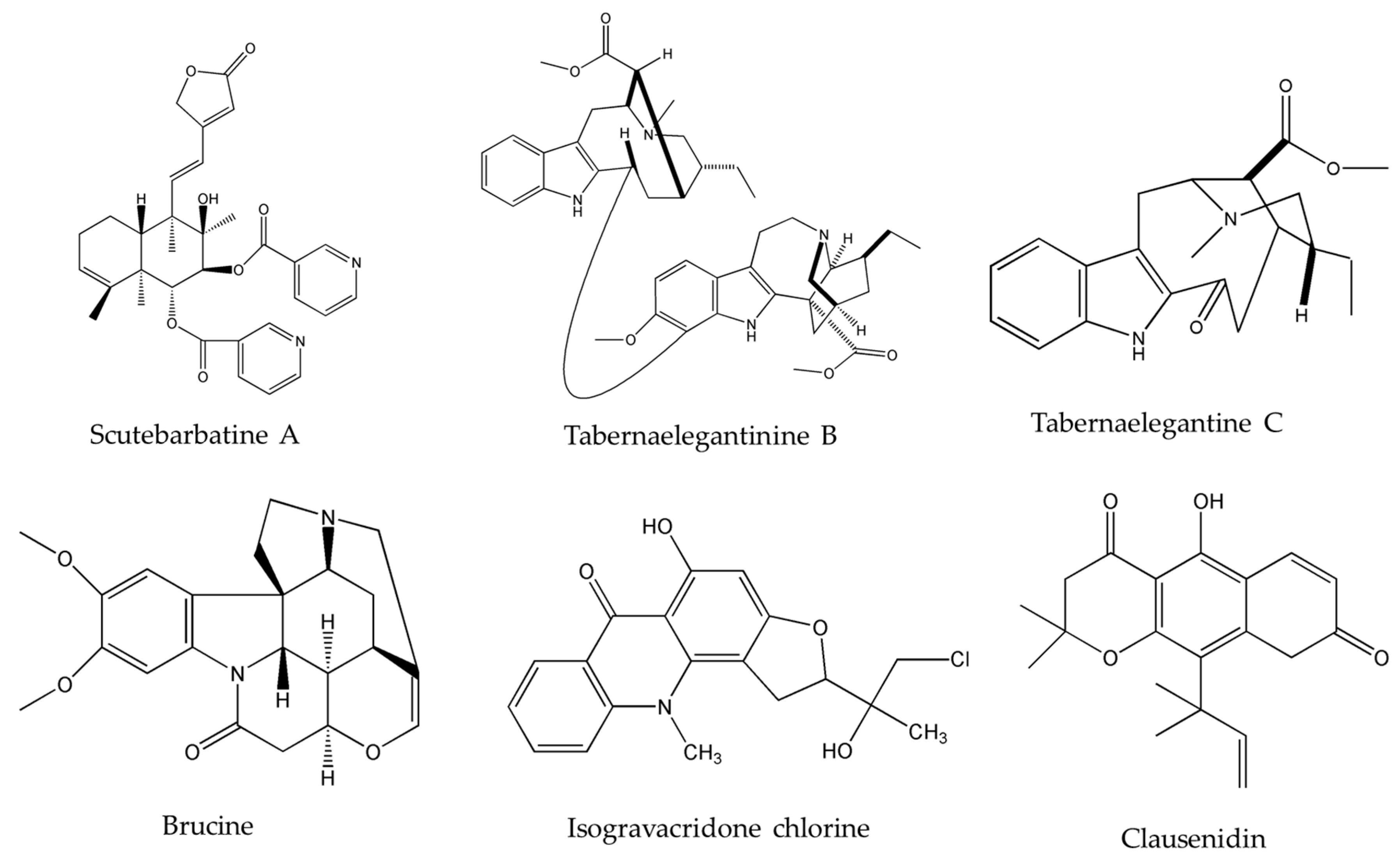
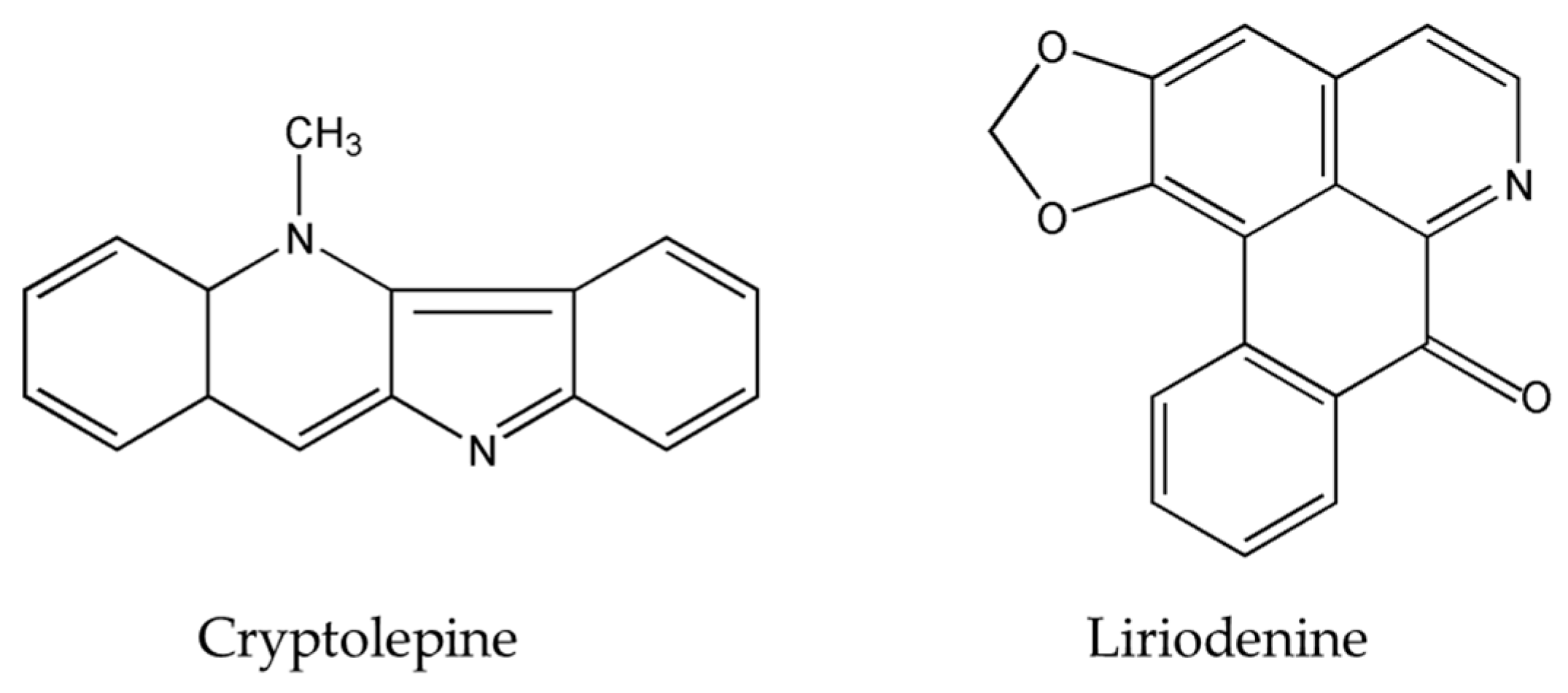
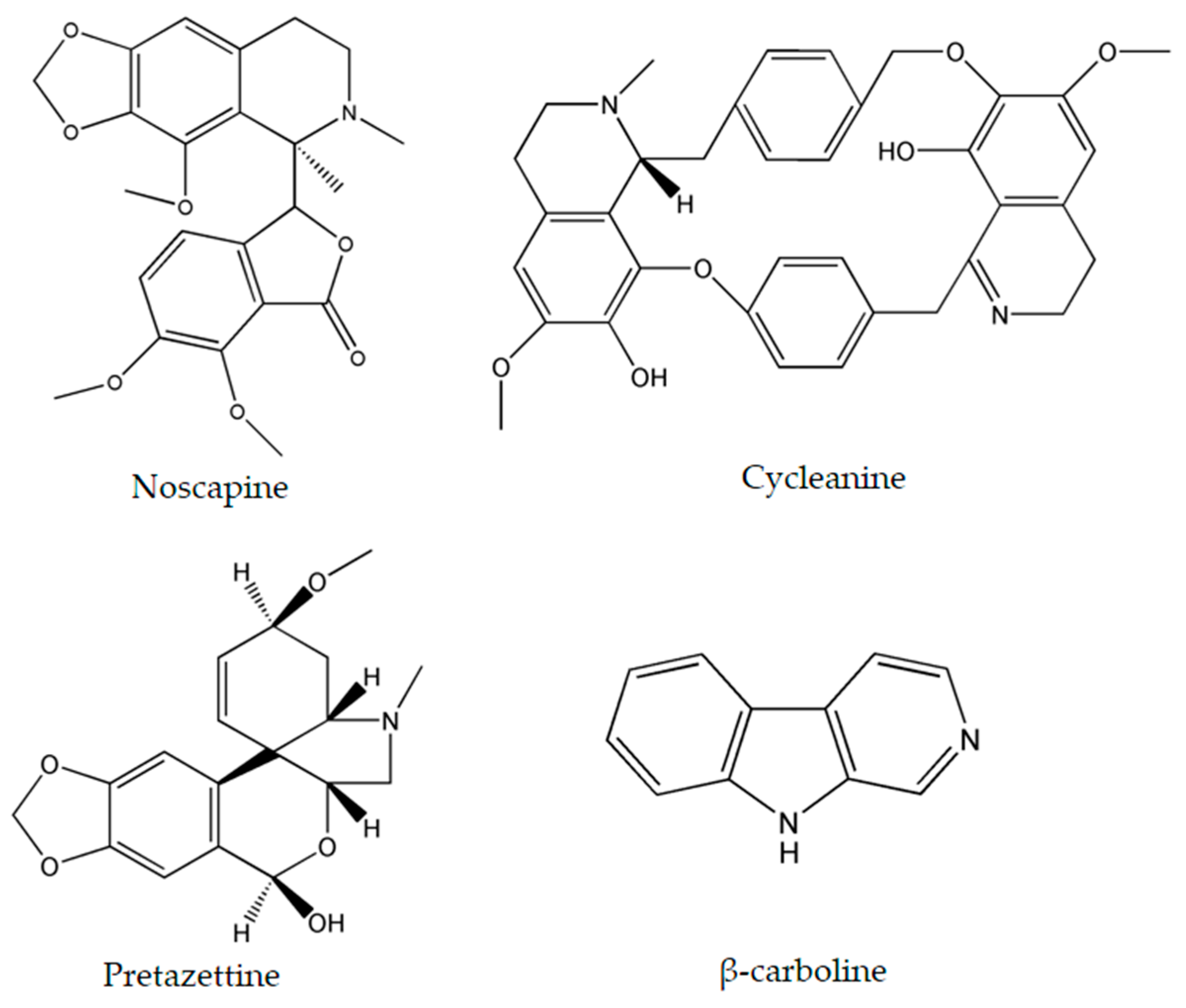
| Alkaloids | Plant Source | Type of Cancer | Cell Lines (IC50) | References |
|---|---|---|---|---|
| Liriodenine | Enicosanthellum pulchrum (King) Heusden | Ovarian | CAOV-3 (37.3 μM) | [25,26] |
| SKOV-3 (68.0 μM) | ||||
| laryngocarcinoma | HEp-2 (2.332 μM) | |||
| Noscapine | Papaver somniferum L. | glioma cell cancer | LN229 (70 μM) | [27,28] |
| A172 (20 μM) | ||||
| U251 (40 μM) | ||||
| neuroblastoma | SK-SY5Y, SH-EP1, SK-N-MC, SK-N-AS, LA1-55N, NB1643, NB1691, SK-N-SH, and IMR32 (IC50 range for all cell lines: from 21 to 100 μM) | [27,28] | ||
| cervical | HeL | |||
| Ca Ski | ||||
| colon | Caco-2 | |||
| T84 | ||||
| ovarian | SK-OV-3 | |||
| and SigC | ||||
| prostate | DU145 | |||
| human lymphoblast | CEM (14.5 μM) | |||
| human cervix | HeLa (24.0 μM) | |||
| lung adenocarcinoma | A549 (72.9 μM) | |||
| breast epithelial | MCF-7 (42.3 μM) | |||
| breast | MDA-MB-231 (20.15 µM) | |||
| MCF-7 (15.47 µM) | ||||
| Cryptolepine | Cryptolepis sanguinolenta Sida acuta Brum.f. Sida cordifolia L. | lung adenocarcinoma | A549 | [29,30,31] |
| Osteosarcoma | MG63 | |||
| T-cell leukemia | CCRF-CEM | |||
| CEM/VM-1 | ||||
| multiple myeloma | RPMI 8226-S | |||
| 8226/Dox | ||||
| 8226/LR5 | ||||
| histiocytic lymphoma | U-937-GTB | |||
| U-937/Vcr | ||||
| small cell lung cancer | NCI-H69 | |||
| H69/AR | ||||
| renal adenocarcinoma | ACHN | |||
| cervical adenocarcinoma | HeLa | |||
