Lipoprotein Lipase Expression in Chronic Lymphocytic Leukemia: New Insights into Leukemic Progression
Abstract
1. Lipoprotein Lipase
LPL Synthesis and Trafficking
2. Chronic Lymphocytic Leukemia
3. LPL in Chronic Lymphocytic Leukemia
3.1. LPL As a Prognostic Marker of Disease Progression
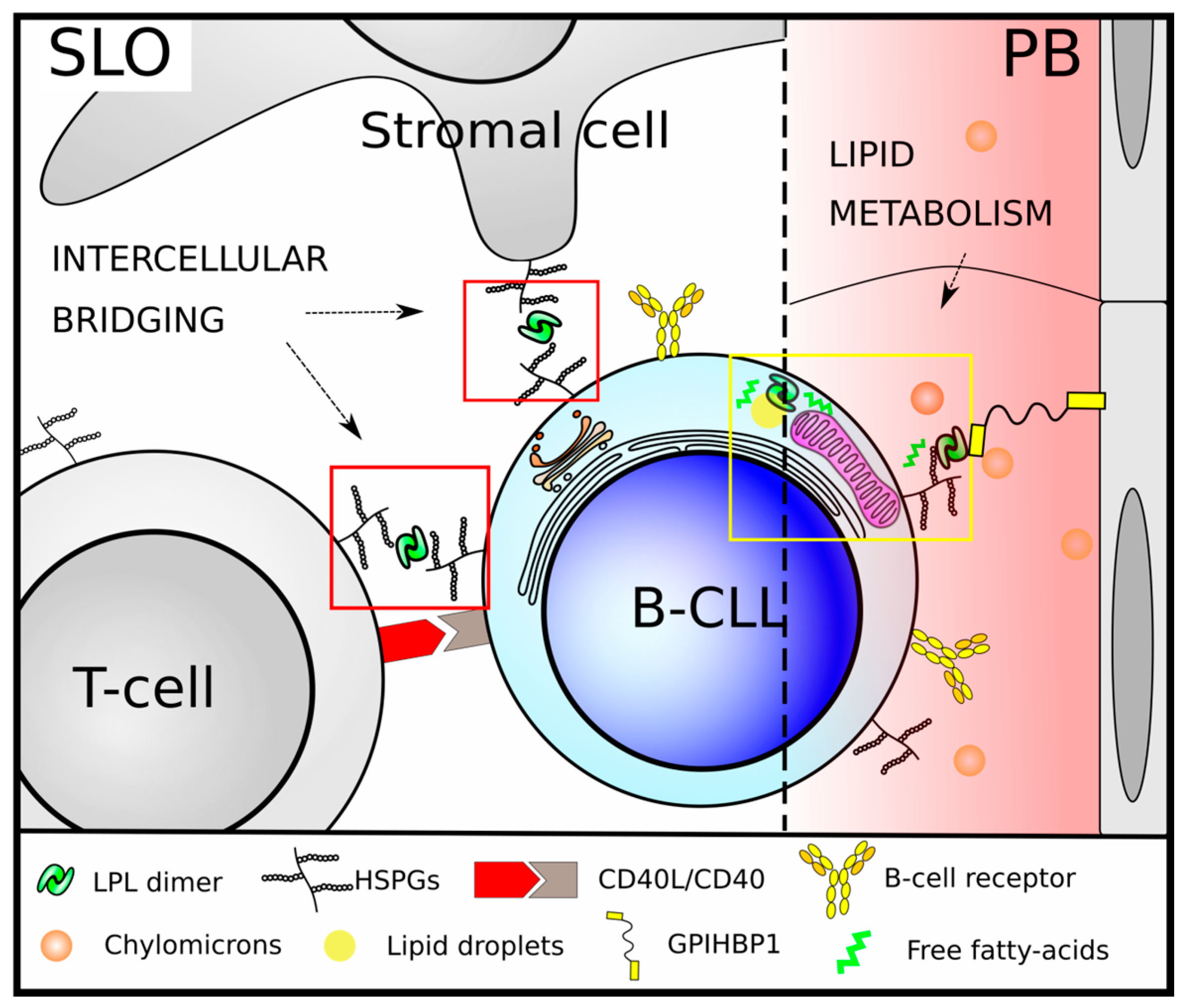
3.2. LPL in CLL B-Cell Metabolism
3.3. Non-Metabolic Roles of LPL in CLL
4. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Chajek-Shaul, T.; Friedman, G.; Knobler, H.; Stein, O.; Etienne, J.; Stein, Y. Importance of the different steps of glycosylation for the activity and secretion of lipoprotein lipase in rat preadipocytes studied with monensin and tunicamycin. Biochim. Biophys. Acta Lipids Lipid Metab. 1985, 837, 123–134. [Google Scholar] [CrossRef]
- Felts, J.M.; Itakura, H.; Crane, R.T. The mechanism of assimilation of constituents of chylomicrons, very low density lipoproteins and remnants—A new theory. Biochem. Biophys. Res. Commun. 1975, 66, 1467–1475. [Google Scholar] [CrossRef]
- Chajek, T.; Stein, O.; Stein, Y. Lipoprotein lipase of cultured mesenchymal rat heart cells. Biochim. Biophys. Acta Lipids Lipid Metab. 1978, 528, 466–474. [Google Scholar] [CrossRef]
- Hahn, P.F. Abolishment of Alimentary Lipemia Following Injection of Heparin. Science 1943, 98, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.S.J.; Beigneux, A.P.; Fong, L.G.; Young, S.G. New wrinkles in lipoprotein lipase biology. Curr. Opin. Lipidol. 2012, 23, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Yang, D.; Hill, J.S.; Davis, R.C.; Nikazy, J.; Schotz, M.C. A molecular biology-based approach to resolve the subunit orientation of lipoprotein lipase. Proc. Natl. Acad. Sci. USA 1997, 94, 5594–5598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, G.; Tate, C.G.; Lookene, A.; Olivecrona, G. Calreticulin promotes folding/dimerization of human lipoprotein lipase expressed in insect cells (Sf21). J. Biol. Chem. 2003, 278, 29344–29351. [Google Scholar] [CrossRef] [PubMed]
- Péterfy, M.; Ben-Zeev, O.; Mao, H.Z.; Weissglas-Volkov, D.; Aouizerat, B.E.; Pullinger, C.R.; Frost, P.H.; Kane, J.P.; Malloy, M.J.; Reue, K.; et al. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat. Genet. 2007, 39, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Péterfy, M. Lipase maturation factor 1: A lipase chaperone involved in lipid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Klinger, S.C.; Glerup, S.; Raarup, M.K.; Mari, M.C.; Nyegaard, M.; Koster, G.; Prabakaran, T.; Nilsson, S.K.; Kjaergaard, M.M.; Bakke, O.; et al. SorLA regulates the activity of lipoprotein lipase by intracellular trafficking. J. Cell Sci. 2011, 124, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Casaroli-Marano, R.P.; García, R.; Vilella, E.; Olivecrona, G.; Reina, M.; Vilaró, S. Binding and intracellular trafficking of lipoprotein lipase and triacylglycerol-rich lipoproteins by liver cells. J. Lipid Res. 1998, 39, 789–806. [Google Scholar] [PubMed]
