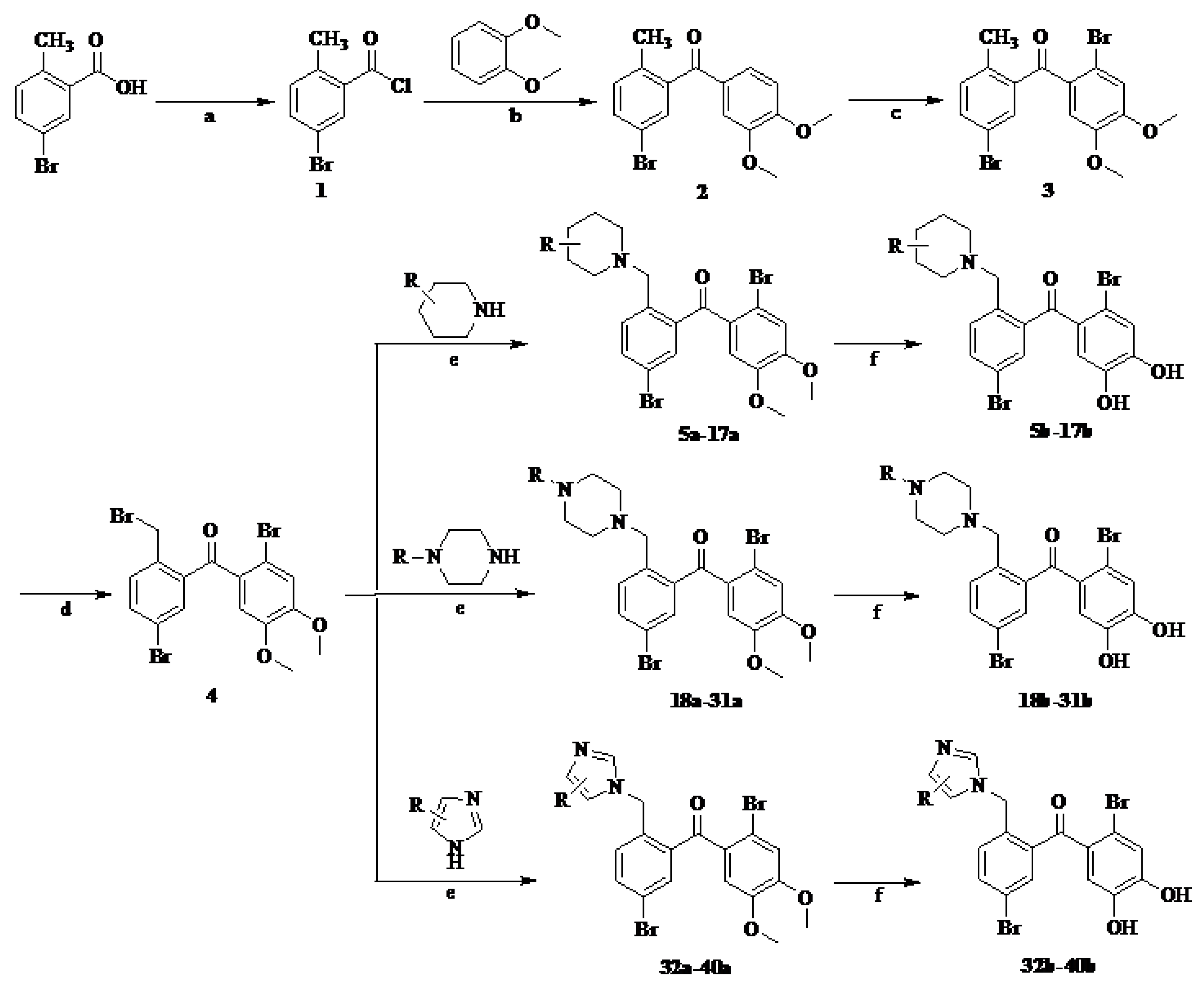
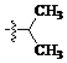
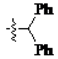
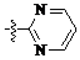
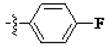
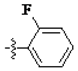
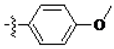
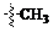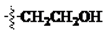The main reagents including 5-bromo-2-methyl benzoic acid, dimethoxybenzene, substituted piperdine, piperazine and imidazole were purchased from J & K Chemical Technology. Other chemical reagents and solvents were commercially available unless otherwise indicated. Dichloromethane was distilled from calcium hydride.
Melting points were taken on a micromelting point apparatus, which were uncorrected. The IR spectra of the compounds were recorded using a Thermo Scientific Nicolet iS 50 Fourier transform IR (FTIR) spectrometer. The 1H- and 13C-NMR spectra were recorded with a Bruker-AV 600 spectrometer in CDCl3 or DMSO-d6 with TMS as reference. Chemical shifts (δ values) and coupling constants (J values) were given in ppm and Hz, respectively. ESI mass spectra were obtained on an API QTRAP 3200 MS spectrometer, and HR-MS were recorded on a Bruker Daltonics Apex IV 70e FTICR-MS (Varian 7.0T).
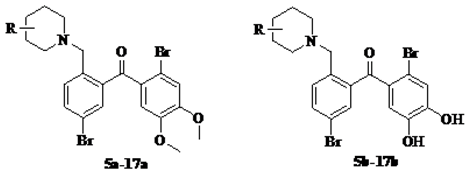
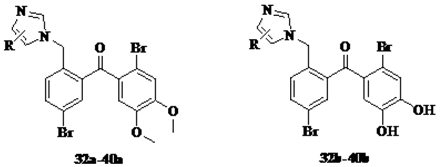
3.1.2. General Procedure for the Synthesis of Intermediate Compounds 5a–40a
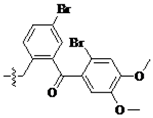
Compound 4 0.2 g (0.41 mmol) and 25 μL piperdine (0.82 mmol) was added to the 1.0 mL dried CH2Cl2. Anhydrous Na2CO3 20 mg was then added to the mixture, which was stirred for 12 h. The mixture was washed with the distilled water, the organic phase was separated and dried over anhydrous Na2SO4, and then concentrated viarotary evaporation. The crude product was purified by silica gel chromatography with petroleum ether–acetone–strong ammonia water (v/v/v, 8/1/0.1) as the eluent to gain 0.18 g yellow solid compound 5a in 90% yield.
Compounds 6a–40a were also obtained from intermediate 4 in a similar manner as for the preparation of 5a in 70–93% yield. Note that the preparation of compound 13a and 14a was requested for the circumstance of heating and refluxing.
Compound 5a: Yellow solid, yield 90%, m.p. 103.3–105.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.34–1.41 (m, 6H, piperidine-3’’,4’’,5’’-H), 2.16 (s, 4H, piperidine-2’’, 6’’-H), 3.37 (s, 2H, Ar-2-CH2-), 3.86 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 7.05 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.29 (s, 1H, Ar-6-H), 7.47 (d, J = 3.0 Hz, 1H, Ar-3-H), 7.51 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 496.02, 498.08, 499.86 ([M + H]+, 78, 100, 98).
Compound 6a: Yellow solid, yield 85%, m.p. 116.0–117.3 °C; 1H-NMR (600 MHz, CDCl3) δ: 0.89 (d, J = 9.6 Hz, 3H, piperidine-2’’-CH3), 1.36–1.95 (m, 6H, piperidine-3’’,4’’,5’’-H), 2.30–2.56 (m, 2H, piperidine-6’’-H), 3.26 (d, J = 9.6 Hz, 1H, piperidine-2’’-H), 3.83 (s, 3H, Ar-4’-OCH3), 3.91 (s, 1H, Ar-2-CH2-), 3.95 (s, 3H, Ar-5’-OCH3), 7.08 (s, 1H, Ar-3’-H), 7.11 (s, 1H, Ar-6’-H), 7.40 (d, J = 3.0 Hz, 1H, Ar-3-H), 7.43 (s, 1H, Ar-6-H), 7.52 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 510.03, 512.07, 513.85 ([M + H]+, 70, 100, 90).
Compound 7a: Yellow solid, yield 87%, m.p. 102.2–103.5 °C; 1H-NMR (600 MHz, CDCl3) δ:0.74 (d, J = 9.0 Hz, 3H, piperidine-3’’-CH3), 0.90 (m, 1H, piperidine-H), 1.35–1.78 (m, 6H, piperidine-H), 2.45 (m, 2H, piperidine-6’’-H), 3.38 (s, 2H, Ar-2-CH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 7.05 (s, 1H, Ar-3’-H), 7.14 (s, 1H, Ar-6’-H), 7.28 (s, 1H, Ar-6-H), 7.47 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.53 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 510.06, 512.09, 513.86 ([M + H]+, 77, 87, 100).
Compound 8a: Yellow solid, yield 87%, m.p. 76.2–78.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 0.83 (d, J = 9.6 Hz, 3H, piperidine-4’’-CH3), 0.88 (d, J = 9.6 Hz, 1H, piperidine-4’’-H), 1.04–2.52 (m, 8H, piperidine-H,), 3.38 (s, 2H, Ar-2-CH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 7.05 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.29 (s, 1H, Ar-6-H), 7.47 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.53 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 510.03, 512.04, 513.90 ([M + H]+, 55, 100, 65).
Compound 9a: Yellow solid, yield 86%, m.p. 82.2–84.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 0.72 (s, 3H, piperidine-3’’-CH3), 0.74 (s, 3H, piperidine-5’’-CH3), 0.86–2.46 (m, 8H, piperidine-H), 3.38 (s, 2H, Ar-2-CH2-), 3.84 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 7.05 (s, 1H, Ar-3’-H), 7.13 (s, 1H, Ar-6’-H), 7.26 (s, 1H, Ar-6-H), 7.47 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.53 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 524.08, 526.14, 527.86 ([M + H]+, 75, 95, 100).