| immortalized normal retinal epithelial cells | hTERT-RPE (mean IC50 of all cell lines: 0.9 μM) | |||
| Clausenidin | Clausena excavata Burum.f. | colon | HT-29 (13.8 μg/mL) | [32] |
| Isogravacridone chlorine | Ruta graveolens L. | breasts | MDA-MB-231 (2.27 μM) | [33] |
| Cycleanine | Triclisia subcordata Oliv. | ovarian | Ovcar-8 (10 μM) | [34] |
| A2780 (7.6 μM) | ||||
| Ovcar-4 (7.2 μM) | ||||
| Igrov-1 (14 μM) | ||||
| Cathachunine | Catharanthus roseus (L.) G.Don. | leukemia | HL60 (9.1 μM) | [35] |
| K562 (9.3) μM | ||||
| Brucine | Strychnos nux-vomica L. | lung | PC-9 | [36,37,38] |
| hepatocellular carcinoma | HepG2 | |||
| SMMC-7721 | ||||
| colon | LoVo (15.1 μM) | |||
| lung | PC-9 | |||
| Subditine | Nauclea subdita (Korth.) Steud. | prostate | LNCaP (12.24 µM) | [39] |
| PC-3 (13.97 µM) | ||||
| Scutebarbatine-A (SBT-A) | Scutellaria barbata D.Don. | lung | A549 (39.21 μg/mL) | [40] |
| Rohitukine | Dysoxylum binectariferum Hook.f. | breast | T47D (50 µM), and | [41] |
| MIDAMB273 (3 µM) | ||||
| MCF7 (15 µM) | ||||
| ovarian | SKOV3 (20 µM) | |||
| lung | A549 (40 µM) | |||
| Tabernaelegantine C | Tabernaemontana elegans Stapf | colon | HCT116 (20 µM) | [42] |
| Muntafara sessilifolia Baker | ||||
| Tabernaelegantinine B | Tabernaemontana elegans Stapf | colon | HCT116 (20 µM) | [42] |
| Muntafara sessilifolia Baker | MRC-5 (0.47 µM) | |||
| Hirsutine | Plants of genus Uncaria | human breast | MDA-MB-453 | [43,44] |
| mouse mammary carcinoma | BT474 | |||
| 4T1 | ||||
