Abstract
Hydroboration-oxidation of 2α,4α-dimethanol-1β,5β-bicyclo[3.3.0]oct-6-en dibenzoate (1) gave alcohols 2 (symmetric) and 3 (unsymmetric) in ~60% yield, together with the monobenzoate diol 4a (37%), resulting from the reduction of the closer benzoate by the intermediate alkylborane. The corresponding alkene and dialdehyde gave only the triols 8 and 9 in ~1:1 ratio. By increasing the reaction time and the temperature, the isomerization of alkylboranes favours the un-symmetrical triol 9. The PDC oxidation of the alcohols gave cleanly the corresponding ketones 5 and 6 and the deprotection of the benzoate groups gave the symmetrical ketone 14, and the cyclic hemiketal 15, all in high yields. The ethylene ketals of the symmetrical ketones 11 and 13 were also obtained. The compounds 5, 6, 11, 13, 14 could be used for synthesis of new (iso)carbacyclin analogues. The structure of the compounds was established by NMR spectroscopy and confirmed by X-ray crystallography.
1. Introduction
The synthesis of hydrogenated pentalene ketone intermediates has found applications in obtaining new drugs, of which the best known are carbacyclin [1] and isocarbacyclin [2,3,4,5,6,7,8] analogues, cioctol [9] and a few antibacterial [10] and antitumor natural products [11,12]. In carbacyclin synthesis, the standard key intermediate I has a symmetric ketone for linking the α-side chain by a Wittig E-olefination (at the ω-side chain) and a R group which finally is transformed into an aldehyde used to introduce the ω-side chain by a stereoselective Wardworth-Emmons E-olefination with a phosphonate (Figure 1) [13,14,15,16].
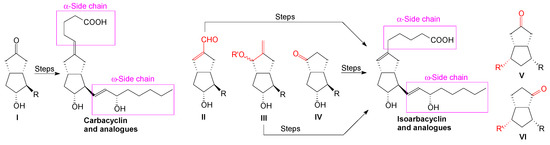
Figure 1.
Key intermediates for synthesis of carbacyclin and isocarbacyclin analogues.
In the synthesis of isocarbacyclin analogues, a few pentalene intermediates with different structures have been used to build the α-side chain (Figure 1). The type II intermediate contains an exocyclic allylic aldehyde for building the α-side chain [17], while the type III intermediate has an allylic alcohol or ester for introducing the α-side chain by a regioselective SN2’ alkylation with zinc-copper organic reagents [18,19,20], and the unsymmetric ketone intermediate of type IV links the α-side chain by different methods [21,22,23,24].
The synthesis of new carbacyclin and isocarbacyclin analogs requires new key intermediate octahydropentalene ketones. In this paper, we describe the synthesis of the ketone-octahydropentalenes of types V and VI useful for obtaining new carbacyclin and isocarbacyclin analogues.
2. Results and Discussion
Retrosynthetic analysis of the key intermediates 5 and 6 (Scheme 1), indicates that these compounds could be obtained by a sequence of two reactions: hydration of the double bond of alkene 1 to an alcohol, followed by the oxidation of the alcohol to the corresponding ketone. The starting compounds 1 were previously synthesized [25] and have already been used for the synthesis of “pseudocarbacyclin” type compounds [26,27], of the corresponding diols by hydroxylation with KMnO4 [28], of the corresponding α and β-epoxides, by epoxidation of the double bond with MPBA [29], and of pentalenofuranic compounds by regioselective reactions [30]. Following our retrosynthetic analysis, we decided to hydrate the double bond of alkene 1 by hydroboration-oxidation with sodium acetoxyborohydride and with borane.
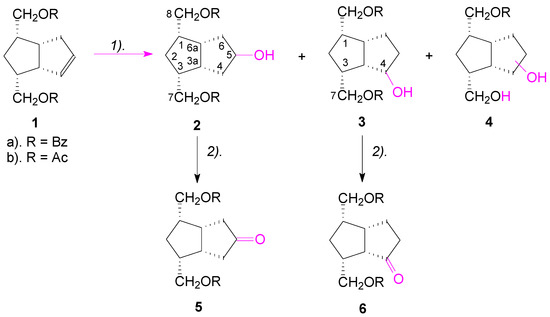
Scheme 1.
Hydroboration-oxidation of the compounds 1 for obtaining the octahydro-pentalenofurane ketones 5 and 6. Reagents and Conditions: (1) (a) Sodium acetoxyborohydride or BH3·THF, r.t, 24 h; (b) 30% H2O2/3 M AcONa or 3 M NaOH, 5 °C, ~35 min. (2) PDC/molecular sieves, r.t., overnight.
Hydroboration-oxidation of alkene 1a with sodium acetoxyborohydride [31] resulted in the formation, in a non-regioselective manner, of both alcohols 2a (34%) and 3a (25%), slightly in favor of the symmetrical alcohol 2. In the reaction, the monobenzoate-triol 4a was also formed in a great quantity (38%) (Scheme 1). Browsing the literature, we found that in the case of the unsaturated esters V, by forming a cyclic intermediate with 5 or 6 atoms VII, hydroboration of the double bond proceeded with concomitant reduction of the ester group (Scheme 2) [32].
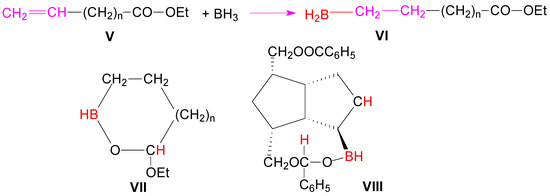
Scheme 2.
Hydroboration mechanism through a cyclic borane ester.
Probably it is the same formation of a similar cyclic ester VIII in the hydroboration of diester compound 1, which favored the reduction of the closer ester group to double bond, this reduction being responsible for the formation of the monoacylated triols 4a and 4b.
In the hydroboration-oxidation of diacetate 1b, the monoacetate-triol 4b was formed also in 38% yield, and this is consistent with the mechanism presented in Figure 2 for the formation of monoesters 4; the corresponding alcohols 2b and 3b were formed in about 1:1 ratio, but their isolation in pure form by low-pressure chromatography (LPC) was more difficult than in the case of the benzoate compounds 2a and 2b.
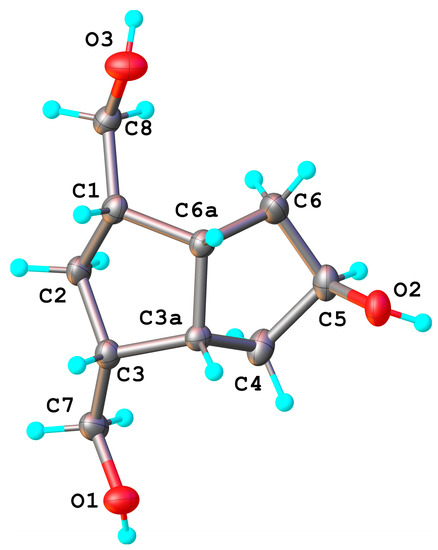
Figure 2.
X-ray molecular structure of the symmetrical triol compound 8 with thermal ellipsoids at 50% probability level. Proposed atom numbering is shown in the scheme attached and will be kept the same for all compounds.
