Antifungal Activity and Action Mode of Cuminic Acid from the Seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. Niveum (FON) Causing Fusarium Wilt on Watermelon
Abstract
:1. Introduction
2. Results
2.1. Effect of Cuminic Acid on FON Colony Growth
2.2. Effect of Cuminic Acid on Mycelial Morphology of FON
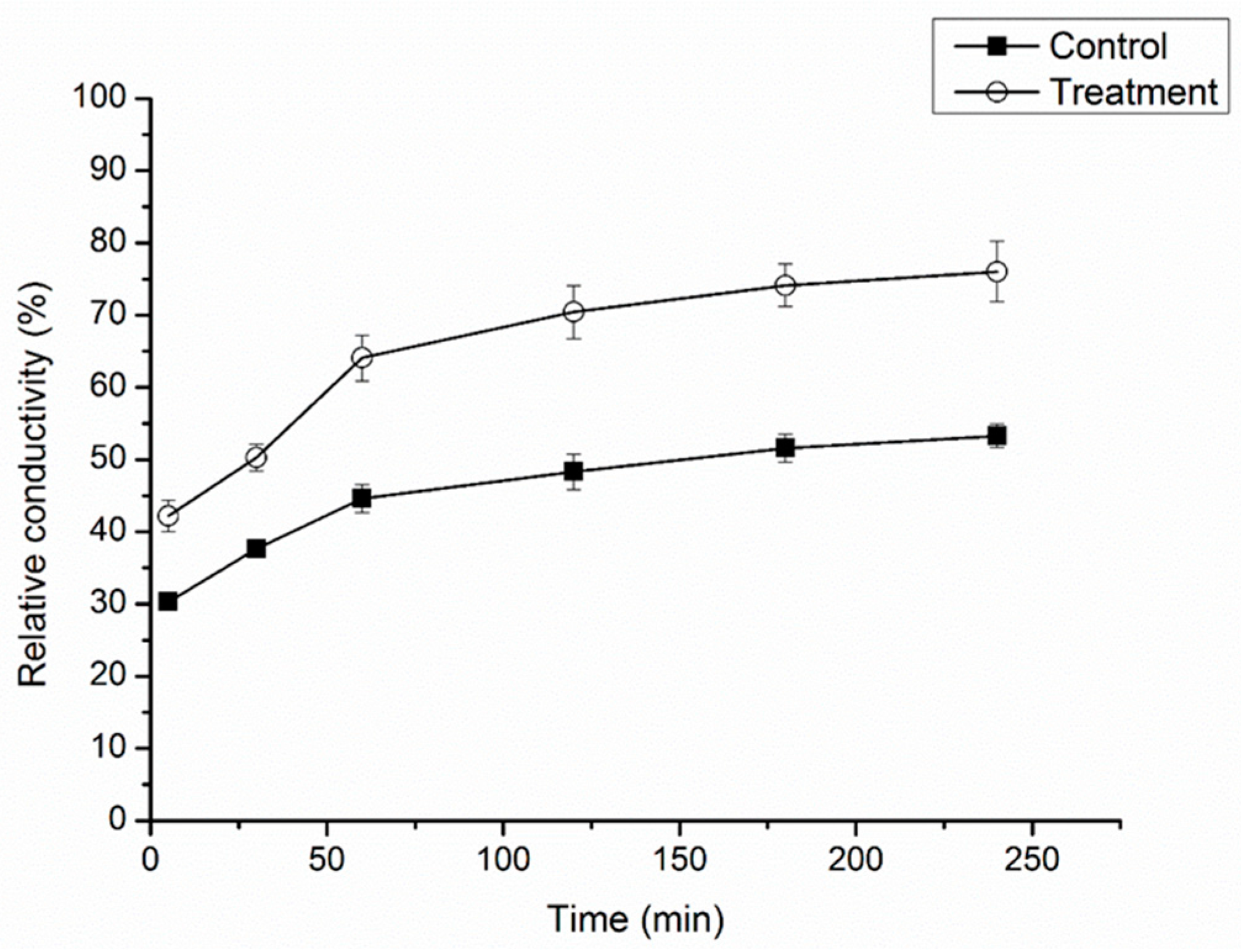
2.3. Effect of Cuminic Acid on Cell Membrane Permeability of FON
2.4. Glycerol Content of Mycelia
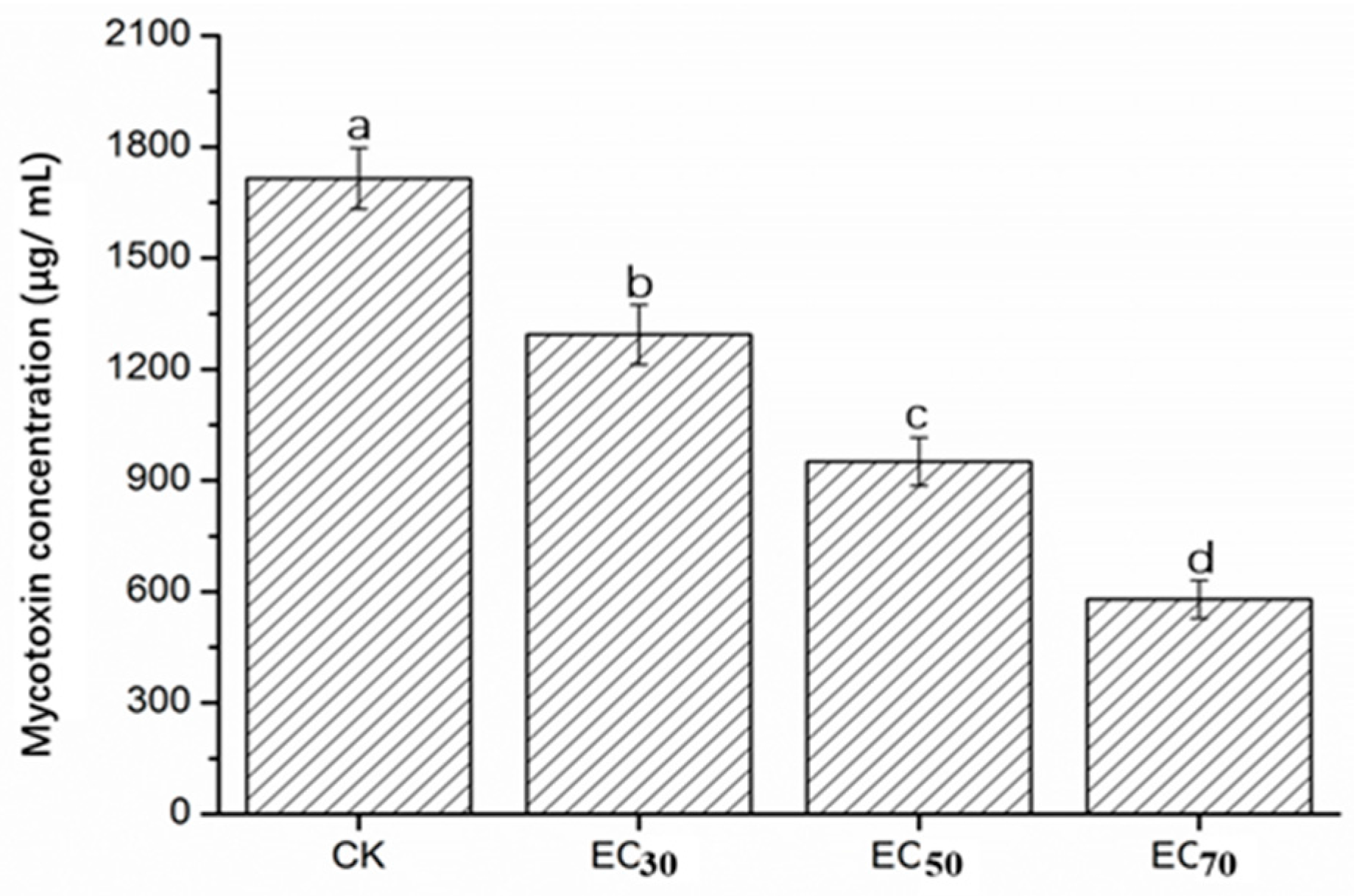
2.5. Mycotoxin Concentration of FON in Liquid Culture
2.6. Greenhouse Experiments
2.7. Assay of Defense Enzyme Activities and Malondialdehyde (MDA) Content
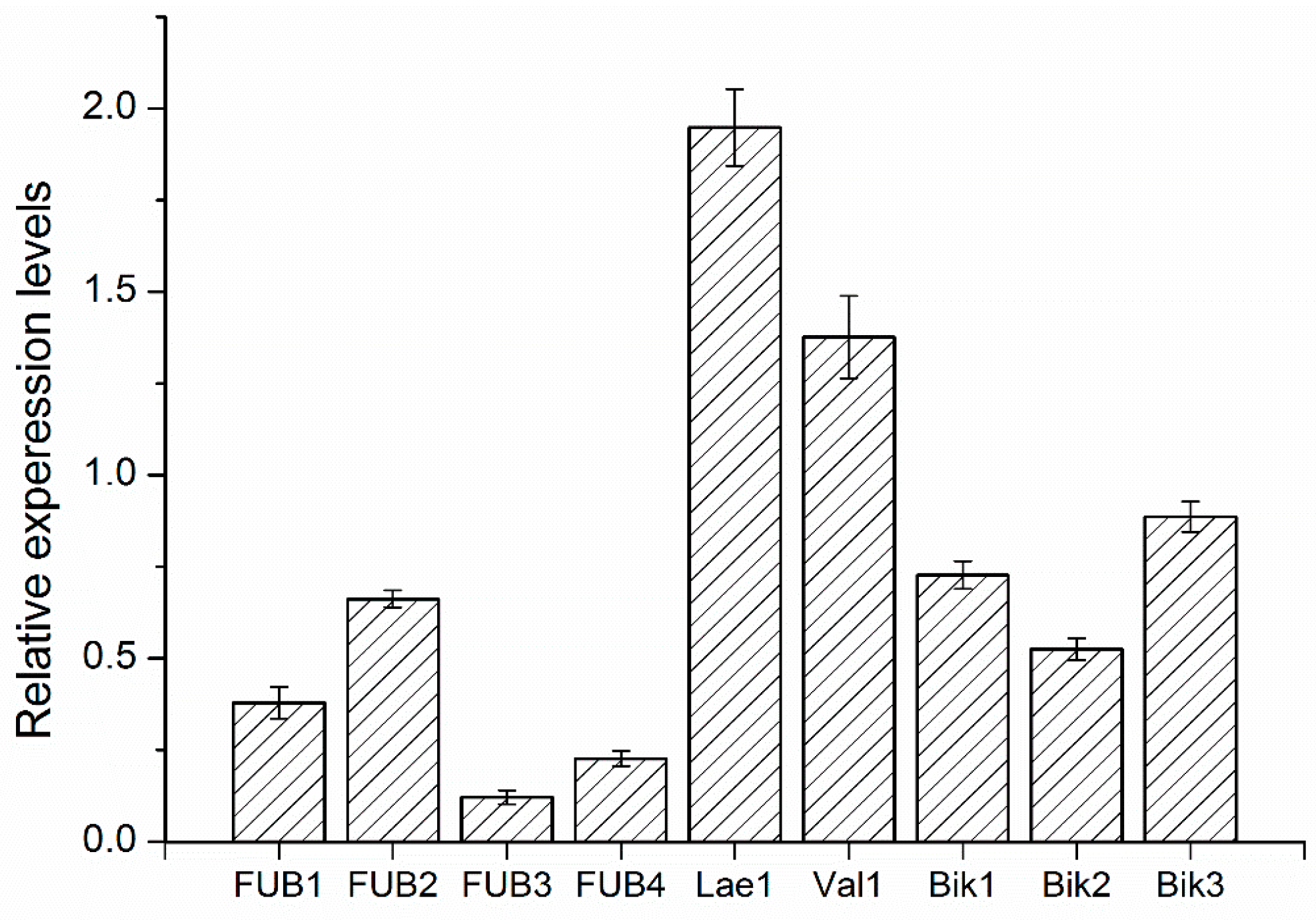
2.8. Quantitative RT-PCR
3. Discussion
4. Materials and Methods
4.1. Pathogen Strains and Fungicides
4.2. Effect of Cuminic Acid on FON Colony Growth
4.3. Effect of Cuminic Acid on Mycelial Morphology of FON
4.4. Effect of Cuminic Acid on Cell Membrane Permeability of FON
4.5. Glycerol Content of Mycelia
4.6. Mycotoxin Conerntration of Mycelia
4.7. Preparation of FON Inoculum and the Watermelon Seedlings
4.8. Greenhouse Experiments
4.9. Assay of Defense Enzymes Activities and Malondialdehyde (MDA) Content
4.10. Quantitative RT-PCR
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shen, Q.R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Limón, M.C.; Rodríguez-Ortiz, R.; Avalos, J. Bikaverin production and applications. Appl. Microbiol. Biotechnol. 2010, 87, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Sieber, C.M.; Von Bargen, K.W.; Studt, L.; Niehaus, E.-M.; Espino, J.J.; Huß, K.; Michielse, C.B.; Albermann, S.; Wagner, D. Deciphering the cryptic genome: Genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, E.-M.; von Bargen, K.W.; Espino, J.J.; Pfannmüller, A.; Humpf, H.-U.; Tudzynski, B. Characterization of the fusaric acid gene cluster in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2014, 98, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Lee, S.