In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States
Abstract
:1. Introduction
2. Results and Discussion
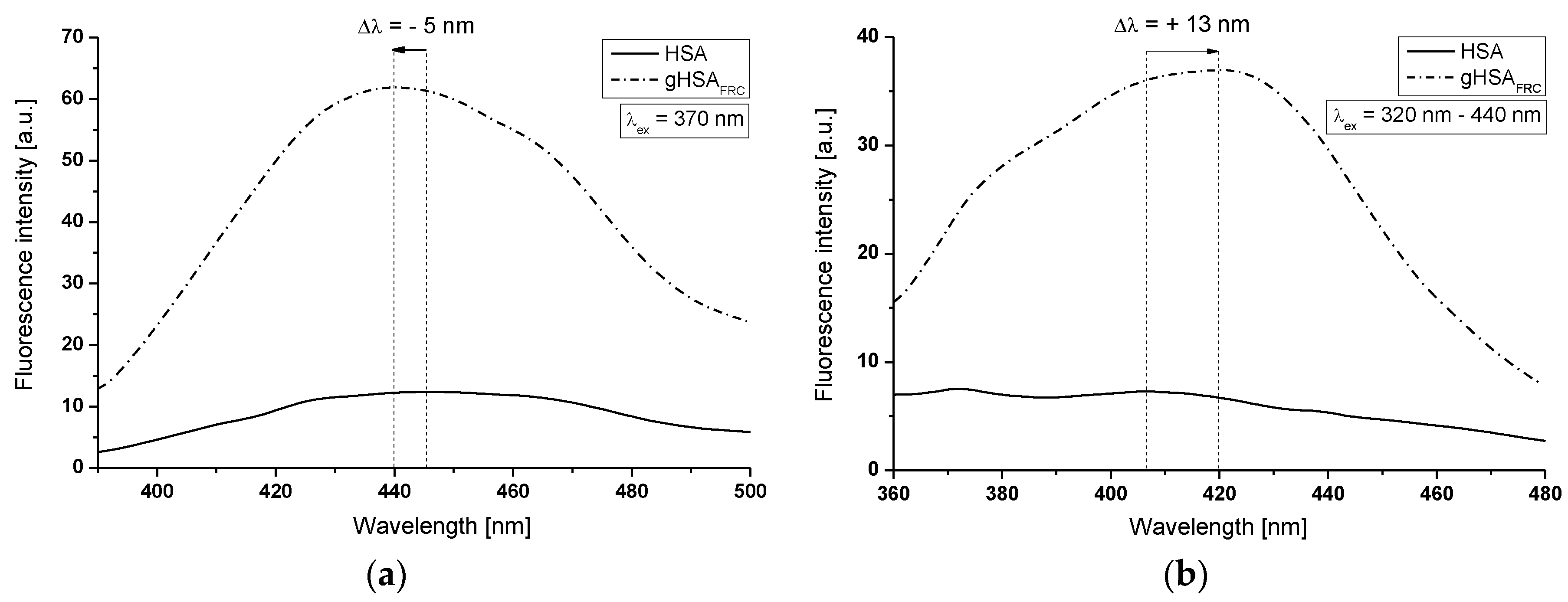
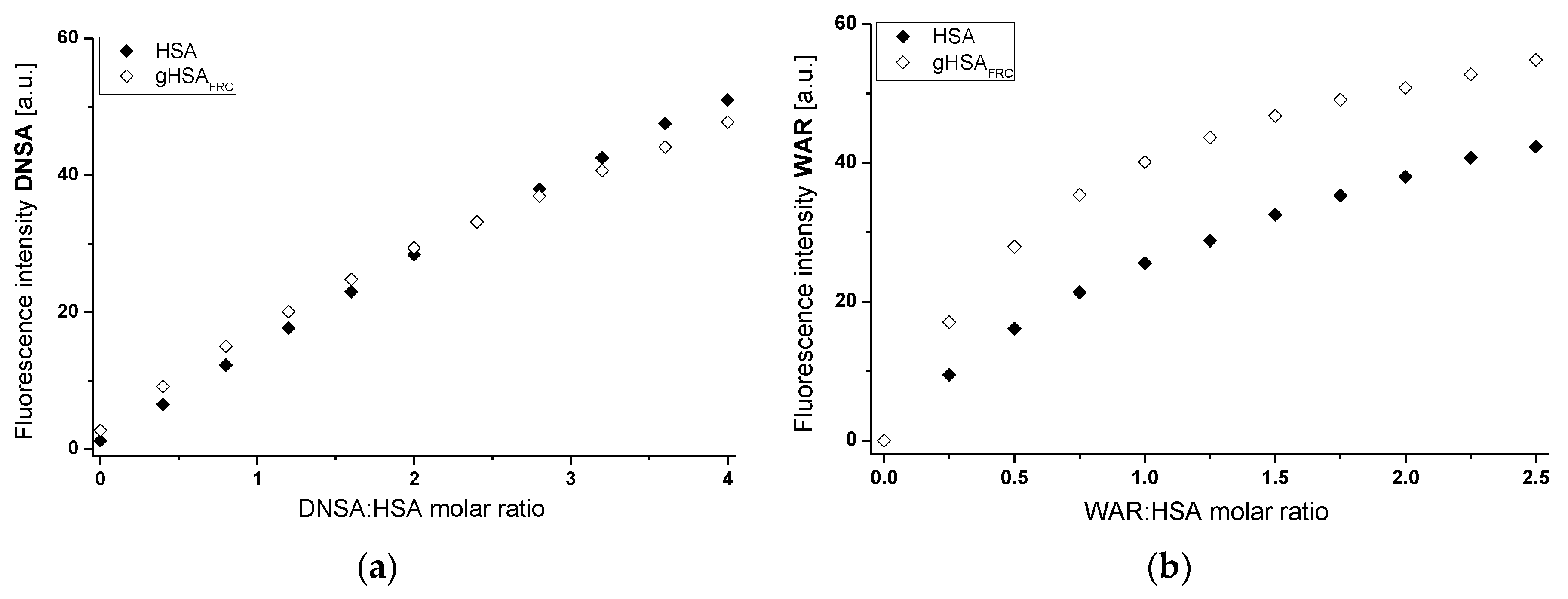
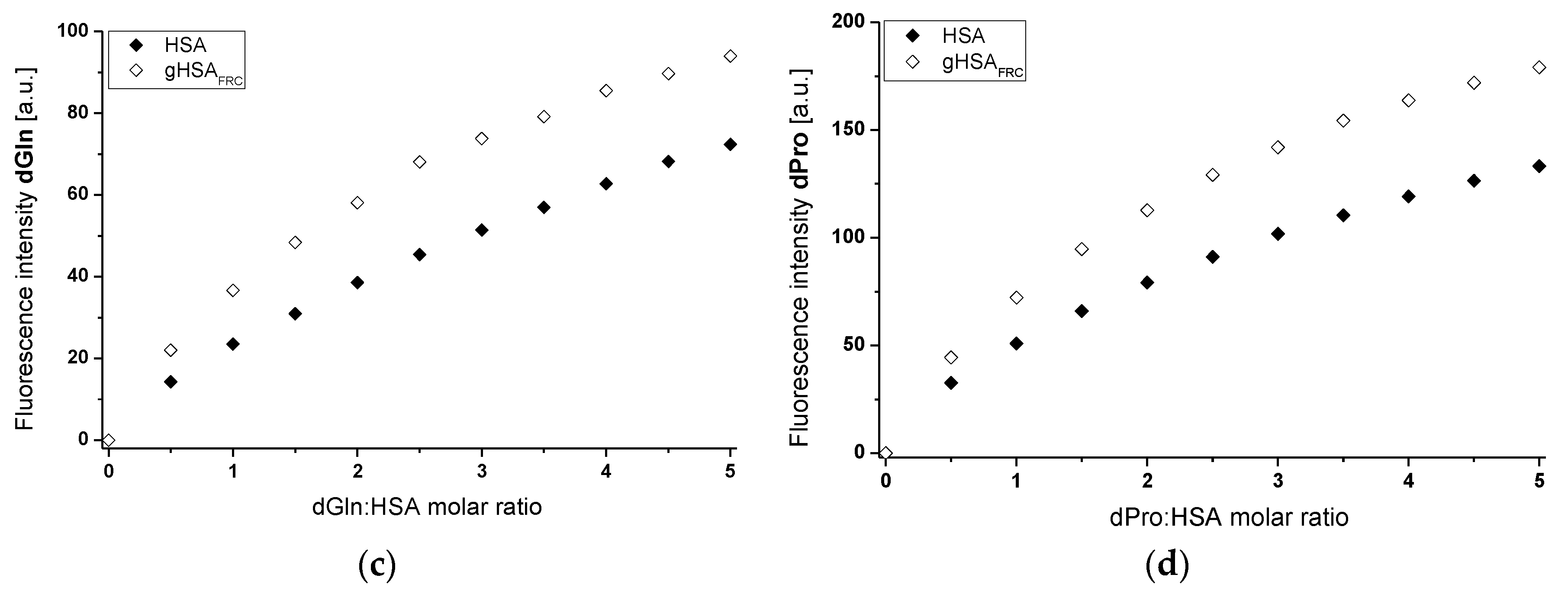
2.1. Effect of Glycation on Serum Albumin Tertiary Structure—Fluorescence Characteristic
2.2. Effect of Glycation on Human Serum Albumin Structure—Analysis of Absorption Spectra—Calculation of Free Sulfhydryl Groups Content in HSA
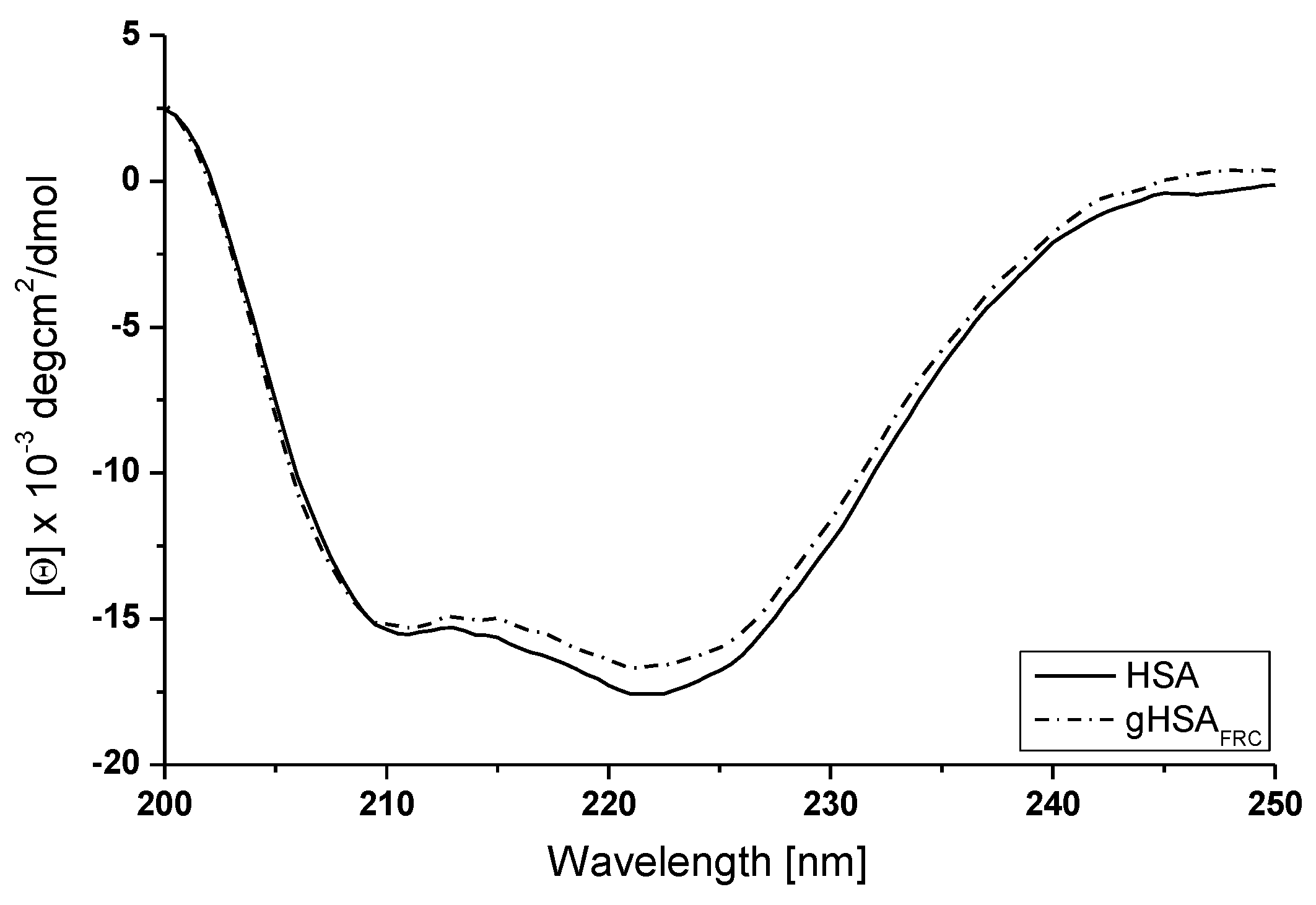
2.3. Effect of Glycation on Human Serum Albumin Secondary Structure—Analysis of Circular Dichroism Spectra
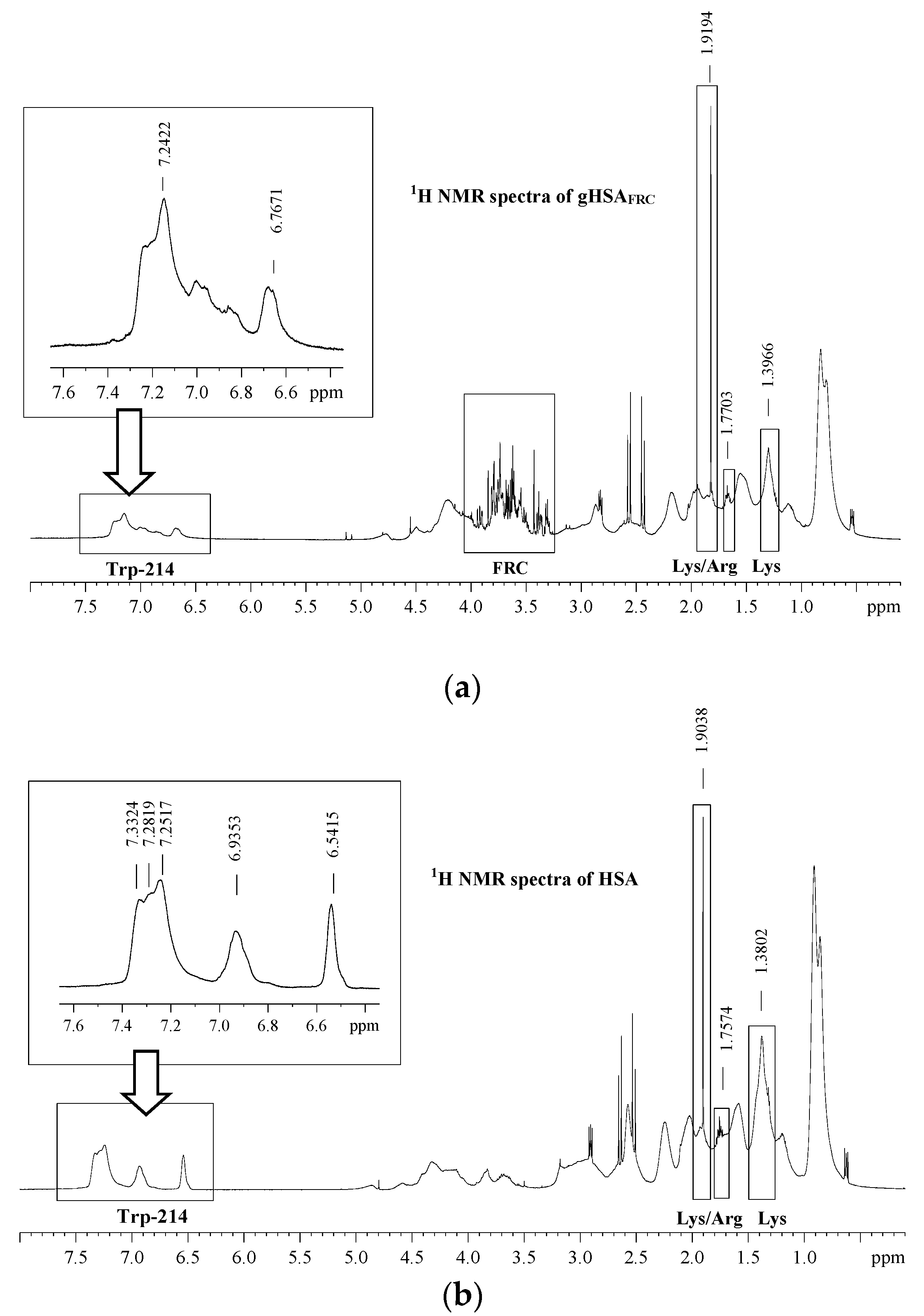
2.4. Effect of Glycation on Human Serum Albumin Structure—Analysis of 1H-NMR Spectra
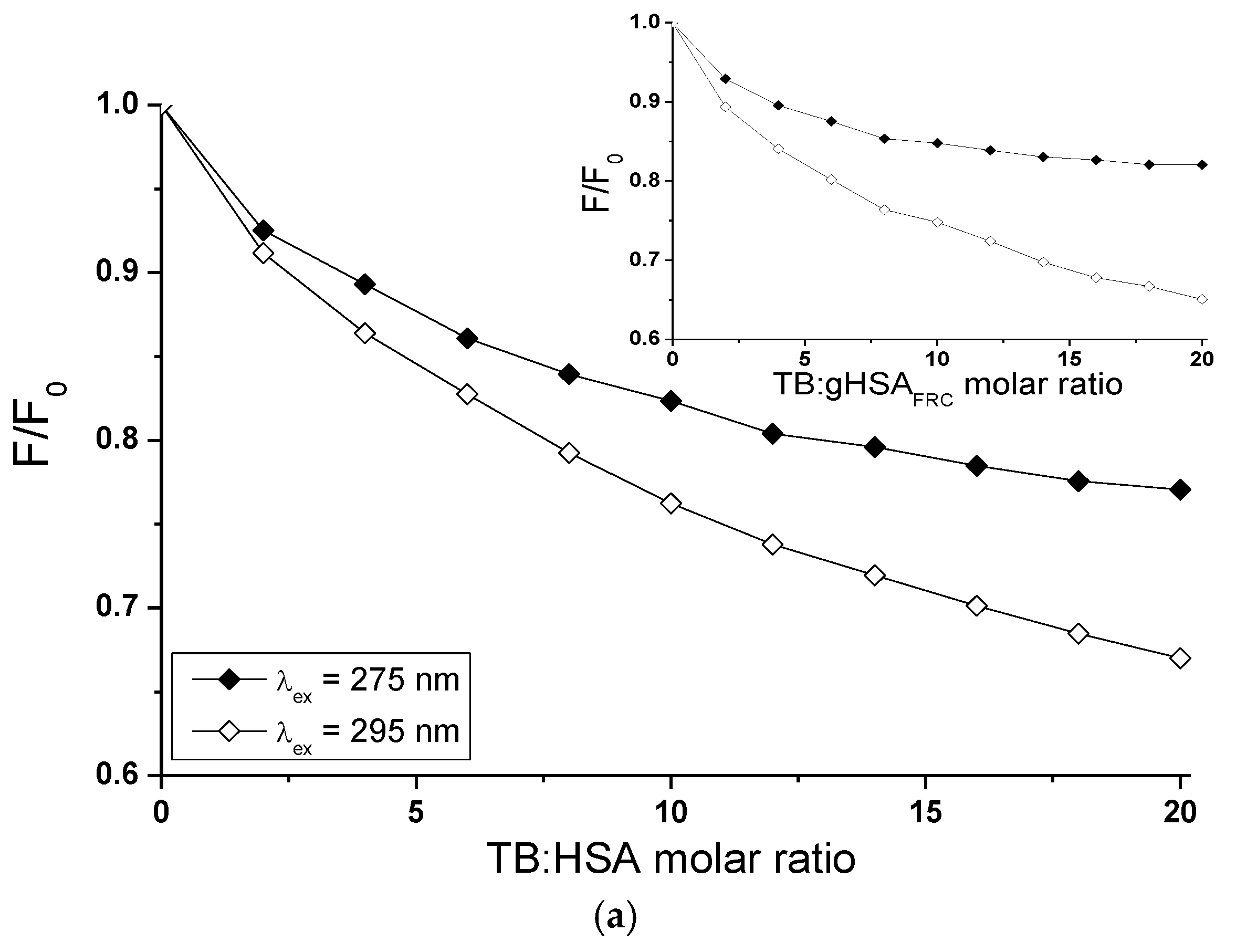
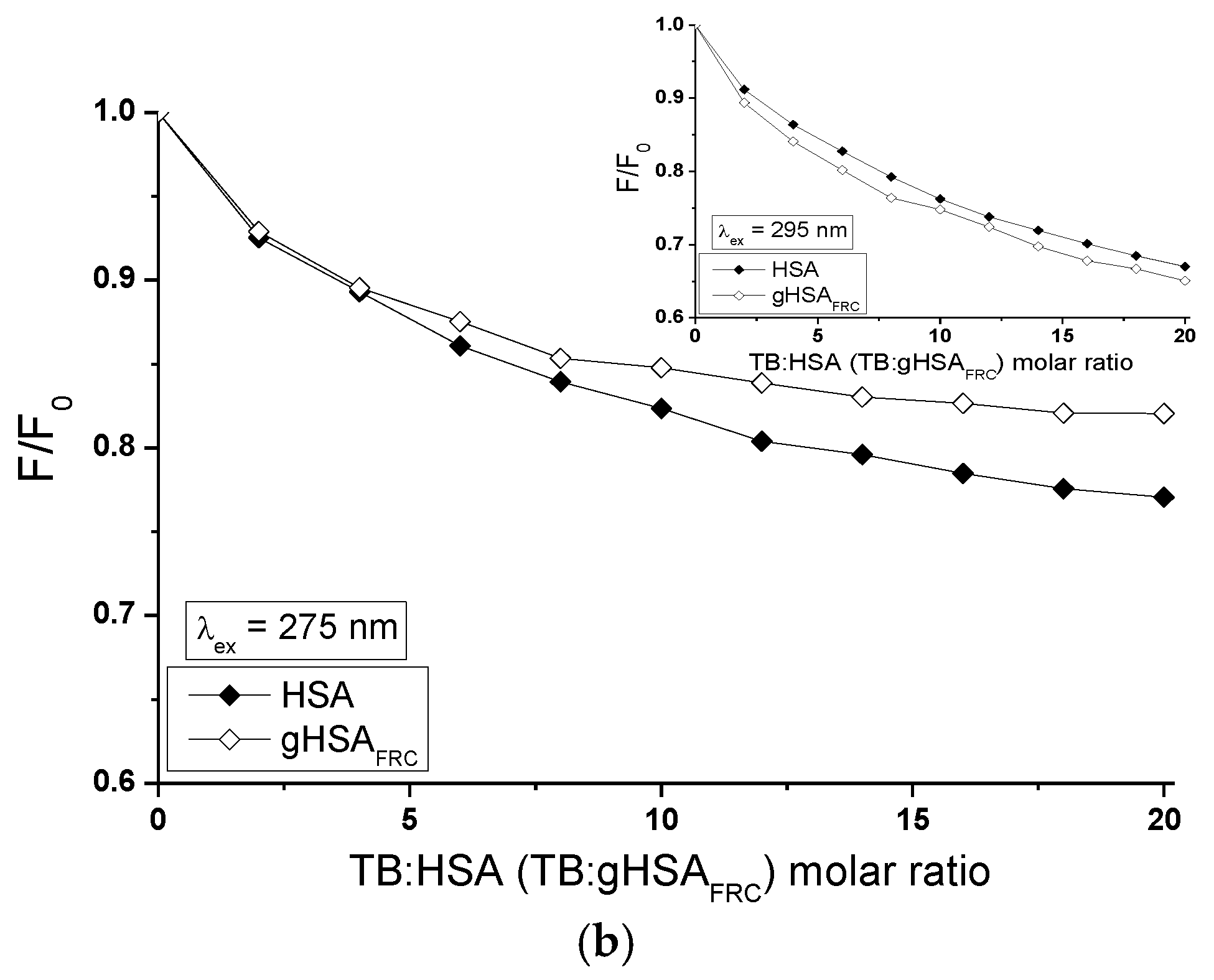
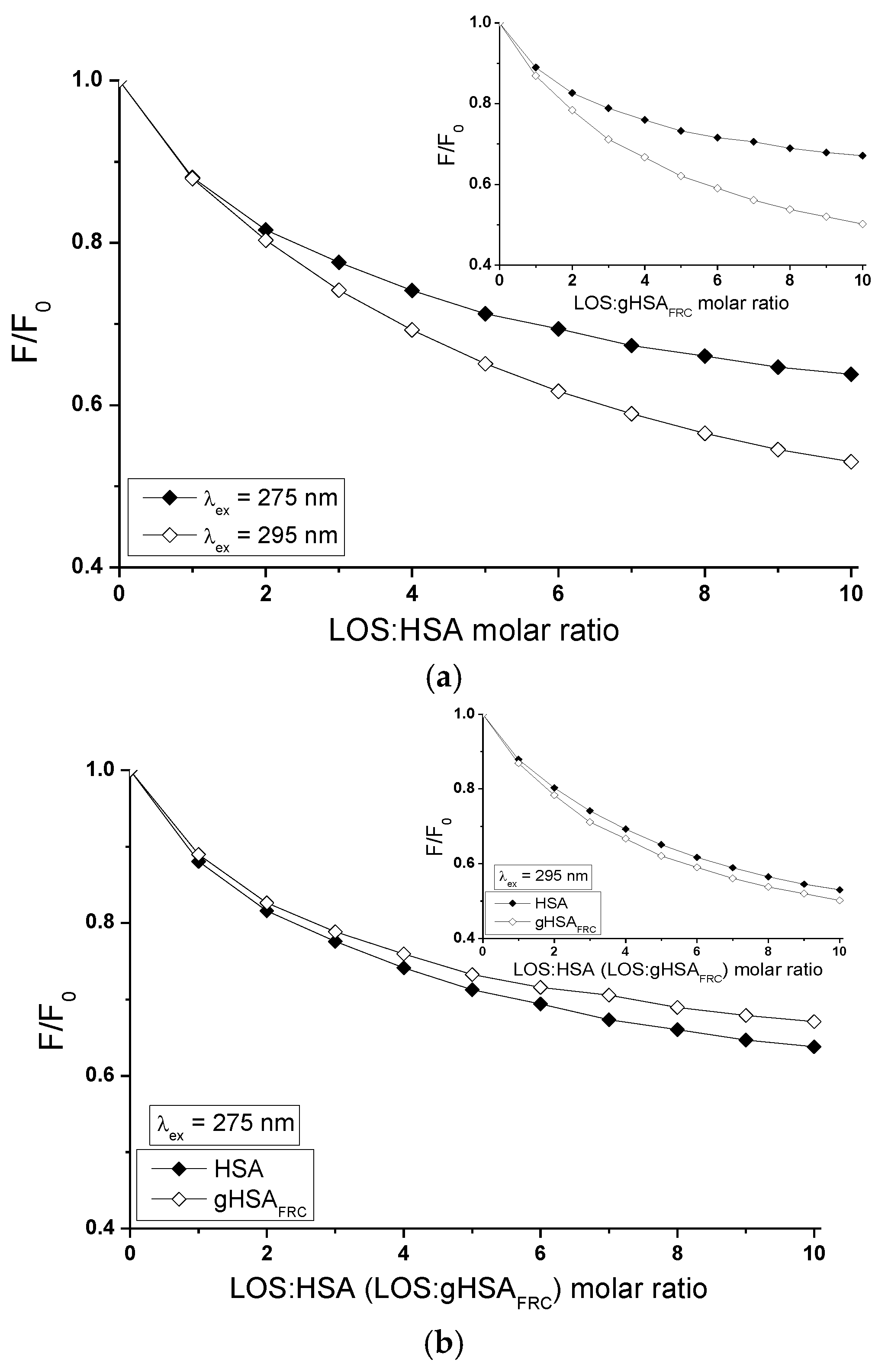
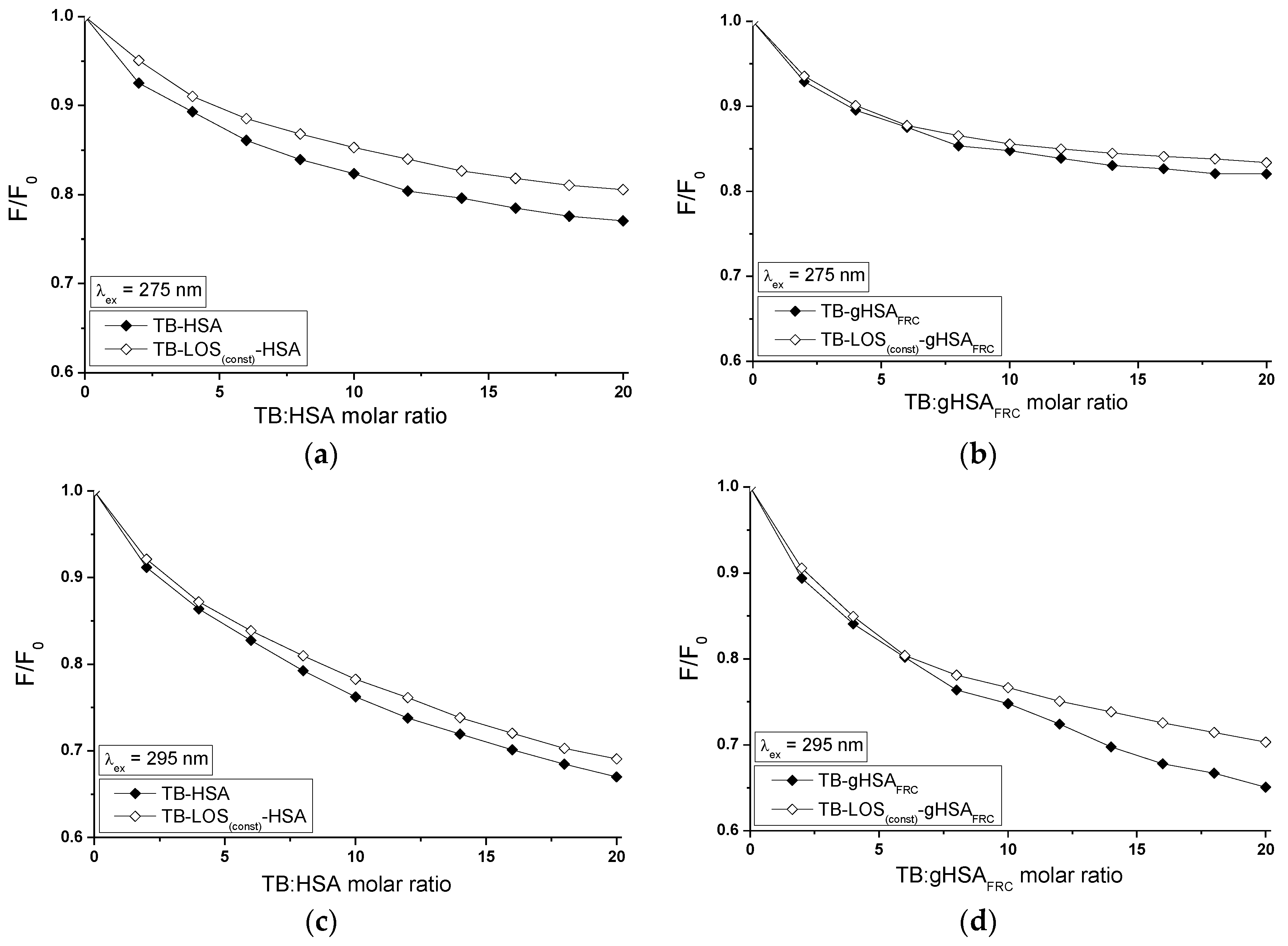
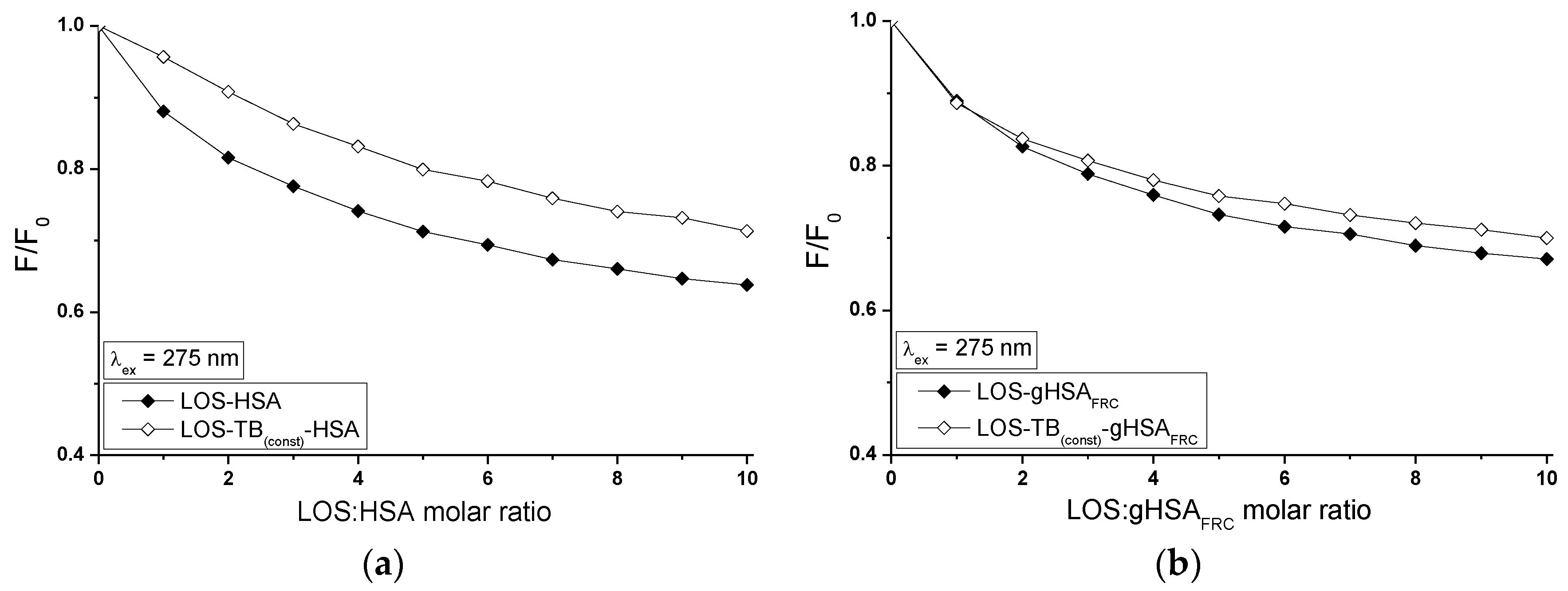
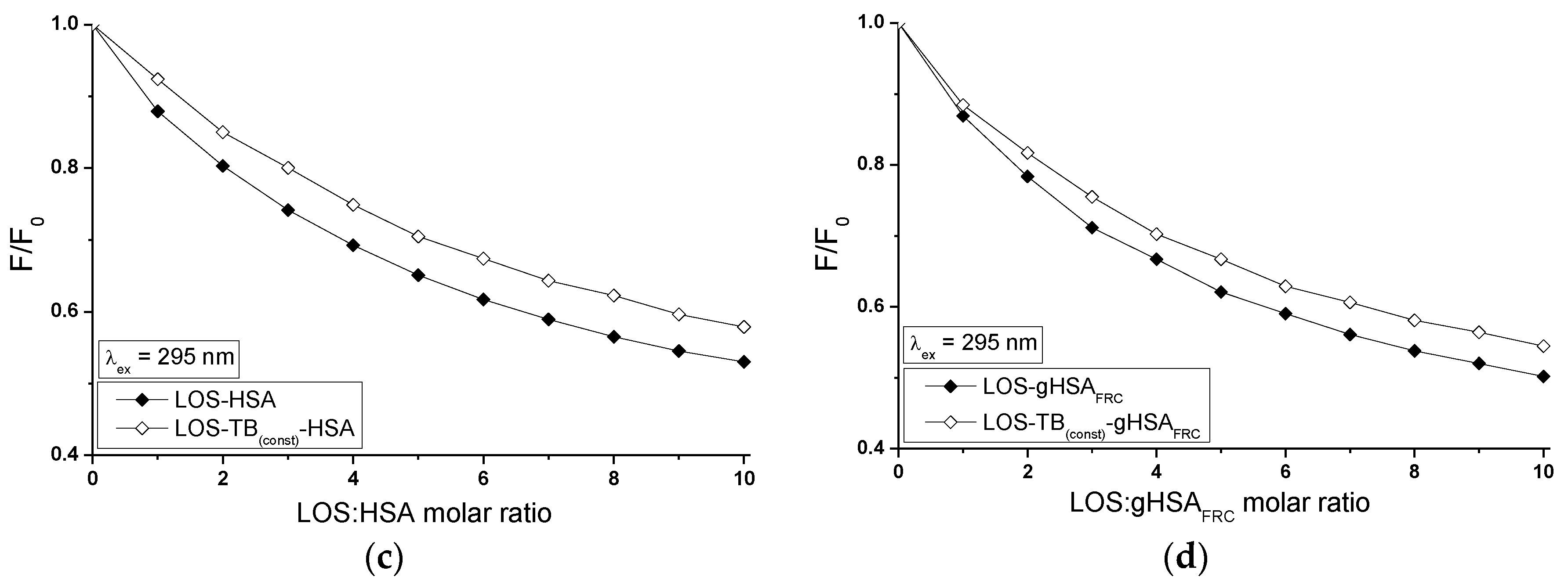
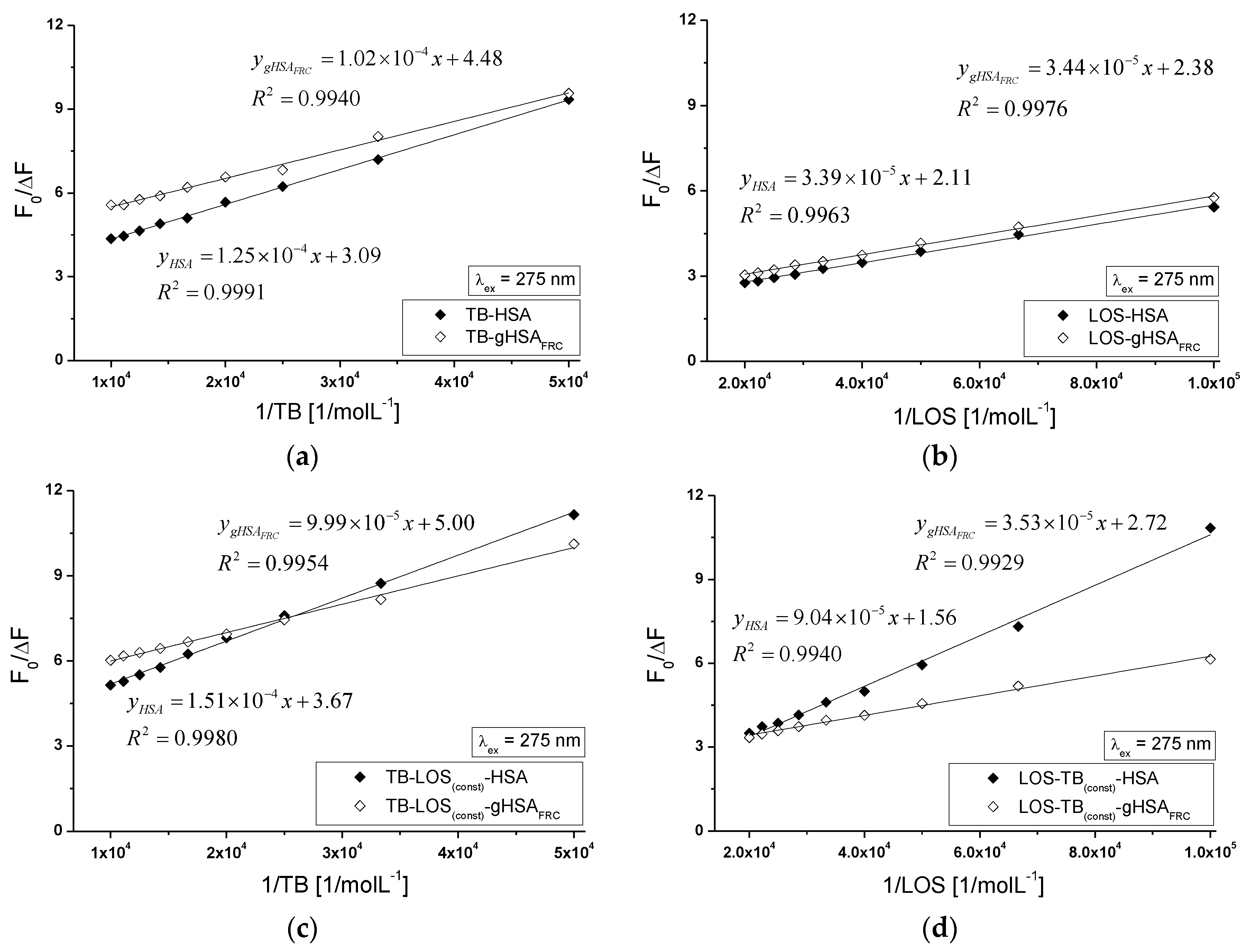
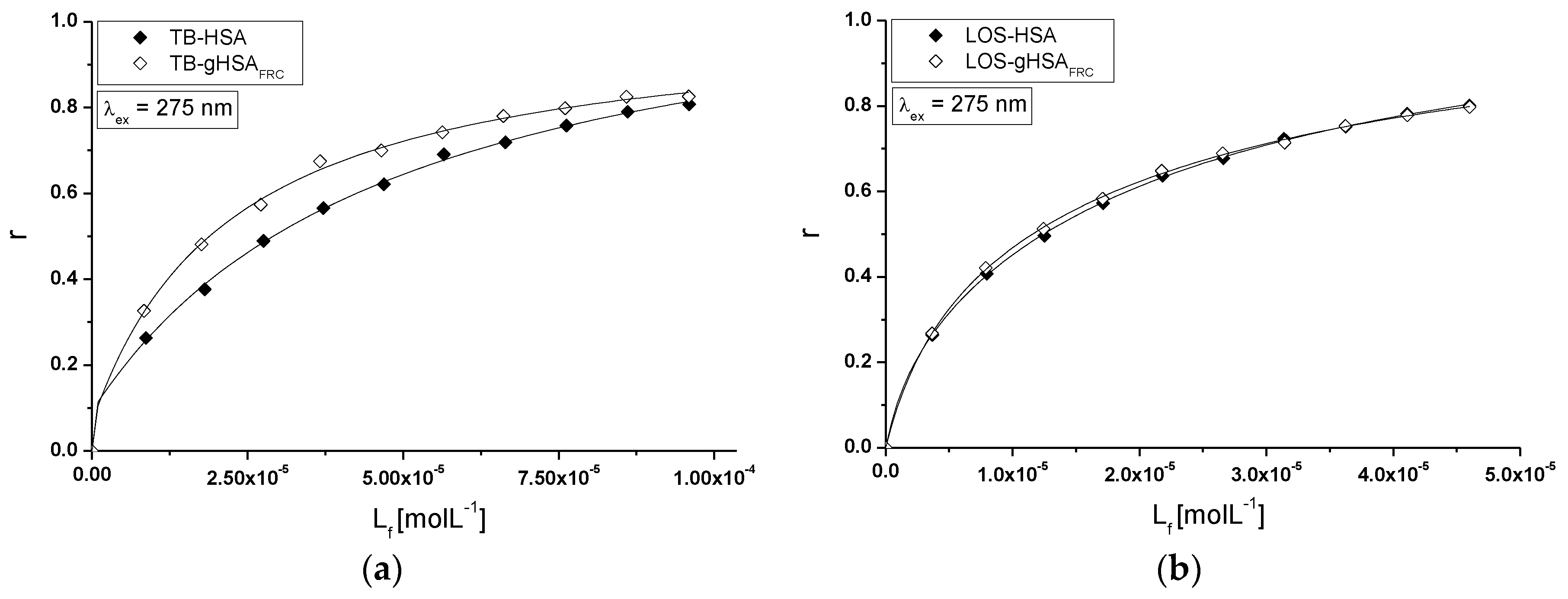
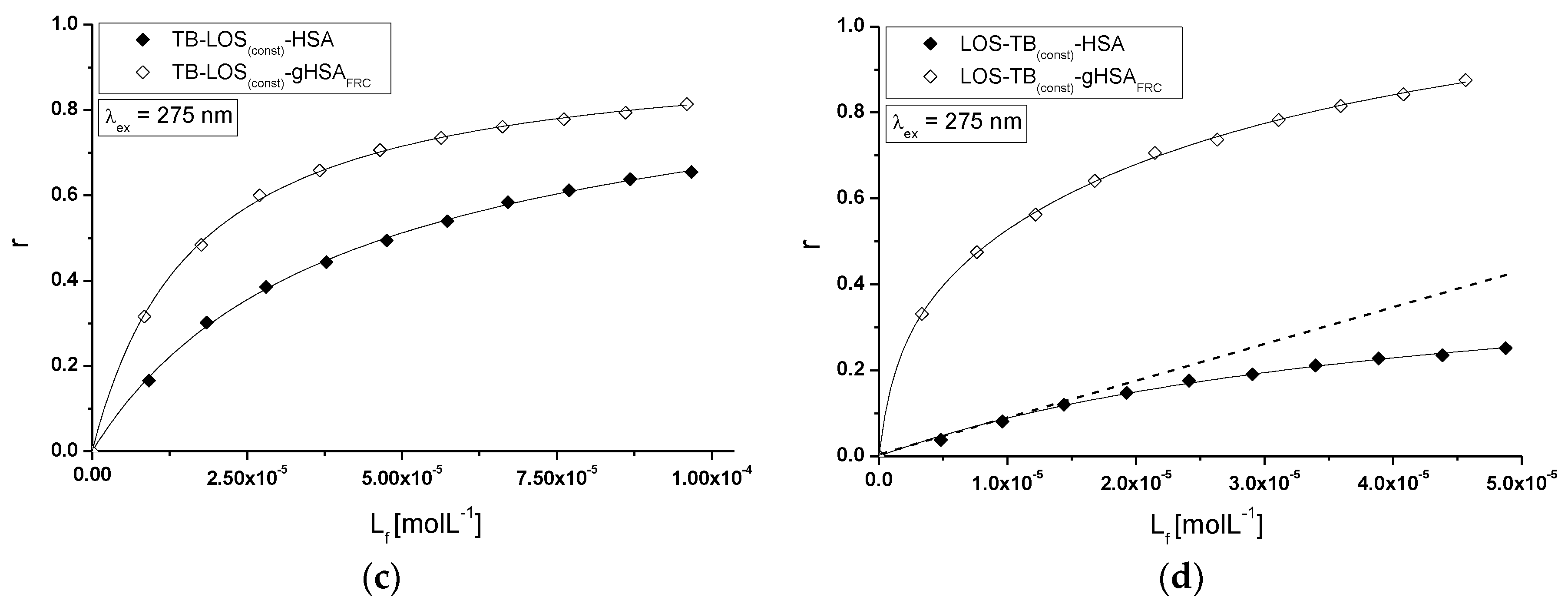
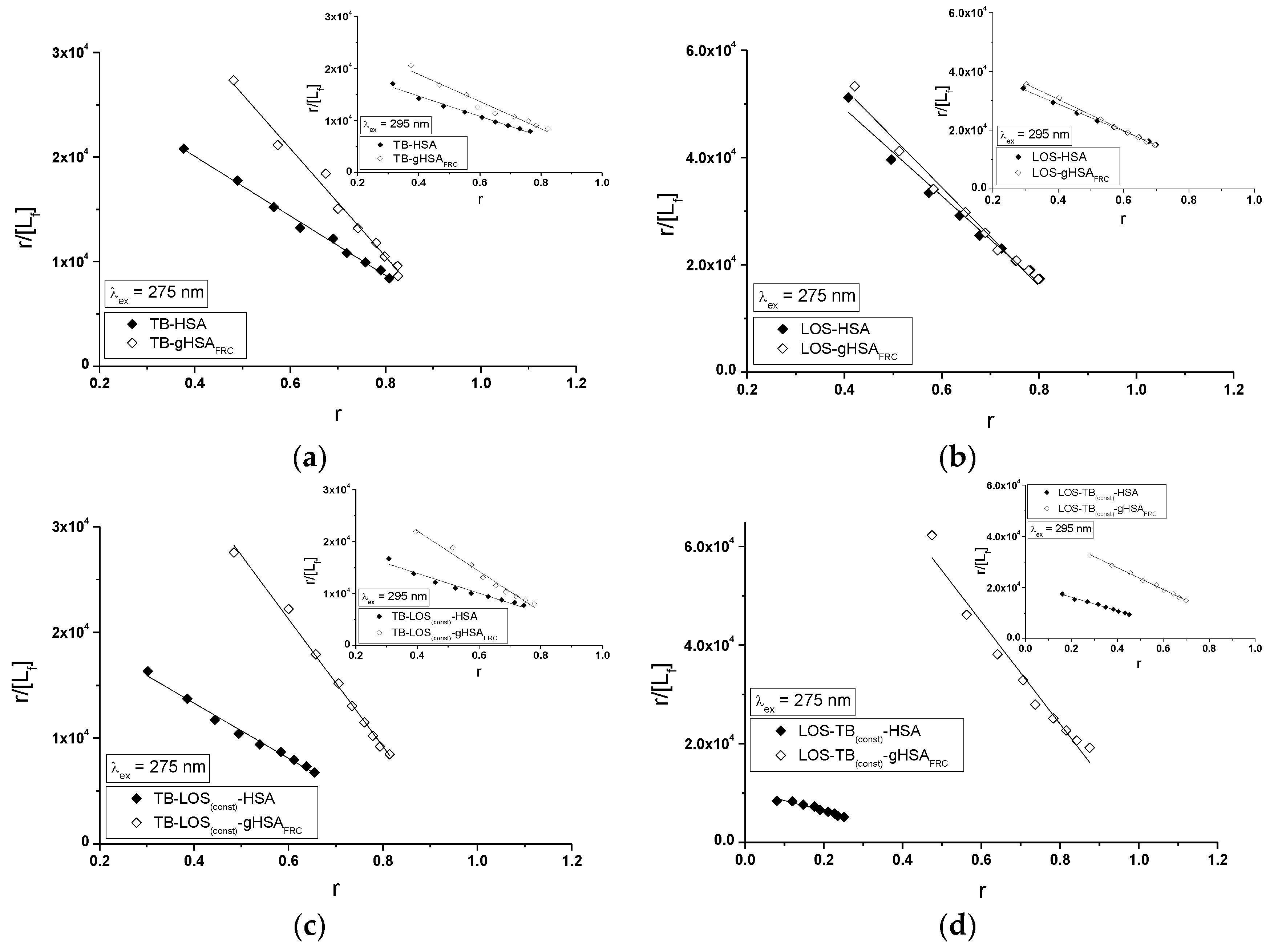
2.5. Fluorescence Quenching of Non-Glycated and Glycated Human Serum Albumin Induced by Tolbutamide and Losartan in the Binary and Ternary Complex
3. Materials and Methods
3.1. Reagents
3.2. In Vitro Modification of Human Serum Albumin
3.3. Analysis of Absorption Spectra—Calculation of Free Sulfhydryl Groups Content in HSA
3.4. Preparation of Samples for Fluorescence Measurements
3.5. Fluorescence, UV-Vis Spectra and Fluorescence Second Derivative Spectra
3.6. Analysis of Fluorescence Spectra—Calculation of the Stern-Volmer and Association Constants in the Binary and Ternary Systems
3.7. Circular Dichroism (CD) Spectra
3.8. Proton Nuclear Magnetic Resonance (1H-NMR) Spectra
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peters, T. All about Albumin. Biochemistry, Genetics and Medical Applications; Academic Press: San Diego, CA, USA, 1995; pp. 1–40. [Google Scholar]
