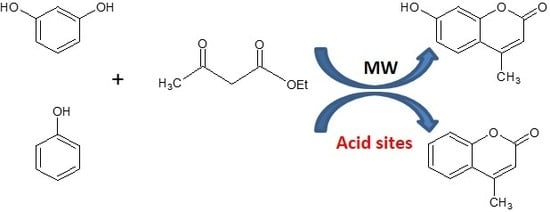
Coumarin Derivatives Solvent-Free Synthesis under Microwave Irradiation over Heterogeneous Solid Catalysts
Abstract
:1. Introduction
2. Results
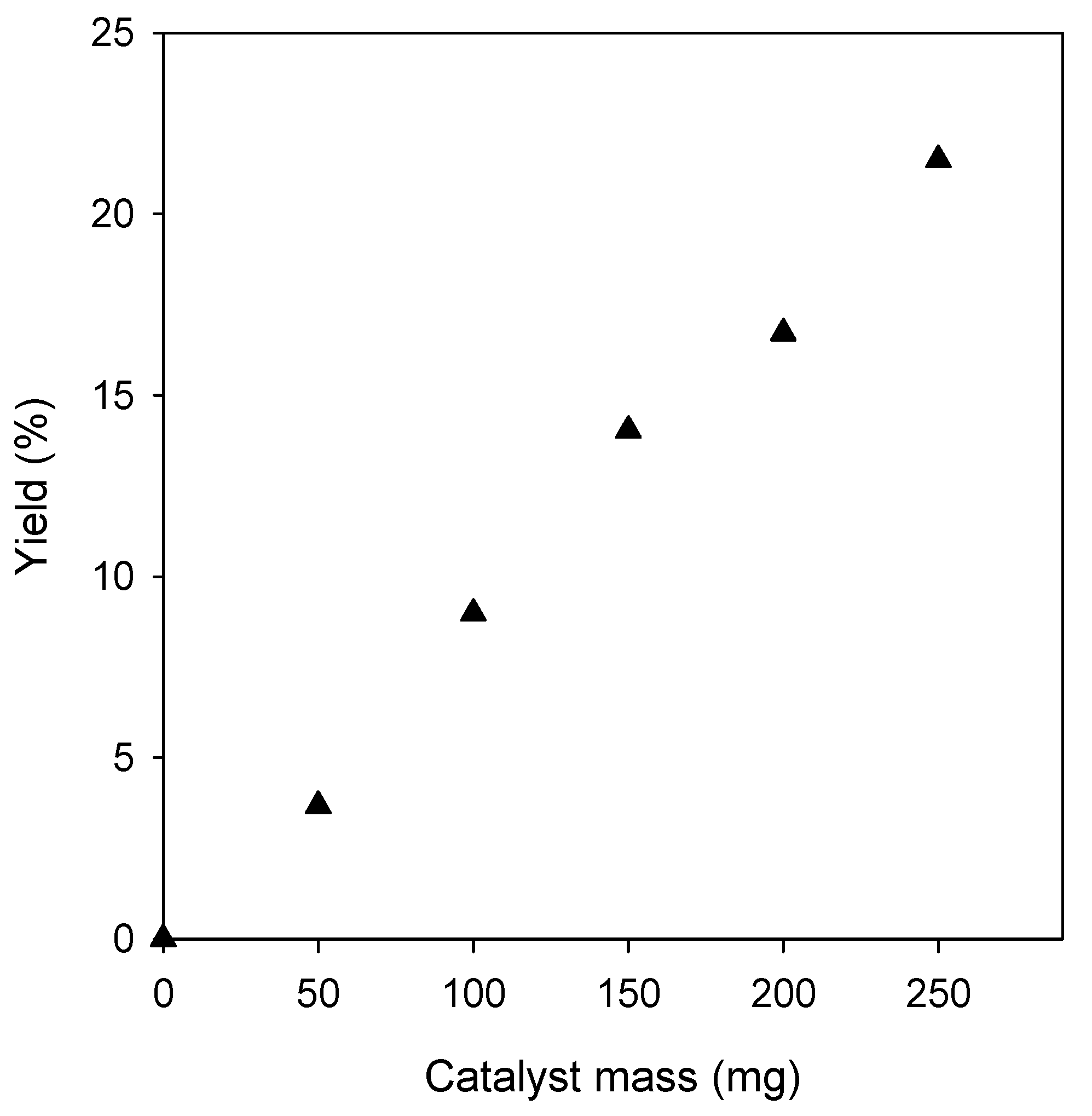
2.1. Optimization of Reaction Conditions
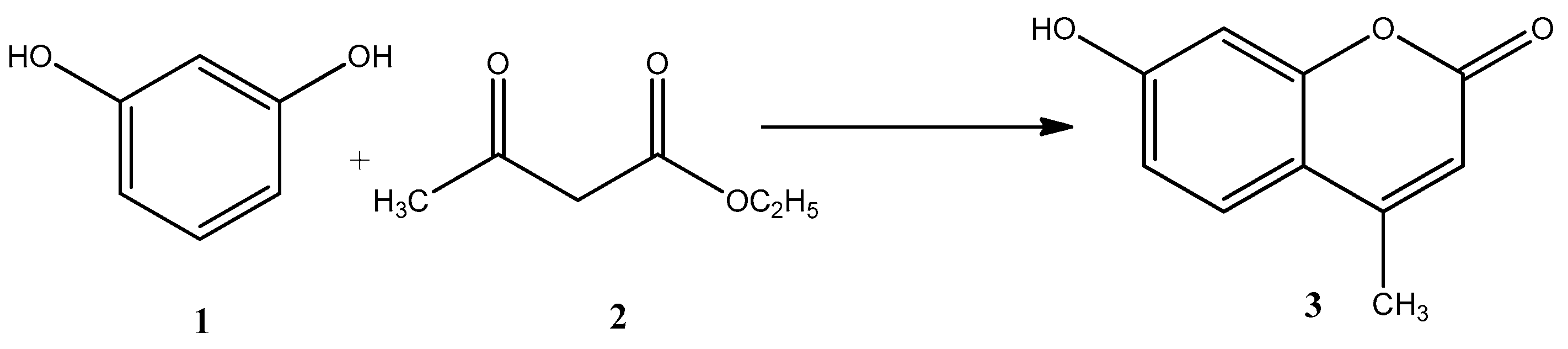
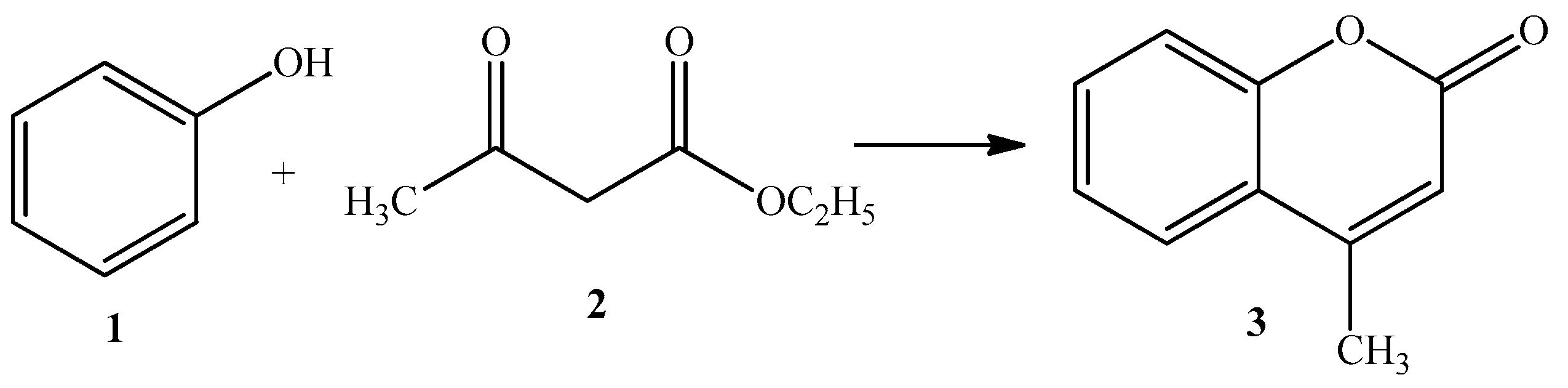
2.2. Reaction Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Sulfonic Acid Functionalized Hybrid Silica (TS-OS-SO3H)
3.3. Microwave Assisted Solvent-Free Synthesis of Coumarin Derivatives
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Al-Haiza, M.; Mostafa, M.; El-Kady, M. Synthesis and biological evaluation of some new coumarin derivatives. Molecules 2003, 8, 275–286. [Google Scholar] [CrossRef]
- Chimenti, F.; Bizzarri, B.; Bolasco, A.; Secci, D.; Chimenti, P.; Granese, A.; Carradori, S.; Rivanera, D.; Zicari, A.; Scaltrito, M.M.; et al. Synthesis, selective anti-helicobacter pylori activity, and cytotoxicity of novel n-substituted-2-oxo-2H-1-benzopyran-3-carboxamides. Bioorg. Med. Chem. Lett. 2010, 20, 4922–4926. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgis, C.A.; Savvoglou, K.; Hadjipavlou-Litina, D.J. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J. Enzym. Inhib. Med. Chem. 2006, 21, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, S.; Figueroa-Guíñez, R.; Matos, M.J.; Santana, L.; Uriarte, E.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Synthesis of coumarin–chalcone hybrids and evaluation of their antioxidant and trypanocidal properties. MedChemComm 2013, 4, 993–1000. [Google Scholar] [CrossRef]
- Van Schie, R.M.; Wadelius, M.; Kamali, F.; Daly, A.K.; Manolopoulos, V.G.; De Boer, A.; Barallon, R.; Verhoef, T.I.; Kirchheiner, J.; Haschke-Becher, E. Genotype-guided dosing of coumarin derivatives: The european pharmacogenetics of anticoagulant therapy (eu-pact) trial design. Pharmacogenomics 2009, 10, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Nofal, Z.; El-Zahar, M.; Abd El-Karim, S. Novel coumarin derivatives with expected biological activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- Bhavsar, D.; Trivedi, J.; Parekh, S.; Savant, M.; Thakrar, S.; Bavishi, A.; Radadiya, A.; Vala, H.; Lunagariya, J.; Parmar, M.; et al. Synthesis and in vitro anti-HIV activity of N-1,3-benzo[d]thiazol-2-yl-2-(2-oxo-2H-chromen-4-yl)acetamide derivatives using MTT method. Bioorg. Med. Chem. Lett. 2011, 21, 3443–3446. [Google Scholar] [PubMed]