- Vergés, M.; Bensadoun, A.; Herz, J.; Belcher, J.D.; Havel, R.J. Endocytosis of Hepatic Lipase and Lipoprotein Lipase into Rat Liver Hepatocytes in Vivo Is Mediated by the Low Density Lipoprotein Receptor-related Protein. J. Biol. Chem. 2004, 279, 9030–9036. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Borja, M.; Bellido, D.; Vilella, E.; Olivecrona, G.; Vilaró, S. Lipoprotein lipase-mediated uptake of lipoprotein in human fibroblasts: Evidence for an LDL receptor-independent internalization pathway. J. Lipid Res. 1996, 37, 464–481. [Google Scholar] [PubMed]
- Hallek, M. Chronic lymphocytic leukemia: 2015 Update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2015, 90, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, G.; Dalla-Favera, R. The molecular pathogenesis of chronic lymphocytic leukaemia. Nat. Rev. Cancer 2016, 16, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Caligaris-Cappio, F.; Ghia, P. Novel insights in chronic lymphocytic leukemia: Are we getting closer to understanding the pathogenesis of the disease? J. Clin. Oncol. 2008, 26, 4497–4503. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Ghia, P.; Rosenwald, A.; Caligaris-Cappio, F. The microenvironment in mature B-cell malignancies: A target for new treatment strategies. Blood 2009, 114, 3367–3375. [Google Scholar] [CrossRef] [PubMed]
- Oppezzo, P.; Dighiero, G. Role of the B-cell receptor and the microenvironment in chronic lymphocytic leukemia. Blood Cancer J. 2013, 3, e149. [Google Scholar] [CrossRef] [PubMed]
- Dighiero, G. Unsolved issues in CLL biology and management. Leukemia 2003, 17, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Damle, R.N.; Wasil, T.; Fais, F.; Ghiotto, F.; Valetto, A.; Allen, S.L.; Buchbinder, A.; Budman, D.; Dittmar, K.; Kolitz, J.; et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999, 94, 1840–1847. [Google Scholar] [PubMed]
- Hamblin, T.J.; Davis, Z.; Gardiner, A.; Oscier, D.G.; Stevenson, F.K. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999, 94, 1848–1854. [Google Scholar] [PubMed]
- Klein, U.; Tu, Y.; Stolovitzky, G.A.; Mattioli, M.; Cattoretti, G.; Husson, H.; Freedman, A.; Inghirami, G.; Cro, L.; Baldini, L.; et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J. Exp. Med. 2001, 194, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Alizadeh, A.A.; Widhopf, G.; Simon, R.; Davis, R.E.; Yu, X.; Yang, L.; Pickeral, O.K.; Rassenti, L.Z.; Powell, J.; et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J. Exp. Med. 2001, 194, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, Y.; De Vos, J.; Vallat, L.; Rème, T.; Lalanne, A.I.; Wanherdrick, K.; Michel, A.; Nguyen-Khac, F.; Oppezzo, P.; Magnac, C.; et al. Gene expression profiling of chronic lymphocytic leukemia can discriminate cases with stable disease and mutated Ig genes from those with progressive disease and unmutated Ig genes. Leukemia 2005, 19, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Oppezzo, P.; Vasconcelos, Y.; Settegrana, C.; Jeannel, D.; Vuillier, F.; Legarff-Tavernier, M.; Kimura, E.Y.; Bechet, S.; Dumas, G.; Brissard, M.; et al. The LPL/ADAM29 expression ratio is a novel prognosis indicator in chronic lymphocytic leukemia. Blood 2005, 106, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Heintel, D.; Kienle, D.; Shehata, M.; Kröber, A.; Kroemer, E.; Schwarzinger, I.; Mitteregger, D.; Le, T.; Gleiss, A.; Mannhalter, C.; et al. High expression of lipoprotein lipase in poor risk B-cell chronic lymphocytic leukemia. Leukemia 2005, 19, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Van’t Veer, M.B.; Brooijmans, A.M.; Langerak, A.W.; Verhaaf, B.; Goudswaard, C.S.; Graveland, W.J.; van Lom, K.; Valk, P.J.M. The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica 2006, 91, 56–63. [Google Scholar] [PubMed]
- Nückel, H.; Hüttmann, A.; Klein-Hitpass, L.; Schroers, R.; Führer, A.; Sellmann, L.; Dührsen, U.; Dürig, J. Lipoprotein lipase expression is a novel prognostic factor in B-cell chronic lymphocytic leukemia. Leuk. Lymphoma 2006, 47, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Sevov, M.; Fahlgren, E.; Tobin, G.; Jondal, M.; Osorio, L.; Roos, G.; Olivecrona, G.; Rosenquist, R. Lipoprotein lipase is differentially expressed in prognostic subsets of chronic lymphocytic leukemia but displays invariably low catalytical activity. Leuk. Res. 2010, 34, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Kaderi, M.A.; Kanduri, M.; Buhl, A.M.; Sevov, M.; Cahill, N.; Gunnarsson, R.; Jansson, M.; Smedby, K.E.; Hjalgrim, H.; Jurlander, J.; et al. LPL is the strongest prognostic factor in a comparative analysis of RNA-based markers in early chronic lymphocytic leukemia. Haematologica 2011, 96, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Porpaczy, E.; Tauber, S.; Bilban, M.; Kostner, G.; Gruber, M.; Eder, S.; Heintel, D.; Le, T.; Fleiss, K.; Skrabs, C.; et al. Lipoprotein lipase in chronic lymphocytic leukaemia—Strong biomarker with lack of functional significance. Leuk. Res. 2013, 37, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Mátrai, Z.; Andrikovics, H.; Szilvási, A.; Bors, A.; Kozma, A.; Ádám, E.; Halm, G.; Karászi, É.; Tordai, A.; Masszi, T. Lipoprotein Lipase as a Prognostic Marker in Chronic Lymphocytic Leukemia. Pathol. Oncol. Res. 2017, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.; Moreno, P.; Palacios, F.; Borge, M.; Morande, P.; Landoni, A.I.; Gabus, R.; Dighiero, G.; Giordano, M.; Gamberale, R.; et al. Methylation status regulates lipoprotein lipase expression in chronic lymphocytic leukemia. Leuk. Lymphoma 2013, 54, 1844–1888. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Abreu, C.; Borge, M.; Palacios, F.; Morande, P.; Pegazzano, M.; Bianchi, S.; Landoni, A.I.; Agrelo, R.; Giordano, M.; et al. Lipoprotein lipase expression in unmutated CLL patients is the consequence of a demethylation process induced by the microenvironment. Leukemia 2013, 27, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Grgurevic, S.; Bueso-Ramos, C.; Harris, D.M.; Li, P.; Liu, Z.; Wu, J.Y.; Jain, P.; Wierda, W.; Burger, J.; et al. Aberrant LPL Expression, Driven by STAT3, Mediates Free Fatty Acid Metabolism in CLL Cells. Mol. Cancer Res. 2015, 13, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Sportoletti, P.; Baldoni, S.; Cavalli, L.; Del Papa, B.; Bonifacio, E.; Ciurnelli, R.; Bell, A.S.; Di Tommaso, A.; Rosati, E.; Crescenzi, B.; et al. NOTCH1 PEST domain mutation is an adverse prognostic factor in B-CLL. Br. J. Haematol. 