Compound 10a: Yellow solid, yield 75%, m.p. 48.3–49.5 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.59–2.33 (m, 9H, piperidine-H), 3.44 (s, 2H, Ar-2-CH2-), 3.87 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 7.05 (s, 1H, Ar-3’-H), 7.16 (s, 1H, Ar-6’-H), 7.30 (s, 1H, Ar-6-H), 7.48 (d, J = 3.0 Hz, 1H, Ar-3-H), 7.55 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H), 10.2 (s, 1H, piperidine-4-COOH); ESI-MS m/z (%) 568.13, 570.15, 571.93 ([M + H]+, 70, 100, 92).
Compound 11a: Yellow solid, yield 70%, m.p. 44.8–46.2 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.20 (t, J = 7.2 Hz, 3H, piperidine-3’’-COOCH2-CH3), 1.58–2.70 (m, 9H, piperidine-H), 3.49 (q, J = 16.8 Hz, 2H, piperidine-3’’-COOCH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 4.07 (s, 2H, Ar-2-CH2-), 7.06 (s, 1H, Ar-3’-H), 7.11 (s, 1H, Ar-6’-H), 7.31 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.46 (s, 1H, Ar-6-H), 7.53 (d, J = 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 568.22, 570.14, 572.29 ([M + H]+, 100, 65, 97).
Compound 12a: Yellow solid, yield 75%, m.p. 62.1–63.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.40–2.51 (m, 9H, piperidine-H), 3.44 (s, 2H, Ar-2-CH2-), 3.62 (brs, 1H, piperidine-OH), 3.85 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 7.06 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.28 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.47 (d, J =1.2 Hz, 1H, Ar-6-H), 7.53 (dd, J = 1.2, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 512.20, 513.56, 516.26 ([M + H]+, 100, 65, 97).
Compound 13a: Yellow solid, yield 75%, m.p. 148.8–150.2 °C; 1H-NMR (600 MHz, CDCl3) δ: 0.92 (d, J = 6.0 Hz, 6H, piperidine-2’’,6’’-CH3), 1.29–1.58 (m, 6H, piperidine-3’’,4’’,5’’-H), 2.48 (m, 2H, piperidine-2’’,6’’-H), 3.86 (s, 3H, Ar-4’-OCH3), 3.89 (s, 2H, Ar-2-CH2-), 3.95 (s, 3H, Ar-5’-OCH3), 7.00 (s, 1H, Ar-3’-H), 7.06 (s, 1H, Ar-6’-H), 7.36 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.58 (dd, J = 1.8, 8.4 Hz, 1H, Ar-3-H), 8.08 (d, J = 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 524.16, 526.19, 528.03 ([M + H]+, 70, 100, 80).
Compound 14a: Yellow solid, yield 90%, m.p. 140.0–142.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 0.85–1.02 (m, 12H, piperidine-2’’,6’’-CH3), 1.53 (m, 6H, piperidine-3’’,4’’,5’’-H), 3.86 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 3.96 (s, 2H, Ar-2-CH2-), 7.00 (s, 1H, Ar-3’-H), 7.07 (s, 1H, Ar-6’-H), 7.36 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.57 (dd, J = 1.8, 8.4 Hz, 1H, Ar-3-H), 8.10 (d, J = 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 552.21, 554.23, 556.03 ([M + H]+, 55, 100, 65).
Compound 15a: Yellow solid, yield 70%, m.p. 138.9–140.5 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.47–1.61 (m, 6H, piperidine-3’’,4’’,5’’-H), 2.03 (t, J = 9.0 Hz, 2H, piperidine-6’’-H), 2.29–2.32 (m, 1H, piperidine-2’’-H), 2.75 (brs, 1H, -OH), 3.31 (d, J = 7.8 Hz, 2H, piperidine-CH2-), 3.81 (s, 2H, Ar-2-CH2-), 3.83 (s, 3H, Ar-4’-OCH3), 3.96 (s, 3H, Ar-5’-OCH3), 7.06 (s, 1H, Ar-3’-H), 7.10 (s, 1H, Ar-6’-H), 7.37 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.40 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.55 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 526.16, 528.23, 530.03 ([M + H]+, 62, 100, 80).
Compound 16a: Yellow solid, yield 72%, m.p. 50.5–51.9 °C; 1H-NMR (600 MHz, CDCl3) δ: 0.88–1.54 (m, 7H, piperidine-3’’,4’’,5’’-H, piperidine-4-CH2-), 1.85 (t, J = 10.8 Hz, 2H, piperidine-2’’-H), 2.01 (brs, 1H, -OH), 2.53 (t, J = 10.8 Hz, 2H, piperidine-6’’-H), 3.40 (s, 2H, Ar-2-CH2-), 3.64 (t, J = 9.6 Hz, 2H, HO-CH2-), 3.84 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 7.06 (s, 1H, Ar-3’-H), 7.13 (s, 1H, Ar-6’-H), 7.29 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.47 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.52 (dd, J = 2.4, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 540.24, 542.26, 544.13 ([M + H]+, 65, 100, 75).
Compound 17a: White solid, yield 65%, m.p. 44.2–45.8 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.09–1.58 (m, 5H, piperidine-3’’,4’’,5’’-H), 1.87 (t, J = 14.4 Hz, 2H, piperidine-2’’-H), 2.05 (brs, 1H, -OH), 2.57 (t, J = 16.8 Hz, 2H, piperidine-6’’-H), 3.42 (d, J = 6.0 Hz, 2H, HO-CH2-), 3.43 (s, 2H, Ar-2-CH2-), 3.84 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 7.06 (s, 1H, Ar-3’-H), 7.13 (s, 1H, Ar-6’-H), 7.28 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.47 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.52 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 526.24, 528.32, 530.16 ([M + H]+, 70, 100, 80).
Compound 18a: White solid, yield 60%, m.p. 200.0–201.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.13–2.24 (m, 8H, piperazine-H), 3.37 (s, 4H, Ar-2-CH2-), 3.84 (s, 6H, Ar-4’-OCH3), 3.94 (s, 6H, Ar-5’-OCH3), 7.04 (s, 2H, Ar-3’-H), 7.11 (s, 2H, Ar-6’-H), 7.24 (d, J = 12.0 Hz, 2H, Ar-3-H), 7.45 (d, J = 3.0 Hz, 2H, Ar-6-H), 7.52 (dd, J = 3.0, 12.0 Hz, 2H, Ar-4-H); ESI-MS m/z (%) 909.12, 911.10, 912.95 ([M + H]+, 65, 100, 75).
Compound 19a: White solid, yield 78%, m.p. 74.3–76.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.03 (t, J = 10.8 Hz, 3H, piperazine-4’’-CH3), 1.70–2.36 (m, 10H, piperazine-4’’-CH2, piperazine-H), 3.43 (s, 2H, Ar-2-CH2-), 3.86 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 7.05 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.29 (s, J = 12.0 Hz, 1H, Ar-3-H), 7.48 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.53 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 525.14, 527.20, 529.00 ([M + H]+, 80, 85, 100).
Compound 20a: Yellow solid, yield 75%, m.p. 65.8–66.9 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.42 (t, J = 7.2Hz, 4H, piperazine-2’’,6’’-H), 3.05 (t, J = 7.2 Hz, 4H, piperazine-3’’,5’’-H), 3.52 (s, 2H, Ar-2-CH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 6.82–6.87 (m, 3H, 3H, piperazine-Ar-3’’’,4’’’,5’’’-H), 7.05 (s, 1H, Ar-3’-H), 7.16 (s, 1H, Ar-6’-H), 7.24 (d, J = 11.4 Hz, 2H, piperazine-Ar-2’’’,6’’’-H), 7.33 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.50 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.57 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 573.10, 575.09, 576.95 ([M + H]+, 48, 100, 58).
Compound 21a: Yellow solid, yield 75%, m.p. 68.2–69.7 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.45 (s, 4H, piperazine-3’’,5’’-H), 2.92 (s, piperazine-2’’, 4’’-H), 3.50 (s, 2H, Ar-2-CH2-), 3.82 (s, 3H, Ar-4’-OCH3), 3.84 (s, 3H, Ar-5’-OCH3), 3.93 (s, 3H, piperazine-Ar-2’’’-OCH3), 6.82 (d, J = 8.4 Hz, 1H, piperazine-Ar-6’’’-H), 6.85 (d, J = 7.8 Hz, 1H, Ar-3’’’-H, Ar-6’’’-H), 6.89 (t, J = 7.8 Hz, 1H, Ar-4’’’-H), 6.97 (t, J = 7.8 Hz, 1H, Ar-5’’’-H), 7.05 (s, 1H, Ar-3’-H), 7.18 (s, 1H, Ar-6’-H), 7.31 (s, J = 7.8 Hz, 1H, Ar-3-H), 7.51 (s, 1H, Ar-6-H), 7.55 (dd, J = 1.2, 7.8 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 603.26, 604.57, 607.42 ([M + H]+, 100, 70, 90).
Compound 22a: Yellow solid, yield 68%, m.p. 64.8–66.5 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.03 (s, 3H, piperazine-4’’-COCH3), 2.24 (t, J = 4.8 Hz, 2H, piperazine-2’’-H), 2.29 (t, J = 5.4 Hz, 2H, piperazine-6’’-H), 3.31 (t, J = 4.8 Hz, 2H, piperazine-3’’-H), 3.48 (t, 2H, J = 5.4 Hz, piperazine-5’’-H), 3.51 (s, 2H, Ar-2-CH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 7.07 (s, 1H, Ar-3’-H), 7.13 (s, 1H, Ar-6’-H), 7.29 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.48 (d, J = 1.8 Hz,1H, Ar-6-H), 7.55 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 539.21, 541.18, 543.14 ([M + H]+, 85, 50, 100).