- Yang, H.L.; Cai, P.; Liu, Q.H.; Yang, X.L.; Li, F.; Wang, J.; Wu, J.J.; Wang, X.B.; Kong, L.Y. Design, synthesis and evaluation of coumarin-pargyline hybrids as novel dual inhibitors of monoamine oxidases and amyloid-beta aggregation for the treatment of alzheimer's disease. Eur. J. Med. Chem. 2017, 138, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Rohini, K.; Srikumar, P. Therapeutic role of coumarins and coumarin-related compounds. J. Thermodyn. Catal. 2014, 5, 1. [Google Scholar] [CrossRef]
- Demyttenaere, J.; Van Syngel, K.; Peter Markusse, A.; Vervisch, S.; Debenedetti, S.; De Kimpe, N. Synthesis of 6-methoxy-4H-1-benzopyran-7-ol, a character donating component of the fragrance of wisteria sinensis. Tetrahedron 2002, 58, 2163–2166. [Google Scholar] [CrossRef]
- Keating, G.; O’kennedy, R. The chemistry and occurrence of coumarins. In Coumarins: Biology, Applications and Mode of Action; John Wiley & Sons, Inc.: New York, NY, USA, 1997; p. 348. [Google Scholar]
- Adronov, A.; Gilat, S.L.; Frechet, J.M.; Ohta, K.; Neuwahl, F.V.; Fleming, G.R. Light harvesting and energy transfer in laser−dye-labeled poly (aryl ether) dendrimers. J. Am. Chem. Soc. 2000, 122, 1175–1185. [Google Scholar] [CrossRef]
- Pechmann, H.V. Neue bildungsweise der cumarine. Synthese des daphnetins. Int. Eur. J. Inorg. Chem. 1884, 147, 929. [Google Scholar]
- Vekariya, R.H.; Patel, H.D. Recent advances in the synthesis of coumarin derivatives via knoevenagel condensation: A review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Cartwright, G. Synthesis of coumarins by flash vacuum pyrolysis of 3-(2-hydroxyaryl) propenoic esters, 1. J. Chem. Res. Synop. 1997, 8, 296–297. [Google Scholar] [CrossRef]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; Carradori, S.; Befani, O.; Turini, P.; Alcaro, S.; Ortuso, F. Synthesis, molecular modeling studies, and selective inhibitory activity against monoamine oxidase of N,N′-bis[2-oxo-2H-benzopyran]-3-carboxamides. Bioorg. Med. Chem. Lett. 2006, 16, 4135–4140. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.; Sapp, J. A new one-step synthesis of substituted coumarins. J. Org. Chem. 1962, 27, 3703–3705. [Google Scholar] [CrossRef]
- Vahabi, V.; Hatamjafari, F. Microwave assisted convenient one-pot synthesis of coumarin derivatives via Pechmann condensation catalyzed by FeF3 under solvent-free conditions and antimicrobial activities of the products. Molecules 2014, 19, 13093–13103. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Reddy, J.J.; Lakshmi, P.S.; Krishna, P.R. An efficient ZrCl4 catalyzed one-pot solvent free protocol for the synthesis of 4-substituted coumarins. Tetrahedron Lett. 2005, 46, 6119–6121. [Google Scholar] [CrossRef]
- Patil, S.B.; Bhat, R.P.; Raje, V.P.; Samant, S.D. Ultrasound-assisted Pechmann condensation of phenols with β-ketoesters to form coumarins, in the presence of bismuth (iii) chloride catalyst. Synth. Commun. 2006, 36, 525–531. [Google Scholar] [CrossRef]
- Stoyanov, E.; Mezger, J. Pechmann reaction promoted by boron trifluoride dihydrate. Molecules 2005, 10, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Gunnewegh, E.A.; Hoefnagel, A.J.; van Bekkum, H. Zeolite catalysed synthesis of coumarin derivatives. J. Mol. Catal. A: Chem. 1995, 100, 87–92. [Google Scholar] [CrossRef]
- Vijayakumar, B.; Ranga Rao, G. Pwa/montmorillonite K10 catalyst for synthesis of coumarins under solvent-free conditions. J. Porous Mater. 2012, 19, 233–242. [Google Scholar] [CrossRef]
- Potdar, M.K.; Mohile, S.S.; Salunkhe, M.M. Coumarin syntheses via pechmann condensation in lewis acidic chloroaluminate ionic liquid. Tetrahedron Lett. 2001, 42, 9285–9287. [Google Scholar] [CrossRef]
- Laufer, M.; Hausmann, H.; Hölderich, W. Synthesis of 7-hydroxycoumarins by pechmann reaction using nafion resin/silica nanocomposites as catalysts. J. Catal. 2003, 218, 315–320. [Google Scholar] [CrossRef]
- Maheswara, M.; Siddaiah, V.; Damu, G.L.V.; Rao, Y.K.; Rao, C.V. A solvent-free synthesis of coumarins via pechmann condensation using heterogeneous catalyst. J. Mol. Catal. A: Chem. 2006, 255, 49–52. [Google Scholar] [CrossRef]