2010, 151, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.; Kristensen, T.; Abildgaard, N.; Royo, C.; Frederiksen, M.; Mourits-Andersen, T.; Campo, E.; Møller, M.B. LPL gene expression is associated with poor prognosis in CLL and closely related to NOTCH1 mutations. Eur. J. Haematol. 2016, 97, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Z.; Tu, J.; Zhu, W.; Ge, J.; Zheng, X.; Yang, L.; Pan, X.; Yan, H.; Zhu, J. MicroRNA-29a regulates pro-inflammatory cytokine secretion and scavenger receptor expression by targeting LPL in oxLDL-stimulated dendritic cells. FEBS Lett. 2011, 585, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bouvy-Liivrand, M.; Heinäniemi, M.; John, E.; Schneider, J.G.; Sauter, T.; Sinkkonen, L. Combinatorial regulation of lipoprotein lipase by microRNAs during mouse adipogenesis. RNA Biol. 2014, 11, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik, S.E.; Iorio, M.V.; Visone, R.; Sever, N.I.; Fabbri, M.; et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 353, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Fulci, V.; Chiaretti, S.; Goldoni, M.; Azzalin, G.; Carucci, N.; Tavolaro, S.; Castellano, L.; Magrelli, A.; Citarella, F.; Messina, M.; et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood 2007, 109, 4944–4951. [Google Scholar] [CrossRef] [PubMed]
- Marton, S.; Garcia, M.R.; Robello, C.; Persson, H.; Trajtenberg, F.; Pritsch, O.; Rovira, C.; Naya, H.; Dighiero, G.; Cayota, A. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia 2008, 22, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, B.; Meuleman, N.; Haibe-kains, B.; Saussoy, P.; Van Den Neste, E.; Michaux, L.; Heimann, P.; Martiat, P.; Bron, D.; Lagneaux, L. microRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification sion levels decreased significantly with progression from Binet stage A to C were significantly lower in poor p. Blood 2009, 113, 5237–5245. [Google Scholar] [PubMed]
- Negrini, M.; Cutrona, G.; Bassi, C.; Fabris, S.; Zagatti, B.; Colombo, M.; Ferracin, M.; D’Abundo, L.; Saccenti, E.; Matis, S.; et al. microRNAome expression in chronic lymphocytic leukemia: Comparison with normal B cell subsets and correlations with prognostic and clinical parameters. Clin. Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Santanam, U.; Zanesi, N.; Efanov, A.; Costinean, S.; Palamarchuk, A.; Hagan, J.P.; Volinia, S.; Alder, H.; Rassenti, L.; Kipps, T.; et al. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc. Natl. Acad. Sci. USA 2010, 107. [Google Scholar] [CrossRef] [PubMed]
- Pekarsky, Y.; Santanam, U.; Cimmino, A.; Palamarchuk, A.; Efanov, A.; Maximov, V.; Volinia, S.; Alder, H.; Liu, C.G.; Rassenti, L.; et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006, 66, 11590–11593. [Google Scholar] [CrossRef] [PubMed]
- Kluiver, J.L.; Chen, C.-Z. MicroRNAs regulate B-cell receptor signaling-induced apoptosis. Genes Immun. 2012, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, N.B.; Rysman, E.; Lombardo, P.S.; Flanagan, A.J.; Lipe, B.C.; Wells, W.A.; Pettus, J.R.; Froehlich, H.M.; Memoli, V.A.; Morganelli, P.M.; et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol. Cancer Ther. 2011, 10, 427–436. [Google Scholar] [CrossRef] [PubMed]