Compound 23a: Yellow solid, yield 60%, m.p. 64.5–66.3 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.44 (t, J = 4.8 Hz, 4H, piperazine-2’’,6’’-H), 3.30 (t, J = 4.8 Hz, piperazine-3’’,5’’-H), 3.56 (s, 2H, Ar-2-CH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 6.75 (d, J = 9.6 Hz, 2H, piperazine-Ar-2’’’,6’’’-H), 7.07 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.34 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.50 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.57 (dd, J = 1.8, 7.8 Hz, 1H, Ar-4-H), 8.10 (d, J = 9.0 Hz, 2H, piperazine-Ar-3’’’,5’’’-H); ESI-MS m/z (%) 618.23, 620.12, 622.02 ([M + H]+, 48, 100, 58).
Compound 24a: Yellow solid, yield 77%, m.p. 125.0–127.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.02 (t, J = 8.4 Hz, 8H, piperazine-H), 2.21 (s, 3H, piperazine-4’’-CH3), 3.44 (s, 2H, Ar-2-CH2-), 3.86 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 7.06 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.28 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.48 (s, 1H, Ar-6-H), 7.53 (d, J = 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 511.12, 513.13, 515.00 ([M + H]+, 50, 100, 60).
Compound 25a: White solid, yield 71%, m.p. 70.0–71.5 °C; 1H-NMR (600 MHz, CDCl3) δ: 0.97 (d, J = 6.6 Hz, 6H, piperazine-4’’-CH3), 2.28 (s, 4H, piperazine-3’’,5’’-H), 2.37 (s, 4H, piperazine-2’’,6’’-H), 2.57 (m, 1H, piperazine-4’’-CH-), 3.42 (s, 2H, Ar-2-CH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 7.06 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.28 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.48 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.52 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 539.02, 541.10, 543.26 ([M + H]+, 50, 100, 50).
Compound 26a: Yellow solid, yield 67%, m.p. 160.0–162.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.26 (s, 8H, piperazine-H), 3.43 (s, 2H, Ar-2-CH2-), 3.81 (s, 3H, Ar-4’-OCH3), 3.93 (s, 3H, Ar-5’-OCH3), 4.15 (s, 1H, piperazine-4’’-CH-), 7.03 (s, 1H, Ar-3’-H), 7.11 (s, 1H, Ar-6’-H), 7.15 (t, J = 7.8 Hz, 2H, piperazine-CH-Ar-4’’’-H), 7.23 (t, J = 7.8 Hz, 4H, piperazine-CH-Ar-3’’’,5’’’-H), 7.27 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.34 (d, J = 7.2 Hz, 4H, piperazine-CH-Ar-2’’’,6’’’-H), 7.45 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.50 (dd, J = 1.8, 7.8 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 663.26, 665.29, 667.10 ([M + H]+, 55, 100, 72).
Compound 27a: Yellow solid, yield 79%, m.p. 56.0–58.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.78 (s, 1H, piperazine-4’’-OH), 2.02 (s, 8H, piperazine-H), 2.47 (t, J = 5.4 Hz, 2H, piperazine-4’’-CH2-), 3.45 (s, 2H, Ar-2-CH2-), 3.56 (t, J = 5.4 Hz, 2H, HO-CH2-), 3.85 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 5.52 (brs, 1H, -OH), 7.06 (s, 1H, Ar-3’-H), 7.14 (s, 1H, Ar-6’-H), 7.28 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.48 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.53 (dd, J = 1.8, 7.8 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 541.15, 543.18, 545.03 ([M + H]+, 60, 100, 70).
Compound 28a: Yellow solid, yield 71%, m.p. 146.0–148.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.34 (t, J = 4.8 Hz, 4H, piperazine-2’’,6’’-H), 3.52 (s, 2H, Ar-2-CH2-), 3.68 (t, J = 4.8 Hz, 4H, piperazine-3’’,5’’-H), 3.85 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 6.45 (t, J = 4.8 Hz, 1H, pyrimidine-5’’’-H), 7.07 (s, 1H, Ar-3’-H), 7.16 (s, 1H, Ar-6’-H), 7.34 (d, 1H, J = 8.4 Hz, Ar-3-H), 7.48 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.55 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H), 8.26 (d, J = 4.8 Hz, 2H, pyrimidine-4’’’,6’’’-H); ESI-MS m/z (%) 575.14, 577.12, 579.02 ([M + H]+, 48, 100, 58).
Compound 29a: Yellow solid, yield 65%, m.p. 115.2–116.1 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.43 (t, J = 4.8 Hz, 4H, piperazine-2’’,6’’-H), 2.97 (t, J = 4.8 Hz, 4H, piperazine-3’’,5’’-H), 3.52 (s, 2H, Ar-2-CH2-), 3.84 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 6.80 (dd, J = 4.8, 9.0 Hz, 2H, Ar-2’’’,6’’’-H), 6.93 (t, J = 9.0 Hz, 2H, Ar-3’’’,5’’’-H), 7.05 (s, 1H, Ar-3’-H), 7.15 (s, 1H, Ar-6’-H), 7.33 (d, 1H, J = 8.4 Hz, Ar-3-H), 7.50 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.55 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 591.16, 593.16, 595.03 ([M + H]+, 48, 100, 58).
Compound 30a: Yellow solid, yield 70%, m.p. 46.2–48.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.44 (t, J = 7.2 Hz, 4H, piperazine-2’’,6’’-H), 2.95 (t, J = 7.2 Hz, 4H, piperazine-3’’,5’’-H), 3.52 (s, 2H, Ar-2-CH2-), 3.84 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 6.84–7.03 (m, 4H, piperazine-Ar-H), 7.06 (s, 1H, Ar-3’-H), 7.16 (s, 1H, Ar-6’-H), 7.32 (d, 1H, J = 12.0 Hz, Ar-3-H), 7.50 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.55 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 591.20, 593.23, 595.10 ([M + H]+, 65, 100, 75).
Compound 31a: Yellow solid, yield 83%, m.p. 129.1–130.8 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.42 (t, J = 7.2 Hz, 4H, piperazine-2’’,6’’-H), 2.94 (t, J = 7.2 Hz, piperazine-3’’,5’’-H), 3.51 (s, 2H, Ar-2-CH2-), 3.75 (s, 3H, piperazine-Ar-OCH3), 3.84 (s, 3H, Ar-4’-OCH3), 3.94 (s, 3H, Ar-5’-OCH3), 6.79–684 (m, 4H, piperazine-Ar-H), 7.05 (s, 1H, Ar-3’-H), 7.16 (s, 1H, Ar-6’-H), 7.30 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.50 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.55 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 603.19, 605.19, 607.08 ([M + H]+, 48, 100, 58).
Compound 32a: Yellow solid, yield 85%, m.p. 150.3–152.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 3.89 (s, 3H, Ar-4’-OCH3), 3.96 (s, 3H, Ar-5’-OCH3), 5.44 (s, 2H, Ar-2-CH2-), 6.90 (d, J = 12.6 Hz, 1H, imidazole-5’’-H), 6.93 (s, J = 12.6 Hz, H, imidazole-4’’-H), 6.94 (d, J = 12.0 Hz,1H, Ar-3-H), 7.06 (s, 1H, Ar-3’-H), 7.10 (s, 1H, Ar-6’-H)), 7.47 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.57 (s, 1H, imidazole-2’’-H), 7.60 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 479.03, 481.07, 482.86 ([M + H]+, 70, 100, 88).
Compound 33a: Yellow solid, yield 84%, m.p. 57.2–59.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.44 (s, 3H, imidazole-2’’-CH3), 3.90 (s, 3H, Ar-4’-OCH3), 3.96 (s, 3H, Ar-5’-OCH3), 5.37 (s, 2H, Ar-2-CH2-), 6.59 (d, J = 8.4 Hz, 1H, imidazole-5’’-H), 6.86 (s,1H, Ar-3’-H), 6.98 (d, J = 7.8 Hz,1H, Ar-3-H), 7.00 (d, J = 7.8 Hz, 1H, imidazole-4’’-H), 7.07 (s, 1H, Ar-6’-H), 7.49 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.57 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 493.14, 495.17, 496.97 ([M + H]+, 75, 100, 95).
Compound 34a: Yellow solid, yield 81%, m.p. 49.5–50.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.22 (s, 3H, imidazole-4’’-CH3), 3.88 (s, 3H, Ar-4’-OCH3), 3.96 (s, 3H, Ar-5’-OCH3), 5.35 (s, 2H, Ar-2-CH2-), 6.63 (s, 1H, imidazole-5’’-H), 6.89 (d, J = 12.0 Hz, 1H, Ar-3-H), 6.94 (s, 1H, Ar-3’-H), 7.06 (s, 1H, Ar-6’-H), 7.44 (s, 1H, imidazole-2’’-H), 7.47 (d, J = 10.8 Hz, 1H, Ar-6-H), 7.59 (d, J = 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 493.12, 495.17, 496.93 ([M + H]+, 75, 100, 98).
Compound 35a: White solid, yield 85%, m.p. 167.0–168.5 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.27 (t, J = 10.8 Hz, 3H, imidazole-2’’-C-CH3), 2.59 (q, J = 10.8 Hz, 2H, imidazole-2’’-CH2-), 3.91 (s, 3H, Ar-4’-OCH3), 3.97 (s, 3H, Ar-5’-OCH3), 5.38 (s, 2H, Ar-2-CH2-), 6.57 (d, J = 12.0 Hz, 1H, Ar-3-H), 6.85 (d, J = 1.8 Hz, 1H, imidazole-5’’-H), 7.00 (s, 1H, Ar-3’-H), 7.04 (d, J = 1.8 Hz, 1H, imidazole-4’’-H), 7.07 (s, 1H, Ar-6’-H), 7.49 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.56 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 507.07, 509.05, 510.97 ([M + H]+, 50, 100, 62).