The hydroboration of 1 with BH3·THF, obtained in situ from NaBH4 and dimethyl sulfate, followed by H2O2 oxidation gave alcohols 2a, 3a and 4a in 31.5%, 28.8% and 34.5% yield; hence, there is no significant difference in the yields and the ratio of the alcohols between the hydroboration with NaBH3OOCCH3 and BH3. It is worth mentioning that the isolation of the alcohols by PC is easier for the benzoate esters than the acetate esters. The formation of the unwanted secondary by-products 4a and 4b could represent also an advantage, because it is easy to selectively protect the primary hydroxyl group, with a group different from benzoate, like an ether, trityl, tert-butyldimethylsilyl or other bulky silyl-protecting group, by the methods known in the art, and thus to obtain different protection of the hydroxymethyl groups, useful for the next steps.
The fact that by-product 4 has the hydroxyl group linked at the C4 atom (see below) means that the hydroboration-oxidation of benzoate 1a gave the C4-alcohol in a total yield of 62–63%.
We then used the hydroboration-oxidation on the diol 7 (Scheme 3). We observed that hydroboration with borane is still a slow reaction and a certain amount of alkene 7 remains unreacted. We used different molar ratios of BH3·THF/7, from 1.2:1 to 4:1, and the results are presented in Table 1.
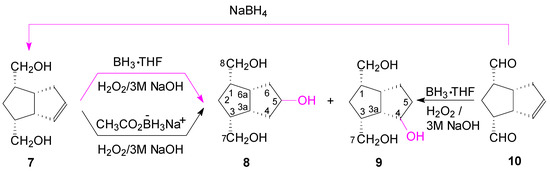
Scheme 3.
Hydroboration-oxidation of alkene-diol 7 with borane and sodium acetoxyborohydride and dialdehyde 10 with borane.

Table 1.
Hydroboration of the alkene-diol 7 with borane.
At a ratio of 2:1 BH3·THF/7, the borane reacted with the hydroxymethyl groups forming two alcoxyborane groups. The alkoxyborane group, closer to the double bond, hydroborated at the nearest carbon atom (C-4) with the formation in excess of the un-symmetrical alcohol 9, through an intermediate of type VIII (Scheme 2); the yield of alcohols 8 and 9 was still low (40.8%). By increasing the ratio of BH3·THF/7 to 3:1 and 4:1, there remained free borane which increased the yield of alcohols to 64.4%, and respectively to 73.3%, but there was no selectivity against 9 and the ratio of alcohols was nearly 1:1 (8/9).
Finally, we performed the hydroboration-oxidation of the double bond, concomitant with the reduction of the aldehyde groups of dialdehyde 10 (from which we previously [25] obtained the alkene-diol 7 by NaBH4 reduction of the aldehyde groups) with 2.2 molar equivalents of BH3·THF (20 h), and a mixture of alkene 7, alcohols 9 and 8 was obtained in a ratio of 1.0:1.1:1.2. When the hydroboration was done with a greater molar ratio BH3·THF:7 of 3:1 (preparative scale on 0.359 M alkene 7) and time was increased to 72 h, the ratio of alcohols (8/9) was 1.0:1.2. Then another reaction was performed for 24 h at r.t. and for 2 h at 45–50 °C and the ratio of the alcohols changed to ~1.0:2.2 (8/9). These suggest that an isomerization of the alkyl-boranes took place from type IX to Xa or Xb (Scheme 4), more thermodinamically stable in the reaction conditions.
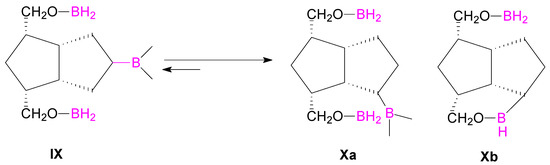
Scheme 4.
Isomerization of alkyl-boranes IX to X.
Such isomerizations between alkyl-boranes are known in the literature [33,34,35,36,37]. The α- or β-configuration of the hydroxyl groups introduced is not important, because the OH is oxidized in the next step to a ketone. Nonetheless, we believe that the secondary hydroxyl group is mainly introduced in a β orientation, because the access of the hydroboration reagent to the double bond ocurrs from the exo-side (the bulky crystallized compounds were only analyzed; the compound(s) remaining in the mother liquors were not analyzed for α/β isomers). The H5 appears in 1H-NMR as a broad singlet and is not a clear evidence for the α-configuration, but the X-ray diffraction analysis (Figure 2) of the crystallized triol 8 confirmed the exo-configuration of the secondary alcohol linked to the C5 position, 5β-OH. Bond distances and angles for compound 8 are listed in Table S1 and crystallographic data, details of data collection and structure refinement parameters in Table S2 (also for compounds 3, 5 and 14, Supplementary Material).
The compound 8 exhibits a molecular crystal structure where the neutral molecules interact through O-H···O hydrogen bonding to form a three-dimensional supramolecular network, as shown in Figure 3. H-bonding parameters are listed in Table 2.
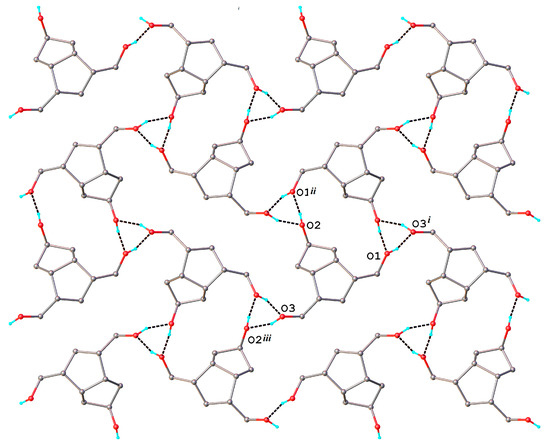
Figure 3.
Three-dimensional supramolecular architecture in the crystal structure 8.

Table 2.
H-bond parameters for compound 8.
The configuration of the 4-OH in 9 was not studied, since the compound was obtained as an oil. For confirming the structure of the unsymmetrical alcohols (OH linked to C4), we synthesized the tri-benzoates of 3 and 4 (and also of 2) to obtain suitable crystals for X-ray analysis, and their preparation is presented in the paper; their characterization by NMR was also done. At least for the fractions used for the analysis of the trisbenzoate obtained from 3, X-ray crystallography confirmed that the secondary 4-OH group are linked exo (β) to the carbon atom, as in the case of 8. The result of single crystal X-ray diffraction study for this compound is shown in Figure 4, while bond distances and angles are summarized in Table S1.
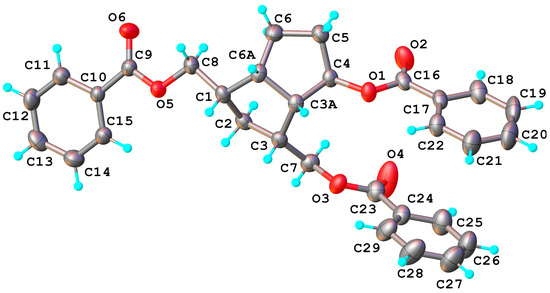
Figure 4.
X-ray molecular structure of non-symmetrical triol tribenzoate compound 3 with atom labeling scheme and thermal ellipsoids at 50% probability level.
Thus, the hydroboration-oxidation of alcohol 7 or dialdehyde 10 gives the symmetrical alcohol 8 in about 38% yield, but by increasing the reaction time and using hydroboration at elevated temperatures, the unsymmetrical alcohol 9 is formed in excess due to isomerization of the intermediary alkylboranes.
