Natural Korean Medicine Dang-Gui: Biosynthesis, Effective Extraction and Formulations of Major Active Pyranocoumarins, Their Molecular Action Mechanism in Cancer, and Other Biological Activities
Abstract
:1. Introduction
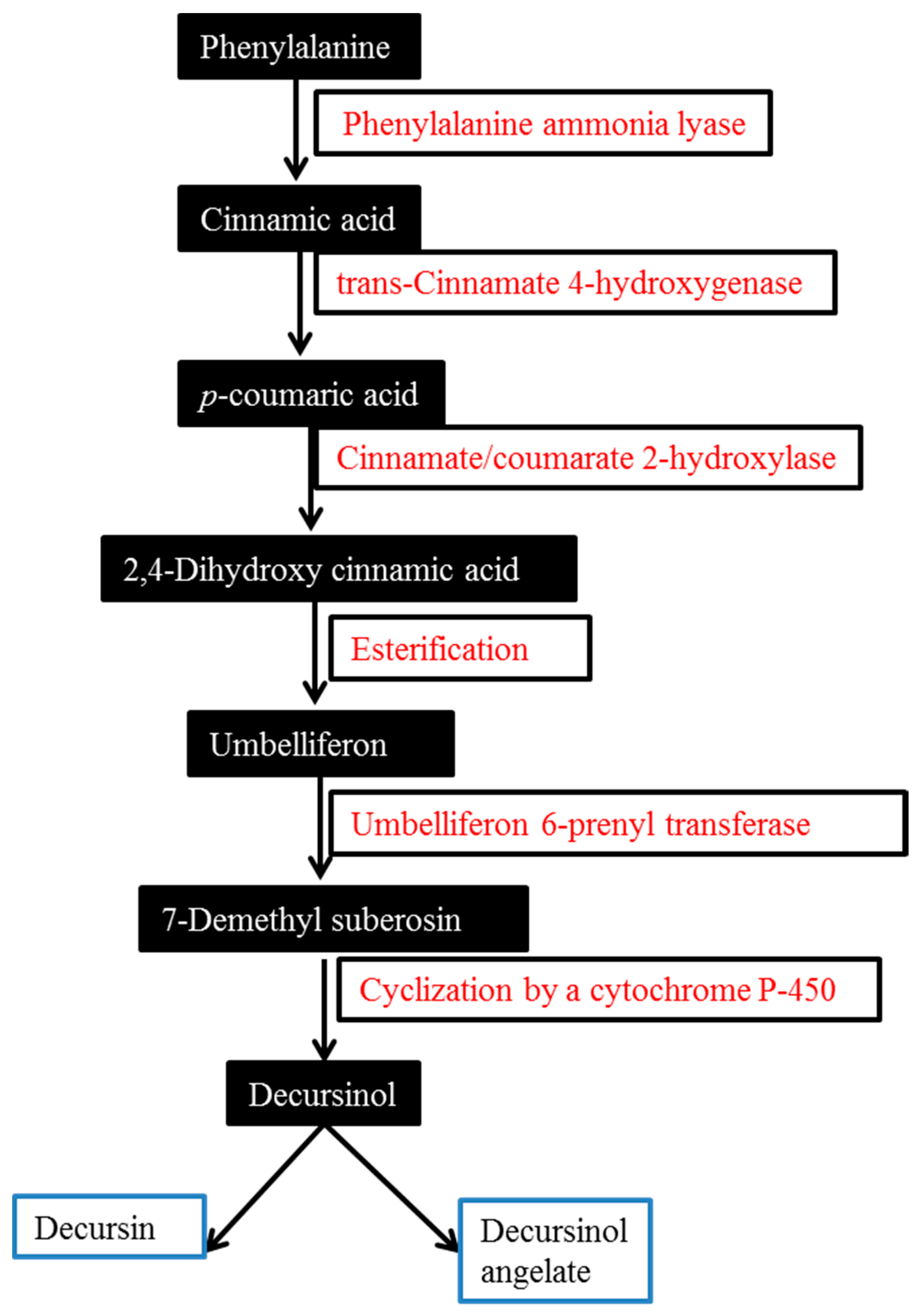
2. Biosynthesis of Major AGNACCs D, DA and DOH
3. Extraction of AGNACCs and Effective Formulation Methods
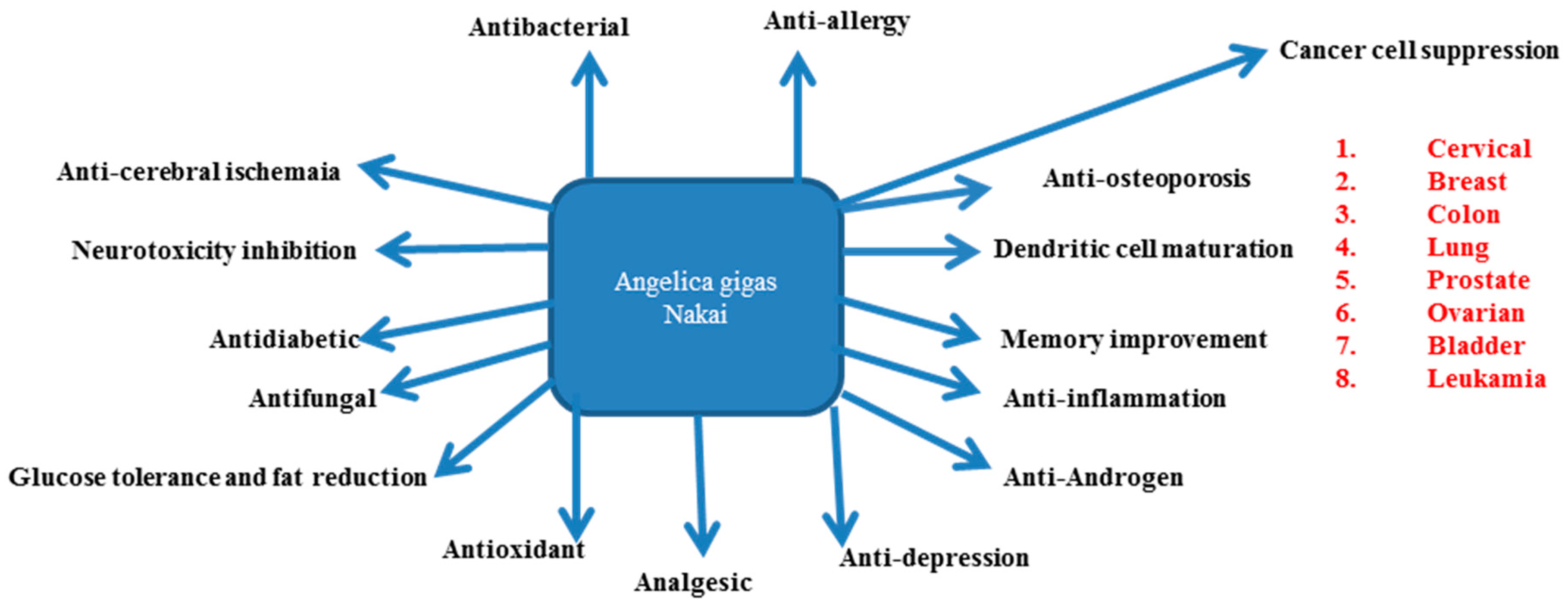
4. AGNACCs and Their Role in Major Biological Activities
5. AGNACCs’ Pharmacokinetics in Rodents and Humans
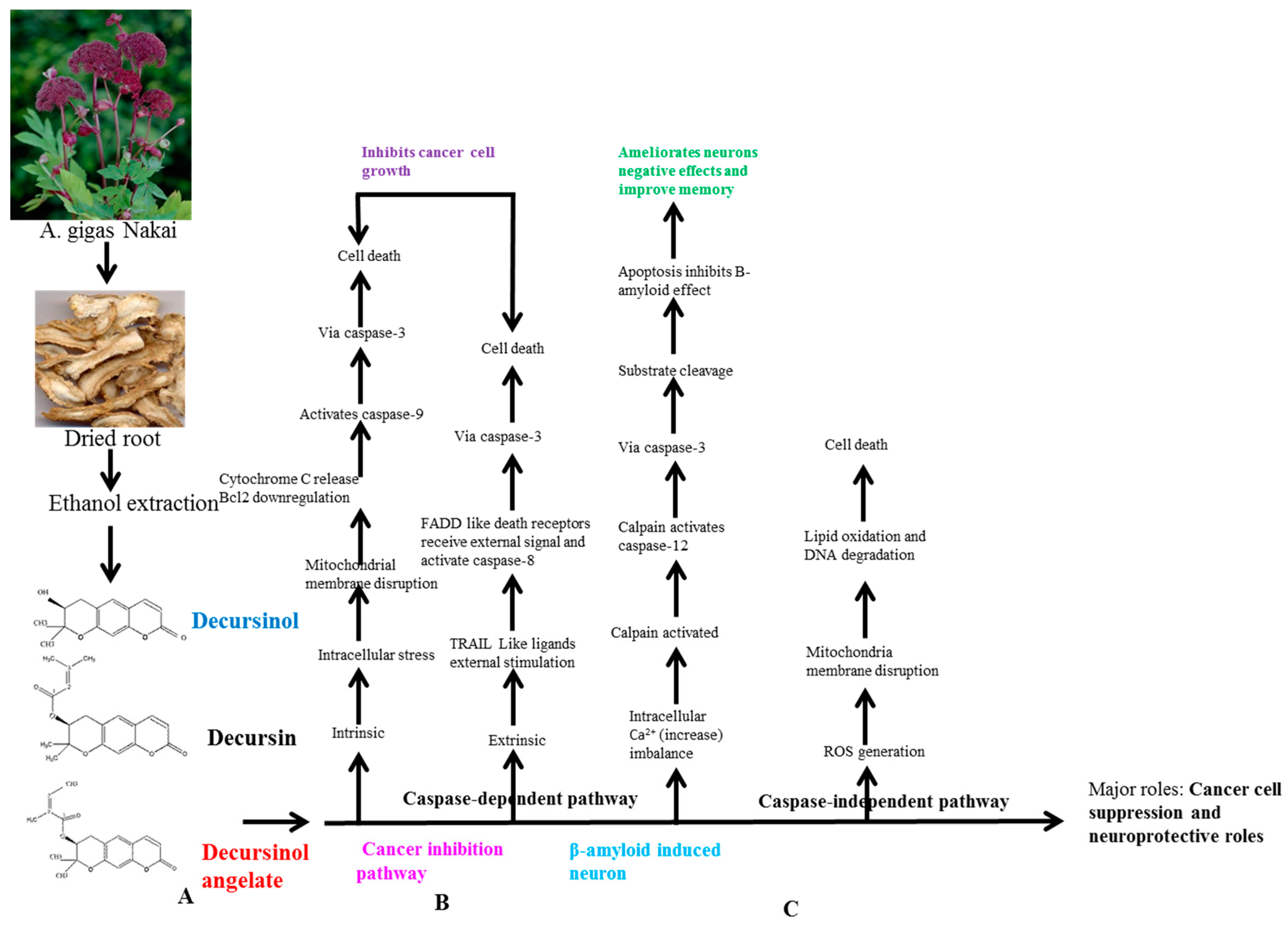
6. AGNACCs’ Molecular Action Mechanism in Cancer
6.1. Basic Principle of AGNACCs’ Action Mechanism in Cancer and Other Biological Activities
6.2. AGNACCs Action Mechanism in Cervical Cancer
6.3. AGNACCs’ Action Mechanism in Prostate Cancer
6.4. AGNACCs’ Action Mechanism in Melanoma
6.5. AGNACCs’ Action Mechanism in Bladder and Colon Cancer
Lung Cancer and Sarcoma
6.6. AGNACCs’ Action Mechanism in Sexual Hormone-Dependent Cancers
7. Other Biological Activities
7.1. AGNACCs’ Molecular Mechanism in Neuroprotective Effects
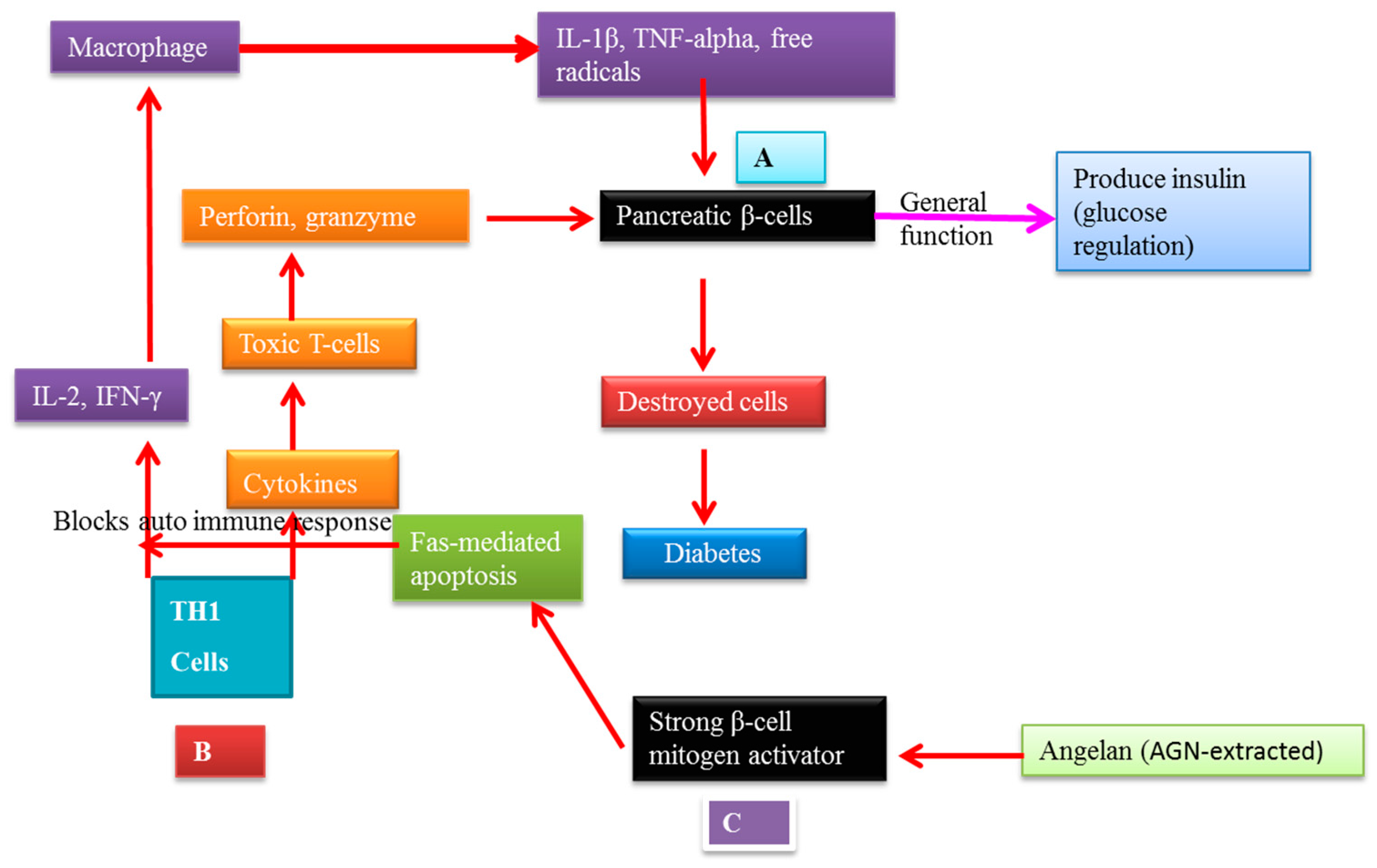
7.2. AGNACCs’ Action Mechanism in Autoimmune Diabetes
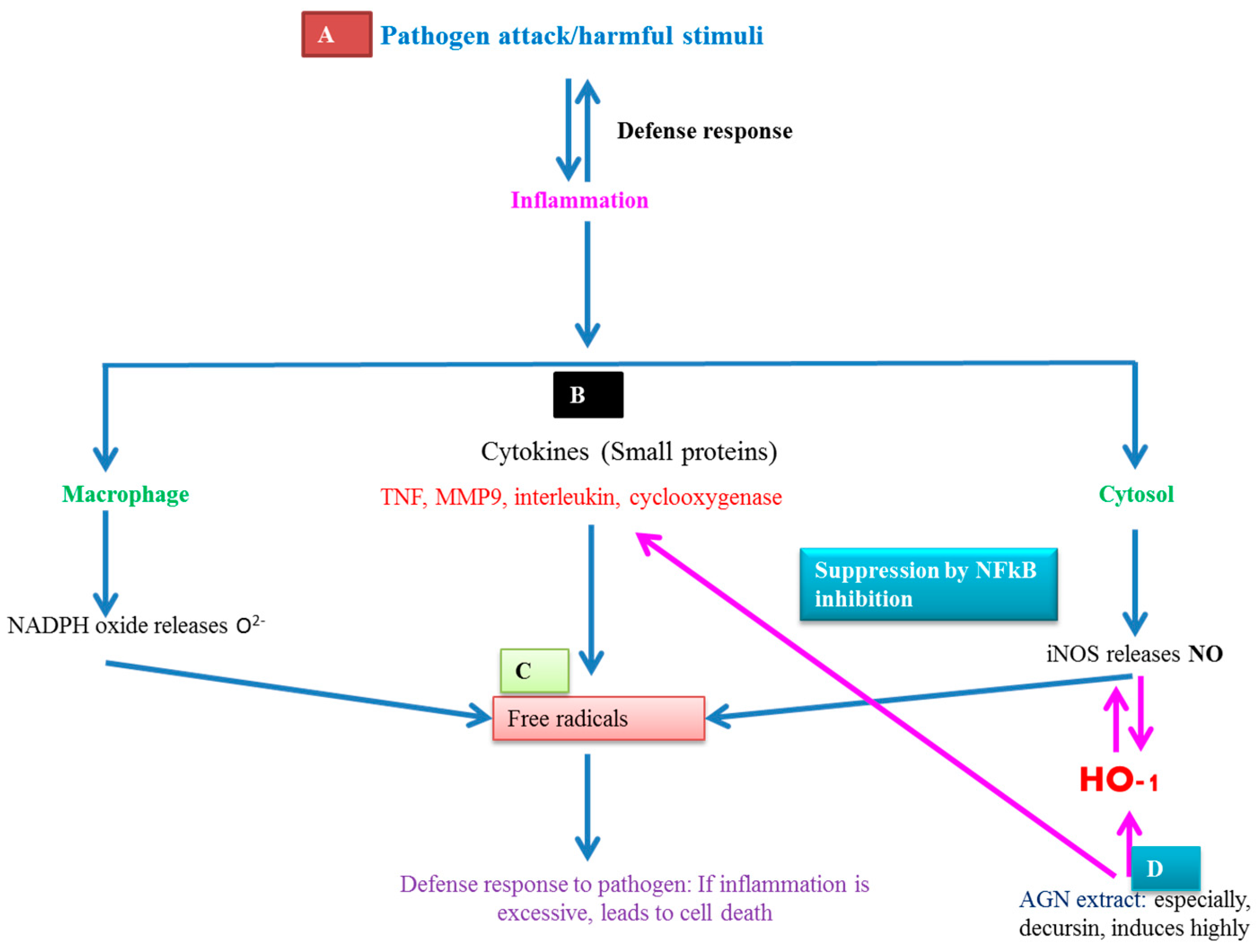
7.3. AGNACCs’ Anti-Inflammatory Activity
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, C.; Guo, J.; Wang, Z.; Xiao, B.; Lee, H.J.; Lee, E.O.; Lu, J. Decursin and decursinol angelate inhibit estrogen-stimulated and estrogen-independent growth and survival of breast cancer cells. Breast Cancer Res. 2007, 9, R77. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, J.K.; Zou, L.Y.; Wu, T.; Lee, Y.W.; Kim, Y.H. Decursin isolated from Angelica gigas Nakai rescues PC12 cells from amyloid-protein-induced neurotoxicity through Nrf2-mediated upregulation of heme oxygenase-1: Potential roles of MAPK. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Natural medicine: The genus Angelica. Curr. Med. Chem. 2004, 11, 1479–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Jiang, C.; Xing, C.; Kim, S.H.; Lu, J. Anti-cancer and other bioactivities of Korean Angelica gigas Nakai (AGN) and its major pyranocoumarin compounds. Anticancer Agents Med. Chem. 2012, 12, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Nam, S.; Park, J.H.; Lee, S.Y.; Jeong, J.Y.; Lee, J.Y.; Cho, H.J. Nanocomposites based on Soluplus and Angelica gigas Nakai extract fabricated by an electrohydrodynamic method for oral administration. J. Colloid Interface Sci. 2016, 484, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Lee, J.H.; Kim, J.Y.; Park, K.W.; Jeong, I.Y.; Shim, K.H.; Seo, K.I. Decursin from Angelicagigas Nakai induces apoptosis in RC-58T/h/SA# 4 primary human prostate cancer cells via a mitochondria-related caspase pathway. Food Chem. Toxicol. 2011, 49, 2517–2523. [Google Scholar] [PubMed]