-H.; Kim, L.-H.; Ryu, J.-G.; Lee, S.; Seo, Y.; Kim, Y.H.; Busman, M.; Yun, S.-H.; Proctor, R.H. Identification of a 12-gene fusaric acid biosynthetic gene cluster in Fusarium species through comparative and functional genomics. Mol. Plant Microbe-Interact. 2015, 28, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Pavlovkin, J.; Mistrik, I.; Prokop, M. Some aspects of the phytotoxic action of fusaric acid on primary Ricinus roots. Plant Soil Environ. 2004, 50, 397–401. [Google Scholar]
- Wu, H.S.; Wang, Y.; Bao, W.; Liu, D.Y.; Raza, W.; Huang, Q.W.; Mao, Z.S.; Shen, Q.R. Responses of Fusarium oxysporum f. sp. niveum to exogenously added sinapic acid in vitro. Biol. Fertil. Soils 2009, 45, 443–447. [Google Scholar] [CrossRef]
- Everts, K.L.; Egel, D.S.; Langston, D.; Zhou, X.-G. Chemical management of Fusarium wilt of watermelon. Crop Prot. 2014, 66, 114–119. [Google Scholar] [CrossRef]
- Himmelstein, J.; Maul, J.; Everts, K. Impact of five cover crop green manures and Actinovate on Fusarium wilt of watermelon. Plant Dis. 2014, 98, 965–972. [Google Scholar] [CrossRef]
- Brimner, T.A.; Boland, G.J. A review of the non-target effects of fungi used to biologically control plant diseases. Agric. Ecosyst. Environ. 2003, 100, 3–16. [Google Scholar] [CrossRef]
- Pascual, J.; Garcia, C.; Hernandez, T.; Lerma, S.; Lynch, J. Effectiveness of municipal waste compost and its humic fraction in suppressing Pythium ultimum. Microb. Ecol. 2002, 44, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.; Hernandez, M.; Garcia, C.; Bernal, A.; Pascual, J. Biopesticide effect of green compost against Fusarium wilt on melon plants. J. Appl. Microbiol. 2005, 98, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytother. 2009, 1, 52–63. [Google Scholar]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Yong, X.; Shen, Q. Formulations can affect rhizosphere colonization and biocontrol efficiency of Trichoderma harzianum SQR-T037 against Fusarium wilt of cucumbers. Biol. Fertil. Soils 2011, 47, 239–248. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Suárez-Estrella, F.; Vargas-Garcia, C.; Lopez, M.; Capel, C.; Moreno, J. Antagonistic activity of bacteria and fungi from horticultural compost against Fusarium oxysporum f. sp. melonis. Crop Prot. 2007, 26, 46–53. [Google Scholar] [CrossRef]
- Bowers, J.H.; Locke, J.C. Effect of botanical extracts on the population density of Fusarium oxysporum in soil and control of Fusarium wilt in the greenhouse. Plant Dis. 2000, 84, 300–305. [Google Scholar] [CrossRef]
- Wu, H.S.; Wang, Y.; Zhang, C.Y.; Bao, W.; Ling, N.; Liu, D.Y.; Shen, Q.R. Growth of in vitro Fusarium oxysporum f. sp. niveum in chemically defined media amended with gallic acid. Biol. Res. 2009, 42, 297–304. [Google Scholar] [PubMed]
- Wu, H.S.; Luo, J.; Raza, W.; Liu, Y.X.; Gu, M.; Chen, G.; Hu, X.F.; Wang, J.H.; Mao, Z.S.; Shen, Q.R. Effect of exogenously added ferulic acid on in vitro Fusarium oxysporum f. sp. niveum. Sci. Hortic. 2010, 124, 448–453. [Google Scholar] [CrossRef]
- Wu, H.S.; Shen, S.H.; Han, J.M.; Liu, Y.D.; Liu, S.D. The effect in vitro of exogenously applied p-hydroxybenzoic acid on Fusarium oxysporum f. sp. niveum. Phytopathol. Mediterr. 2010, 48, 439–446. [Google Scholar]
- Hu, L.F.; Feng, J.T.; Zhang, X. Isolation and Structure Detection of Fungicidal Components from Cuminum cyminum Seed. Chin. J. Pestic. Sci. 2007, 4, 5. [Google Scholar]
- Wang, Y.; Sun, Y.; Zhang, Y.; Zhang, X.; Feng, J.T. Antifungal Activity and Biochemical Response of Cuminic Acid against Phytophthora capsici Leonian. Molecules 2016, 21, 756. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Chen, C.Z.; Yi, X.H.; Feng, J.T.; Zhang, X. Inhibition of p-isopropyl Benzaldehyde and p-isopropyl Benzoic Acid extracted from Cuminum cyminum against Plant Pathogens. Acta Bot. Boreal. Occident. Sin. 2008, 11, 42. [Google Scholar]