The regioisomers 8 (crystallized, m.p. 98–99 °C) and 9 (oil) were separated by LPC; their use in the next steps requires the selective protection of the primary hydroxyl groups, as exemplified for the obtaining of 2c (R = Tr) by treating 8 with trityl chloride; the following sequence is similar to the one of 2a (Scheme 5).
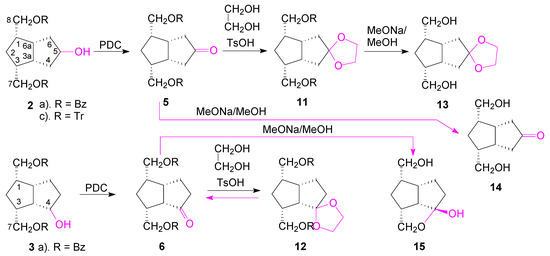
Scheme 5.
Synthesis of carbonyl compounds from alcohols 2 and 3.
The secondary alcohols of 2 and 3 were oxidized with PDC to the corresponding ketones 5 and 6 and then the benzoate protecting groups were cleanly removed by transesterification with MeONa in MeOH (Scheme 5). The structure of ketone 5 is easily established by NMR, just like the structure of the symmetrical alcohols 2 and 8, and that of the following compounds obtained from it: the ketone 14 and the ethylene ketal compounds 11 and 13. The X-ray diffraction investigation has confirmed that compound 5 crystalizes in P21/n space group of monoclinic system and its molecular crystal consists of isolated neutral molecules, as illustrated in Figure 5. Bond distances and angles are summarized in Table S1.
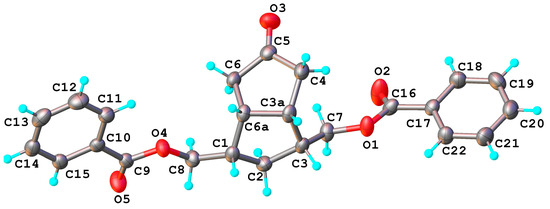
Figure 5.
X-ray molecular structure of symmetric ketone bis-benzoate compound 5 with atom labeling scheme and thermal ellipsoids at 50% probability level.
The structure of ketone 6 was also established by NMR spectroscopy. By transesterification (MeONa/MeOH), the benzoate groups were removed and the symmetrical ketone bis-hydroxymethyl compound 14 was obtained from ketone 5. In the case of the unsymmetrical ketone 6, during the transesterification of the benzoate groups, the closer hydroxymethyl reacted with the ketone C4=O and gave a cyclic hemiketal 15. Its molecular structure was also confirmed by X-ray crystallography (Figure 6), showing the formation of the tetrahydrofuran ring with exo-linked 4-hydroxyl of the hemiketal. According to X-ray crystallography, compound 15 crystallizes in P21/n space group of monoclinic system with two crystallographically independent but chemically identical units, denoted as molecules A and B. In Figure 6 only the structure of molecule B is shown.
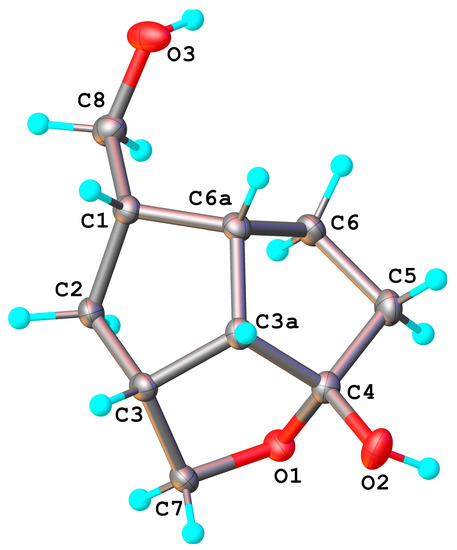
Figure 6.
X-ray molecular structure of the un-symmetrical ketone, as internal hemiketal compound 15 (molecule B). Thermal ellipsoids are drawn at 50% probability level.
The crystal packing shows a parallel arrangement of two-dimensional supramolecular layers extended in the 101 plane. A partial view of the crystal structure is shown in Figure 7. Each layer involves both independent molecules A and B connected by O-H···O hydrogen bonds, the formation of which is completely realized in the crystal. The corresponding hydrogen bond parameters are listed in Table 3.
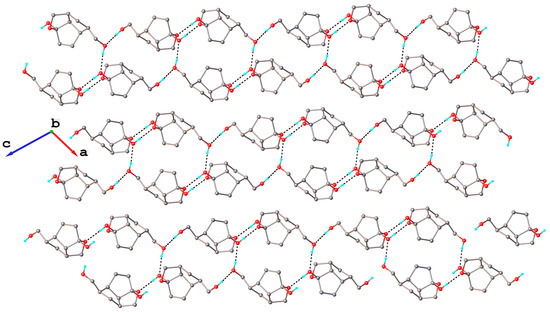
Figure 7.
A fragment of the crystal structure of compound 15 viewed along the b crystallographic axis.

Table 3.
H-bond parameters for compound 15.
Both ketone groups of compounds 5 and 6 were transformed into the corresponding ethylene ketals by standard treatment with ethylene glycol (in C6H6 at reflux, TsOH catalyst). In the first case, we obtained compound 11 which by a similar transesterification reaction, gave compound 13, with the ketone protected as an ethylene ketal, a group useful for the next reactions for discrimination between the two hydroxymethyl groups. In the case of compound 12, though the reaction proceeded until all 6 reacted (TLC), during work-up or column chromatography purification, only the starting compound 6 was isolated, indicating that slightly acid conditions favored the deprotection of the ethylene ketal group. The applications of the symmetric-ketone compounds in the synthesis of new carbacyclin analogues are in progress.
3. Materials and Methods
3.1. General Information
Melting points (uncorrected) were determined in open capillaries on an OptiMelt apparatus (MPA 100, Stanford Research System, Inc., Sunnyvale, CA, USA). The progress of the reactions was monitored by TLC on silica gel 60F254 plates (Merck, Darmstadt, Germany) in solvent systems: I (benzene–ethyl acetate–hexane, 5:3:2), II (cyclohexane–ethyl acetate, 5:1), III (ethyl acetate–methanol–acetic acid, 90:13:1), IV (acetone–hexanes, 2:1), V (hexane–ethyl acetate–acetic acid, 5:3:0.1), VI (hexane–ethyl acetate–acetic acid, 5:3:0.1), VII (ethyl acetate–hexane–acetic acid, 5:1:0.1). Spots were visualized in UV or with 15% H2SO4 in MeOH (heating at 110 °C, 10 min) and 2,4-dinitrophenylhydrazine reagent for ketones. The compounds were purified by low pressure chromatography (<2 atm) (LPC), on a glass column, in the solvent systems presented at experimental. IR spectra were recorded on an FT-IR spectrometer 100 (Perkin Elmer, Shelton, CT, USA) and frequencies are expressed in cm−1. MS were recorded on a 1200 L/MS/MS triple-quadrupole instrument (Varian, Inc., Walnut Creek, CA, USA) equipped with an ESI interface, fragments obtained by collision with Ar and relative abundances (%) are given in parenthesis. 1H-NMR and 13C-NMR spectra were recorded on a Varian Gemini 300 BB spectrometer (300 MHz for 1H and 75 MHz for 13C, Varian, Inc., Palo Alto, CA, USA). Chemical shifts are given in ppm relative to TMS as an internal standard. Complementary spectra: 2D-NMR and decoupling were done for correct assignment of NMR signals. The numbering of the carbon atoms in the compounds is presented in the Schemes.