- Choi, S.S.; Han, K.J.; Lee, H.K.; Han, E.J.; Suh, H.W. Antinociceptive profiles of crude extract from roots of Angelica gigas NAKAI in various pain models. Biol. Pharm. Bull. 2003, 26, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Park, J.H.; Shin, B.N.; Ahn, J.H.; Kim, I.H.; Lee, J.C.; Lee, Y.L. Neuroprotective effect of a new synthetic aspirin-decursinol adduct in experimental animal models of ischemic stroke. PLoS ONE 2013, 8, e74886. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jung, J.M.; Lee, H.E.; Lee, Y.W.; Kim, D.H.; Kim, J.M.; Jang, D.S. The memory ameliorating effects of INM-176, an ethanolic extract of Angelica gigas, against scopolamine-or Aβ 1–42-induced cognitive dysfunction in mice. J. Ethnopharmacol. 2012, 143, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, J.S.; Park, S.K.; Lee, K.; Kim, J.Y.; Kim, Y.J.; Han, S.B. Antidiabetic activity of angelan isolated from Angelica gigas Nakai. Arch. Pharm. Res. 2008, 31, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.S.; Park, D.; Shin, S.; Jeon, J.H.; Kim, T.K.; Choi, Y.J.; Lee, D.I. Anti-allergic effects and mechanisms of action of the ethanolic extract of Angelica gigas in dinitrofluorobenzene-induced inflammation models. Environ. Toxicol. Pharmacol. 2010, 30, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kwon, J.E.; Cho, Y.; Kim, I.; Kang, S.C. Anti-inflammatory effect of Angelica gigas via heme oxygenase (HO)-1 expression. Nutrients 2015, 7, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Kim, Y.H.; Lee, C.W.; Park, S.M.; Lee, H.Y.; Ahn, K.S.; Kim, H.M. Characteristic immunostimulation by angelan isolated from Angelica gigas Nakai. Immunopharmacology 1998, 40, 39–48. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, C.; Wang, Z.; Lee, H.J.; Hu, H.; Malewicz, B.; Kim, D.K. A novel class of pyranocoumarin anti–androgen receptor signaling compounds. Mol. Cancer Ther. 2007, 6, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Heo, B.G.; Park, Y.S.; Chon, S.U.; Lee, S.Y.; Cho, J.Y.; Gorinstein, S. Antioxidant activity and cytotoxicity of methanol extracts from aerial parts of Korean salad plants. BioFactors 2007, 30, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Kim, S.H.; Hur, H.J.; Kim, H.J.; Park, J.H.; Sung, M.J.; Kim, M.S. Decursin, an Active Compound Isolated from Angelica gigas, Inhibits Fat Accumulation, Reduces Adipocytokine Secretion and Improves Glucose Tolerance in Mice Fed a High-Fat Diet. Phytother. Res. 2012, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Park, J.H.; Kim, J.D.; Lee, J.C.; Kim, I.H.; Yim, Y.; Yoo, K.Y. Protective effects of a novel synthetic alpha-lipoic acid-decursinol hybrid compound in experimentally induced transient cerebral ischemia. Cell. Mol. Neurobiol. 2012, 32, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y.; Kim, Y.S.; Ryu, S.Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. In vitro and in vivo antifungal activities of decursin and decursinol angelate isolated from Angelica gigas against Magnaporthe oryzae, the causal agent of rice blast. Pestic. Biochem. Physiol. 2011, 101, 118–124. [Google Scholar] [CrossRef]