| β-carboline | Peganum harmala L. | human promyelocytic leukemia | HL-60 (3.48 μg/mL) | [45,46] |
| prostate | PC-3 (10.59 μg/mL) | |||
| gastric | SGC-7901 (11.53 μg/mL) | |||
| Pretazettine | Amaryllidaceae (genus Amaryllis L.) | breast | MCF7 (7.869 µM) | [47] |
| cervical | HeLa (8.853 µM) | |||
| skin epidermoid carcinoma | A431 (5.373 µM) | |||
| α-tomatine | Lycopersicon esculentum Mill. | human lung adenocarcinoma | A549 cells | [48,49] |
| human prostatic adenocarcinoma | PC-3 Cells (1.67 µM) |
| Alkaloid | Type of Cancers It Protects against | Exact Pathway | References |
|---|---|---|---|
| Cathachunine | leukemia | ↑ROS levels | [35] |
| Subditine | prostate | ↑ROS levels | [39] |
| Rohitukine | breast, ovarian, lung | ↑ROS levels | [41] |
| Hirsutine | human, breast, cancer, mouse mammary carcinoma | Damaging DNA | [43,44] |
| ↑γH2AX | |||
| Suppression of Akt Pathways |
| Alkaloid | Mechanisms of Action | References |
|---|---|---|
| Liriodenine | Cleavage of caspases-3 and -9 | [25] |
| Efflux of cytochrome c | ||
| ↑Bax, ↑p53 expression, ↓Bcl-2 and ↓survivin | ||
| Cryptolepine | ↑p53 and p21Cip1/WAF1 | [29,31] |
| Clausenidin | Cleavage of caspases-3 and -9 | [32] |
| Efflux of cytochrome c | ||
| ↑Bax and ↑Apaf-1 | ||
| Isogravacridone chlorine | Cleavage of caspase-9 | [33] |
| Cathachunine | Cleavage of caspases-3, -9 and PARP | [35] |
| Disruption of mitochondrial membrane potential | ||
| Efflux of cytochrome c | ||
| activation of caspases-3 and -9 | ||
| ↑Bax and ↓Bcl-2 | ||
| Brucine | ↑Bax and ↓Bcl-2 expression | [37] |