Compound 36a: Yellow solid, yield 77%, m.p. 162.0–163.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.25 (d, J = 6.6 Hz, 6H, imidazole-2’’-C-CH3), 2.89 (m, 1H, imidazole-2’’-CH-), 3.91 (s, 3H, Ar-4’-OCH3), 3.97 (s, 3H, Ar-5’-OCH3), 5.41 (s, 2H, Ar-2-CH2-), 6.57 (d, J = 8.4 Hz, 1H, Ar-3-H), 6.81 (d, 1H, J = 1.2 Hz, imidazole-5’’-H), 7.00 (s, 1H, Ar-3’-H), 7.06 (d, J = 1.2 Hz, 1H, imidazole-4’’-H), 7.07 (s, 1H, Ar-6’-H), 7.49 (d, J = 1.2 Hz, 1H, Ar-6-H), 7.56 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 521.17, 523.05, 525.04 ([M + H]+, 85, 98, 100).
Compound 37a: Yellow solid, yield 72%, m.p. 32.8–34.5 °C; 1H-NMR (600 MHz, CDCl3) δ: 1.24 (s, 3H, imidazole-2’’-C-CH3), 2.23 (s, 3H, imidazole-4’’-CH3), 2.58 (q, 2H, imidazole-2’’-CH2-), 3.90 (s, 3H, Ar-4’-OCH3), 3.97 (s, 3H, Ar-5’-OCH3), 5.30 (s, 2H, Ar-2-CH2-), 6.54 (s, 1H, imidazole-5’’-H), 6.63 (d, J = 8.4 Hz, 1H, Ar-3-H), 6.99 (s, 1H, Ar-3’-H), 7.07 (s, 1H, Ar-6’-H), 7.48 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.57 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 521.18, 523.22, 525.06 ([M + H]+, 80, 100, 95).
Compound 38a: White solid, yield 70%, m.p. 183.0–185.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 3.89 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 5.54 (s, 2H, Ar-2-CH2-), 6.73 (d, J = 8.4 Hz, 1H, Ar-3-H), 6.96 (s, 1H, Ar-3’-H), 7.02 (s, 1H, Ar-6’-H), 7.03 (s, 1H, imidazole-4’’-H), 7.24 (s, 1H, imidazole-5’’-H), 7.35–7.36 (m, 3H, imidazole-2’’-Ph-H), 7.48 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.49–7.50 (m, 2H, imidazole-2’’-Ph-H), 7.59 (dd, J = 1.8, 8.4 Hz, 1H, Ar-4-H); 555.14, 557.17, 558.99 ([M + H]+, 52, 100, 48).
Compound 39a: White solid, yield 79%, m.p. 164.2–165.1 °C; 1H-NMR (600 MHz, CDCl3) δ: 2.20 (s, 3H, imidazole-4’’-CH3), 2.26 (s, 3H, imidazole-2’’-CH3), 3.90 (s, 3H, Ar-4’-OCH3), 3.97 (s, 3H, Ar-5’-OCH3), 5.29 (s, 2H, Ar-2-CH2-), 6.55 (s, imidazole-5’’-H), 6.64 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.01 (s, 1H, Ar-3’-H), 7.07 (s, 1H, Ar-6’-H), 7.48 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.58 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H); ESI-MS m/z (%) 507.22, 509.25, 511.10 ([M + H]+, 68, 100, 78).
Compound 40a: Yellow solid, yield 63%, m.p. 130.0–132.0 °C; 1H-NMR (600 MHz, CDCl3) δ: 3.78 (s, 3H, Ar-4’-OCH3), 3.95 (s, 3H, Ar-5’-OCH3), 5.68 (s, 2H, Ar-2-CH2-), 6.83 (s, 1H, Ar-3’-H), 6.89 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.05 (s, 1H, Ar-6’-H), 7.23–7.24 (m, 2H, imidazole-Ar-H), 7.29–7.30 (m, 1H, imidazole-Ar-H), 7.49 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.55 (dd, J = 3.0, 12.0 Hz, 1H, imidazole-Ar-H), 7.83 (d, J = 12.6 Hz, 1H, Ar-4-H ), 7.97 (s, 1H, imidazole-2’’-H); ESI-MS m/z (%) 529.16, 531.20, 533.04 ([M + H]+, 68, 100, 80).
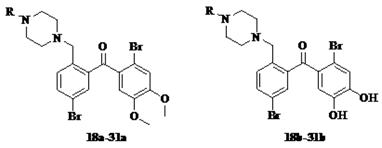
3.1.3. General Procedure for the Synthesis of Target Compounds 5b–40b
BBr3 solution (BBr3/CH2Cl2, v/v, 1/9) 1.5 mL was dropwise added to a cooled (−78 °C) solution of 0.259 g (0.52 mmol) compound 5a in 5 mL dried CH2Cl2. The mixture was allowed to warm to room temperature and stirred for 2 h, and poured into 30 mL ice-water. The precipitate was filtered, washed with a little distilled water and dried CH2Cl2, respectively, and dried in a vacuum drying oven to obtain 0.183 g yellow solid compound 5b in 75% yield. The total yield of target compound 5b was 18.8%.
Target compounds 6b–40b were obtained from 6a to 40a in a similar manner as for the preparation of 5b in 38–85% yield, the total yields of which were 7.7–23%。
Compound 5b: Yellow solid, final yield 18.8 %, m.p. 148.0–150.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 1.73–1.82 (m, 6H, piperidine-3’’,4’’,5’’-H), 3.08 (t, J = 10.2 Hz 2H, piperidine-2’’-H), 3.43 (t, J = 12 Hz, 2H, piperidine-6’’-H), 4.37 (s, 2H, Ar-2-CH2-), 6.95 (s, 1H, Ar-6’-H), 7.08 (s, 1H, Ar-3’-H), 7.58 (s, 1H, Ar-6-H), 7.77 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.98 (d, J = 8.4 Hz, 1H, Ar-4-H), 9.73 (brs, 1H, Ar-4’-OH), 10.41 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) δ: 21.6 × 2, 22.7 × 2, 53.1 × 2, 56.9, 110.7, 119.7, 120.9, 123.1, 129.0, 129.7, 133.8, 135.4, 135.6, 141.4, 145.3, 151.0, 194.9; ESI-MS m/z (%): 468.11, 470.13, 471.96 ([M + H]+, 78, 100, 98).
Compound 6b: Yellow solid, final yield 17.3%, m.p. 172.5–174.3 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 1.48 (d, J = 6.0 Hz, 3H, piperidine-2’’-CH3), 1.69–1.92 (m, 6H, piperidine-3’’,4’’,5’’-H), 2.90–2.96 (m, 1H, piperidine-2’’-H), 3.12 (t, J = 4.8 Hz, 2H, piperidine-6’’-H), 4.83 (s, 1H, Ar-2-CH2-), 6.95 (s, 1H, Ar-6’-H), 7.08 (s, 1H, Ar-3’-H), 7.61 (d, J = 23.4 Hz, 1H, Ar-6-H), 7.79 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.97 (d, J = 8.4 Hz, 1H, Ar-4-H), 9.72 (brs, 1H, Ar-4’-OH), 10.40 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) δ: 18.1, 22.4, 28.0, 30.9, 51.3, 57.6, 61.6, 110.8, 119.8, 121.0, 123.0, 128.9, 129.9, 133.8, 135.4, 135.9, 141.5, 145.2, 151.0, 194.8; ESI-MS m/z (%): 482.16, 484.19, 486.05 ([M + H]+, 82, 80, 100).
Compound 7b: Yellow solid, final yield 16.5%, m.p. 174.0–175.3 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 0.88 (d, J = 6.6 Hz, 3H, piperidine-3’’-CH3), 1.06–1.13 (m, 1H, piperidine-3’’-H), 1.71–1.84 (m, 4H, piperidine-4’’,5’’-H), 2.66–2.71 (m, 2H, piperidine-2’’-H), 3.32–3.45 (m, 2H, piperidine-6’’-H), 4.38 (s, 2H, Ar-2-CH2), 6.96 (s, 1H, Ar-6’-H), 7.08 (s, 1H, Ar-3’-H), 7.58 (s, 1H, Ar-6-H), 7.80 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.98 (d, J = 8.4 Hz, 1H, Ar-4-H), 9.71 (brs, 1H, Ar-4’-OH), 10.40 (brs, 1H, Ar-5’-OH); 13 C-NMR (150 MHz, DMSO-d6) δ: 19.0, 22.8, 29.1, 30.2, 52.7, 57.3, 58.4, 110.7, 119.8, 121.0, 123.2, 128.9, 129.5, 133.7, 135.4, 135.7, 141.5, 145.3, 151.0, 194.8; ESI-MS m/z (%): 482.15, 484.16, 485.98 ([M + H]+, 80, 95, 100).
Compound 8b: Yellow solid, final yield 15.9%, m.p. 103.3–105.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 0.92 (d, J = 9.6 Hz, 3H, piperidine-4’’-CH3), 1.01–1.03 (m, 1H, piperidine-4’’-H), 1.38–1.47 (m, 2H, piperidine-3’’-H), 1.78–1.81 (m, 2H, piperidine-5’’-H), 3.09 (t, J = 18.6 Hz, 2H, piperidine-2’’-H), 3.44 (t, J = 16.2 Hz, 2H, piperidine-6’’-H), 4.37 (s, 2H, Ar-2-CH2-), 6.95 (s, 1H, Ar-6’-H), 7.08 (s, 1H, Ar-3’-H), 7.58 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.79 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.97 (dd, J = 12 Hz, 3.0 Hz, 1H, Ar-4-H), 9.75 (brs, 1H, Ar-4’-OH), 10.42 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 21.5, 28.4, 31.2 × 2, 53.0 × 2, 57.2, 110.8, 113.8, 119.7, 120.9, 123.1, 129.8, 133.8, 135.6, 141.5, 145.3, 151.0, 156.3, 195.7; ESI-MS m/z (%): 481.99, 484.02, 485.92 ([M + H]+, 50, 100, 58).