- Yim, D.; Singh, R.P.; Agarwal, C.; Lee, S.; Chi, H.; Agarwal, R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005, 65, 1035–1044. [Google Scholar] [PubMed]
- Zhang, J.; Li, L.; Tang, S.; Hale, T.W.; Xing, C.; Jiang, C.; Lü, J. Cytochrome P450 isoforms in the metabolism of decursin and decursinol angelate from Korean angelica. Am. J. Chin. Med. 2015, 43, 1211–1230. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; El-Aty, A.A.; Choi, J.H.; Kim, M.R.; Shim, J.H. Optimized conditions for the extraction of secondary volatile metabolites in Angelica roots by accelerated solvent extraction. J. Pharm. Biomed. Anal. 2007, 44, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Nancy, L.P. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Bum, H.; Soo, U.K. Determination of biosynthetic pathway of decursin in hairy root culture of Angelica gigas. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 258–262. [Google Scholar] [CrossRef]
- Park, J.H.; Park, N.I.; Xu, H.; Park, S.U. Cloning and characterization of phenylalanine ammonia-lyase and cinnamate 4-hydroxylase and pyranocoumarin biosynthesis in Angelica gigas. J. Nat. Prod. 2010, 73, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Park, N.I.; Park, J.H.; Park, S.U. Overexpression of Cinnamate 4-Hydroxylase Gene Enhances Biosynthesis of Decursinol Angelate in Angelica gigas Hairy Roots. Mol. Biotechnol. 2012, 50, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.Y.; Lee, J.Y.; Kwak, B.Y.; Lee, H.G. Estrogenic Effects of Various Extracts from Chamdanggui (Angelica gigas Nakai) and Sogdan (Phlomis umbrosa Turcz). Food Sci. Biotechnol. 2011, 20, 1113–1118. [Google Scholar] [CrossRef]
- Choi, K.O.; Lee, I.; Paik, S.Y.R.; Kim, D.E.; Lim, J.D.; Kang, W.S.; Ko, S. Ultrafine Angelica gigas powder normalizes ovarian hormone levels and has antiosteoporosis properties in ovariectomized rats: Particle size effect. J. Med. Food 2012, 15, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Lee, J.Y.; Weon, J.B.; Ma, C.; Ko, H.J.; Kim, D.D.; Cho, H.J. Angelica gigas Nakai and Soluplus-based solid formulations prepared by hot-melting extrusion: Oral absorption enhancing and memory ameliorating effects. PLoS ONE 2015, 10, e0124447. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.S.; Park, J.H.; Kang, H.J.; Choe, J.H.; Goh, M.S.; Kim, D.D.; Cho, H.J. Poly (d,l-lactic acid)-glycerol-based nanoparticles for curcumin delivery. Int. J. Pharm. 