Crystallographic measurements were carried out with an XCALIBUR E CCD diffractometer (Oxford-Diffraction, Ltd., Abingdon, Oxfordshire, UK) equipped with graphite-monochromated Mo-Kα radiation. Single crystals were positioned at 40 mm from the detector and 481, 258, 251, and 774 frames were measured each for 20, 10, 5, and 20 s over 1° scan width for 8, 3, 5 and 15, respectively. The unit cell determination and data integration were carried out using the CrysAlis package of Oxford Diffraction [38]. The structures were solved by direct methods using the Olex2 [39] software (OlexSys Ltd., Durham University, UK) with the SHELXS structure solution program and refined by full-matrix least-squares method on F2 with SHELXL-97 [40]. The atomic displacements for the non-hydrogen atoms were refined using an anisotropic model. Hydrogen atoms were placed in fixed, idealized positions and refined as rigidly bonded to the corresponding atoms. Positional parameters of the H attached to the O atoms were obtained from difference Fourier syntheses and verified by the geometric parameters of the corresponding hydrogen bonds. In the absence of significant anomalous scattering, the absolute configuration for 8 could not be reliably determined. Friedel pairs were merged and any references to the Flack parameter were removed. The molecular plots were obtained using the Olex2 program. CCDC: 1566141 (for 8), 1566142 (for 3), 1566143 (for 5) and 1566145 (for 15). These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or deposit@ccdc.ca.ac.uk).
3.2. Hydroboration of 2α,4α-Dimethanol-1β,5β-bicyclo[3.3.0]oct-6-en dibenzoate with Sodium Acetoxy-Borohydride
To a suspension of 98% NaBH4 (772 mg, 20 mmol) in anhydrous THF (60 mL), cooled on an ice-water bath, 1.14 mL (1.2 g, 20 mmol) 99.9% acetic acid were added in Ar atmosphere, under stirring for 10 min. and stirring was continued for 20 min. A solution of 1a (7.53 g, 20 mmol) in THF (15 mL) was added during 20 min, stirring was continued overnight, monitoring the reaction by TLC (I, Rf1b = 0.91, Rf3a = 0.47, Rf2a = 0.35, Rf4a = 0.05). Water (5 mL) was added, the reaction mixture was cooled on an ice-water bath, then the alkylboranes were oxidized with 30% H2O2 (6 mL) and 3 M sodium acetate (10 mL) (instead of 3 M NaOH to prevent the hydrolysis of the benzoate protecting groups) both added at the same time during ~15 min, and stirred for 20 min. Ethyl ether (50 mL) was added, phases were separated, aqueous phase was extracted with ether (3 × 50 mL), organic phases were washed with sat. soln. NaHCO3 (50 mL), dried (MgSO4) and concentrated, obtaining 7.77 of crude product, which was purified by low-pressure chromatography (LPC) (eluent: hexanes, then solvent system: benzene–hexane–ethyl acetate, 5:3:2). The following fractions were eluted:
- (a)
- 1.6 g (21.2%) of unreacted 1a, (the yields below are based on the reacted 1a).
- (b)
- 1.54 g (24.8%, based on the reacted alkene) of pure unsymmetrical alcohol (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,5,6,6a-octahydro-4-hydroxy-1,3-diyl)bis(methylene) dibenzoate (3a) as an oil, IR (2% CHCl3): 3550–3400, 3350, 3035, 2910–2885, 2850, 1695, 1595, 1580, 1440, 1350, 1270–1200, 1090, 1055, 1040, 945 cm−1, 1H-NMR (DMSO-d6, δ ppm, J Hz): 7.98 (dd, 2H, 8.0, 1.2, H-o), 7.95 (dd, 2H, 8.0, 1.2, H-o), 7.67–7.61 (m, 2H, 8.0, 1.2, H-p), 7.51 (apparent t, 2H, 8.0, H-m), 7.50 (t, 2H, 8.0, H-m), 4.60 (d, 1H, OH, exchangeable with D2O, 4.8), 4.51 (dd,1H, 11.1, 5.0, H-7), 4.18–4.45 (m, 3H, H-7, H-8), 3.82 (m, 1H, H-4), 2.70 (m, 1H, H-3a), 2.20–2.40 (m, 3H, H-6a, H-1, H-3), 1.05–1.90 (m, 6H, H-5, H-6, H-2), 13C-NMR (DMSO-d6, δ ppm): 165.75, 165.58 (COO), 133.19, 133.08 (C-p), 130.04, 129.81 (C-q), 129.12, 129.03 (C-o), 128.71, 128.61 (C-m); 72.98 (C-4), 65.60 (C-7 or C-8), 65.21 (C-8 or C-7), 51.58 (C-3a), 42.61 (C-1), 40.42 (C-3 or C-6a), 40. 40 (C-6a or C-3), 35.85 (C-5), 31.02 (C-2), 23.01 (C-6), elem. anal. calcd for C24H26O5 (%), C: 73.07, H: 6.64, found: C: 72.80, H: 6.42.
- (c)
- 2.11 g (34%) of pure symmetrical more polar alcohol, (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,5,6,6a-5-hydroxyoctahydropentalene-1,3-diyl)bis(methylene) dibenzoate (2a) which was crystallized (1.69 g) by ethyl acetate-gas extraction, m.p. 115–117 °C. A fraction recrystallized twice had m.p. 119.5–120.5 °C, 1H-NMR (CDCl3, δ ppm, J Hz): 8.03 (dd, 4H, 7.1, 1.4, H-o), 7.56 (tt, 2H, 7.1, 1.4, H-p), 7.44 (t, 4H, 7.1, H-m), 4.41 (dd, 2H, 11.0, 7.1, H-7, H-8), 4.31 (dd, 2H, 11.0, 8.2, H-7, H-8), 4.11 (tt, 1H, 5.9, 10.4, H-5), 2.69–2.64 (m, 2H, H-3a, H-6a), 2.48–2.38 (m, 2H, 8.2, 7.1, H-1, H-3), 2.05–1.97 (m, 2H, 10.4, 5.9, H-4, H-6), 1.89 (dt, 1H, 11.1, 5.4, H-2), 1.34 (q, 1H, 11.1, H-2), 1.36–1.26 (m, 2H, 10.4, 5.9, H-4, H-6), 13C-NMR (CDCl3, δ ppm): 166.45 (COO), 132.88 (2C-p), 130.24 (2C-q), 129.48 (4C-o), 128.32 (4C-m), 73.33 (C5), 65.58 (C-7, C-8), 41.05 (C-1, C-3 or C-3a, C-6a), 40.85 (C-3a, C-6a or C-1, C-3), 35.98 (C-4, C-6), 30.01 (C-2), elem. anal. Calcd. for C24H26O5, C:d 73.07, H: 6.64, found: C: 72.88, H: 6.52.