- Kragh-Hansen, U.; Minchiotti, L.; Galiano, M.; Peters, T. Human serum albumin isoforms: Genetic and molecular aspects and functional consequences. Biochim. Biophys. Acta 2013, 1830, 5405–5417. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Pea, F.; Lipman, J. The Clinical Relevance of Plasma Protein Binding Changes. Clin. Pharmacokinet. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The Characterization of Two Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum Albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Qiu, Y.; Burlingame, A.; Benet, L. Mechanisms for covalent binding of benoxaprofen glucuronide to human serum albumin. Drug Metab. Dispos. 1998, 26, 246–256. [Google Scholar] [PubMed]
- Laleman, W. Hemodynamic effects of albumin dialysis in patients with liver failure: For better or for worse? Ther. Apher. Dial. 2009, 13, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Ahmad, J.; Alam, K. Nonenzymatic glycosylation of human serum albumin and its effect on antibodies profile in patients with diabetes mellitus. PLoS ONE 2017, 12, e0176970. [Google Scholar] [CrossRef] [PubMed]
- Guerin-Dubourg, A.; Catan, A.; Bourdon, E.; Rondeau, P. Structural modifications of human albumin in diabetes. Diabetes Metab. 2012, 38, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, S.C.; Gurachevsky, A.; Matthes, G.; Muravsky, V. Electron spin resonance spectroscopy of serum albumin: A novel new test for cancer diagnosis and monitoring. Clin. Chem. 2006, 52, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Katsuzaki, T.; Miyazaki, S.; Momoi, T.; Akiba, T.; Miyazaki, T.; Nokura, K.; Hayase, F.; Tatemichi, N.; Takei, Y. Amyloid β2-microglobulin is modified with imidazolone, a novel advanced glycation end product, in dialysis-related amyloidosis. Kidney Int. 1997, 51, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J. Peptide mapping of human serum albumin modified minimally by methylglyoxal in vitro and in vivo. Ann. N. Y. Acad. Sci. 2005, 1043, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Bucala, R.; Striker, L. Pathogenic effects of advanced glycosylation: Biochemical, biologic, and clinical implications for diabetes and aging. Lab. Investig. 1994, 70, 138–151. [Google Scholar] [PubMed]
