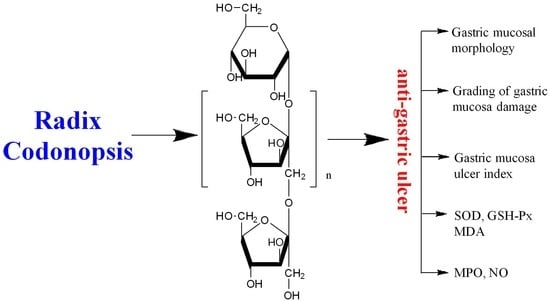
Structure Features and Anti-Gastric Ulcer Effects of Inulin-Type Fructan CP-A from the Roots of Codonopsis pilosula (Franch.) Nannf.
Abstract
:1. Introduction
2. Results and Discussion
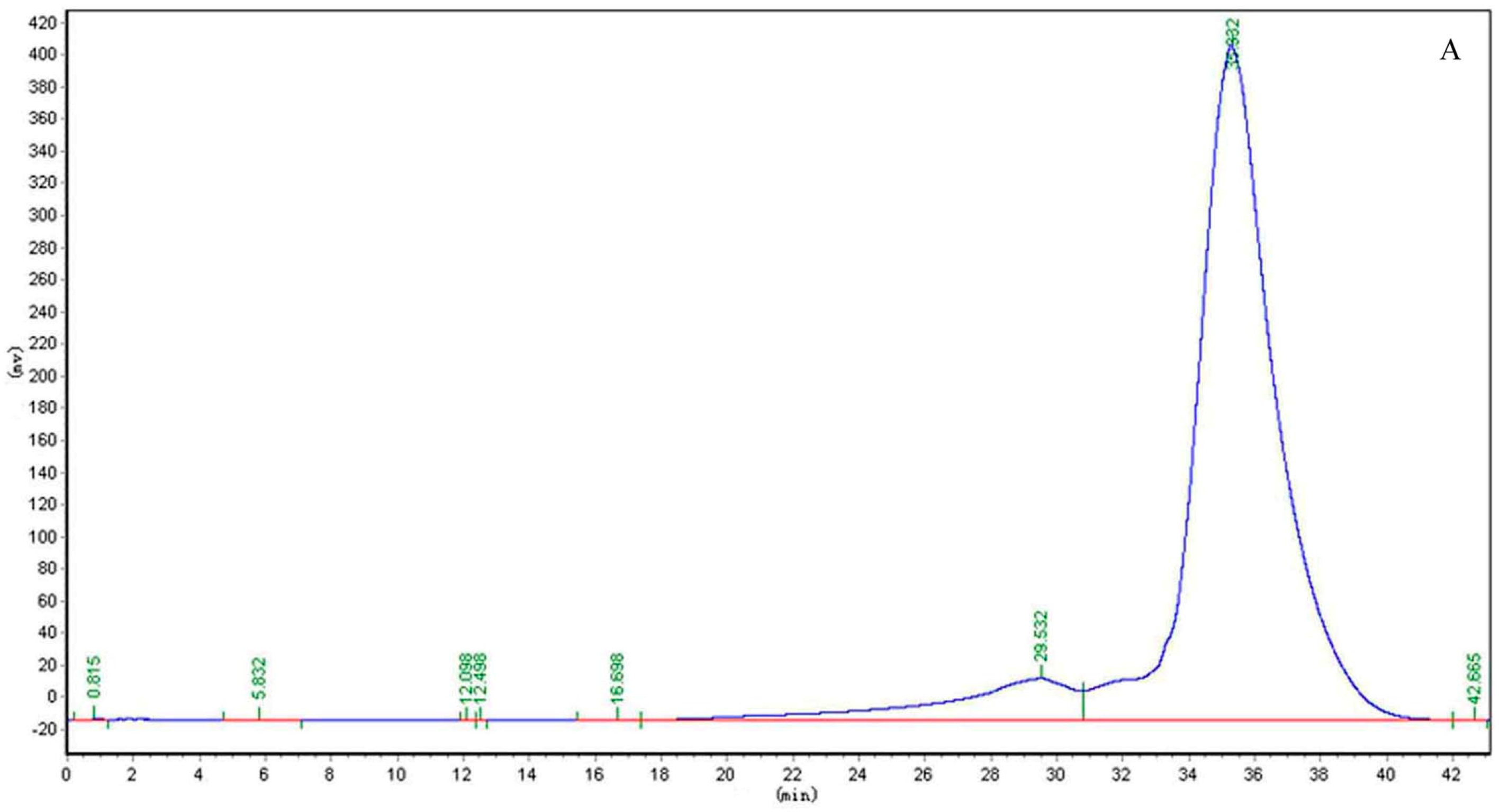
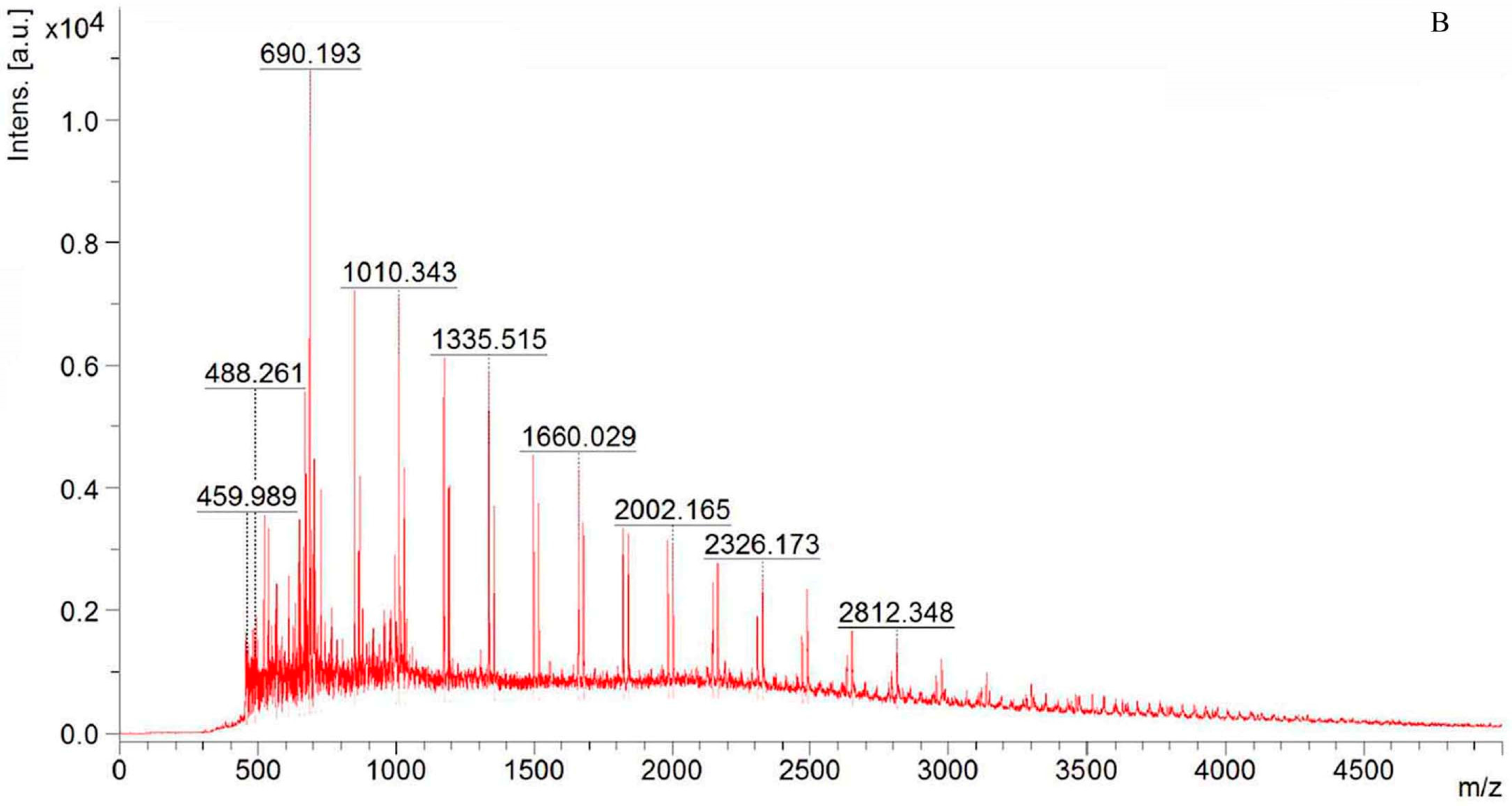
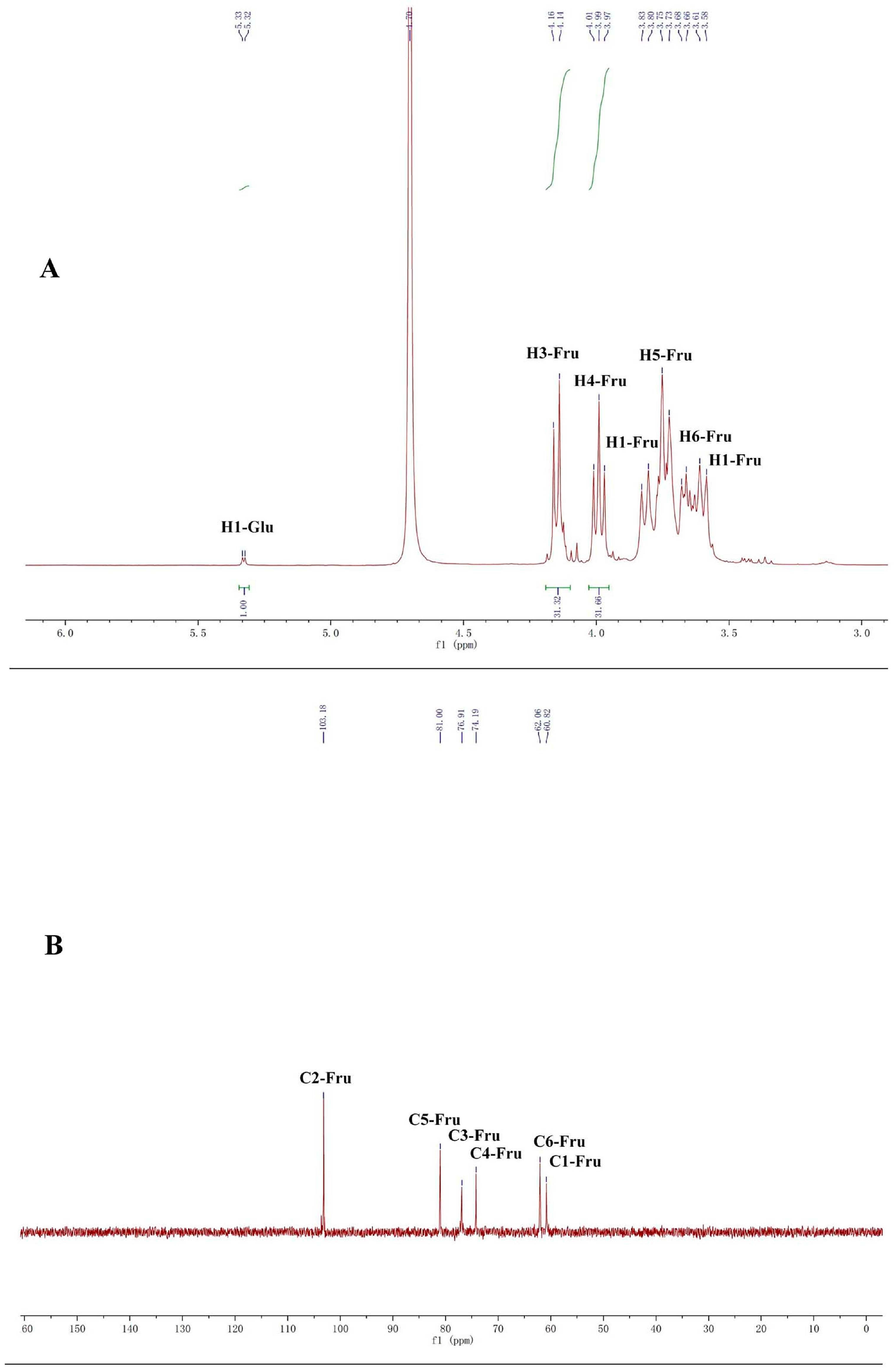
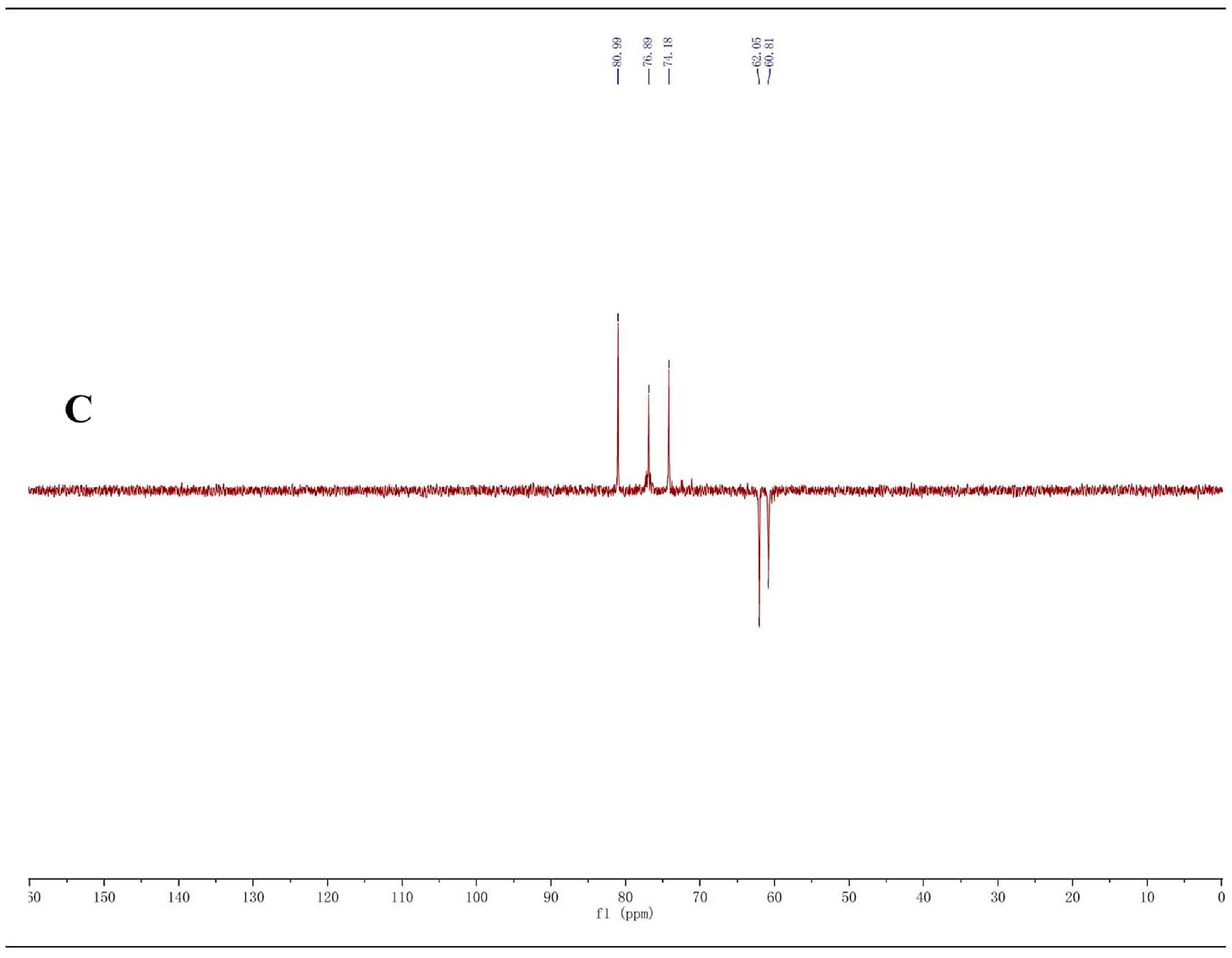
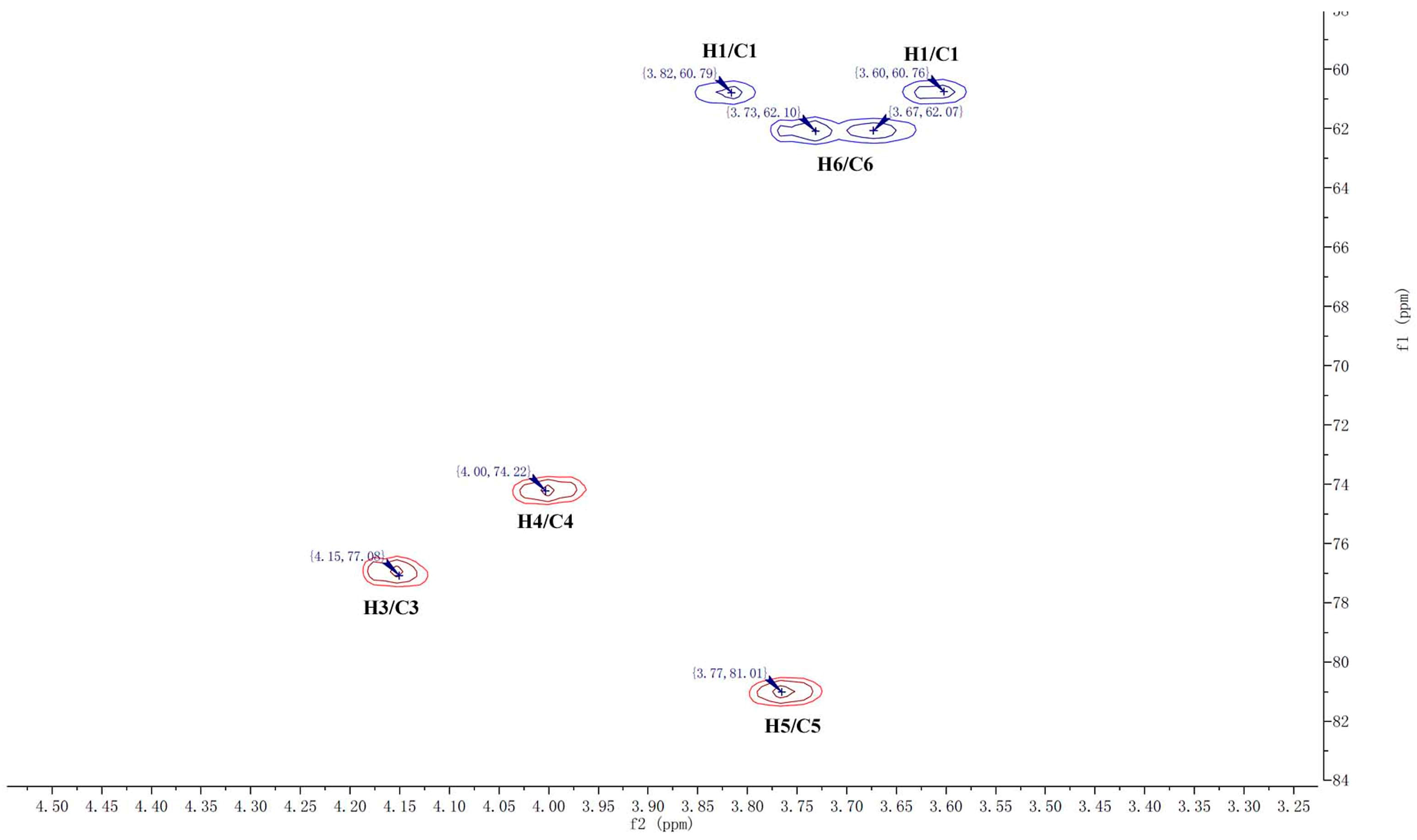
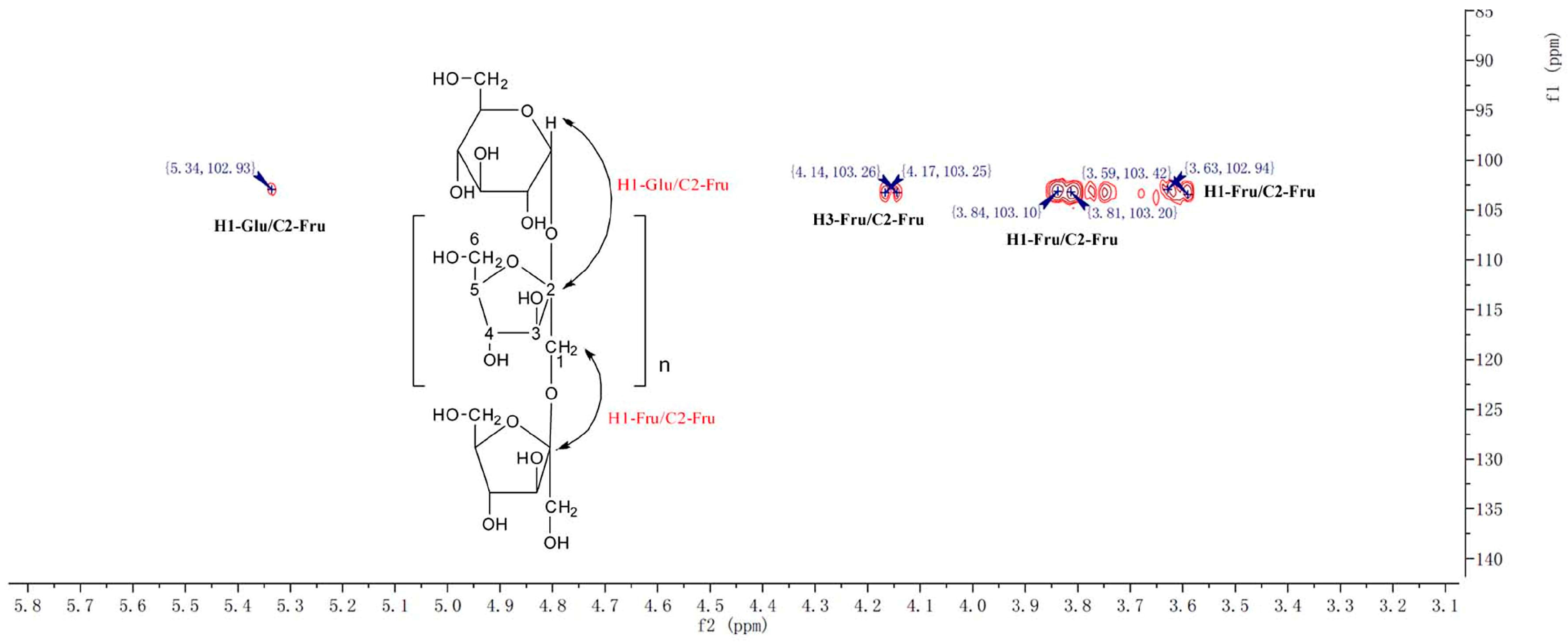
2.1. Structure Identification of CP-A
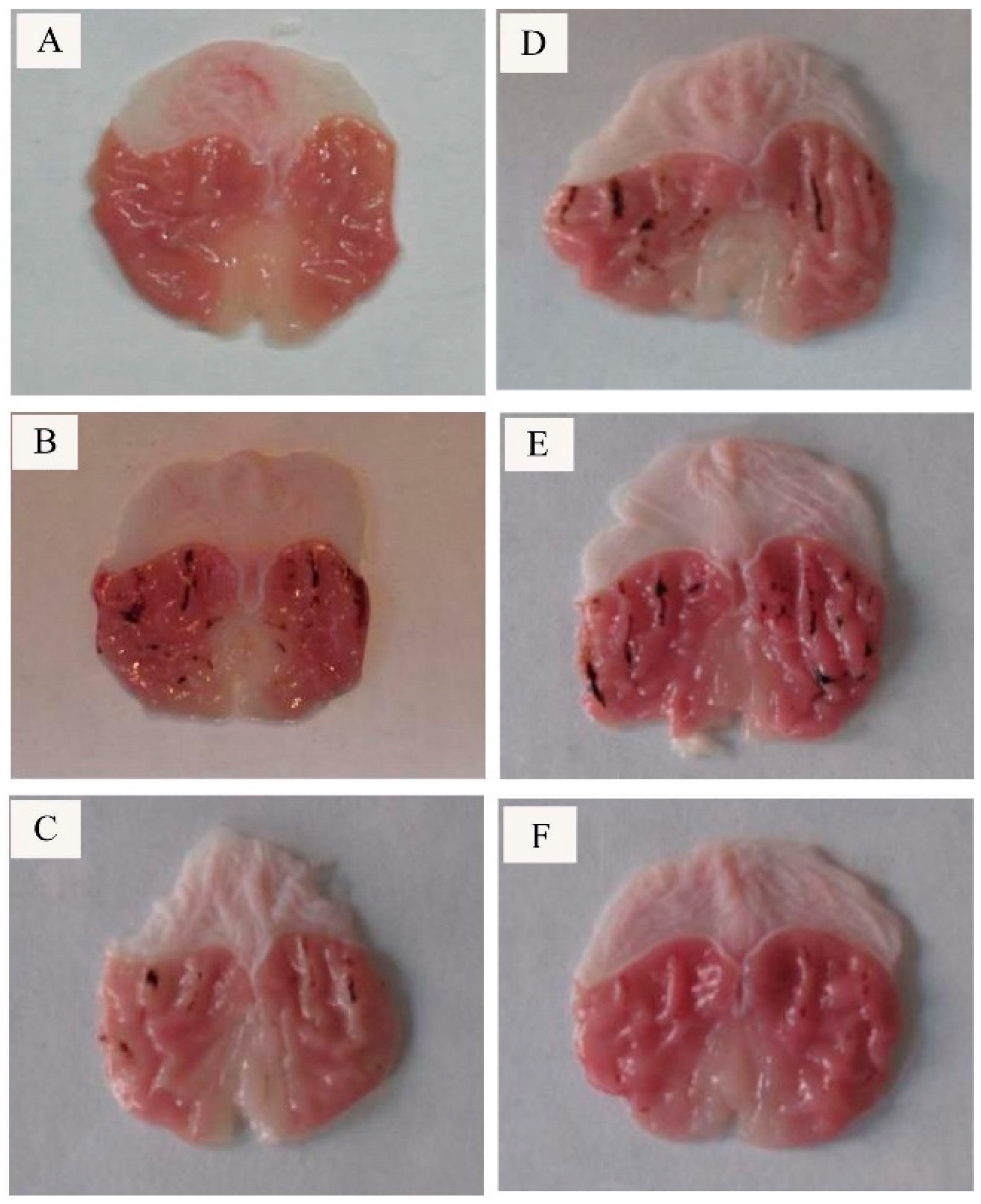
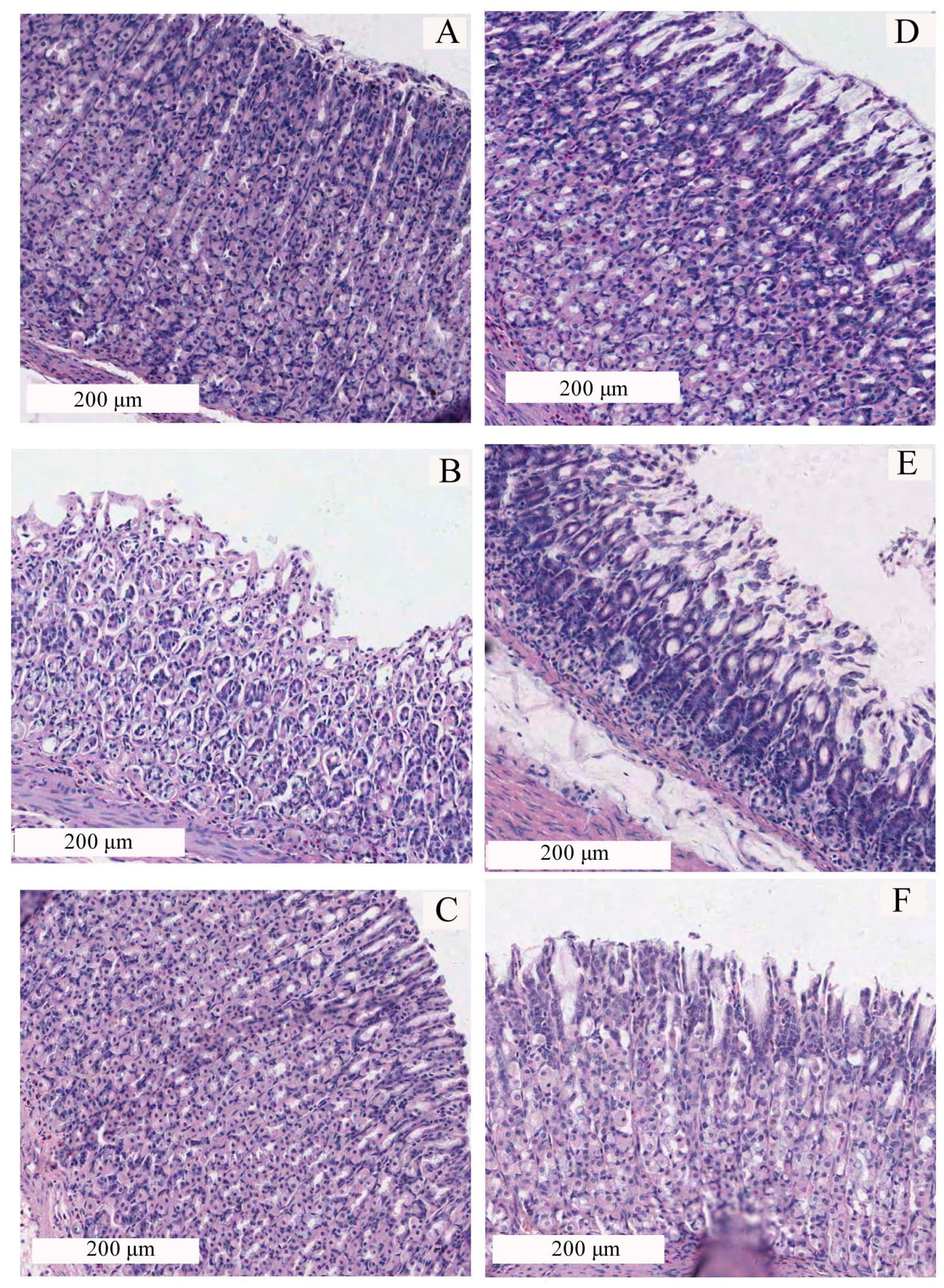
2.2. Effect of CP-A on Rat Gastric Mucosal Morphology
2.3. Effects of CP-A on Rat Gastric Mucosa Damage
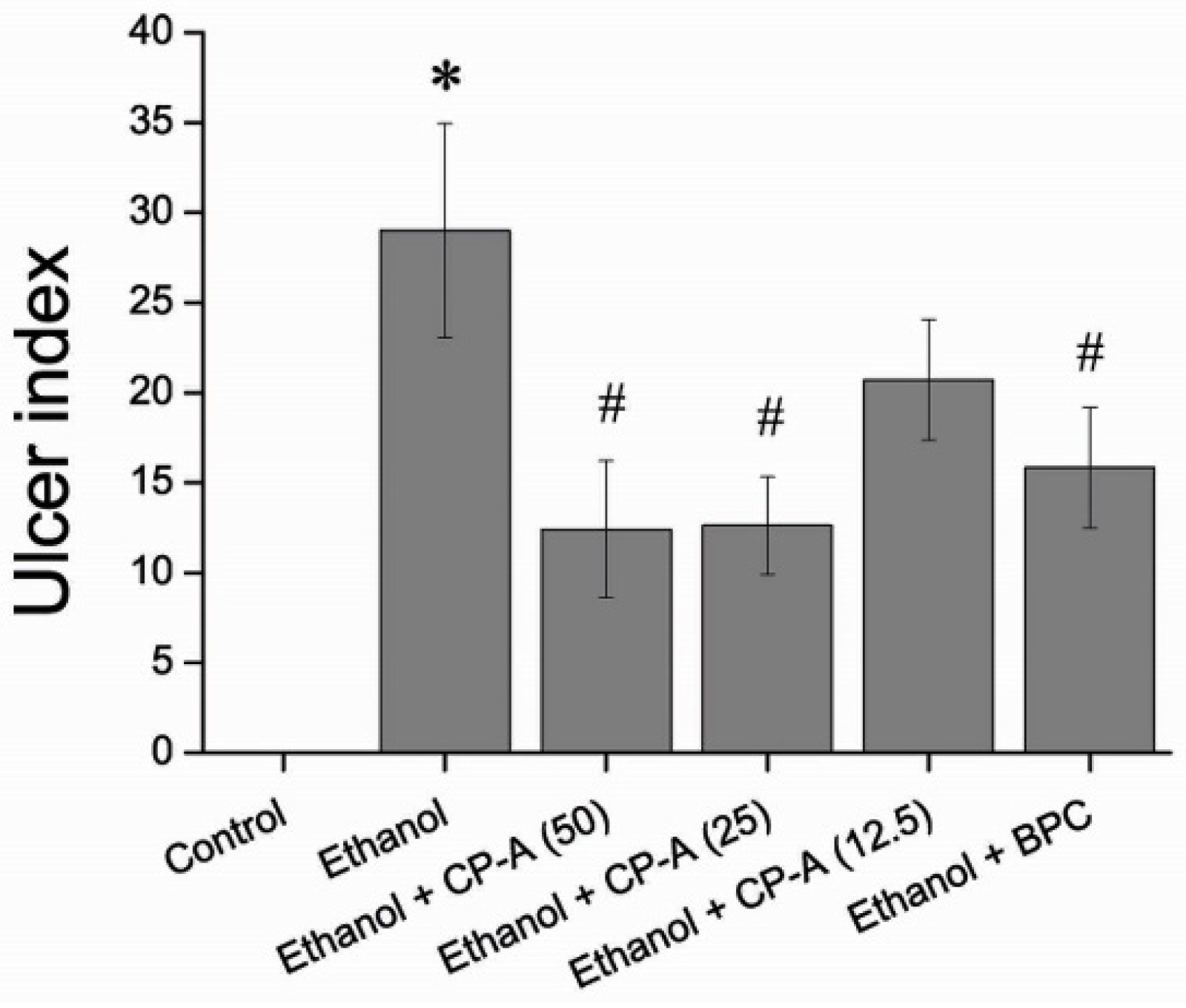
2.4. Effects of CP-A on Rat Gastric Mucosa Ulcer Index
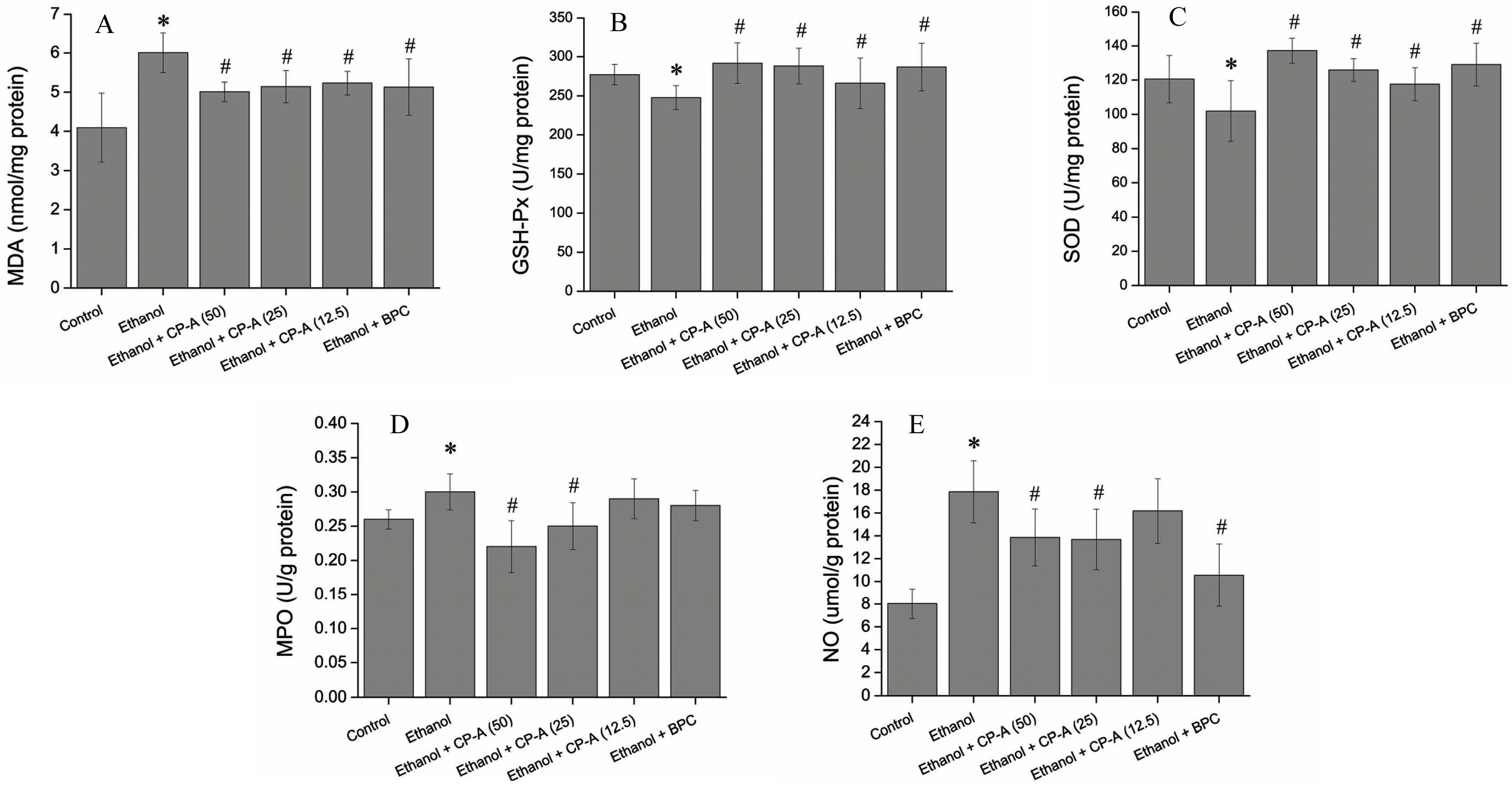
2.5. Effects of CP-A on the Activities of MPO, SOD, GSH-Px and the Contents of MDA, NO in Gastric Tissue
3. Material and Methods
3.1. Materials and Chemicals
3.2. Extraction and Purification of CP-A
3.3. Homogeneity and Molecular Weight Determination of CP-A