- (d)
- 1.72 g (37.6%) pure (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,5,6,6a-4-hydroxy-3-(hydroxymethyl)-octahydropentalen-1-yl)methyl benzoate (4a), which was crystallized by ethyl acetate-gas extraction to give 1.12 g of product, m.p. 74–77 °C, 1H-NMR (CDCl3, δ ppm, J Hz): 8.02 (dd, 2H, 1.4, 7.4, H-o), 7.53 (t, 1H, 1.4, 7.4, H-p), 7.44 (t, 2H, 7.4, 2H-m), 4.36 (dd, 1H, 7.1, 11.0, H-8), 4.27 (dd, 1H, 8.0, 11.0, H-8), 4.02 (ddd, 1H, 5.5, 8.2, 10.4, H-4), 3.85 (dd, 1H, 5.8, 11.0, H-7), 3.76 (t, 1H, 11.0, H-7), 3.14–3.00 (br d, 2H, OH), 2.86 (qv, 1H, 9.9, H-6a), 2.51 (dt, 1H, 8.2, 9.9, H-3a), 2.44–2.28 (m, 2H, H-1, H-3), 1.98 (dt, 1H, 5.5, 11.0, H-5), 1.75 (m, 1H, H-6), 1.67 (dt, 1H, 5.5, 12.6, H-2), 1.47 (dd, 1H, 5.8, 11.0, H-5), 1.27 (m, 1H, H-6), 0.99 (q, 1H, 12.6, H-2), 13C-NMR (CDCl3, δ ppm): 166.68 (COO), 133.06 (C-p), 130.45 (C-q), 129.65 (C-o), 128.51 (C-m), 73.99 (C-4), 65.56 (C-8), 63.50 (C-7), 52.09 (C-3a), 43.68 (C-1), 42.98 (C-6a), 41.11 (C-3), 35.10 (C-5), 30.43 (C-2), 23.76 (C-6).
3.3. Hydroboration of 2α,4α-Dimethanol-1β,5β-bicyclo[3.3.0]oct-6-en diacetate with Sodium Acetoxy-Borohydride
Hydroboration of diacetate 1b (20 mmol) was realized as for 1a, affording 5.23 g of crude product which was similarly purified. The following fractions were collected: 0.86 g (3.41 mmol) of starting alkene 1b, (16.9%); 0.13 g slightly impure unsymmetrical alcohol 3b, as an oil; 1.62 g of a mixture of alcohols 2b and 3b, as an oil; 0.26 g of slightly impure symmetric alcohol 2b, as an oil and 1.74 g (7.6 mmol, 38%) of (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,5,6,6a-4-hydroxy-3-(hydroxymethyl)octahydro-pentalen-1-yl)methyl acetate (4b) which gave 1.32 g (28.8%) as of the title product as needles, m.p. 58–59.5 °C, upon crystallization from ethyl acetate-hexane; 1H-NMR (DMSO-d6, δ ppm, J Hz): 3.97 (d, 2H, 7.7, H-8), 3.77 (dd + TFA, 1H, 3.3, 6.3, H-4), 3.62 (dd, 1H, 7.6, 10.8, H-7), 3.54–3.40 (br, 2H, OH), 3.45 (dd, 1H, 7.4, 10.8, H-7), 2.57 (dt + TFA, 1H, 6.2, 9.2, H-6a), 2.20 (dt, 1H, 6.3, 8.8, H-3a), 2.08–1.98 (m, 2H, H-1, H-3), 1.98 (s, 3H, CH3), 1.68 (m, 1H, H-5), 1.61 (dt, 1H, 6.5, 12.8, H-2), 1.49 (m, 1H, H-6), 1.28 (m, 1H, H-5), 1.13 (m, 1H, H-6), 0.85 (q, 1H, 12.8, H-2), 13C-NMR (CDCl3, δ ppm): 170.40 (COO), 72.72 (C-4), 64.69 (C-8), 61.97 (C-7), 51.83 (C-3a), 43.83 (C-1), 42.53 (C-6a), 40.50 (C-3), 35.40 (C-5), 31.03 (C-2), 23.73 (C-6), 20.73 (CH3), elem. anal. calcd. for C12H20O4 (%), C: 63.12, H: 8.83, found: C: 63.20, H: 8.65.
3.4. Hydroboration of (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6a-Hexahydro-1,3-pentalenodimethanol Dibenzoate with Borane (BH3·THF)
To a suspension of 98% NaBH4 (0.3 g, 7.8 mmol) in anhydrous THF (25 mL), dimethyl sulfate (DMS, 0.76 mL, 1.014 g, 7.8 mmol) was added dropwise under mechanical stirring under an Ar atmosphere. The reaction mixture was stirred for 2 h at room temperature (r.t.) and for 30 min at 50–60 °C, cooled under −10 °C, and a solution of alkene dibenzoate 1a (7.58 g, 20 mmol; alkene/borane molar ratio of 2.56:1, for forming the trialkylborane) in THF (15 mL) was added dropwise and stirred for 20 h at r.t., following the reaction by TLC. An equal quantity of borane was prepared and added to the reaction mixture at 10–13 °C, then stirred overnight at r.t. Though all the alkene was not consumed, the alkylboranes formed were oxidized, after cooling on an ice-water bath, with 30% H2O2 (4.4 mL) and 1 N NaOH (8.1 mL), added dropwise at the same time. The stirring was continued then for 20 min at r.t., 40 mL water were added and the mixture was extracted with dichloromethane (3 × 100 mL). Organic phases were washed with brine (2 × 50 mL), dried (MgSO4), concentrated and the crude product (7.2 g) was purified as in ex. 3.2, resulting 1.25 g of unreacted alkene (16.6%), 1.90 g (28.8%) 3a, 2.07 g (31.5%) 2a and 1.67 g (34.5%) 4a.
3.5. Hydroboration of Alkene-Diol 7 with Sodium Acetoxyborohydride
2α,4α-Dimethanol-1β,5β-bicyclo[3.3.0]octene-6 7 (6.72 g, 40 mmol) dissolved in anhydrous THF (50 mL), was hydroborated with acetoxyborohydride (as described in Section 3.2), obtained from NaBH4 (4.63 g, 120 mmol) and 99.9% acetic acid (7.2 mL, 120 mmol) in THF (100 mL) for 3 h at 0–5 °C, and over a weekend at r.t. TLC (III, Rf7 = 0.84, Rf9 = 0.62, Rf8 = 0.41). The alkylborane was oxidized with 30% H2O2 (33 mL) and 3 N NaOH (60 mL) added dropwise in the same time. The solvent was removed under reduced pressure, the concentrate was co-evaporated with anhydrous ethanol (3 × 50 mL), extracted with ethanol, resulting in an 8.9 g of a mixture of triols 8 and 9. The pure products were obtained by LPC (silica gel, eluent: acetone–hexanes, 1:2, then 1:1), resulting in 2.57 g (34.5%) of the symmetric alcohol 8 as an oil and 3.58 g (48%) of pure unsymmetric alcohol 9 as a slightly yellow oil (the isolated alcohols were obtained in a 1.0:1.4 ratio of 8/9).