| Subditine | Cleavage of caspases-3 and -9 | [40] |
| Efflux of cytochrome c | ||
| ↑Bax, ↑p53 expression, ↓Bcl-2, and ↓Bcl-x | ||
| Scutebarbatine A (SBT-A) | Cleavage of caspases-3 and -9 | [40] |
| Efflux of cytochrome c | ||
| ↑Bax and ↓Bcl-2 | ||
| Rohitukine | Cleavage of caspases-3 and -9 | [41] |
| Efflux of cytochrome c | ||
| ↓Bcl-2 | ||
| Tabernaelegantinine B | Cleavage of caspases-3 and -8 | [42] |
| Tabernaelegantine C |
| Alkaloid | Plant Source | Type of Cancer | Cell Lines (IC50/ED50) | Mechanism of Action | References |
|---|---|---|---|---|---|
| Oxymatrine | Sophora flavescens Ait. | breast | MCF7 | ↑Bax and ↓Bcl-2 | [82] |
| ovarian | OVCAR-3 | Cleavage of caspase-3, ↑miR-29b and ↓matrix metalloproteinase-2 (MMP2) | [83] | ||
| prostate | DU145, PC-3 | ↑Bax, ↑p53, and ↓Bcl-2 | [84] | ||
| colorectal | RKO HCT116 SW480 | Regulation of EMT markers (↑E-cadherin, ↓Snail and ↓N-cadherin) Inhibition of NF-κB activation, ↓p65 | [85] | ||
| lung | A549 | ↑Bax and ↓Bcl-2 | [86] | ||
| gastric | MKN-45 BGC823 SGC7901 HEK293 | G1 cell cycle arrest Disruption of mitochondrial membrane potential Inhibition of EGFR (p-Tyr845) ↓CyclinD1, ↓CDK4/6 ↑Bax and ↓Bcl-2 ↑ caspases-3 and -9 mRNA level ↓phospho-Cofilin (Ser3), phospho-LIMK1 (Thr508) levels, and ↓MMP2 | [87] | ||
| cervical | CaSki | G0/G1 and S cell cycle arrest ↓HPV16E7 | [94] | ||
| cervical | HeLa | ↓IMPDH2 ↓intracellular GTP | [88] | ||
| human hepatoma carcinoma | Hep-G2 (1.32 mg/mL) SMMC-7721 (1.21 mg/mL) | ↑Bax and ↓Bcl-2 and ↑caspase-3 mRNA level | [90] | ||