- Kuarm, B.S.; Madhav, J.V.; Laxmi, S.V.; Rajitha, B.; Reddy, Y.T.; Reddy, P.N.; Crooks, P.A. Expeditious pechmann condensation by using biodegradable cellulose sulfuric acid as a solid acid catalyst. Synth. Commun. 2010, 40, 3358–3364. [Google Scholar] [CrossRef]
- Karami, B.; Kiani, M. ZrOCl2. 8H2O/SiO2: An efficient and recyclable catalyst for the preparation of coumarin derivatives by Pechmann condensation reaction. Catal. Commun. 2011, 14, 62–67. [Google Scholar]
- Opanasenko, M.; Shamzhy, M.; Čejka, J. Solid acid catalysts for coumarin synthesis by the Pechmann reaction: MOFs versus zeolites. ChemCatChem 2013, 5, 1024–1031. [Google Scholar] [CrossRef]
- Niknam, K.; Sajadi, S.A.; Hosseini, R.; Baghernejad, M. Silica-bonded n-propyldiethylenetriamine sulfamic acid as a recyclable solid acid catalyst for the synthesis of coumarin and biscoumarin derivatives. Iran J. Catal. 2014, 4, 163–173. [Google Scholar]
- Jafari, F.; Khodabakhshi, S.; Shirazi, S.G. Correction: Zinc oxide nanorods: A new application as a powerful catalyst for the green one-pot synthesis of new warfarin analogs. RSC Adv. 2014, 4, 64215. [Google Scholar] [CrossRef]
- Manhas, M.S.; Ganguly, S.N.; Mukherjee, S.; Jain, A.K.; Bose, A.K. Microwave initiated reactions: Pechmann coumarin synthesis, biginelli reaction, and acylation. Tetrahedron Lett. 2006, 47, 2423–2425. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [PubMed]
- Li, S.; Qi, X.; Huang, B. Synthesis of 7-hydroxy-4-methylcoumarin via the Pechmann reaction with PVP-supported phosphotungstic acid catalyst. Catal. Today 2016, 276, 139–144. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Jiménez, C.; Marinas, J.M.; Romero, F.J.; Urbano, F.J. Catalytic use of zeolites in the Prins reaction of arylalkenes. Catal. Lett. 2001, 73, 203–206. [Google Scholar] [CrossRef]
- Sethna, S.; Phadke, R. The Pechmann reaction. Org. React. 1953, 7, 1–58. [Google Scholar]
- Tyagi, B.; Mishra, M.K.; Jasra, R.V. Microwave-assisted solvent free synthesis of hydroxy derivatives of 4-methyl coumarin using nano-crystalline sulfated-zirconia catalyst. J. Mol. Catal. A Chem. 2008, 286, 41–46. [Google Scholar] [CrossRef]