- Maciążek-Jurczyk, M.; Szkudlarek, A.; Chudzik, M.; Pożycka, J.; Sułkowska, A. Alteration of human serum albumin binding properties induced by modifications: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 188, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, J. Metabolic syndrome and mental illness. Am. J. Manag. Care 2007, 13, 170–177. [Google Scholar]
- Kirchheiner, J.; Bauer, S.; Meineke, I.; Rohde, W.; Prang, V.; Meisel, C.; Roots, I.; Brockmöller, J. Impact of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and the insulin and glucose response in healthy volunteers. Pharmacogenetics 2002, 12, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Christ, D.D. Human plasma protein binding of the angiotensin II receptor antagonist losartan potassium (DuP 753/MK 954) and its pharmacologically active metabolite EXP3174. J. Clin. Pharmacol. 1995, 35, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.; Raghavan, C.T.; Nahomi, R.; Nagaraj, R.H.; Kessel, L. Effects of photobleaching on selected advanced glycation end products in the human lens. BMC Res. Notes 2015, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Shaklai, N.; Garlick, R.L.; Bunn, H.F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J. Biol. Chem. 1984, 259, 3812–3817. [Google Scholar] [PubMed]
- Szkudlarek, A.; Pentak, D.; Ploch, A.; Pożycka, J.; Maciążek-Jurczyk, M. Effect of Temperature on Tolbutamide Binding to Glycated Serum Albumin. Molecules 2017, 22, 569. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N. Recent advances in molecular luminescence analysis. Proc. Anal. Div. Chem. Soc. 1979, 16, 203–208. [Google Scholar]
- Varlan, A.; Hillebrand, M. Bovine and Human Serum Albumin Interactions with 3-Carboxyphenoxathiin Studied by Fluorescence and Circular Dichroism Spectroscopy. Molecules 2010, 15, 3905–3919. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.; Ladokhin, A. Red-edge-excitation fluorescence spectroscopy of indole and tryptophan. Eur. Biophys. J. 1988, 15, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Takahashi, H.; Tsuchiya, S. New fluorescence of nonenzymatically glucosylated human serum albumin. FEBS Lett. 1984, 176, 27–31. [Google Scholar] [CrossRef]