| laryngeal squamous cell carcinoma | Hep-2 (7 mg/mL) | G0/G1 cell cycle arrest ↓HPV16E7 gene | [91] | ||
| Pancreatic | PANC-1 (1 mg/mL) | Inhibition of NF-κB activity, ↓VEGF | [89] | ||
| osteosarcoma | MNNG/HOS (72.50 μg/mL) | ↑Bax and ↓Bcl-2 Disruption of mitochondrial membrane potential Efflux of cytochrome c Cleavage of caspases-3 and -9 Inactivation of PI3K/Akt pathway ↑Bax and ↓Bcl-2 | [92] | ||
| osteosarcoma | MG-63 (0.75 mg/mL) | Disruption of mitochondrial membrane potential Cleavage of caspases-3 and -9 ↑Bax and ↓Bcl-2 | [116] | ||
| hemangioma | HDEC | ↓HIF-1ɑ, ↓VEGF, ↑Bax and ↑p53, and ↓Bcl-2 G0/G1 cell cycle arrest and ↓cyclinD1 | [93] | ||
| breast | MCF-7 | ↓SP and ↓Wnt/β-catenin signaling pathways | [96] | ||
| Piperine | Piper nigrum L. Piper longum L. | colon | CaCo-2 (54 μM) SW480 (126 μM) HCT116 (118 μM) HT-29 (53 μM) | G1 cell cycle arrest Disruption of mitochondrial membrane potential Cleavage of caspases-3, -9 and PARP ↑ROS Induction of endoplasmic reticulum stress | [98] |
| triple-negative breast cancer | MDA-MB-468 T-47D MCF-7 | Disruption of mitochondrial membrane potential Efflux of cytochrome c G1/S and G2/M cell cycle arrest Induction of caspase-dependent apoptosis ↓p-Akt, ↑p21Waf1/Cip1 ↓MMP-2/-9 mRNA levels | [99] | ||
| HER2-overexpressing breast cancer | SKBR3 (50 μM) BT-474 (50 μM) MCF-7 (200 μM) MDA-MB-231 | Cleavage of caspase-3 and PARP ↓SREBP-1 and ↓FAS mRNA levels ↓HER2 Inhibition of Akt, MAPK, AP-1 and NF-κB activation Suppression of migration | [104] | ||