3.4. NMR and TOF-MS Analysis of CP-A
3.5. Anti-Gastric Ulcer Effect of CP-A in Ethanol-Induced Acute Gastric Ulcer Rats
3.6. Statistic
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BPC | bismuth potassium citrate |
| DEPT | distortionless enhancement by polarization transfer |
| DMSO | dimethyl sulphoxide |
| DP | degree of polymerization |
| GSH-Px | glutathione peroxidase |
| HMBC | heteronuclear multiple bond correlation |
| HPGPC | high performance gel permeation chromatography |
| HSQC | heteronuclear singular quantum correlation |
| MDA | malondialdehyde |
| MS | mass spectrometer |
| MPO | myeloperoxidase |
| NMR | nuclear magnetic resonance spectrometer |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- He, J.Y.; Ma, N.; Zhu, S.; Komatsu, K.; Li, Z.Y.; Fu, W.M. The genus Codonopsis (Campanulaceae): A review of phytochemistry, bioactivity and quality control. J. Nat. Med. 2015, 69, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Liu, J.C. Structural characterization of a water soluble polysaccharide from the roots of Codonopsis pilosula and its immunity activity. Int. J. Biol. Macromol. 2008, 43, 279–282. [Google Scholar]
- Zhang, Y.J.; Zhang, L.X.; Yang, J.F.; Liang, Z.Y. Structure analysis of water-soluble polysaccharide CPPS3 isolated from Codonopsis pilosula. Fitoterapia 2010, 81, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gou, Y.; Chen, J.; An, J.; Chen, W.; Hu, F. Structural characterization and antitumor activity of a pectic polysaccharide from Codonopsis pilosula. Carbohydr. Polym. 2013, 98, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.N.; Hu, Y.L.; Wang, D.Y.; Liu, J.Z.; Guo, L.W. The comparison of immune-enhancing activity of sulfated polysaccharidses from Tremella and Condonpsis pilosula. Carbohydr. Polym. 2013, 98, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Chen, X.F.; Malterud, K.E.; Rise, F.; Barsett, H.; Inngjerdingen, K.T. Structural features and complement fixing activity of polysaccharidesfrom Codonopsis pilosula Nannf. var. modesta L.T.Shen roots. Carbohydr. Polym. 2014, 113, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Li, E.T.; Fan, Q.; Wang, D.Y.; Li, P.; Li, X.; Chen, X.; Qiu, S.; Gao, Z.; et al. The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int. Immunopharmacol. 