- Mendez, D.L.; Jensen, R.A.; McElroy, L.A.; Pena, J.M.; Esquerra, R.M. The effect of non-enzymatic glycation on the unfolding of human serum albumin. Arch. Biochem. Biophys. 2005, 444, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nakajou, K.; Watanabe, H.; Kragh-Hansen, U.; Maruyama, T.; Otagiri, M. The effect of glycation on the structure, function and biological fate of human serum albumin as revealed by recombinant mutants. Biochim. Biophys. Acta 2003, 1623, 88–97. [Google Scholar] [CrossRef]
- Demchenko, A.P. Site-selective excitation: A new dimension in protein and membrane spectroscopy. Trends Biochem. Sci. 1988, 13, 374–377. [Google Scholar] [CrossRef]
- Chattopadhyay, A. Exploring membrane organization and dynamics by the wavelength-selective fluorescence approach. Chem. Phys. Lipids 2003, 122, 3–17. [Google Scholar] [CrossRef]
- Maciążek-Jurczyk, M.; Sułkowska, A.; Równicka-Zubik, J. Alteration of methotrexate binding to human serum albumin induced by oxidative stress. Spectroscopic comparative study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 537–550. [Google Scholar]
- Parkhomenko, T.; Klicenko, O.; Shavlovski, M.; Kuznetsova, I.; Uversky, V.; Turoverov, K. Biophysical characterization of albumin preparations from blood serum of healthy donors and patients with renal diseases. Part I: Spectrofluorometric analysis. Med. Sci. Monit. 2002, 8, 261–265. [Google Scholar]
- Mozo-Villarías, A. Second derivative fluorescence spectroscopy of tryptophan in proteins. J. Biochem. Biophys. Methods 2002, 50, 163–178. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, V.K.; Kalonia, D.S. Second derivative tryptophan fluorescence spectroscopy as a tool to characterize partially unfolded intermediates of proteins. Int. J. Pharm. 2005, 294, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Weber, G. Fluorescent indicators of adsorption in aqueous solution and on the solid phase. Biochem. J. 1954, 56, 31. [Google Scholar]