- Wu, H.S.; Raza, W.; Fan, J.Q.; Sun, Y.G.; Bao, W.; Shen, Q.R. Cinnamic acid inhibits growth but stimulates production of pathogenesis factors by in vitro cultures of Fusarium oxysporum f. sp. niveum. J. Agric. Food Chem. 2008, 56, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.; Porter, J.; Norred, W.; Leslie, J. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 1996, 62, 4039–4043. [Google Scholar] [PubMed]
- Gaumann, E. Fusaric acid as a wilt toxin. Phytopathology 1957, 47, 342–357. [Google Scholar]
- Bell, A.A.; Wheeler, M.H.; Liu, J.; Stipanovic, R.D.; Puckhaber, L.S.; Orta, H. United States Department of Agriculture-Agricultural Research Service studies on polyketide toxins of Fusarium oxysporum f. sp. Vasinfectum: potential targets for disease control. Pest Manag. Sci. 2003, 59, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Brown, D.W.; Kleigrewe, K.; Bok, J.W.; Keller, N.P.; Humpf, H.U.; Tudzynski, B. FfVel1 and FfLae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol. Microbiol. 2010, 77, 972–994. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, S.Q.; Xie, B.J.; Sun, Z.D.; Li, X.Y.; Miao, W.H. Characterization of polyphenol oxidase from litchi pericarp using (−)-epicatechin as substrate. J. Agric. Food Chem. 2007, 55, 7140–7143. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Choi, G.J.; Lee, H.J.; Cho, K.Y. Lipid peroxidation and membrane disruption by vinclozolin in dicarboximide-susceptible and-resistant isolates of Botrytis cinerea. Pestic. Biochem. Physiol. 1996, 55, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Vesentini, D.; Steward, D.; Singh, A.P.; Ball, R.; Daniel, G.; Franich, R. Chitosan-mediated changes in cell wall composition, morphology and ultrastructure in two wood-inhabiting fungi. Mycol. Res. 2007, 111, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.T.; Yang, S.Z.; Cheng, Y.J.; Chen, F.; Pan, S.Y.; Fan, G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]