| osteosarcoma | HOS (72 μM) U2OS (126 μM) | G2/M cell cycle arrest ↓cyclinB1, ↑p-CDK1, ↑p-Chk2 Inhibition of p-Akt Activation of c-JNK, p38MAPK- ↑TIMP-1/-2 and ↓MMP-2/-9 | [100] | ||
| prostate | DU145 (74.4 μM) PC-3 (226.6 μM) LNCaP (111 μM) | G0/G1 cell cycle arrest Cleavage of caspase-3 Induction of autophagy via ↑LC3B-II and formation of LCb3 puncta ↑p21Cip1, ↑p27Kip1, ↓cyclin D1, ↓cyclin A | [101] | ||
| lung | A549 (122 μg/mL) | G2/M phase cell cycle arrest Cleavage of caspases-3 and -9 ↑Bax, ↑p53, and ↓Bcl-2 | [102] | ||
| rectal | HRT-18 | G0/G1cell cycle arrest ↑ROS | [103] | ||
| Piperlon-gumine | Piper longum L. | gastric | SGC-7901 (2.3 μM) BGC-823 (3.9 μM) KATO III: (6.0 μM) | G2/M cell cycle arrest ↓MDM-2, ↓Cyclin B1, and ↓Cdc2 ↑ROS and ↓TrxR1 Cleavage of caspase-3 ↑Bax and ↓Bcl-2 Induction of ROS-dependent endoplasmic reticulum stress Induction of ROS-dependent mitochondrial dysfunction | [113] |
| head and neck | AMC-HN SNU HN30 HN31 | ↑ROS ↑p53, ↑PUMA, ↑p-JNK, ↓GSTP1 and ↑p21Waf1/Cip1 Cleavage of PARP | [114] | ||
| glioma | HGG 1123 HGG MD13 | ↑ROS levels ↓PRDX4 | [107] | ||
| large B cell lymphoma ABC-DLBCL | OCI-Ly10 U2932 DB | Inhibition of TNF-α and p65 nuclear import ↓NF-κB activity ↓survivin, Bcl-2, ↑Bax, nd ↑p21 Cleavage of caspases-3 and -9 | [109] | ||
| breast myeloid leukemia | MCF7 (0.9 μM) MDA-MB-453 (0.9 μM) T-47D (2.7 μM), Kasumi-1 (3.7 μM) | ↓ p-STAT3 Inhibition of STAT3 binding to its immobilized phosphopeptide ligand Cleavage of caspase-3 ↑p53, ↓survivin, ↓Bcl-2, ↓Bcl-x, ↓XIAP, and ↓CIAP mRNA levels | [108] | ||