Compound 9b: Yellow solid, final yield 15.3%, m.p. 173.0–175.3 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 0.88 (d, J = 6.6 Hz, 6H, piperidine-3’’,5’’-CH3), 1.74 (t, J = 12.6 Hz, 2H, piperidine-4’’-H), 1.93–1.94 (m, 2H, piperidine-3’’,5’’-H), 2.59–2.65 (m, 2H, piperidine-2’’-H), 3.29–3.37 (m, 2H, piperidine-6’’-H), 4.37 (s, 2H, Ar-2-CH2-), 6.97 (s, 1H, Ar-3’-H), 7.08 (s, 1H, Ar-6’-H), 7.59 (s, 1H, Ar-6-H), 7.81 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.98 (d, J = 8.4 Hz, 1H, Ar-4-H), 9.71 (brs, 1H, Ar-4’-OH), 10.41 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 18.3 × 2, 28.3, 38.4 × 2, 56.7 × 2, 57.4, 110.3, 119.3, 120.5, 122.7, 128.3, 128.9, 133.2, 134.9, 135.4, 141.1, 144.7, 150.5, 194,3; ESI-MS m/z (%) 496.17, 498.42, 500.07 ([M + H]+, 82, 78, 100).
Compound 10b: Yellow solid, final yield 12.3%, m.p. 158.3–159.5 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 1.79–2.12 (m, 5H, piperidine-3’’,4’’,5’’-H,), 3.14 (t, J = 12.6 Hz, 2H, piperdine-2’’-H), 3.50 (t, 2H, J = 12.0 Hz, 2H, piperidine-6’’-H), 4.38 (s, 2H, Ar-2-CH2-), 6.95 (s, 1H, Ar-3’-H), 7.08 (s, 1H, Ar-6’-H), 7.58 (s, 1H, Ar-6-H), 7.78 (d, J = 8.4 Hz, 1H, Ar-3-H), 7.98 (d, J = 7.8 Hz, 1H, Ar-4-H), 9.72 (s, 1H, Ar-4’-OH), 10.40 (s, 1H, Ar-5’-OH), 12.57 (s, 1H, piperidine-4’’-COOH); 13C-NMR (150 MHz, DMSO-d6) 25.7, 38.1 × 2, 52.1 × 2, 57.1, 110.7, 120.0, 120.9, 123.2, 129.0, 129.6, 133.8, 135.4, 135.6, 141.3, 145.3, 151.0, 175.0, 194.8; ESI-MS m/z (%) 512.08, 514.12, 515.93 ([M + H]+, 65, 100, 75).
Compound 11b: Yellow solid, final yield 15.7%, m.p. 180.0–181.3 °C; FT-IR (ATR) υ (cm−1): 3191, 2979, 2878, 1716, 1652, 1586, 1401, 1287, 1188, 1012, 796, 639; 1H-NMR (600 MHz, DMSO-d6) δ: 1.20 (t, J = 10.8 Hz, 3H, piperidine-3’’-COOCH2-CH3), 1.50–2.04 (m, 4H, piperidine-5’’,6’’-H), 3.07–3.60 (m, 5H, piperidine-2’’,3’’,4’’-H), 4.10 (q, 2H, piperidine-3’’-COOCH2-), 4.45 (s, 2H, Ar-2-CH2-), 6.96 (s, 1H, Ar-3’-H), 7.08 (s, 1H, Ar-6’-H), 7.59 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.81 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.98 (d, J = 12.0 Hz, 1H, Ar-4-H), 9.74 (brs, 1H, Ar-4’-OH), 10.43 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 14.4, 22.2, 25.1, 52.6, 55.4, 57.7, 61.2, 61.5, 110.8, 113.8, 119.7, 120.9, 123.3, 129.0, 133.8, 135.7, 141.5, 145.3, 151.0, 156.3, 171.4, 194.8; ESI-MS m/z (%) 539.96, 541.95, 543.78 ([M + H]+, 52, 100, 70); HR-MS (ESI) calcd for C22H23Br2NO5 [M − H]−: 539.9840; found: 539.9835.
Compound 12b: Yellow solid, final yield 19.0%, m.p. 184.0–186.0 °C; FT-IR (ATR) υ (cm−1): 3191, 2960, 2764, 1649, 1586, 1411, 1286, 1191, 1152, 1010, 796, 638; 1H-NMR (600 MHz, DMSO-d6) δ: 1.75–1.99 (m, 4H, piperidine-3’’,5’’-H), 3.12-3.45 (m, 4H, piperidine-2’’,6’’-H), 3.94 (s, 1H, piperidine-4’’-OH), 3.67–3.72 (m, 1H, piperidine-4’’-H), 4.39 (s, 2H, Ar-2-CH2-), 6.96 (s, 1H, Ar-3’-H), 7.09 (s, 1H, Ar-6’-H), 7.58 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.84 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.98 (d, J = 12.6 Hz, 1H, Ar-4-H), 9.78 (s, 1H, Ar-4’-OH), 10.37 (s, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 29.8 × 2, 48.1 × 2, 59.9, 64.2, 110.6, 119.6, 120.9, 123.2, 129.1, 129.7, 133.9, 135.5, 135.8, 141.4, 145.3, 150.9, 195.0; ESI-MS m/z (%) 483.74, 485.71, 487.62 ([M + H]+, 48, 100, 58); HR-MS (ESI) calcd for C19H19Br2NO4 [M + H]+: 485.9730; found: 485.9692.
Compound 13b: Yellow solid, final yield 15.3%, m.p. 98.0–99.5 °C; FT-IR (ATR) υ (cm−1): 3191, 2975, 2941, 1655, 1586, 1409, 1279, 1191, 1118, 1009, 805, 637; 1H-NMR (600 MHz, DMSO-d6) δ: 1.20 (d, J = 9.0 Hz, 6H, piperidine-2’’,6’’-CH3), 3.40–3.50 (m, 6H, piperidine-3’’,4’’,5’’-H), 4.63–4.67 (m, 2H, piperidine-2’’,6’’-H), 5.76 (s, 2H, Ar-2-CH2-), 6.98 (s, 1H, Ar-3’-H), 7.10 (s, 1H, Ar-6’-H), 7.58 (d, J = 2.4 Hz, 1H, Ar-3-H), 7.79 (d, J = 5.4 Hz, 1H, Ar-6-H), 7.98 (d, J = 3.0 Hz, 1H, Ar-4-H), 9.75 (brs, 1H, Ar-4’-OH), 10.48 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 18.4, 19.5 × 2, 30.1 × 2, 52.8 × 2, 55.4, 110.8, 119.8, 121.1, 122.2, 128.9, 132.7, 133.7, 134.3, 135.3, 139.4, 145.1, 151.0, 195.1; ESI-MS m/z (%) 494.20, 496.18, 498.15 ([M − H]−, 50, 100, 50); HR-MS (ESI) calcd for C21H23Br2NO3 [M − H]−: 495.9940, found: 495.9961.
Compound 14b: Yellow solid, final yield 5.3%, m.p. 176.0–177.3 °C; FT-IR (ATR) υ (cm−1): 2951, 2921, 2850, 1654, 1606, 1442, 1394, 1283, 1048, 999, 864, 686; 1H-NMR (600 MHz, DMSO-d6) δ: 0.87 (s, 12H, piperidine-2’’,6’’-CH3), 1.23–1.29 (m, 6H, piperidine-3’’,4’’,5’’-H), 3.77 (s, 2H, Ar-2-CH2-), 6.67 (s, 1H, Ar-3’-H), 6.92 (s, 1H, Ar-6’-H), 7.34 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.69 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.95 (d, J = 12.6 Hz, 1H, Ar-4-H), 9.74 (brs, 1H, Ar-4’-OH), 10.38 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 17.8, 41.2 × 4, 45.5 × 2, 54.8 × 2, 54.9, 109.7, 110.3, 113.5, 118.5, 125.0, 129.6, 131.3, 131.4, 133.7, 139.2, 144.9, 155.8, 196.5; ESI-MS m/z (%) 523.98, 525.96, 527.82 ([M + H]+, 48, 100, 58); HR-MS (ESI) calcd for C25H31Br2NO4-H: 524.026, found: 524.0268.
Compound 15b: Yellow solid, final yield 12.7%, m.p. 122.5–124.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 1.52–1.90 (m, 6H, piperidine-3’’, 4’’, 5’’-H), 3.03–3.13 (m, 2H, piperidine-6’’-H), 3.34 (s, 1H, piperidine-2’’-H), 3.74–3.92 (m, 2H, piperidine-2’’-CH2-), 4.37 (s, 2H, Ar-2-CH2-), 6.96 (s, 1H, Ar-3’-H), 7.08 (s, 1H, Ar-6’-H), 7.56 (s, 1H, Ar-6-H), 7.78 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.99 (d, J = 12 Hz, 1H, Ar-4-H), 9.78 (brs, 1H, Ar-4’-OH), 10.46 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 20.8, 21.7, 25.4, 50.7, 53.3, 55.4, 59.6, 111.0, 119.9, 121.0, 123.1, 128.8, 129.8, 133.8, 135.4, 135.9, 141.7, 145.2, 151.1, 195.2; ESI-MS m/z (%) 497.74, 499.67, 501.64 ([M + H]+, 75, 100, 70).
Compound 16b: Yellow solid, final yield 16.2%, m.p. 50.0–51.0 °C; FT-IR (ATR) υ (cm−1): 3360, 2920, 2851, 1631, 1588, 1500, 1362, 1289, 1237, 1153, 1012, 878, 798; 1H-NMR (600 MHz, DMSO-d6) δ: 1.04–1.46 (m, 7H, piperidine-3’’,4’’,5’’-H, piperidine-4’’-CH2), 3.03–3.45 (m, 6H, piperidine-2’’,6’’-H, HO-CH2-), 4.37 (s, 2H, Ar-2-CH2-), 6.96 (s, 1H, Ar-3’-H), 7.08 (s, 1H, Ar-6’-H), 7.57 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.81 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.98 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 9.74 (brs, 1H, Ar-4’-OH), 10.41 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 29.3, 30.2 × 2, 38.8 × 2, 53.1, 57.1, 58.4, 110.7, 119.7, 120.9, 123.1, 129.0, 129.7, 133.7, 135.4, 135.6, 141.5, 145.3, 150.9, 194.8; ESI-MS m/z (%) 511.96, 513.95, 515.79 ([M + H]+, 55, 100, 70).