- (a)
- (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,5,6,6a-5-Hydroxyoctahydropentalene-1,3-diyl)dimethanol (8). The symmetric alcohol was crystallized from acetone-hexanes giving 1.35 g 8 as needle crystals, m.p. 98–99 °C. IR (KBr): 3380–3180, 2945, 2980, 2850, 1480, 1460, 1440, 1350, 1200, 1080, 1020, 960, 870 cm−1, 1H-NMR (DMSO-d6, δ ppm, J Hz): 4.26 (t, 2H, 5.0, HO-C7,8, exchangeable with D2O), 4.16 (d, 1H, 3.0, HO-C5, exchangeable with D2O), 4.10 (br s, 1H, H-5), 3.34 (dd, 4H, 7.3, 5.0, H-7, H-8), 2.67–2.60 (m, 2H, H-3a, H-6a), 1.99–1.89 (m, 2H, 7.3, 5.5, H-1, H-3), 1.56 (dt, 1H, 12.1, 5.5, H-2), 1.52–1.44 (m, 2H, H-4, H-6), 1.30–1.20 (m, 2H, H-4, H-6), 0.71 (q, 1H, 12.1, H-2), 13C-NMR (DMSO-d6, δ ppm): 72.92 (C-5), 61.97 (C-7, C-8), 44.31 (C-1, C-3 or C-3a, C-6a), 41.98 (C-3a, C-6a or C-1, C-3), 35.30 (C-4, C-6), 30.62 (C-2), MS, m/z: 168 (PM), 150, 137 (68%), 109 (73%), 95, 93, 91, 79 (PB).
- (b)
- (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,5,6,6a-4-Hydroxyoctahydropentalene-1,3-diyl)dimethanol (9). IR: practically identical to that of 8, 1H-NMR (DMSO-d6, δ ppm, J Hz): 4.65 (dd, 1H, 3.8, 0.5, HO-C4, exchangeable with D2O), 4.43 (t, 1H, 5.6, HO-C7, exchangeable with D2O), 4.37 (t, 1H, 4.7, HO-C8, exchangeable with D2O), 3.76 (dt, 1H, 6.7, 7.3, H-4), 3.62 (dd, 1H, 10.7, 7.7, H-7), 3.46 (dd, 1H, 10.7, 7.5, H-7), 3.37 (d, 2H, 6.0, H-8), 2.56 (d, 1H, 9.0, H-6a), 2.20 (q, 1H, 8.8, H-3a), 2.00 (m, 1H, H-3), 1.90 (m, 1H, H-1), 1.69–1.44 (m, 3H, H-2, H-6, H-5), 1.28 (m, 1H, H-5), 1.17 (m, 1H, H-6), 0.74 (q, 1H, 12.3, H-2), 13C-NMR (DMSO-d6, δ ppm): 72.61 (C-4), 61.88 (C-7), 61.37 (C-8), 51.71 (C-3a), 44.30 (C-1), 43.62 (C-3), 42.45 (C-6a), 35.24 (C-5), 31.01 (C-2), 22.83 (C-6), MS, m/z: 168 (PM), 150, 138, 119, 106, 93, 91, 83 (PB).
3.6. Hydroboration of Alkene-Diol 7 with Borane
Alkene 7 (3.26 mmol) was hydroborated with borane in the conditions mentioned in Section 3.4 and using the ratios of borane/7 mentioned in Table 1. The purification of the crude mixtures was performed as in Section 3.5 and the yields are presented in Table 1.
3.7. Hydroboration of Alkene-Dialdehyde 10 with Borane (at a 0.359 M Scale)
To a solution of BH3·THF, prepared from 98% NaBH4 (41 g, 1.056 mol) and 97% dimethyl sulfate (105 mL, 1.056 mol) in THF (500 mL), cooled to <−10 °C, a solution of 2α,4α-diformyl-1β,5β-bicyclo[3.3.0]octene-6 (0.359 mol, previously co-evaporated with benzene) in THF (150 mL) was added dropwise in 90 min, maintaining the temperature under −5 °C. The temperature was left to reach the r.t. and the reaction mixture was further stirred over weekend. TLC (IV, Rf7 = 0.43, Rf9 = 0.23, Rf8 = 0.09). The crude product (75 g) was purified (3×) by LPC (acetone–hexanes, 1:1, then 2:1), resulting 7.55 g (12.5%) alkene 7, 29.77 g (44.5%) 9 as an oil and 25.1 g (37.5%) 8, crystallized in mass, m.p. 102–104 °C (twice recrystallized from acetone). The ratio of 9/8 was 1.185/1.0.
3.8. Benzoylation of Symmetrical Alcohol 2a
The dibenzoate alcohol 2a (160 mg, 0.4 mmol) in pyridine (5 mL) was benzoylated with benzoyl chloride (0.24 mL) in usual conditions. TLC (V, Kieselgel plastifollien, Merck, Rf2a = 0.57, Rf2a-triBz = 0.93). The crude product was purified by LPC, resulting 190 mg (95.2%) of symmetrical tribenzoate 2a, m.p. 85–87 °C (crystallized from hexane–ethyl acetate), 1H-NMR (CDCl3, δ ppm, J Hz): 8.05–7.99 (m, 6H, H-o), 7.58–7.53 (m, 3H, H-p), 7.46–7.40 (m, 6H, H-m), 5.57 (t, 1H, 3.3, H-5), 4.43 (dd, 2H, 7.1, 11.0, H-7, H-8), 4.33 (dd, 2H, 8.0, 11.0, H-7, H-8), 3.12–3.03 (m, 2H, H-3a, H-6a), 2.60–2.51 (m, 2H, H-1, H-3), 2.11–2.04 (m, 2H, H-4, H-6), 1.95 (dt, 1H, 5.5, 12.4, H-2), 1.73–1.62 (m, 2H, H-4, H-6), 1.26 (q, 1H, 12.4, H-2), 13C-NMR (CDCl3, δ ppm): 166.63 (COO), 133.09, 132.97 (3C-p), 130.24 (3C-q), 129.71, 129.67 (6C-o), 128.53, 128.46 (6C-m), 79.06 (C-5), 65.58 (C-7, C-8), 42.97 (C-1, C-3 or C-3a, C-6a), 40.99 (C-3a, C-6a or C-1, C-3), 33.52 (C-4, C-6), 30.59 (C-2).
3.9. Benzoylation of Unsymmetrical Alcohol 3a
The dibenzoate alcohol 3a (160 mg, 0.4 mmol) was benzoylated as above; 150 mg (73%) of un-symmetrical tribenzoate 3a, m.p. 82–83 °C (hexane-ethyl acetate) were obtained, 1H-NMR (CDCl3, δ ppm, J Hz): 8.05 (dd, 2H, 1.4, 7.8, H-o), 7.94 (dd, 2H, 1.4, 7.8, H-o), 7.87 (dd, 2H, 1.4, 7.4, H-o), 7.57 (tt, 1H, 1.4, 7.8, H-p-in 4-O-Bz), 7.50–7.23 (m, 8H, 2H-p, 6H-m), 5.33 (dt, 1H, 5.5, 8.2, H-4), 4.51 (dd, 1H, 6.9, 11.3, H-7 or H-8), 4.51 (dd, 1H, 8.0, 11.3, H-7 or H-8), 4.43 (dd, 1H, 6.9, 11.0, H-8 or H-7), 4.37 (dd, 1H, 8.0, 11.0, H-8 or H-7), 3.00–2.89 (m, 2H, H-3a, H-6a), 2.59–2.42 (m, 2H, H-1, H-3), 2.23 (ddt, 1H, 3.6, 5.8, 12.1, H-5), 1.95 (dt, 1H, 5.5, 12.6, H-2), 1.87 (m, 1H, H-6), 1.68 (m, 1H, H-5), 1.51 (m, 1H, H-6), 1.26 (q, 1H, 12.6, H-2), 13C-NMR (CDCl3, δ ppm): 166.63, 166.45, 166.19 (3COO), 133.11 (C-p), 132.85 (2C-p), 130.45 (2-C-q), 130.24 (C-q), 129.65 (6C-o), 128.55, 128.31, 128.27 (6C-m), 76.58 [C-4, with Cr(acac)3], 65.49 (C-7 or C-8), 64.94 (C-8 or C-7), 49.67 (C-3a), 43.38 (C-1), 41.09, 40.98 (C-3, C-6a), 33.26 (C-5), 31.43 (C-2), 24.23 (C-6).