2015, 24, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B.; Delzenne, N.M. Dietary fructans. Annu. Rev. Nutr. 1998, 18, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. Inulin-type prebiotics—A review Part 1. Altern. Med. Rev. 2008, 13, 315–329. [Google Scholar] [PubMed]
- Kelly, G. Inulin-type prebiotics—A review Part 2. Altern. Med. Rev. 2009, 14, 36–55. [Google Scholar] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Caleffi, E.R.; Krausova, G.; Hyrlova, I.; Paredes, L.L.R.; Santos, M.M.; Guilherme Lanzi Sassaki, G.L.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Isolation and prebiotic activity of inulin-type fructan extracted from Pfaffia glomerata (Spreng) Pedersen roots. Int. J. Biol. Macromol. 2015, 80, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Guth, P.H.; Aures, D.; Paulsen, G. Topical aspirin HCl gastric lesions in the rat, cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology 1979, 76, 88–93. [Google Scholar] [PubMed]
- Xu, J.; Chen, D.; Liu, C.; Wu, X.Z.; Dong, C.X.; Zhou, J. Structural characterization and anti-tumor effects of an inulin-typefructan from Atractylodes chinensis. Int. J. Biol. Macromol. 2016, 82, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, S.; Kervarec, N.; Pichon, R.; Magne, C.; Bessieresa, M.A.; Deslandes, E. NMR characterisation of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima (L.). Carbohydr. Res. 2004, 339, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Lacy, E.R.; Ito, S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology 1982, 83, 619–625. [Google Scholar] [PubMed]
- Stewart, D.J.; Ackroyd, R. Peptic ulcers and their complications. Surgery 2011, 29, 491–495. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.S.A.; Maghrabi, I.A. Diosmin Protects against Ethanol-Induced Gastric Injury in Rats: Novel Anti-Ulcer Actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, X.; Zhang, H.; He, Z.; Zhi, W.; Liu, F.; Wang, Y.; Niu, X. Anti-ulcerogenic effect of cavidine against ethanol-induced acute gastric ulcer in mice and possible underlying mechanism. Int. Immunopharmacol. 