Compound 17b: White solid, fianl yield 13.5%, m.p. 113.0–115.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 1.42–1.85 (m, 5H, piperidine-3’’,4’’,5’’-H), 3.04–3.13 (m, 2H, piperidine-2’’-H), 3.28 (d, J = 8.4 Hz, 2H, HO-CH2-), 3.42–3.57 (m, 2H, piperidine-6’’-H), 4.39 (s, 2H, Ar-2-CH2-), 5.76 (s, 1H, -OH), 6.96 (d, J = 5.4 Hz, 1H, Ar-3’-H), 7.08 (s, 1H, Ar-6’-H), 7.57 (s, 1H, Ar-6-H), 7.78 (dd, J = 3.6, 12.6 Hz, 1H, Ar-3-H), 7.98 (d, J = 12.6 Hz, 1H, Ar-4-H), 9.75 (brs, 1H, Ar-4’-OH), 10.42 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 26.2 × 2, 36.1, 52.8×2, 57.2, 65.2, 110.8, 119.7, 120.9, 123.1, 129.0, 129.7, 133.8, 135.4, 135.5, 141.5, 145.2, 151.0, 194.8; ESI-MS m/z (%) 497.91, 499.94, 501.79 ([M + H]+, 60, 100, 75).
Compound 18b: White solid, final yield 10.0%, m.p. 183.0–185.0 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 3.34 (s, 8H, piperazine-2’’,3’’,5’’,6’’-H), 4.29 (s, 4H, Ar-2-CH2-), 6.96 (s, 2H, Ar-3’-H), 7.09 (s, 2H, Ar-6’-H), 7.57 (s, 2H, Ar-6-H), 7.76 (d, J = 9.6 Hz, 2H, Ar-3-H), 7.94 (d, J = 9.0 Hz, 2H, Ar-4-H), 9.90 (brs, 4H, Ar-4’-OH, Ar-5’-OH); 13 C-NMR (150 MHz, DMSO-d6) 49.6 × 4, 57.1 × 2, 110.7 × 2, 119.7 × 2, 121.0 × 2, 122.9 × 2, 126.3 × 2, 128.7 × 2, 133.5 × 2, 135.1 × 2, 141.5 × 2, 145.2 × 2, 150.9 × 2, 156.2 × 2, 194.7 × 2; ESI-MS m/z (%) 850.69, 852.83, 854.92 ([M + H]+, 52, 100, 48).
Compound 19b: Yellow solid, final yield 18.4%, m.p. 195.8–197.5 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 1.20–1.28 (m, 5H, piperazine-CH2CH3), 3.16 (m, 4H, piperazine- 3’’,5’’-H), 3.53 (m, 4H, piperazine-2’’,6’’-H), 3.88 (s, 2H, Ar-2-CH2-), 6.95 (s, 1H, Ar-3’-H), 7.06 (s, 1H, Ar-6’-H), 7.09 (s, 1H, Ar-3-H), 7.14 (s, 1H, Ar-4-H), 7.23 (s, 1H, Ar-6-H), 9.02 (brs, 1H, Ar-4’-OH), 9.67 (brs, 1H, Ar-5’-OH); 13 C-NMR (150 MHz, DMSO-d6) 9.3, 25.7, 31.7 × 2, 50.5 × 2, 52.3, 110.7, 116.9, 119.8, 121.2, 128.5, 132.9, 134.8, 141.6, 145.1, 148.5, 150.8, 152.5, 194.5; ESI-MS m/z (%) 497.11, 499.11, 500.96 ([M + H]+, 48, 100, 55).
Compound 20b: Yellow solid, fianl yield 18.1%, m.p. 219.5–220.8 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 3.15 (s, 2H, piperazine-2’’-H), 3.35 (s, 2H, piperazine-6’’-H), 3.54 (s, 2H, piperazine-3’’-H), 3.84 (s, 2H, piperazine-5’’-H), 4.51 (s, 2H, Ar-2-CH2-), 6.87-7.01 (m, 5H, piperazine-C6H5), 7.27 (s, 2H, Ar-3’,6’-H), 7.60 (s, 1H, Ar-3-H), 7.83 (s, 1H, Ar-4-H), 8.00 (s, 1H, Ar-6-H), 9.78 (brs, 1H, Ar-4’-OH), 10.38 (brs, 1H, Ar-5’-H); 13C-NMR (150 MHz, DMSO-d6) 45.5 × 2, 51.5 × 2, 56.9, 110.7, 111.7, 118.4 × 2, 119.7, 120.9, 123.3, 129.0, 129.6, 132.2 × 2, 133.9, 135.5, 135.7, 141.4, 145.3, 149.1, 151.0, 195.0; ESI-MS m/z (%) 545.11, 547.15, 548.96 ([M + H]+, 60, 100, 80).
Compound 21b: White solid, fianl yield 15.9%, m.p. 156.0–157.8 °C; FT-IR (ATR) υ (cm−1): 3189, 2636, 2251, 2261, 1654, 1588, 1500, 1411, 1191, 1014, 752, 637; 1H-NMR (600 MHz, DMSO-d6) δ: 3.02–3.53 (m, 8H, piperazine-2’’,3’’,5’’,6’’-H), 3.80 (s, 3H, Ar-OCH3), 4.50 (s, 2H, Ar-2-CH2-), 6.92–7.04 (m, 5H, piperazine-C6H4-, Ar-3’-H), 7.09 (s, 1H, Ar-6’-H), 7.60 (s, 1H, Ar-6-H), 7.82 (d, J = 12 Hz, 1H, Ar-6-H), 8.01 (d, J = 12.6 Hz, 1H, Ar-4-H), 9.29 (brs, 2H, Ar-4’-OH, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 47.3, 52.2 × 2, 52.3 × 2, 56.0, 110.7, 112.5, 115.5, 118.9, 119.7, 120.9, 121.4, 123.3, 124.1, 129.0, 129.4, 133.9, 135.5, 135.9, 141.1, 145.3, 150.9, 152.3, 195.0; ESI-MS m/z (%) 560.71, 562.71, 564.86 ([M + H]+, 45, 100, 55); HR-MS (ESI) calcd for C25H24Br2N2O4 [M − H]−: 575.0000, found: 575.0241.
Compound 22b: Yellow solid, fianl yield 14.2%, m.p. 208.0–209.0 °C; FT-IR (ATR) υ (cm−1): 3193, 2770, 2708, 2262, 1649, 1587, 1410, 1280, 1191, 1151, 1010, 796, 638; 1H-NMR (600 MHz, DMSO-d6) δ: 2.05 (s, 3H, piperazine-CO-CH3), 3.08–3.50 (m, 8H, piperazine-2’’,3’’, 5’’,6’’-H,), 4.48 (s, 2H, Ar-2-CH2-), 6.99 (s, 1H, Ar-3’-H), 7.10 (s, 1H, Ar-6’-H), 7.58 (d, J = 3.6 Hz, 1H, Ar-6-H), 7.86 (d, J = 12.6 Hz, 1H, Ar-3-H), 8.00 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 9.08 (brs, 1H, Ar-4’-OH), 9.62 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 21.4, 42.9 × 2, 52.0 × 2, 56.9, 110.8, 119.7, 120.9, 123.3, 129.0, 129.3, 133.9, 135.5, 135.7, 141.4, 145.3, 151.0, 169.1, 194.9; ESI-MS m/z (%) 510.72, 512.69, 514.70 ([M + H]+, 48, 100, 52); HR-MS (ESI) calcd for C20H20Br2N2O4 [M − H]−: 510.969, found: 510.9676.
Compound 23b: Yellow solid, final yield 8.3%, m.p. 64.5–66.3 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 3.35–3.88 (m, 8H, piperazine-2’’,3’’,5’’,6’’-H), 4.49 (s, 2H, Ar-2-CH2-), 6.96 (s, 1H, Ar-3’-H), 7.07–8.20 (m, 8H, piperazine-C6H4-, Ar-6’,3,4,6-H), 9.75 (brs, 1H, Ar-4’-OH), 10.43 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 19.0 × 2, 51.5 × 2, 56.5, 110.8 × 2, 112.9 × 2, 113.9, 119.8, 120.9, 123.4, 126.2, 129.0, 129.4, 132.2, 133.9, 135.6, 135.7, 141.5, 145.2, 150.9, 195.0; ESI-MS m/z (%) 590.19, 591.91, 593.79 ([M + H]+, 70, 100, 50).
Compound 24b: Yellow solid, final yield 16.1%, m.p. 217.0–218.5 °C; FT-IR (ATR) υ (cm−1): 3363, 3010, 2699, 1633, 1585, 1500, 1411, 1365, 1298, 1119, 1031, 798, 639;1H-NMR (600 MHz, DMSO-d6) δ: 2.86 (s, 3H, piperazine-4’’-CH3), 3.64–3.77 (m, 8H, piperazine-2’’,3’’, 5’’,6’’-H), 4.60 (s, 2H, Ar-2-CH2-), 6.97 (s, 1H, Ar-3’-H), 7.10 (s, 1H, Ar-6’-H), 7.55 (s, 1H, Ar-6-H), 7.91 (d, J = 9.0 Hz,1H, Ar-3-H), 7.98 (d, J = 10.2 Hz, 1H, Ar-4-H), 10.06 (brs, 2H, Ar-4’-OH, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 19.0, 42.5, 49.1 × 2, 56.5 × 2, 110.6, 119.8, 121.1, 122.3, 128.5, 132.5, 132.8, 134.5, 141.7, 145.2, 150.8, 158.3, 194.4; ESI-MS m/z (%) 482.75, 484.62, 486.57 ([M + H]+, 50, 100, 40); HR-MS (ESI) calcd for C19H20Br2N2O3 [M + H]+: 484.9894, found: 484.9927.