| gastric | AGS HGC27 | ↑ROS Cleavage of caspases-3, -7, -9 and PARP G2/M cell cycle arrest and ↑GADD45ɑ ↓CyclinB1, ↓cdc2, ↓XIAP, and ↑p21 ↓telomerase reverse transcriptase gene Induction of endoplasmic reticulum stress | [106] | ||
| renal carcinoma | 786-O PNX0010 (ED50: 1.6, 2.3 μM respectively) | ↓c-Met ↑ROS Inhibition of Erk/MAPK, STAT3, Akt/mTOR and NF-κB pathways | [110] | ||
| prostate | PC-3 DU-145 (ED50: 4.9, 3.4 μM respectively) | Inhibition of TNF-α and p65 nuclear import Inhibition of NF-κB pathway ↓Il-6, ↓IL-8, ↓MMP-9 Inhibition surface expression of ICAM-1 | [111] | ||
| colon | HT-29 (10.1 μM) HCT 116 (6.4 μM) | Cleavage of caspase-3 Induction of ERK signaling pathway via ↑p-ERK | [112] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms. Molecules 2017, 22, 250. https://doi.org/10.3390/molecules22020250
Habli Z, Toumieh G, Fatfat M, Rahal ON, Gali-Muhtasib H. Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms. Molecules. 2017; 22(2):250. https://doi.org/10.3390/molecules22020250
Chicago/Turabian StyleHabli, Zeina, Georgio Toumieh, Maamoun Fatfat, Omar Nasser Rahal, and Hala Gali-Muhtasib. 2017. "Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms" Molecules 22, no. 2: 250. https://doi.org/10.3390/molecules22020250
APA StyleHabli, Z., Toumieh, G., Fatfat, M., Rahal, O. N., & Gali-Muhtasib, H. (2017). Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms. Molecules, 22(2), 250. https://doi.org/10.3390/molecules22020250