- McCaw, L.; Shi, Y.; Wang, G.; Li, Y.-J.; Spaner, D.E. Low Density Lipoproteins Amplify Cytokine-signaling in Chronic Lymphocytic Leukemia Cells. EBioMedicine 2017, 15, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Spaner, D.E.; Lee, E.; Shi, Y.; Wen, F.; Li, Y.; Tung, S.; McCaw, L.; Wong, K.; Gary-Gouy, H.; Dalloul, A.; et al. PPAR-alpha is a therapeutic target for chronic lymphocytic leukemia. Leukemia 2013, 27, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Buckstein, R.; Spaner, D.E. A link between hypercholesterolemia and chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mozessohn, L.; Earle, C.; Spaner, D.; Cheng, S.Y.; Kumar, M.; Buckstein, R. The association of dyslipidemia with chronic lymphocytic leukemia: A population-based study. J. Natl. Cancer Inst. 2017, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bilban, M.; Heintel, D.; Scharl, T.; Woelfel, T.; Auer, M.M.; Porpaczy, E.; Kainz, B.; Kröber, A.; Carey, V.J.; Shehata, M.; et al. Deregulated expression of fat and muscle genes in B-cell chronic lymphocytic leukemia with high lipoprotein lipase expression. Leukemia 2006, 20, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Pallasch, C.P.; Schwamb, J.; Königs, S.; Schulz, A.; Debey, S.; Kofler, D.; Schultze, J.L.; Hallek, M.; Ultsch, A.; Wendtner, C.-M. Targeting lipid metabolism by the lipoprotein lipase inhibitor orlistat results in apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia 2008, 22, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Hazan-Halevy, I.; Barzilai, M.; Keating, M.J.; Estrov, Z. Metabolism pathways in chronic lymphocytic leukemia. Leuk. Lymphoma 2016. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Kako, Y.; Radner, H.; Cho, I.S.; Ramasamy, R.; Brunzell, J.D.; Goldberg, I.J.; Breslow, J.L. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: Direct evidence that lipoprotein lipase bridging occurs in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 13841–13846. [Google Scholar] [CrossRef] [PubMed]
- Beisiegel, U.; Weber, W.; Bengtsson-Olivecrona, G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA 1991, 88, 8342–8346. [Google Scholar] [CrossRef] [PubMed]
- Goulbourne, C.N.; Gin, P.; Tatar, A.; Nobumori, C.; Hoenger, A.; Jiang, H.; Grovenor, C.R.M.; Adeyo, O.; Esko, J.D.; Goldberg, I.J.; et al. The GPIHBP1-LPL Complex Is Responsible for the Margination of Triglyceride-Rich Lipoproteins in Capillaries. Cell Metab. 2014, 19, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Mamputu, J.C.; Desfaits, A.C.; Renier, G. Lipoprotein lipase enhances human monocyte adhesion to aortic endothelial cells. J. Lipid Res. 1997, 38, 1722–1729. [Google Scholar] [PubMed]