Compound 25b: Yellow solid, fianl yield 14.4%, m.p. 208.0–210.0 °C; FT-IR (ATR) υ (cm−1): 3479, 3194, 3006, 2622, 2519, 2260, 1657, 1604, 1406, 1284, 1190, 1013, 798, 639; 1H-NMR (600 MHz, DMSO-d6) δ: 1.27 (d, J = 9.6 Hz, 6H, piperazine-4’’-CH3), 3.18–3.58 (m, 9H, piperazine-2’’,3’’,5’’,6’’-H, piperazine-CHMe2), 4.17 (s, 2H, Ar-2-CH2-), 6.97 (s, 1H, Ar-3’-H), 7.10 (s, 1H, Ar-6’-H), 7.56 (s, 1H, Ar-6-H), 7.75 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.91 (d, J = 10.2 Hz, 1H, Ar-4-H), 10.00 (brs, 2H, Ar-4’-OH, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 16.7×2, 45.9, 49.2×2, 56.7, 57.8 × 2, 110.7, 120.0, 121.2, 122.7, 126.0, 128.4, 130.7, 132.9, 134.7, 141.6, 145.1, 150.9, 194.5; ESI-MS m/z (%) 510.70, 512.77, 514.75 ([M + H]+, 55, 100, 57); HR-MS (ESI) calcd for C21H24Br2N2O3 [M + H]+: 513.0210, found: 513.0218.
Compound 26b: White solid, final yield 13.9%, m.p. 160.0–162.0 °C; FT-IR (ATR) υ (cm−1): 3194, 2798, 2524, 2361, 1652, 1588, 1412, 1292, 1192, 1015, 883, 639;1H-NMR (600 MHz, DMSO-d6) δ: 3.12–3.44 (m, 9H, piperazine-2’’,3’’,5’’,6’’-H, Ph2CH-), 4.39 (s, 2H, Ar-2-CH2-), 6.95 (s, 1H, Ar-3’-H), 7.09 (s, 1H, Ar-6’-H), 7.33–7.74 (m, 12H, Ar-H, Ar-4’’’-H), 7.92 (d, J = 10.2 Hz, 1H, Ar-4-H), 9.00 (brs, 2H, Ar-4’-OH, Ar-5’-OH); 13 C-NMR (150 MHz, DMSO-d6) 40.5 × 2, 48.9 × 2, 56.5, 73.6, 110.6, 112.8, 119.6, 120.9 × 2, 126.7, 127.2, 128.5 × 4, 128.9, 129.6 × 4, 130.0, 133.2, 133.7, 135.2×2, 141.3, 145.2, 150.8, 194.8; ESI-MS m/z (%) 634.80, 636.74, 638.50 ([M + H]+, 55, 100, 45); HR-MS (ESI) calcd for C31H28Br2N2O3 [M−H]−: 635.0370, found: 635.0389.
Compound 27b: Yellow solid, final yield 15.1%, m.p. 56.0–58.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 2.22–2.31 (m, 10H, piperazine-2’’,3’’,5’’,6’’-H, Ar-2-CH2-), 3.31 (t, J = 10.8 Hz, 2H, piperazine-4’’-CH2-), 3.45 (t, J = 10.2 Hz, 2H, HO-CH2-), 6.92 (s, 1H, Ar-3’-H), 7.04 (s, 1H, Ar-6’-H), 7.37 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.41 (d, J = 1.8 Hz, 1H, Ar-6-H), 7.66 (d, J = 12.6 Hz, 1H, Ar-4-H), 9.83 (brs, 2H, Ar-4’-OH, Ar -5’-OH); 13C-NMR (150 MHz, DMSO-d6) 52.9 × 2, 53.2 × 2, 58.8, 59.5, 60.6, 110.6, 111.6, 113.9, 120.3, 127.9, 131.7, 132.2, 133.2, 137.7, 142.5, 150.7, 155.9, 194.5; ESI-MS m/z (%) 512.92, 514.96, 516.81 ([M + H]+, 60, 100, 70).
Compound 28b: Yellow solid, final yield 18.6%, m.p. 114.0–115.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 3.28–3.57 (m, 6H, piperazine-H), 4.49 (s, 2H, Ar-2-CH2-), 4.72 (d, J = 14.4 Hz, 2H, piperazine-H), 6.80 (t, J = 7.2 Hz, 1H, pyrimidine-5’’’-H), 7.00 (s, 1H, Ar-3’-H), 7.10 (s, 1H, Ar-6’-H), 7.58 (d, J = 2.4 Hz, 1H, Ar-6-H), 7.91 (d, 1H, J = 12.6 Hz, Ar-3-H), 8.01 (dd, J = 12.6 Hz, 1H, Ar-4-H), 8.47 (d, J = 7.2 Hz, 2H, pyrimidine-4’’’,6’’’-H), 9.70 (brs, 2H, Ar-4’-OH, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 40.8 × 2, 51.5 × 2, 57.0, 110.7, 111.8, 119.7, 120.9, 123.3, 129.0, 129.4, 133.8, 135.5, 135.7, 141.5, 145.3, 150.9, 158.6 × 2, 160.8, 194.9; ESI-MS m/z (%) 546.72, 548.73, 550.59 ([M + H]+, 48, 100, 60).
Compound 29b: White solid, final yield 21.1%, m.p. 193.0–195.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 3.08–3.77 (m, 8H, piperazine-2’’,3’’,5’’,6’’-H), 4.50 (s, 2H, Ar-2-CH2-), 6.97 (s, 1H, Ar-3’-H), 7.03 (dd, J = 6.6, 13.8 Hz, 2H, piperazine-Ar-2’’’,6’’’-H), 7.09 (s, 1H, Ar-6’-H), 7.12 (d, J = 13.2 Hz, 2H, piperazine-Ar-3’’’,5’’’-H), 7.60 (d, 1H, J = 2.4 Hz, Ar-6-H), 7.83 (d, J = 12.6 Hz, 1H, Ar-3-H), 8.01 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 9.39 (brs, 2H, Ar-4’-OH, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 46.5 × 2, 51.7 × 2, 56.7, 110.7, 116.1 × 2, 118.4, 119.7, 120.9, 123.3, 129.0, 129.4, 133.9, 135.5 × 2, 141.5, 145.3, 146.7, 151.0, 156.3, 157.9, 194.9; ESI-MS m/z (%) 562.96, 564.95, 566.77 ([M + H]+, 48, 100, 60).
Compound 30b: Yellow solid, final yield 19.4%, m.p. 190.0–191.5 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 3.14–3.57 (m, 8H, piperazine-2’’,3’’,5’’ 6’’,-H), 4.52 (s, 2H, Ar-2-CH2-), 6.97 (s, 1H, Ar-3’-H), 7.05–7.22 (m, 5H, Ar-6’-H, piperazine-H), 7.60 (s, 1H, Ar-6-H), 7.80 (dd, J = 6.6, 12.6 Hz, 1H, Ar-3-H), 8.01 (d, J = 12.6 Hz, 1H, Ar-4-H), 9.35 (brs, 2H, Ar-4’-OH, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 47.4 × 2, 52.0 × 2, 57.0, 110.7, 116.7, 119.7, 120.2, 120.9, 123.3, 124.0, 125.5, 129.1, 129.4, 134.0, 135.8, 138.7, 141.4, 145.3, 151.0, 154.5, 156.2, 195.0; ESI-MS m/z (%) 562.97, 564.95, 566.79 ([M + H]+, 60, 100, 75).
Compound 31b: White solid, final yield 17.5%, m.p. 185.0–186.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 3.04–3.56 (m, 8H, piperazine-2’’,3’’,5’’,6’’-H), 4.49 (s, 2H, Ar-2-CH2-), 6.72 (d, 2H, J = 12.0 Hz,2H, piperazine-Ar-2’’’,6’’’-H), 6.88 (d, J = 12.0 Hz, 2H, piperazine-Ar-3’’’,5’’’-H), 6.97 (s, 1H, Ar-3’-H), 7.09 (s, 1H, Ar-6’-H), 7.59 (s, 1H, Ar-6-H), 7.80 (s, J = 12.0 Hz, 1H, Ar-3-H), 7.99 (d, J = 12.0 Hz, 1H, Ar-4-H), 9.32 (brs, 3H, Ar-4’-OH, Ar-5’-OH, Ar-4’’’-OH); 13C-NMR (150 MHz, DMSO-d6) 40.5 × 2, 48.9 × 2, 56.5, 110.7, 116.1 × 2, 119.9, 120.9, 124.4, 125.4, 126.1, 136.7, 137.1, 138.0, 141.5, 145.3, 150.2 × 2, 150.9, 152.3, 153.4, 184.5; ESI-MS m/z (%) 560.98, 562.97, 564.81 ([M + H]+, 45, 100, 60).
Compound 32b: Yellow solid, final yield 20.5%, m.p. 205.8–207.3 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 5.58 (s, 2H, Ar-2-CH2-), 6.93 (s, 1H, Ar-3’-H), 7.06 (s, 1H, Ar-6’-H), 7.45 (d, J = 7.8 Hz, 1H, Ar-3-H), 7.52 (s, 1H, Ar-6-H), 7.71 (t, J = 11.4 Hz, 2H, imidazole-4’’,5’’-H), 7.88 (d, J = 7.8 Hz, 1H, Ar-4-H), 9.14 (t, J = 11.4 Hz, 1H, imidazole-2’’-H), 9.70 (brs, 1H, Ar-4’-OH), 10.39(brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 49.7, 110.5, 119.4, 119.9, 120.6, 120.8, 122.3, 122.8, 129.0, 133.2, 133.5, 135.6, 136.5, 140.0, 145.2, 150.8, 194.7; ESI-MS m/z (%) 451.00, 453.06, 454.88 ([M + H]+, 70, 100, 80).