- Duan, Y.B.; Ge, C.Y.; Liu, S.G.; Chen, C.J.; Zhou, M.G. Effect of phenylpyrrole fungicide fludioxonil on morphological and physiological characteristics of Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2013, 106, 61–67. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.; Prévost, D.; Brar, S.K.; Pouleur, S.; Surampalli, R. Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot. 2010, 29, 1452–1459. [Google Scholar]
- Zhao, S.; Du, C.M.; Tian, C.Y. Suppression of Fusarium oxysporum and induced resistance of plants involved in the biocontrol of Cucumber Fusarium Wilt by Streptomyces bikiniensis HD-087. World J. Microbiol. Biotechnol. 2012, 28, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Garcı́a-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Dı́az, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Sun, D.Q.; Lu, X.H.; Hu, Y.L.; Li, W.M.; Hong, K.Q.; Mo, Y.W.; Cahill, D.M.; Xie, J.H. Methyl jasmonate induced defense responses increase resistance to Fusarium oxysporum f. sp. cubense race 4 in banana. Sci. Hortic. 2013, 164, 484–491. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



| Compounds | Regression Equation | EC50 (μg·mL−1) | Confidence Interval of EC50 (p < 0.05) | χ2 |
|---|---|---|---|---|
| Cuminic acid | Y = 3.83 + 0.86X | 22.53 | 17.85–25.96 | 4.83 |
| Compound | Concentration | Disease Index (%) | Efficacy (%) |
|---|---|---|---|
| Cuminic acid | 1000 μg/mL | 38.8 ± 2.5b | 54.5 ± 2.3b |
| 2000 μg/mL | 21.4 ± 1.51c | 74.5 ± 1.5a | |
| 3000 μg/mL | 24.8 ± 1.15c | 71.9 ± 1.22a | |
| Carbendazim | 1000 μg/mL | 23.2 ± 1.18c | 72.8 ± 1.4a |
| Water | ___ | 85.5 ± 3.5a | ___ |
| Gene Name | Accession Number | Primer | Sequence (5′-3′) |
|---|---|---|---|
| Bike1 | AJ278141 | Forward | CGGTATCTGTGGTGGTGTC |
| Reverse | TCGGGAGGTGATGTTGTG | ||
| Bike2 | AM229668 | Forward | TGCCTGCTCCACAGTCTACG |
| Reverse | GCCAATCTTGACCGCCAC | ||
| Bike3 | AM229667 | Forward | CGCCAAAGTCATCAAGGA |