3.10. Benzoylation of Monobenzoate Alcohol 4a
The monobenzoate alcohol 4a (145 mg, 0.5 mmol) was benzoylated as above; 230 mg (73%) of tribenzoate 4a, m.p. 83–85 °C (hexane–ethyl acetate); 1H-NMR (CDCl3, δ ppm, J Hz): 8.05 (dd, 2H, 1.4, 7.4, H-o), 7.94 (dd, 2H, 1.4, 7.4, H-o), 7.87 (dd, 2H, 1.4, 7.4, H-o), 7.57 (tt, 1H, 1.4, 7.8, H-p-in 4-O-Bz), 7.50–7.42 (m, 2H-p), 7.35–7.20 (m, 6H-m), 5.33 (dt, 1H, 5.5, 8.2, H-4), 4.51 (dd, 1H, 6.9, 11.3, H-7 or 8), 4.46 (dd, 1H, 8.0, 11.3, H-7 or H-8), 4.43 (dd, 1H, 6.9, 11.0, H-8 or H-7), 4.37 (dd, 1H, 8.0, 11.0, H-8 or H-7), 3.00–2.88 (m, 2H, H-3a, H-6a), 2.60–2.39 (m, 2H, H-1, H-3), 2.23 (ddt, 1H, 3.5, 5.8, 12.1, H-5), 1.94 (dt, 1H, 5.5, 12.6, H-2), 1.87 (m, 1H, H-6), 1.68 (m, 1H, H-5), 1.51 (m, 1H, H-6), 1.26 (q, 1H, 12.6, H-2), 13C-NMR (CDCl3, δ ppm): 166.62, 166.43, 166.18 (3COO), 133.10, 132.85, 132.81 (3C-p), 130.445 (2-C-q), 130.25 (C-q), 129.70, 129.65 (6C-o), 128.55, 128.31, 128.27 (6C-m), 76.76, 65.49 (C-7 or C-8), 64.94 (C-8 or C-7), 49.70 (C-3a), 43.38 (C-1), 41.09, 40.99 (C-3, C-6a), 33.27 (C-5), 31.43 (C-2), 24.23 (C-6); mixt. m.p. tri-Bz-2a + triBz-3a = 69–72 °C; mixt. m.p. tri-Bz-2a + triBz-4a = 72–74 °C; mixt. m.p. tri-Bz-3a + triBz-4a = 83–85 °C.
3.11. Tritylation of Symmetric Triol 8
The symmetric triol 7 (3.17 g, 17.02 mmol) was dissolved in pyridine (40 mL) and 97% trityl chloride (10.2 g, 35.6 mmol) was added in portions over 30 min at r.t. under stirring. Stirring was continued over weekend (3 days) and TLC (IV, Rf7 = 0.11, Rf2c = 0.53, Rf2c-tris-Tr = 0.80) still showed the presence of a minor quantity of the starting triol. Another 1 g of trityl chloride was added and the solution was stirred for 3 h at 45–50 °C. The cooled reaction mixture was poured over crushed ice, stirred for 1 h, the product was extracted with dichloromethane (3 × 100 mL), the unified organic solutions were washed with sat. soln. NaHCO3 (2 × 100 mL), brine (100 mL), dried (MgSO4), filtered, concentrated and co-evaporated with toluene. The crude product was crystallized from methanol and recrystallized from methanol-ethyl acetate, resulting 3.84 g of pure compound 2c, m.p. 98–101 °C [By LPC purification of the concentrated mother liquors (eluent, hexanes–ethyl acetate, 3:1), 6.65 g (total yield: 92%) of pure compound 2c and 700 mg of almost pure tristrityl derivative (4.5%) were obtained], IR (KBr): 3600–3300, 3040–3020, 2920–2850, 1585, 1480, 1440, 1150, 1055, 740, 695 cm−1, 1H-NMR (CDCl3, δ ppm, J Hz): 7.43 (dd, 12H, 1.7, 8.5, H-o), 7.28 (t, 12H, 8.5, H-m), 7.21 (td, 6H, 1.7, 8.5, H-p), 4.21 (br s, 1H, H-5), 3.14 (dd, 2H, 6.5, 8.6, H-7, H-8), 2.96–2.85 (m, 2H, H-3a, H-6a), 2.80 (t, 2H, 8.6, H-7, H-8), 2.42–2.34 (m, 2H, 6.5, H-1, H-3), 1.71 (dt, 1H, 12.2, 5.1, H-2), 1.55–1.44 (m, 2H, 6.5, 13.1, H-4, H-6), 1.03–0.95 (m, 2H, H-4, H-6), 0,66 (q, 1H, 12.2, H-2), 13C-NMR (CDCl3, δ ppm): 144.42 (6C-q), 128.70 (12C, C-o), 127.65 (12C, C-m), 126.79 (6C, C-p), 86.22 (2C-q), 75.10 (C-5), 64.26 (2C, C-7, C-8), 42.34 (2C, C-3a, C-6a), 42.14 (2C, C-1, C-3), 35.91 (2C, C-4, C-6), 30.87 (C-2), elem. anal. calcd for C48H46O3: th. C: 85.93, H: 6.91, found: C: 85.10 (−0.83, due to traces of solvent, not determined), H: 6.79.
3.12. PDC Oxidation of Alcoholdibenzoate 2a
To alcohol 2a (1.2 g, 2.84 mmol) dissolved in dichloromethane (60 mL), PDC (1.65 g, 4.26 mM) and molecular sieve (2 g) were added and stirred overnight, monitoring the reaction by TLC (I, Rf2a = 0.40, Rf5a = 0.66). The reaction mixture was diluted with ethyl ether, filtered of a anh. MgSO4 bed, the bed was washed with ether, the filtrate was concentrated and the crude product was purified by LPC (hexanes–ethyl acetate, 3:1 or benzene–ethyl acetate–hexane 5:3:2) and the pure ketone, (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6a-5-oxooctahydropentalene-1,3-diyl)bis(methylene) dibenzoate (5) was obtained in more than 90% yield as an oil, which crystallized in time, m.p. 79.5–81.5 °C (ethyl ether–hexane), IR (1% in CHCl3): 3030, 2910–1880, 1700–1690, 1595, 1580, 1450, 1250–1200, 1100, 950 cm−1, 1H-NMR (CDCl3, δ ppm, J Hz): 8.00 (d, 4H, 7.1, H-o), 7.57 (t, 2H, 7.1, H-p), 7.44 (d, 4H, 7.1, H-m), 4.39 (dd, 2H, 11.4, 6.3, H-7, H-8), 4.30 (dd, 2H, 11.4, 7.6, H-7, H-8), 3.10–3.03 (m, 2H, H-1, H-3), 2.38 (dd, 2H, 18.6, 6.2, H-4, H-6), 2.30 (dd, 2H, 18.6, 6.5, H-4, H-6), 2. 08 (dt, 1H, 12.3, 6.5, H-2), 1.46 (q, 1H, 12.3, H-2), 13C-NMR (CDCl3, δ ppm): 218.50 (C-5), 166.26 (COO), 133.01 (C-p), 129.87 (C-q), 129.42 (C-o), 128.36 (C-m), 65.25 (2C, C-7, C-8), 41.09 (2C, C-3a, C-6a), 40.29 (2C, C-1, C-3), 38.71 (2C, C-4, C-6), 30.47 (C-2), elem. anal. calcd. for C24H24O5, C: 73.45, H: 6.16, found: C: 73.49, H: 6.22.