Compound 33b: White solid, final yield 21.1%, m.p. 217.0–218.5 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 2.53 (s, 3H, imidazole-2’’-CH3), 5.53 (s, 2H, Ar-2-CH2-), 6.95 (s, 1H, Ar-3’-H), 7.08 (s, 1H, Ar-6’-H), 7.19 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.50 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.53 (d, J = 3.0 Hz, 1H, imidazole-5’’-H), 7.62 (d, J = 3.0 Hz, 1H, imidazole-4’’-H), 7.85 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 9.75 (brs, 1H, Ar-4’-OH), 10.39 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 11.1, 48.4, 110.5, 118.8, 119.5, 120.9, 121.9, 123.0, 128.8, 131.9, 132.0, 133.5, 133.7, 135.5, 139.4, 145.3, 150.9, 194.6; ESI-MS m/z (%) 464.69, 466.67, 468.57 ([M + H]+, 50, 100, 60).
Compound 34b: White solid, final yield 17.3%, m.p. 170.0–172.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 2.20 (s, 3H, imidazole-4’’-CH3), 5.45 (s, 2H, Ar-2-CH2-), 6.90 (s, 1H, imidazole-5’’-H), 7.05 (s, 1H, Ar-3’-H), 7.27 (s, 1H, Ar-6’-H), 7.37 (d, J = 12.0 Hz, 1H, Ar-3-H), 7.50 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.87 (dd, J = 3.0, 12.0 Hz, 1H, Ar-4-H), 8.76 (s, 1H, imidazole-2’’-H), 9.72 (brs, 1H, Ar-4’-OH), 10.42 br (s, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 10.6, 49.3, 110.5, 118.9, 119.5, 120.9, 122.1, 128.9, 131.3, 133.0, 133.2, 134.2, 135.4, 135.9, 140.0, 145.2, 150.9, 194.7; ESI-MS m/z (%) 464.86, 466.88, 498.75 ([M + H]+, 54, 100, 65).
Compound 35b: White solid, final yield 20.5%, m.p. 235.2–236.9 °C; FT-IR (ATR) υ (cm−1): 3184, 3159, 2789, 2673, 2260, 1667, 1598, 1500, 1383, 1194, 1012, 882, 801, 640; 1H-NMR (600 MHz, DMSO-d6) δ: 1.20 (t, J = 11.4 Hz, 3H, imidazole-2’’-CH3), 2.90 (q, J = 11.4 Hz, 2H, imidazole-2’’-CH2-), 5.56 (s, 2H, Ar-2-CH2-), 6.94 (s, 1H, Ar-3’-H), 7.07 (s, 1H, Ar-6’-H), 7.16 (d, J = 6.0 Hz, 1H, Ar-6-H), 7.52 (d, J = 3.0 Hz, 1H, imidazole-5’’-H), 7.54 (d, J = 3.6 Hz, 1H, imidazole-4’’-H), 7.66 (d, J = 3.0 Hz, 1H, Ar-3-H), 7.84 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 9.72 (brs, 1H, Ar-4’-OH), 10.35 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 11.2, 18.5, 48.4, 110.5, 119.0, 119.5, 120.9, 121.9, 123.1, 128.8, 131.7, 133.6, 133.9, 135.5, 139.3, 145.3, 149.2, 150.9, 194.5; ESI-MS m/z (%) 478.78, 480.63, 482.73 ([M + H]+, 48, 100, 45); HR-MS (ESI) calcd for C19H16Br2N2O3 [M−H]−: 478.9420, found: 478.9469.
Compound 36b: Yellow solid, final yield 17.8%, m.p. 140.0–141.5 °C; FT-IR (ATR) υ (cm−1): 3327, 3149, 3115, 3028, 2975, 1673, 1590, 1415, 1283, 1201, 1008, 786, 635; 1H-NMR (600 MHz, DMSO-d6) δ: 1.24 (d, J = 10.2 Hz, 6H, imidazole-2’’-CH3), 3.36 (m, 1H, imidazole-2’’-CHMe2), 5.61 (s, 2H, Ar-2-CH2-), 6.94 (s, 1H, Ar-3’-H), 7.07 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.10 (s, 1H, Ar-6’-H), 7.53 (s, 1H, imidazole-5’’-H), 7.55 (s, 1H, imidazole-4’’-H), 7.72 (s, 1H, Ar-6-H), 7.85 (d, J = 12.6 Hz, 1H, Ar-4-H), 9.75 (brs, 1H, Ar-4’-OH), 10.42 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 20.8×2, 25.2, 48.5, 110.5, 119.3, 119.5, 120.9, 121.9, 122.9, 128.8, 131.5, 133.6, 134.1, 135.6, 139.2, 145.3, 150.9, 152.3, 194.5; ESI-MS m/z(%) 492.72, 494.71, 496.60 ([M + H]+, 48, 100, 58); HR-MS (ESI) calcd for C20H18Br2N2O3 [M−H]–: 492.9580, found: 492.9537.
Compound 37b: White solid, final yield 18.3%, m.p. 283.0–285.0 °C; FT-IR (ATR) υ (cm−1): 3074, 2985, 2887, 2780, 1664, 1586, 1504, 1408, 1279, 1229, 1151, 1013, 813, 623; 1H-NMR (600 MHz, DMSO-d6) δ: 1.19 (t, J = 11.4 Hz, 3H, imidazole-2’’-CH3), 2.20 (s, 3H, imidazole-4’’-CH3), 2.86 (q, J = 1.4 Hz, 2H, imidazole-2’’-CH2- ), 5.46 (s, 2H, Ar-2-CH2-), 6.90 (s, 1H, Ar-3’-H), 7.06 (s, 1H, Ar-6’-H), 7.16 (s, 1H, imidazole-5’’-H), 7.20 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.54 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.84 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 9.73 (brs, 1H, Ar-4’-OH), 10.43 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 10.0, 11.2, 18.4, 48.1, 110.6, 119.3, 119.7, 121.0, 122.0, 128.5, 128.6, 132.0, 133.3, 133.9, 135.4, 139.6, 145.2, 148.2, 150.9, 194.4; ESI-MS m/z (%) 491.13, 493.12, 495.14 ([M − H]−, 48, 100, 45); HR-MS (ESI) calcd for C20H18Br2N2O3 [M − H]−: 492.9580, found: 492.9518.
Compound 38b: White solid, final yield 15.7%, m.p. 210.3–212.0 °C; FT-IR (ATR) υ (cm−1): 3415, 3336, 3144, 2927, 2791, 2681, 1654, 1587, 1496, 1416, 1284, 1153, 1012, 700, 635; 1H-NMR (600 MHz, DMSO-d6) δ: 5.57 (s, 2H, Ar-2-CH2-), 6.83 (s, 1H, Ar-3’-H), 7.01 (s, 1H, Ar-6’-H), 7.10 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.49 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.59-7.65 (m, 5H, imidazole-Ar-H), 7.76 (d, J = 3.0 Hz, 1H, imidazole-5’’-H), 7.79 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 7.89 (d, J = 3.0 Hz, 1H, imidazole-4’’-H), 9.70 (brs, 1H, Ar-4’-OH), 10.39 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 49.5, 110.4, 119.3, 120.6, 120.8, 121.9, 123.1, 124.3, 128.8, 129.8 × 2, 130.0 × 2, 131.4, 132.6, 133.6, 134.1, 135.5, 139.0, 145.2, 145.3, 150.8, 194.4; ESI-MS m/z (%) 525.13, 527.13, 529.13 ([M − H]−, 50, 100, 48); HR-MS (ESI) calcd for C23H16Br2N2O3 [M − H]−: 526.9403, found: 526.9444.
Compound 39b: Yellow solid, final yield 19.1%, m.p. 190.0–192.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 2.18 (s, 3H, imidazole-4’’-CH3), 2.49 (s, 3H, imidazole-2’’-CH3), 5.43 (s, 2H, Ar-2-CH2-), 6.90 (s, 1H, imidazole-5’’-H), 7.06 (s, 1H, Ar-3’-H), 7.14 (d, J = 13.2 Hz, 1H, Ar-3-H), 7.22 (s, 1H, Ar-6’-H), 7.54 (d, J = 3.0 Hz, 1H, Ar-6-H), 7.85 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 9.72 (brs, 1H, Ar-4’-OH), 10.41 (brs, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 9.9, 11.0, 48.2, 110.6, 119.3, 119.7, 121.0, 122.0, 128.2, 128.5, 132.2, 133.3, 133.7, 135.3, 139.7, 144.1, 145.2, 150.9, 194.5; ESI-MS m/z (%) 478.92, 480.90, 482.75 ([M + H]+, 50, 100, 60).
Compound 40b: White solid, final yield 23%, m.p. 162.0–163.0 °C; 1H-NMR (600 MHz, DMSO-d6) δ: 5.88 (s, 2H, Ar-2-CH2-), 6.93 (s, 1H, Ar-3’-H), 7.03 (s, 1H, Ar-6’-H), 7.32 (d, J = 12.6 Hz, 1H, Ar-3-H), 7.55 (s, 1H, Ar-6-H), 7.59 (t, J = 10.8 Hz, 2H, imidazole-Ar-H), 7.72 (d, J = 10.8 Hz, 1H, imidazole-Ar-H), 7.81 (dd, J = 3.0, 12.6 Hz, 1H, Ar-4-H), 7.87 (d, J = 10.8 Hz, 1H, imidazole-Ar-H), 9.47 (s, 1H, imidazole-2’-H), 9.72 (brs, 1H, Ar-4’-OH), 10.38 (s, 1H, Ar-5’-OH); 13C-NMR (150 MHz, DMSO-d6) 48.1, 110.4, 113.8, 115.8, 119.5, 120.8, 122.1, 126.5, 126.8, 128.8, 131.6, 132.3, 132.4, 133.4, 133.6, 135.5, 139.6, 143.2, 145.3, 150.9, 194.7; ESI-MS m/z(%) 500.88, 502.89, 504.73 ([M + H]+, 50, 100, 60).