2015, 488, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.; Janero, D.R.; Amiji, M. Engineering of an ω-3 polyunsaturated fatty acid-containing nanoemulsion system for combination C6-ceramide and 17β-estradiol delivery and bioactivity in human vascular endothelial and smooth muscle cells. Nanomedicine 2013, 9, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Ku, W.S.; Termsarasab, U.; Yoon, I.; Chung, C.W.; Moon, H.T.; Kim, D.D. Development of udenafil-loaded microemulsions for intranasal delivery: In vitro and in vivo evaluations. Int. J. Pharm. 2012, 423, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Doh, H.J.; Jung, Y.; Balakrishnan, P.; Cho, H.J.; Kim, D.D. A novel lipid nanoemulsion system for improved permeation of granisetron. Colloids Surf. B Biointerfaces 2013, 101, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Park, J.H.; Lee, J.Y.; Jeong, J.Y.; Lee, S.Y.; Yoon, I.S.; Cho, H.J. Omega-3 fatty acids incorporated colloidal systems for the delivery of Angelica gigas Nakai extract. Colloids Surf. B Biointerfaces 2016, 140, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Williams, M.A.; Jones, D.S.; Andrews, G.P. Hot-melt extrusion technology and pharmaceutical application. Ther. Deliv. 2012, 3, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Abd El-Aty, A.M.; Kim, I.S.; Shim, J.H. Determination of volatile flavor components in danggui cultivars by solvent free injection and hydrodistillation followed by gas chromatographic-mass spectrometric analysis. J. Chromatogr. 2006, 1116, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kim, S.H.; Jiang, C.; Lee, H.; Guo, J. Oriental herbs as a source of novel anti-androgen and prostate cancer chemopreventive agents. Acta Pharmacol. Sin. 2007, 28, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Yang, H.J.; Ma, J.Y.; Ma, C.J. A HPLC-DAD method for the simultaneous determination of five marker components in the traditional herbal medicine Bangpungtongsungsan. Pharmacogn. Mag. 2011, 7, 60–64. [Google Scholar] [PubMed]
- Won, J.B.; Ma, J.Y.; Um, Y.R.; Ma, C.J. Simultaneous determination of five marker constituents in Ssanghwa tang by HPLC/DAD. Pharmacogn. Mag. 2010, 6, 111–115. [Google Scholar] [PubMed]
- Seo, J.S.; Yun, J.H.; Baek, I.S.; Leem, Y.H.; Kang, H.W.; Cho, H.K.; Lyu, Y.S.; Son, H.J.; Han, P.L. Oriental medicine Jangwonhwan reduces A beta (1–42) level and beta-amyloid deposition in the brain of Tg-APPswe/PS1dE9 mouse model of Alzheimer disease. J. Ethnopharmacol. 2010, 128, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Ma, J.Y.; Weon, J.B.; Ma, C.J. Simultaneous determination of eight marker compounds in the traditional herbal medicine, sipjundaebo-tang by HPLC-DAD. Arch. Pharm. Res. 2011, 34, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Shaik, A.A.; Zhang, Y.; Wang, L.; Xing, C.; Kim, S.H.; Lu, J. Quantitative Determination of Decursin, Decursinol Angelate, and Decursinol in Mouse Plasma and Tumor Tissue Using