- Allan, C.M.; Larsson, M.; Jung, R.S.; Ploug, M.; Bensadoun, A.; Beigneux, A.P.; Fong, L.G.; Young, S.G. Mobility of “HSPG-bound” LPL explains how LPL is able to reach GPIHBP1 on capillaries. J. Lipid Res. 2017, 58, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, F.; Pede, V.; Janssens, A.; Callewaert, F.; Offner, F.; Verhasselt, B.; Philippé, J. Lipoprotein lipase mRNA expression in whole blood is a prognostic marker in B cell chronic lymphocytic leukemia. Clin. Chem. 2007, 53, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Rombout, A.; Verhasselt, B.; Philippé, J. Lipoprotein lipase in chronic lymphocytic leukemia: Function and prognostic implications. Eur. J. Haematol. 2016, 97, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.C.; Reich, D.; Tandon, A.; Akylbekova, E.; Patterson, N.; Waliszewska, A.; Kathiresan, S.; Sarpong, D.; Taylor, H.A.; Wilson, J.G. Genetic differences between the determinants of lipid profile phenotypes in African and European Americans: The Jackson Heart Study. PLoS Genet. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Nettleton, J.A.; Rotllan, N.; Tanaka, T.; Smith, C.E.; Lai, C.Q.; Parnell, L.D.; Lee, Y.C.; Lahti, J.; Lemaitre, R.N.; et al. Gain-of-function lipoprotein lipase variant rs13702 modulates lipid traits through disruption of a MicroRNA-410 seed site. Am. J. Hum. Genet. 2013, 92, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Badia-Villanueva, M.; Carulla, P.; Carrascal, M.; Abián, J.; Llobera, M.; Casanovas, A.; Dolores López-Tejero, M. Lipoprotein lipase isoelectric point isoforms in humans. Biochem. Biophys. Res. Commun. 2014, 445, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Kolset, S.O.; Salmivirta, M. Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell. Mol. Life Sci. 1999, 56, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Borge, M.; Nannini, P.R.; Galletti, J.G.; Morande, P.E.; Sanchez Avalos, J.; Bezares, R.F.; Giordano, M.; Gamberale, R. CXCL12-induced chemotaxis is impaired in T cells from ZAP-70-chronic lymphocytic leukemia patients. Haematologica 2010, 95, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Palacios, F.; Moreno, P.; Morande, P.; Abreu, C.; Correa, A.; Porro, V.; Landoni, A.I.; Gabus, R.; Giordano, M.; Dighiero, G.; et al. High expression of AID and active class switch recombination might account for a more aggressive disease in unmutated CLL patients: Link with an activated microenvironment in CLL disease. Blood 2010, 115, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, D.; Oppezzo, P. Lipoprotein Lipase Expression in Chronic Lymphocytic Leukemia: New Insights into Leukemic Progression. Molecules 2017, 22, 2083. https://doi.org/10.3390/molecules22122083
Prieto D, Oppezzo P. Lipoprotein Lipase Expression in Chronic Lymphocytic Leukemia: New Insights into Leukemic Progression. Molecules. 2017; 22(12):2083. https://doi.org/10.3390/molecules22122083
Chicago/Turabian StylePrieto, Daniel, and Pablo Oppezzo. 2017. "Lipoprotein Lipase Expression in Chronic Lymphocytic Leukemia: New Insights into Leukemic Progression" Molecules 22, no. 12: 2083. https://doi.org/10.3390/molecules22122083
APA StylePrieto, D., & Oppezzo, P. (2017). Lipoprotein Lipase Expression in Chronic Lymphocytic Leukemia: New Insights into Leukemic Progression. Molecules, 22(12), 2083. https://doi.org/10.3390/molecules22122083