2016, 38, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Abdulla, M.A.; Hajrezaie, M.; Bader, A.; Shahzad, N.; Al-Ghamdi, S.S.; Gushash, A.; Hasanpourghadi, M. The gastroprotective effects of hydroalcoholic extract of Monolluma quadrangula against ethanol-induced gastric mucosal injuries in Sprague Dawley rats. Drug Des. Dev. Ther. 2015, 10, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.V.; Gadelha, G.G.; Lima, S.J.; Garcia, J.A.; Soares, P.M.; Santos, A.A.; Brito, G.A.C.; Ribeiro, R.A.; Souza, M.H.L.P. Role of the NO/cGMP/KATP pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats. Br. J. Pharmacol. 2008, 153, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.V. Molecular targets of oxidative stress. Biochem. J. 2011, 434, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta 2007, 380, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cadirci, E.; Suleyman, H.; Aksoy, H.; Halici, Z.; Ozgen, U.; Koc, A.; Ozturk, N. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem. Biol. Interact. 2007, 170, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Jimenez, M.D.; Motilva, V. New issues about nitric oxide and its effects on the gastrointestinal tract. Curr. Pharm. Des. 2001, 7, 881–908. [Google Scholar] [CrossRef] [PubMed]
- Dursun, H.; Bilici, M.; Albayrak, F.; Ozturk, C.; Saglam, M.B.; Alp, H.H.; Suleyman, H. Antiulcer activity of fluvoxamine in rats and its effect on oxidant and antioxidant parameters in stomach tissue. BMC Gastroenterol. 2009, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.Y.; Feng, X.; Zhu, K.; Wang, C.; Zhao, X.; Kan, J.Q. Shuidouchi (Fermented Soybean) Fermented in Different Vessels Attenuates HCl/Ethanol-Induced Gastric Mucosal Injury. Molecules 2015, 20, 19748–19763. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Ohta, Y.; Ishiguro, I. Contribution of NO synthases to neutrophil infiltration in the gastric mucosal lesions in rats with water immersion restraint stress. FEBS Lett. 1998, 425, 243–248. [Google Scholar] [CrossRef]
- Takeuchi, K.; Yasuhiro, T.; Asada, Y.; Sugawa, Y. Role of nitric oxide in pathogenesis of aspirin-induced gastric mucosal damage in rats. Digestion 1998, 59, 298–307. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound CP-A is available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, T.; Zhu, Z.; Yang, F.; Cao, L.; Gao, J. Structure Features and Anti-Gastric Ulcer Effects of Inulin-Type Fructan CP-A from the Roots of Codonopsis pilosula (Franch.) Nannf. Molecules 2017, 22, 2258. https://doi.org/10.3390/molecules22122258
Li J, Wang T, Zhu Z, Yang F, Cao L, Gao J. Structure Features and Anti-Gastric Ulcer Effects of Inulin-Type Fructan CP-A from the Roots of Codonopsis pilosula (Franch.) Nannf. Molecules. 2017; 22(12):2258. https://doi.org/10.3390/molecules22122258
Chicago/Turabian StyleLi, Jiankuan, Tao Wang, Zhichuan Zhu, Fengrong Yang, Lingya Cao, and Jianping Gao. 2017. "Structure Features and Anti-Gastric Ulcer Effects of Inulin-Type Fructan CP-A from the Roots of Codonopsis pilosula (Franch.) Nannf." Molecules 22, no. 12: 2258. https://doi.org/10.3390/molecules22122258
APA StyleLi, J., Wang, T., Zhu, Z., Yang, F., Cao, L., & Gao, J. (2017). Structure Features and Anti-Gastric Ulcer Effects of Inulin-Type Fructan CP-A from the Roots of Codonopsis pilosula (Franch.) Nannf. Molecules, 22(12), 2258. https://doi.org/10.3390/molecules22122258