Liquid-Liquid Extraction and HPLC. Planta Medica 2012, 78, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Xing, C.; Kim, S.H.; Lu, J. Single Oral Dose Pharmacokinetics of Decursin, Decursinol Angelate, and Decursinol in Rats. Planta Medica 2013, 79, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Xing, C.; Kim, S.H.; Jiang, C. In Vitro Metabolism of Pyranocoumarin Isomers. Decursin and Decursinol Angelate by Liver Microsomes from Man and Rodents. Planta Medica 2013, 79, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Hale, T.W.; Chee, W.; Xing, C.; Jiang, C.; Lü, J. Single oral dose pharmacokinetics of decursin and decursinol angelate in healthy adult men and women. PLoS ONE 2015, 10, e0114992. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Coggeshall, M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Nesslany, F.; Simar-Meintières, S.; Ficheux, H.; Marzin, D. Aloe-emodin-induced DNA fragmentation in the mouse in vivo comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 678, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lewanski, C.R.; Gullick, W.J. Radiotherapy and cellular signaling. Lancet Oncol. 2001, 2, 366–370. [Google Scholar] [CrossRef]
- Debatin, K.M.; Poncet, D.; Kroemer, G. Chemotherapy: Targeting the mitochondrial cell death pathway. Oncogene 2002, 21, 8786–8803. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.H.; Lee, J.H.; Cho, W.K.; Yang, M.C.; Kwak, D.H.; Ma, J.Y. Decursin and decursinol angelate from Angelica gigas Nakai induce apoptosis via induction of TRAIL expression on cervical cancer cells. Eur. J. Integr. Med. 2011, 3, e299–e307. [Google Scholar] [CrossRef]
- Kim, B.S.; Seo, H.; Kim, H.J.; Bae, S.M.; Son, H.N.; Lee, Y.J.; Nam, J.O. Decursin from Angelica gigas Nakai Inhibits B16F10 Melanoma Growth Through Induction of Apoptosis. J. Med. Food 2015, 18, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Lee, C.W.; Kang, M.R.; Yoon, Y.D.; Kang, J.S.; Lee, K.H.; Kim, H.M. Pectic polysaccharide isolated from Angelica gigas Nakai inhibits melanoma cell metastasis and growth by directly preventing cell adhesion and activating host immune functions. Cancer Lett. 2006, 243, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Lee, S.J.; Choi, Y.D.; Moon, S.K. Decursin inhibits growth of human bladder and colon cancer cells via apoptosis, G1-phase cell cycle arrest and extracellular signal-regulated kinase activations. Int. J. Mol. Med. 2010, 25, 635. [Google Scholar] [PubMed]