- Dockal, M.; Carter, D.C.; Rüker, F. The three recombinant domains of human serum albumin. Structural characterization and ligand binding properties. J. Biol. Chem. 1999, 274, 29303–29310. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Maruyama, T.; Kragh-Hansen, U.; Otagiri, M. Characterization of site I on human serum albumin: Concept about the structure of a drug binding site. Biochim. Biophys. Acta 1996, 1295, 147–157. [Google Scholar] [CrossRef]
- Chen, R.F. Fluorescence of dansyl amino acids in organic solvents and protein solution. Arch. Biochem. Biophys. 1967, 120, 609–620. [Google Scholar] [CrossRef]
- Ellman, G. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Maciążek-Jurczyk, M.; Szkudlarek, A. Spectroscopic analysis of the impact of oxidative stress on the structure of human serum albumin (HSA) in terms of its binding properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Aubin-Tam, M.E.; Hamad-Schifferli, K. Gold nanoparticle cytochrome c complexes: The effect of nanoparticle ligand charge on protein structure. Langmuir 2005, 21, 12080–12084. [Google Scholar] [CrossRef] [PubMed]
- Trynda-Lemiesz, L.; Wiglusz, K. Effects of glycation on meloxicam binding to human serum albumin. J. Mol. Struct. 2011, 995, 35–40. [Google Scholar] [CrossRef]
- Szkudlarek, A.; Maciążek-Jurczyk, M.; Chudzik, M.; Równicka-Zubik, J.; Sułkowska, A. Alteration of human serum albumin tertiary structure induced by glycation. Spectroscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: London, UK; Weinheim, Germany, 2002. [Google Scholar]
- Eftink, M.R.; Ghiron, C.A. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry 1976, 15, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.B.; Naik, L.R.; Kadadevarmath, J.S.; Pal, H.; Rao, V.J. Fluorescence quenching of anthrylvinyl acetate by carbon tetrachloride. Photochem. Photobiol. A 2010, 214, 145–151. [Google Scholar] [CrossRef]
- Geethanjali, H.S.; Nagaraja, D.; Melavanki, R.M.; Kusanur, R.A. Fluorescence quenching of boronic acid derivatives by aniline in alcohols—A Negative deviation from Stern-Volmer equation. J. Lumin. 2015, 167, 216–221. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 130–135. [Google Scholar]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Joseph, K.S.; Anguizola, J.; Hage, D.S. Binding of tolbutamide to glycated human serum albumin. J. Pharm. Biomed. Anal. 2011, 54, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Riener, C.K.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.P. Fluorescence Instrumentation and Methodology. In Excited States of Proteins and Nucleic Acids; Steiner, R.F., Weinryb, I., Eds.; Springer: Boston, MA, USA, 1971. [Google Scholar]
- Rub, M.A.; Khan, J.M.; Asiri, A.M.; Khan, R.H.; Din, K. Study on the interaction between amphiphilic drug and bovine serum albumin: A thermodynamic and spectroscopic description. J. Lumin. 2014, 155, 39–46. [Google Scholar] [CrossRef]
- Curry, S.; Brick, P.; Franks, N. Fatty acid binding to human serum albumin: New insights from crystallographic studies. Biochim. Biophys. Acta 1999, 1441, 131–140. [Google Scholar] [CrossRef]
- Taira, Z.; Terada, H. Specific and non-specific ligand binding to serum albumin. Biochem. Pharmacol. 1985, 34, 1999–2005. [Google Scholar] [PubMed]
- Lehrer, S.S. Solute Perturbation of Protein Fluorescence. The Quenching of the Tryptophyl Fluorescence of Model Compounds and of Lysozyme by Iodide Ion. Biochemistry 1971, 10, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, T. Conformational changes in the 23-kilodalton NH2-terminal peptide segment of myosin ATPase associated with ATP hydrolysis. J. Biol. Chem. 1990, 265, 18786–18790. [Google Scholar] [PubMed]
- Klotz, I.M.; Hunston, D.L. Properties of graphical representations of multiple classes of binding sites. Biochemistry 1971, 10, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
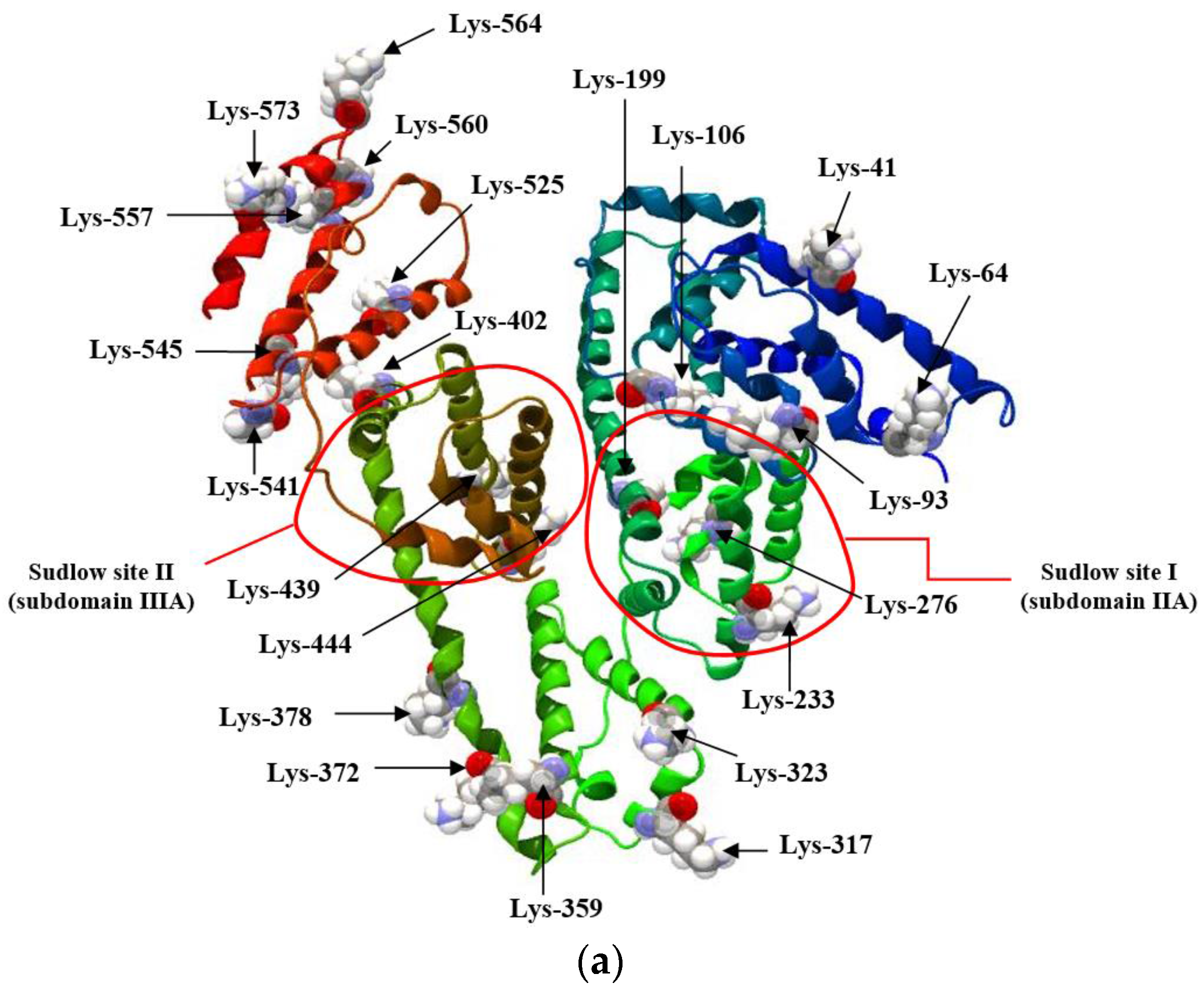
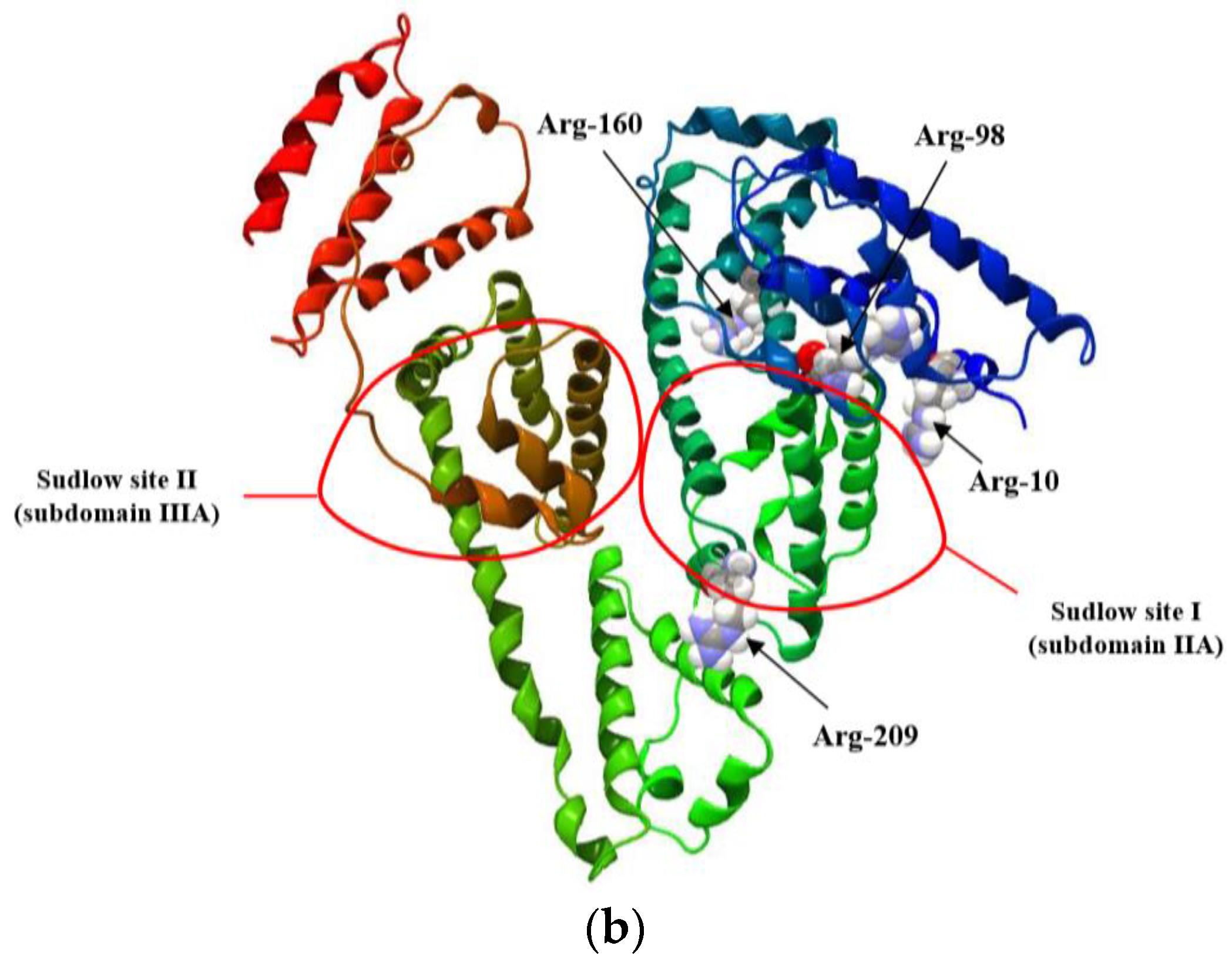
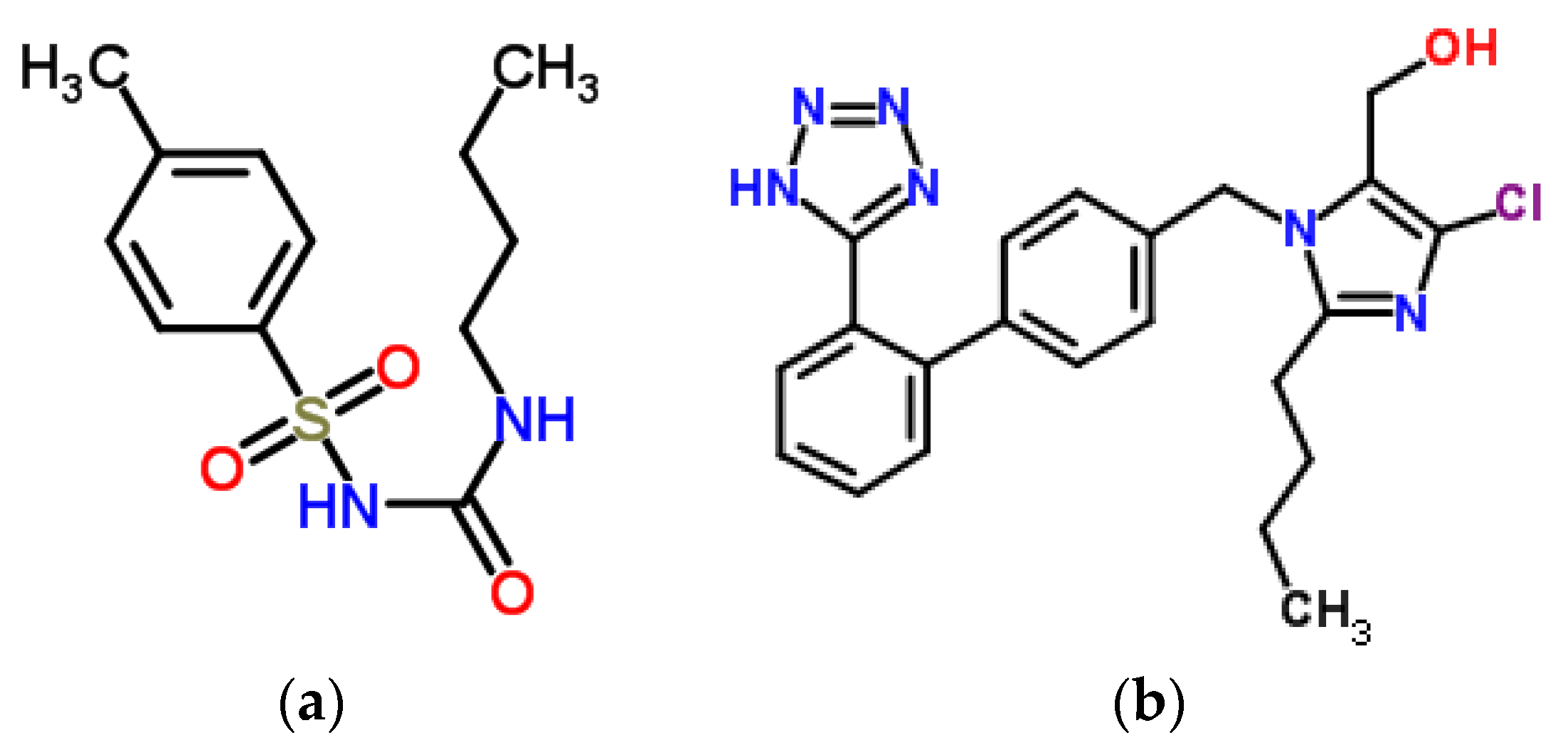
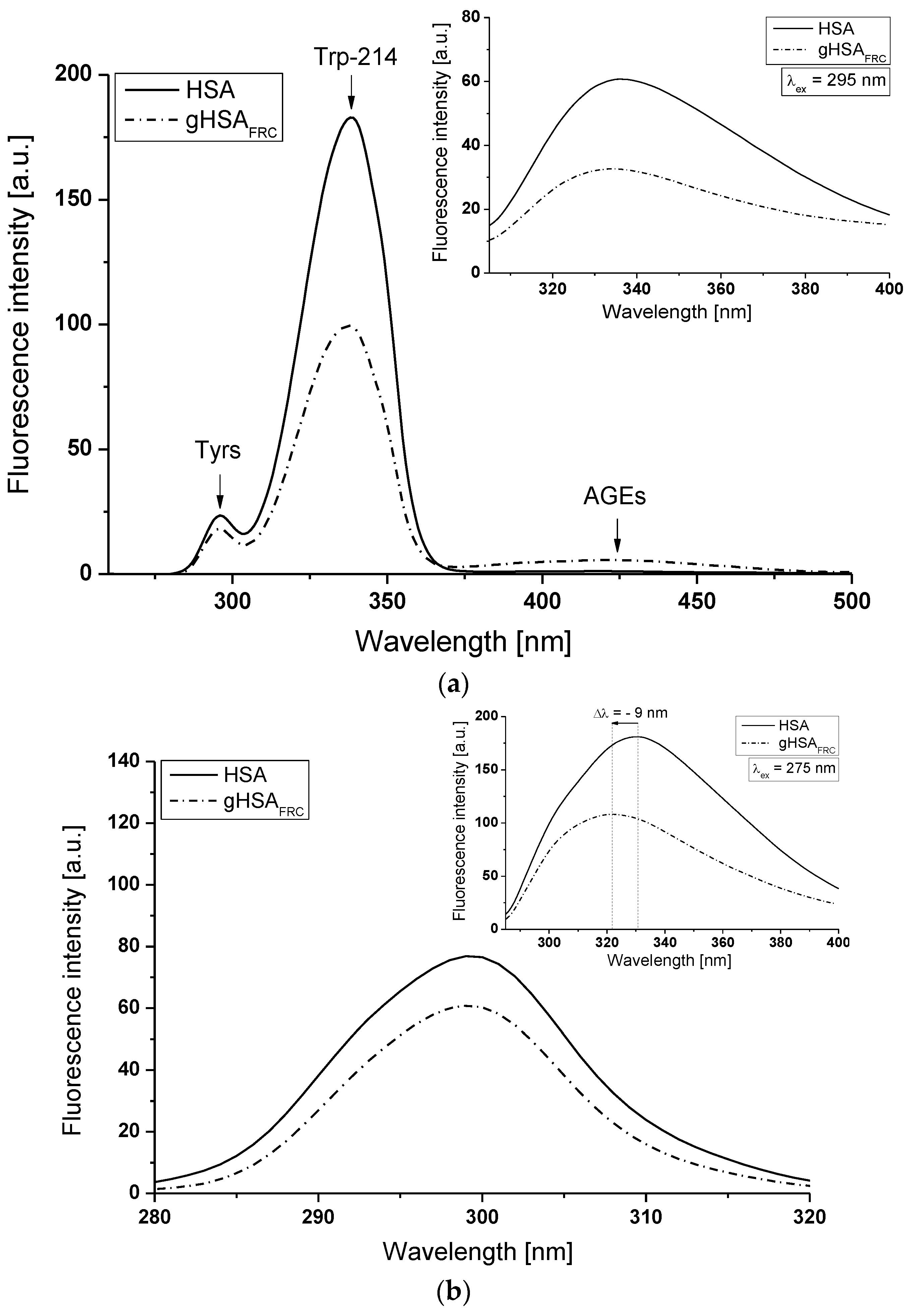
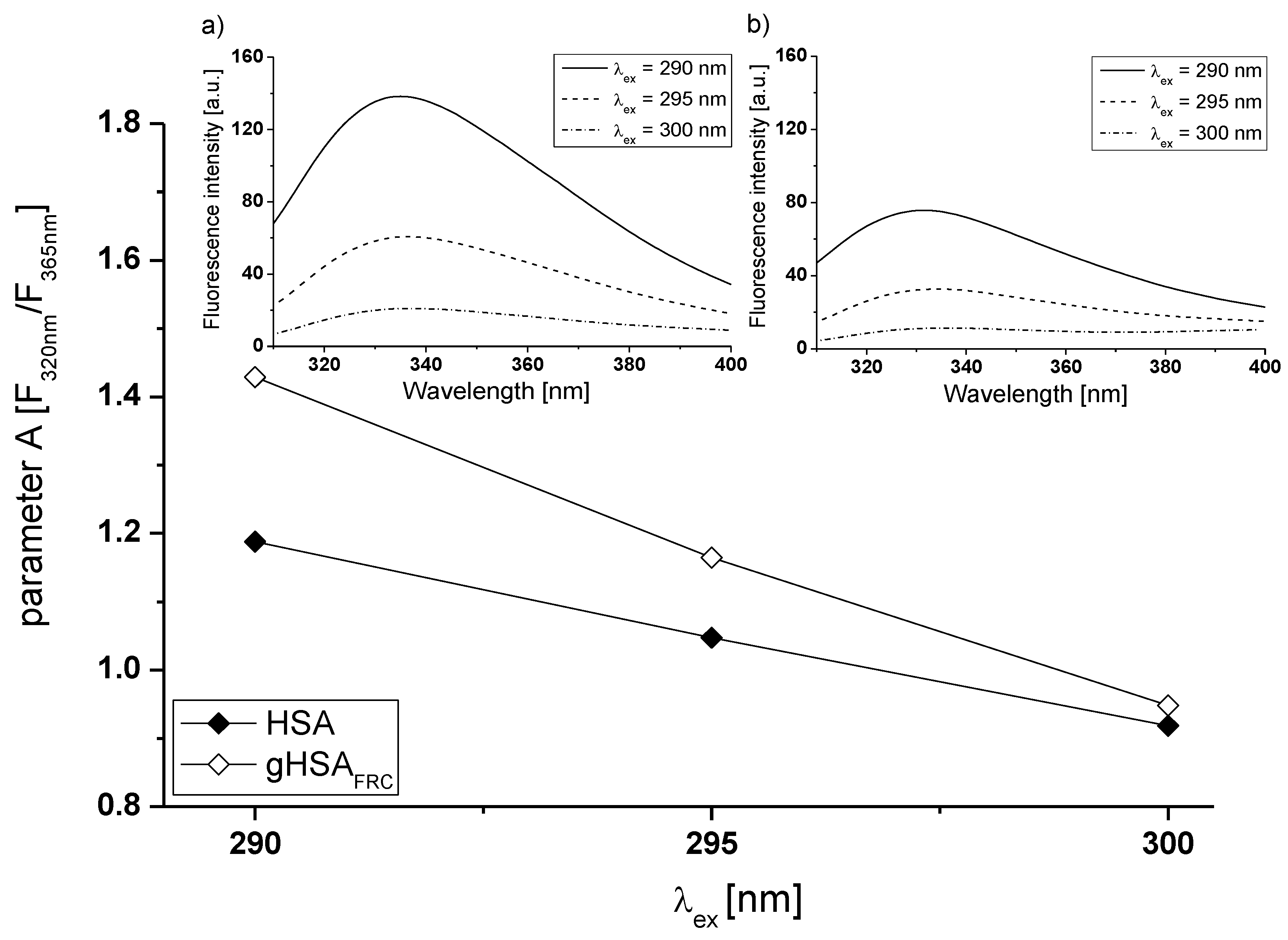
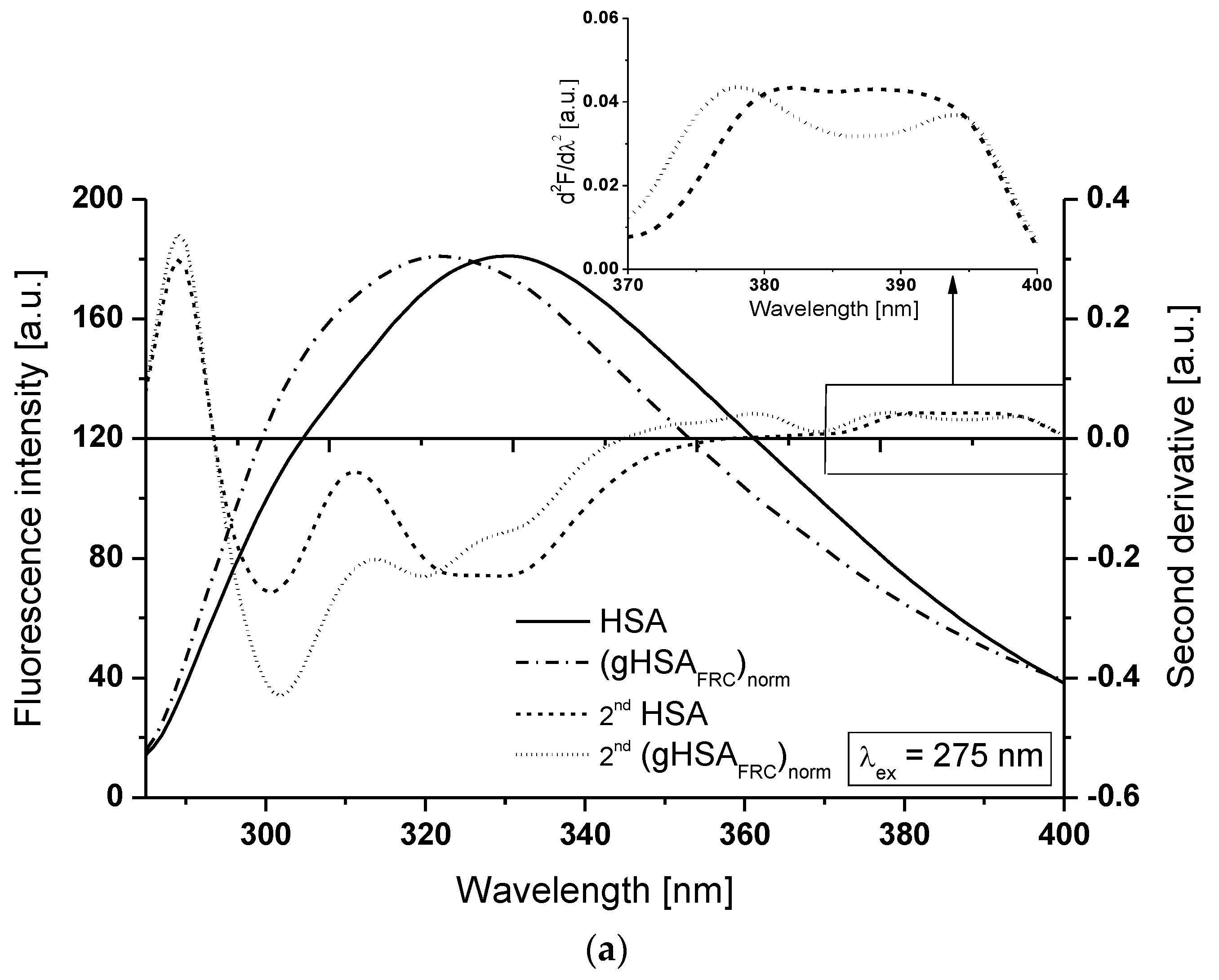
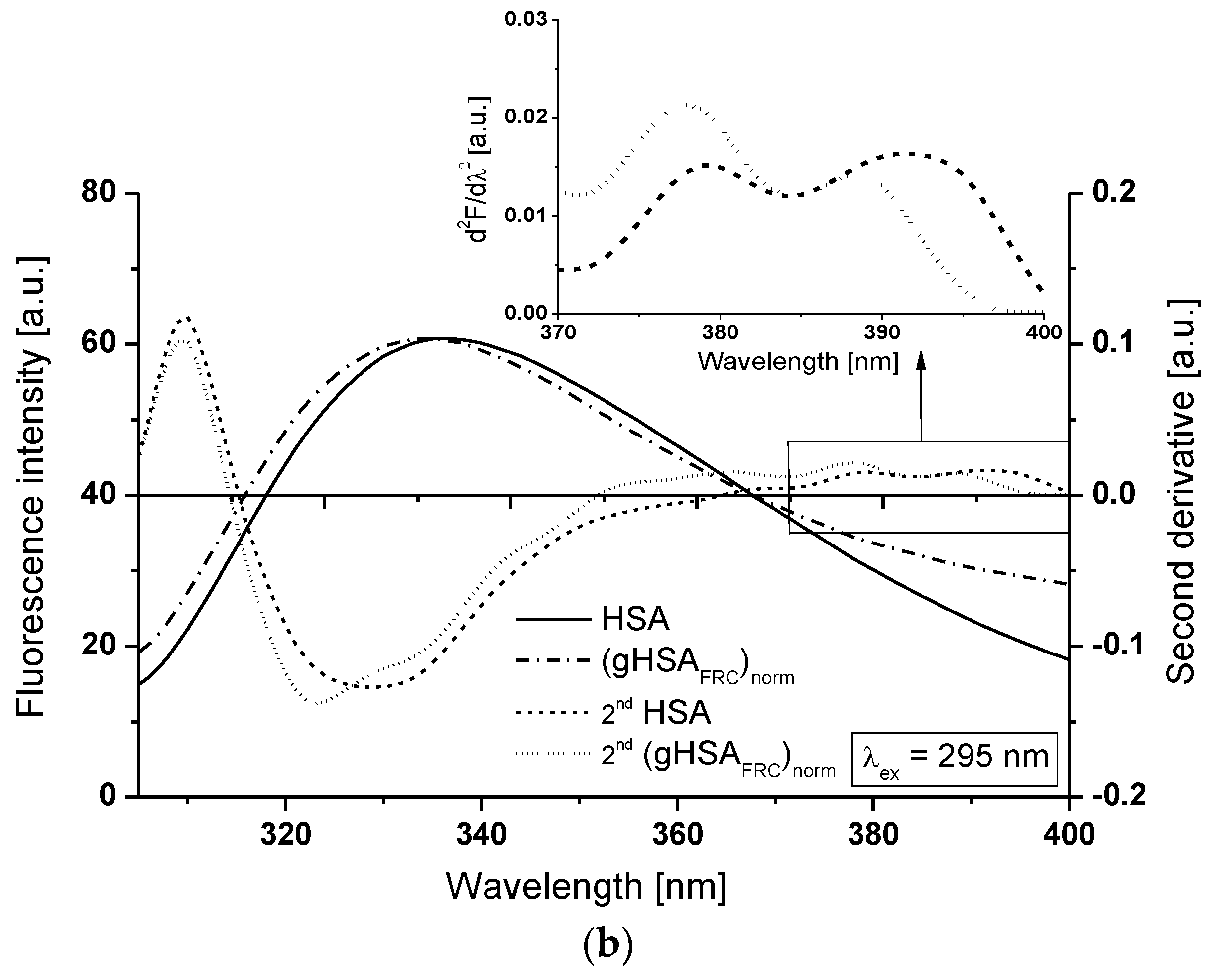
| H275nm | λmin (nm) | λmax (nm) | H295nm | λmin (nm) | λmax (nm) | |
|---|---|---|---|---|---|---|
| HSA | 0.201 | 301 | 311 | 0.004 | 385 | 392 |
| (gHSAFRC)norm | 0.228 | 302 | 314 | 0.002 | 385 | 389 |
| at 222 nm (deg·cm2·dmol−1) | % α-Helix | % β-Sheet | % Other | |