- Robertson, A.; Waters, R.B.; Jones, E.T. 223. Hydroxy-carbonyl compounds. Part VII. Coumarins and 1: 4-benzopyrones derived from m-cresol. J. Chem. Soc. 1932, 1681–1688. [Google Scholar] [CrossRef]
- López, M.I.; Esquivel, D.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Application of sulfonic acid functionalised hybrid silicas obtained by oxidative cleavage of tetrasulfide bridges as catalysts in esterification reactions. ChemCatChem 2013, 5, 1002–1010. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Catalyst | SBET 2 (m2g−1) | Acidity (mmol g−1) | Yield (%) |
|---|---|---|---|
| Amberlyst-15 | 43 | 4.30 | 97 |
| H-β | 530 | 1.01 | 21 |
| TS-OS-SO3H | 448 | 1.24 | 44 |
| Catalyst | Yield (%) |
|---|---|
| Amberlyst-15 | 43 |
| H-β | 13 |
| TS-OS-SO3H | 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouasla, S.; Amaro-Gahete, J.; Esquivel, D.; López, M.I.; Jiménez-Sanchidrián, C.; Teguiche, M.; Romero-Salguero, F.J. Coumarin Derivatives Solvent-Free Synthesis under Microwave Irradiation over Heterogeneous Solid Catalysts. Molecules 2017, 22, 2072. https://doi.org/10.3390/molecules22122072
Bouasla S, Amaro-Gahete J, Esquivel D, López MI, Jiménez-Sanchidrián C, Teguiche M, Romero-Salguero FJ. Coumarin Derivatives Solvent-Free Synthesis under Microwave Irradiation over Heterogeneous Solid Catalysts. Molecules. 2017; 22(12):2072. https://doi.org/10.3390/molecules22122072
Chicago/Turabian StyleBouasla, Souad, Juan Amaro-Gahete, Dolores Esquivel, M. Isabel López, César Jiménez-Sanchidrián, Mabrouk Teguiche, and Francisco J. Romero-Salguero. 2017. "Coumarin Derivatives Solvent-Free Synthesis under Microwave Irradiation over Heterogeneous Solid Catalysts" Molecules 22, no. 12: 2072. https://doi.org/10.3390/molecules22122072
APA StyleBouasla, S., Amaro-Gahete, J., Esquivel, D., López, M. I., Jiménez-Sanchidrián, C., Teguiche, M., & Romero-Salguero, F. J. (2017). Coumarin Derivatives Solvent-Free Synthesis under Microwave Irradiation over Heterogeneous Solid Catalysts. Molecules, 22(12), 2072. https://doi.org/10.3390/molecules22122072