| Reverse | AGGCTCAGGCACCACAAA | ||
| FUB1 | FFUJ_02105 | Forward | ACTTCGCCTCGTCATCTC |
| Reverse | GAACCCAGCATCAAACTTAT | ||
| FUB4 | FFUJ_02108 | Forward | CACCCTTGCTCATCACAG |
| Reverse | CGTAAAAATATCCTTCCGAATAATC | ||
| FUB2 | FFUJ_02106 | Forward | GCCAACTGCTGTCACTAT |
| Reverse | TTCCGAGGTGGAGATTAG | ||
| FUB3 | FFUJ_02107 | Forward | CCCGATACACCATACCCT |
| Reverse | CCAACTTCTTGCCGTGAG | ||
| Lae1 | FVEG_00539 | Forward | TATTGGTACGGGCACAGG |
| Reverse | GGCATAAAGCCAGGAGGA | ||
| Vel1 | FN548142 | Forward | CTACTAAGGAGGAAAGGGACT |
| Reverse | TCCATCAAACCAGGAAACT | ||
| Related actin gene | Foxq13729 | Forward | GAGGGACCGCTCTCGTCGT |
| Reverse | GGAGATCCAGACTGCCGCTCAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, Y.; Han, L.R.; Zhang, X.; Feng, J.T. Antifungal Activity and Action Mode of Cuminic Acid from the Seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. Niveum (FON) Causing Fusarium Wilt on Watermelon. Molecules 2017, 22, 2053. https://doi.org/10.3390/molecules22122053
Sun Y, Wang Y, Han LR, Zhang X, Feng JT. Antifungal Activity and Action Mode of Cuminic Acid from the Seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. Niveum (FON) Causing Fusarium Wilt on Watermelon. Molecules. 2017; 22(12):2053. https://doi.org/10.3390/molecules22122053
Chicago/Turabian StyleSun, Yang, Yong Wang, Li Rong Han, Xing Zhang, and Jun Tao Feng. 2017. "Antifungal Activity and Action Mode of Cuminic Acid from the Seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. Niveum (FON) Causing Fusarium Wilt on Watermelon" Molecules 22, no. 12: 2053. https://doi.org/10.3390/molecules22122053
APA StyleSun, Y., Wang, Y., Han, L. R., Zhang, X., & Feng, J. T. (2017). Antifungal Activity and Action Mode of Cuminic Acid from the Seeds of Cuminum cyminum L. against Fusarium oxysporum f. sp. Niveum (FON) Causing Fusarium Wilt on Watermelon. Molecules, 22(12), 2053. https://doi.org/10.3390/molecules22122053