|---|---|---|---|---|
| HSA | −11,770.59 | 36.10 | 47.60 | 16.30 |
| gHSAFRC | −11,108.69 | 43.30 | 43.80 | 12.90 |
| λex = 275 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | a ± RSD *) × 1012 (mol−1∙L∙s−1) |
| TB-HSA | 2.47 ± 0.01 | 0.32 ± 0.01 | 3.98 ± 0.02 |
| TB-gHSAFRC | 4.39 ± 0.05 | 0.22 ± 0.01 | 7.08 ± 0.08 |
| LOS-HSA | 6.24 ± 0.02 | 0.47 ± 0.01 | 10.06 ± 0.03 |
| LOS-gHSAFRC | 6.92 ± 0.03 | 0.42 ± 0.01 | 11.16 ± 0.05 |
| λex = 295 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | a ± RSD *) × 1012 (mol−1∙L∙s−1) |
| TB-HSA | 1.83 ± 0.02 | 0.50 ± 0.02 | 2.95 ± 0.03 |
| TB-gHSAFRC | 2.45 ± 0.01 | 0.48 ± 0.02 | 3.95 ± 0.02 |
| LOS-HSA | 3.71 ± 0.01 | 0.72 ± 0.01 | 5.98 ± 0.02 |
| LOS-gHSAFRC | 4.15 ± 0.02 | 0.74 ± 0.01 | 6.69 ± 0.03 |
| λex = 275 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | a ± RSD *) × 1012 (mol−1∙L∙s−1) |
| TB-LOS(const)-HSA | 2.43 ± 0.01 | 0.27 ± 0.01 | 3.92 ± 0.02 |
| TB-LOS(const)-gHSAFRC | 4.99 ± 0.06 | 0.20 ± 0.01 | 8.05 ± 0.10 |
| LOS-TB(const)-HSA | 1.73 ± 0.09 | 0.64 ± 0.05 | 2.79 ± 0.14 |
| LOS-TB(const)-gHSAFRC | 7.71 ± 0.09 | 0.37 ± 0.01 | 12.44 ± 0.14 |
| λex = 295 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | a ± RSD *) × 1012 (mol−1∙L∙s−1) |
| TB-LOS(const)-HSA | 1.88 ± 0.02 | 0.46 ± 0.02 | 3.03 ± 0.03 |
| TB-LOS(const)-gHSAFRC | 3.36 ± 0.02 | 0.38 ± 0.01 | 5.42 ± 0.03 |
| LOS-TB(const)-HSA | 2.33 ± 0.03 | 0.79 ± 0.02 | 3.76 ± 0.05 |
| LOS-TB(const)-gHSAFRC | 3.36 ± 0.02 | 0.73 ± 0.01 | 5.42 ± 0.03 |
| Scatchard Method | Klotz Method | Hill Method | |||
|---|---|---|---|---|---|
| λex = 275 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) |
| TB-HSA | 2.84 ± 0.07 | 1.11 ± 0.04 | 2.88 ± 0.01 | 1.10 ± 0.01 | 1.17 ± 0.03 |
| TB-gHSAFRC | 5.17 ± 0.23 | 1.00 ± 0.08 | 5.24 ± 0.10 | 1.00 ± 0.01 | 1.00 ± 0.03 |
| TB-LOS(const)-HSA | 2.61 ± 0.10 | 0.91 ± 0.05 | 2.73 ± 0.01 | 0.89 ± 0.02 | 0.91 ± 0.01 |
| TB-LOS(const)-gHSAFRC | 6.03 ± 0.17 | 0.95 ± 0.05 | 5.97 ± 0.06 | 0.95 ± 0.01 | 0.88 ± 0.02 |
| λex = 295 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) |
| TB-HSA | 1.94 ± 0.09 | 1.16 ± 0.08 | 2.06 ± 0.02 | 1.12 ± 0.04 | 1.19 ± 0.04 |