- Andriole, G.; Bruchovsky, N.; Chung, L.W.; Matsumoto, A.M.; Rittmaster, R.; Roehrborn, C.; Tindall, D. Dihydrotestosterone and the prostate: The scientific rationale for 5α-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urol. 2004, 172, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Lee, S.H.; Ahn, E.M.; Lee, Y.M. Decursin and decursinol angelate inhibit VEGF-induced angiogenesis via suppression of the VEGFR-2-signaling pathway. Carcinogenesis 2009, 30, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Shin, K.H.; Kim, B.K.; Kang, S.S. Anti-tumor activities of decursinol angelate and decursin from Angelica gigas. Arch. Pharm. Res. 2003, 26, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Taplin, M.E.; Balk, S.P. Androgen receptor: A key molecule in the progression of prostate cancer to hormone independence. J. Cell. Biochem. 2004, 91, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Y.; Lee, J.H.; Cho, M.; Park, B.S.; Kim, D.E.; Oh, S. Decursin suppresses human androgen-independent PC3 prostate cancer cell proliferation by promoting the degradation of β-catenin. Mol. Pharmacol. 2007, 72, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sim, W.S.; Kim, I.H. Detection of anticancer activity from the root of angelica-gigas in-vitro. J. Microbiol. Biotechnol. 1995, 5, 105–109. [Google Scholar]
- Ahn, K.S.; Sim, W.S.; Kim, I.H. Decursin: A cytotoxic agent and protein kinase C activator from the root of Angelica gigas. Planta Medica 1996, 62, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sim, W.S.; Lee, I.K.; Seu, Y.B.; Kim, I.H. Decursinol angelate: A cytotoxic and protein kinase C activating agent from the root of Angelica gigas. Planta Medica 1997, 63, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Ahn, K.S.; Han, H.Y.; Choung, S.Y.; Choi, S.Y.; Kim, I.H. Decursin and PDBu: Two PKC activators distinctively acting in the megakaryocytic differentiation of K562 human erythroleukemia cells. Leuk. Res. 2005, 29, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Bang, S.S.; Choi, J.S.; Han, H.; Kim, I.H. Involvement of PKC and ROS in the cytotoxic of anti-leukemic decursin and its derivatives mechanism and their structure-activity relationship in human K562 erythroleukemia and U937 myeloleukemia cells. Cancer Lett. 2005, 223, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Q.; Jeong, S.J.; Lee, H.J.; Kwon, H.Y.; Han, I.; Kim, H.S.; Lee, E.O.; Ahn, K.S.; Jung, M.H.; Zhu, S.D.; et al. Inhibition of cyclooxygenase-2-dependent survivin mediates decursin-induced apoptosis in human KBM-5 myeloid leukemia cells. Cancer Lett. 2010, 298, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.M.; Park, K.R.; Jang, H.J.; Na, Y.S.; Ahn, K.S.; Kim, S.H. Decursin chemosensitizes human multiple myeloma cells through inhibition of STAT3 signaling pathway. Cancer Lett. 2011, 301, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jeong, S.J.; Kwon, H.Y.; Jung, J.H.; Sohn, E.J.; Lee, H.J.; Kim, S.H. Decursin and doxorubicin are in synergy for the induction of apoptosis via STAT3 and/or mTOR pathways in human multiple myeloma cells. J. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Islam, S.U.; Ahn, E.M.; Lee, Y.M.; Lee, Y.S. Decursinol angelate inhibits PGE2-induced survival of the human leukemia HL-60 cell line via regulation of the EP2 receptor and NF-κB pathway. Cancer Boil. Ther. 2016, 17, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeon, S.J.; Jung, J.W.; Lee, S.; Yoon, B.H.; Shin, B.Y.; Ko, K.H. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur. J. Pharmacol. 2007, 574, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. New trends in cholinergic therapy for Alzheimer disease: Nicotinic agonists or cholinesterase inhibitors? Prog. Brain Res. 1996, 109, 311–323. [Google Scholar] [PubMed]
- Kang, S.Y.; Lee, K.Y.; Sung, S.H.; Park, M.J.; Kim, Y.C. Coumarins Isolated from Angelica gigas Inhibit Acetylcholinesterase: Structure−Activity Relationships. J. Nat. Prod. 2001, 64, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Lee, K.Y.; Park, M.J.; Kim, Y.C.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. Decursin from Angelica gigas mitigates amnesia induced by scopolamine in mice. Neurobiol. Learn. Mem. 2003, 79, 11–18. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, Y.C. Decursinol and decursin protect primary cultured rat cortical cells from glutamate-induced neurotoxicity. J. Pharm. Pharmacol. 2007, 59, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Funkenstein, H.H.; Albert, M.S.; Scherr, P.A.; Cook, N.R.; Chown, M.J.; Taylor, J.O. Prevalence of Alzheimer’s disease in a community population of older persons: Higher than previously reported. JAMA 1989, 262, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Kim, D.H.; Moon, Y.S.; Jung, J.S.; Ahn, E.M.; Baek, N.I.; Song, D.K. Protection against β-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 25–30. [Google Scholar] [CrossRef]
- Park, S.J.; Jung, H.J.; Son, M.S.; Jung, J.M.; Kim, D.H.; Jung, I.H.; Cho, Y.B.; Lee, E.H.; Ryu, J.H. Neuroprotective effects of INM-176 against lipopolysaccharide-induced neuronal injury. Pharmacol. Biochem. Behav. 2012, 101, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Reddy, P.H. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: Implications for early intervention and therapeutics. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, J.; Zou, L.; Xia, H.; Wu, T.; Kim, Y.; Lee, Y. The Neuroprotective Effects of Decursin Isolated from Angelica gigas Nakai against Amyloid β-Protein-Induced Apoptosis in PC 12 Cells via a Mitochondria-Related Caspase Pathway. Neurochem. Res. 2015, 40, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.H.; Kim, J.S.; Zou, Y.; Yoon, C.S.; Lim, H.W.; Ahn, J.; Lee, H.Y. Enhancement of pheochromocytoma nerve cell growth by consecutive fractionization of Angelica gigas Nakai extracts. Cytotechnology 2010, 62, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Wang, S.; Zong, H.; Zheng, G.; Chen, H.; Tao, J.; Tao, Y. Anti-inflammatory effects of ethanol extract from Melilotus suaveolens Ledeb: Involvement of pro- and anti-inflammatory cytokines and mediators. J. Med. Plants Res. 2012, 6, 516–525. [Google Scholar] [CrossRef]