| TB-gHSAFRC | 2.61 ± 0.19 | 1.12 ± 0.13 | 2.83 ± 0.01 | 1.09 ± 0.04 | 1.21 ± 0.07 |
| TB-LOS(const)-HSA | 1.90 ± 0.14 | 1.13 ± 0.12 | 2.13 ± 0.02 | 1.06 ± 0.05 | 1.15 ± 0.05 |
| TB-LOS(const)-gHSAFRC | 3.86 ± 0.20 | 0.97 ± 0.09 | 3.92 ± 0.04 | 0.96 ± 0.02 | 0.95 ± 0.03 |
| Scatchard Method | Klotz Method | Hill Method | |||
|---|---|---|---|---|---|
| λex = 275 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) |
| LOS-HSA | 8.13 ± 0.41 | 1.00 ± 0.08 | 8.64 ± 0.11 | 0.98 ± 0.02 | 1.03 ± 0.03 |
| LOS-gHSAFRC | 9.21 ± 0.40 | 0.97 ± 0.07 | 9.63 ± 0.08 | 0.96 ± 0.02 | 0.97 ± 0.02 |
| LOS-TB(const)-HSA | 2.10 ± 0.18 | 0.50 ± 0.06 | 1.85 ± 0.09 | 0.55 ± 0.04 | 0.80 ± 0.03 |
| LOS-TB(const)-gHSAFRC | 10.37 ± 0.70 | 1.03 ± 0.12 | 11.17 ± 0.27 | 1.01 ± 0.02 | 1.12 ± 0.06 |
| λex = 295 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) |
| LOS-HSA | 4.62 ± 0.07 | 1.02 ± 0.02 | 4.69 ± 0.01 | 1.02 ± 0.01 | 1.03 ± 0.01 |
| LOS-gHSAFRC | 5.31 ± 0.11 | 0.97 ± 0.03 | 5.34 ± 0.01 | 0.97 ± 0.01 | 0.97 ± 0.01 |
| LOS-TB(const)-HSA | 2.64 ± 0.09 | 0.82 ± 0.04 | 2.66 ± 0.03 | 0.81 ± 0.02 | 0.90 ± 0.02 |
| LOS-TB(const)-gHSAFRC | 4.26 ± 0.07 | 1.05 ± 0.03 | 4.24 ± 0.02 | 1.05 ± 0.01 | 1.05 ± 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudlarek, A.; Pentak, D.; Ploch, A.; Pożycka, J.; Maciążek-Jurczyk, M. In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States. Molecules 2017, 22, 2249. https://doi.org/10.3390/molecules22122249
Szkudlarek A, Pentak D, Ploch A, Pożycka J, Maciążek-Jurczyk M. In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States. Molecules. 2017; 22(12):2249. https://doi.org/10.3390/molecules22122249
Chicago/Turabian StyleSzkudlarek, Agnieszka, Danuta Pentak, Anna Ploch, Jadwiga Pożycka, and Małgorzata Maciążek-Jurczyk. 2017. "In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States" Molecules 22, no. 12: 2249. https://doi.org/10.3390/molecules22122249
APA StyleSzkudlarek, A., Pentak, D., Ploch, A., Pożycka, J., & Maciążek-Jurczyk, M. (2017). In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States. Molecules, 22(12), 2249. https://doi.org/10.3390/molecules22122249