- Vazquez-Torres, A.; Jones-Carson, J.; Mastroeni, P.; Ischiropoulos, H.; Fang, F.C. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 2000, 192, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jung, J.Y.; Jung, Y.J.; Choi, J.H.; Jeong, W.S.; Song, Y.S.; Kang, J.S.; Bi, K.; Kim, M.J. Anti-inflammatory activities of coumarins isolated from Angelica gigas Nakai on LPS-stimulated RAW 264.7 cell. J. Food Sci. Nutr. 2009, 14, 179–187. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Durante, W.; Kroll, M.H.; Christodoulides, N.; Peyton, K.J.; Schafer, A.I. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ. Res. 1997, 80, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, G.; Yoshida, T.; Noguchi, M. Heme oxygenase and heme degradation. Biochem. Biophys. Res. Commun. 2005, 338, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.K.; Lianos, E.A. Nitric oxide induces heme oxygenase-1 gene expression in mesangial cells. Kidney Int. 1999, 55, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- De Montellano, P.R.O. The mechanism of heme oxygenase. Curr. Opin. Chem. Biol. 2000, 4, 221–227. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, C.S.; Kim, S.C.; Hur, M.; Kim, Y.B.; Park, C.G.; Lee, W.M.; Jang, J.K.; Koo, S.C. Natural Korean Medicine Dang-Gui: Biosynthesis, Effective Extraction and Formulations of Major Active Pyranocoumarins, Their Molecular Action Mechanism in Cancer, and Other Biological Activities. Molecules 2017, 22, 2170. https://doi.org/10.3390/molecules22122170
Reddy CS, Kim SC, Hur M, Kim YB, Park CG, Lee WM, Jang JK, Koo SC. Natural Korean Medicine Dang-Gui: Biosynthesis, Effective Extraction and Formulations of Major Active Pyranocoumarins, Their Molecular Action Mechanism in Cancer, and Other Biological Activities. Molecules. 2017; 22(12):2170. https://doi.org/10.3390/molecules22122170
Chicago/Turabian StyleReddy, Chinreddy Subramanyam, Seong Cheol Kim, Mok Hur, Yeon Bok Kim, Chun Geon Park, Woo Moon Lee, Jae Ki Jang, and Sung Cheol Koo. 2017. "Natural Korean Medicine Dang-Gui: Biosynthesis, Effective Extraction and Formulations of Major Active Pyranocoumarins, Their Molecular Action Mechanism in Cancer, and Other Biological Activities" Molecules 22, no. 12: 2170. https://doi.org/10.3390/molecules22122170
APA StyleReddy, C. S., Kim, S. C., Hur, M., Kim, Y. B., Park, C. G., Lee, W. M., Jang, J. K., & Koo, S. C. (2017). Natural Korean Medicine Dang-Gui: Biosynthesis, Effective Extraction and Formulations of Major Active Pyranocoumarins, Their Molecular Action Mechanism in Cancer, and Other Biological Activities. Molecules, 22(12), 2170. https://doi.org/10.3390/molecules22122170