3.13. PDC Oxidation of Alcoholdibenzoate 3a
Dibenzoate alcohol 3a (0.81 g, 2.84 mmol) in dichloromethane (50 mL), was oxidized as before with PDC (1.20 g, 3.19 mmol) and molecular sieve (1.35 g), overnight. TLC (I, Rf3a = 0.50, Rf6a = 0.68) showed still the presence of 3a and more PDC (0.35 g) were added and stirring was continued for 3 days. After work-up and purification as above, 0.67 g (83.3%) of pure ketone, (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6a-4-oxooctahydropentalene-1,3-diyl)bis(methylene) dibenzoate (6) were obtained as an oil, which crystallized overnight in the refrigerator, m.p. 61–63 °C, IR (1% CHCl3), practically the same as those of ketone 5: 3030, 2910–2880, 1700–1690, 1595, 1580, 1450, 1250–1200, 1100, 1060, 950 cm−1, 1H-NMR (CDCl3, δ ppm, J Hz): 8.05 (dd, 2H, 8.3, 1.4, H-o), 7.98 (dd, 2H, 8.3, 1.4, H-o), 7.58 (tt, 1H, 8.3, 1.4, H-p), 7.55 (tt, 1H, 8.3, 1.4, H-p), 7.45 (t, 2H, 8.3, H-m), 7.43 (t, 2H, 8.3, H-m), 4.55 (dd, 1H, 11.4, 3.6, H-7 or H-8), 4.48 (dd, 1H, 6.9, 11.1, H-8 or H-7), 4.44 (dd, 1H, 11.1, 6.9, H-7 or H-8), 4.43 (dd, 1H, 11.4, 7.9, H-8 or H-7), 3.01 (m, 1H, H-1), 2.83 (t, 1H, 10.8, H-3a), 2.78 (m, 1H, H-3), 2.65 (m, 1H, H-6a), 2.20–2.28 (m, 2H, H-5), 2.00–2.15 (m, 2H, H-2, H-6), 1.80 (dq, 1H, 13.0, 10.2, H-6), 1.62 (q, 1H, 12.5, H-2), 13C-NMR (CDCl3, δ ppm): 219.83 (C-6), 166.30, 166.05 (COO), 132.95, 132.84 (C-p), 129.98, 129.93 (C-q), 129.40, 129.22 (C-o), 128.31, 128.28 (C-m), 64.81 (C-8 or C-7), 63.73 (C-7 or C-8), 51.90 (C-3a), 43.83 (C-1), 41.29, 41.20 (2C, C-3, C-6a), 39.66 (C-5), 30.76 (C-2), 22.16 (C-6), elem. anal. calcd for C24H24O5, C: 73.45, H: 6.16, found: C: 73.90, H: 6.24.
3.14. PDC Oxidation of Alcohol 2c
The compound 2c (3.42 g, 5.1 mmol) was oxidized (as in 3.12) [CH2Cl2 (100 mL), PDC (3 g), molecular sieves (3.8 g), r.t., 2 days]; TLC (II, Rf2c = 0.32, Rf5c = 0.57); 3.31 g (97%) of pure 5c were obtained. By recrystallization from ethyl ether, 2.35 g of crystallized (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6a-1,3-bis((trityloxy)methyl)hexahydropentalen-5-one (5) were obtained, m.p. 189–191 °C, IR (KBr): 3020, 2910, 2860, 1730, 1590, 1480, 1445, 1150, 1060, 750, 700 cm−1, 1H-NMR (CDCl3, δ ppm, J Hz): 7.40 (dd, 12H, 7.9, 1.2, H-o), 7.28 (t, 12H, 7.9, H-m), 7.23 (tt, 6H, 7.9, 1.2, H-p), 3.13 (dd, 2H, 8.8, 5.8, H-7, H-8), 3.04–2.95 (m, 2H, H-3a, H-6a), 2.80 (t, 2H, 8.8, H-7, H-8), 2.57–2.45 (m, 2H, H-1, H-3), 2.06 (dd, 2H, 19.1, 9.5, H-4, H-6), 1.87 (dd, 2H, 19.1, 5.9, H-4, H-6), 1.82 (dt, 1H, 12.4, 6.2, H-2), 1.00 (q, 1H, 12.4, H-2), 13C-NMR (CDCl3, δ ppm): 220.42 (C-5), 144.05 (6 Cq, Tr), 128.53 (12 C-m), 127.72 (12 C-o), 126.93 (6 C-p), 86.40 (2Cq, Tr), 64.30 (C-7, C-8), 41.75 (2C, C-3a, C-6a or C-1, C-3), 40.88 (2C, C-1, C-3 or C-3a, C-6a), 38.76 (2C, C-4, C-6), 30.89 (C-2).
3.15. Synthesis of (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6,6a-Hexahydro-1H-spiro[pentalene-4,4′-[1,3]dioxolane]-1,3-diyl)bis(methylene)dibenzoate (11) from Ketone 5a
The symmetric ketone 5a (0.93g, 2.37 mmol) was dissolved in benzene (25 mL), ethylene glycol (0.4 mL) and TsOH (10 mg) were added, and refluxed for 5 h (Dean-Stark trap for removing the water) monitoring the reaction by TLC (VI, Rf5a = 0.69, Rf11 = 0.77). The reaction mixture was cooled, washed with sat. sol. NaHCO3 (2 × 20 mL), brine (20 mL), concentrated, and ketone 11 was obtained in quantitative yield by crystallization from benzene-hexane; the product has m.p. 99–100 °C, IR (KBr): 3045, 2945, 2870, 1690, 1590, 1580, 1445, 1260, 1160, 1090, 1060, 1015, 960, 940, 870, 695 cm−1, 1H-NMR (CDCl3, δ ppm, J Hz): 8.04 (dd, 4H, 8.3, 1.2, H-o), 7.75 (tt, 2H, 7.2, 1.2, H-p), 7.44 (td, 4H, 8.3, 7.2, H-m), 4.40 (dd, 2H, 11.1, 6.8, H-7, H-8), 4.27 (dd, 2H, 11.1, 8.4, H-7, H-8), 3.92 (apparent t, 4H, O-CH2CH2-O), 2.90–2.83 (m, 2H, H-3a, H-6a), 2.55–2.46 (m, 2H, 6.8, 8.4, H-1, H-3), 1.91 (dt, 1H, 12.5, 5.3, H-2), 1.82 (dd, 2H, 12.7, 8.6, H4, H-6), 1.68 (dd, 2H, 12.7, 9.6, H-4, H-6), 1.32 (q, 1H, 12.5, H-2), 13C-NMR (CDCl3, δ ppm): 166.46 (COO), 132.93 (C-p), 130.54 (C-q), 129.60 (C-o), 128.40 (C-m), 117.86 (C-5), 65.54 (C-7, C-8), 64.91, 64.26 (OCH2CH2O), 41.14 (C-3a, C-6a or C-1, C-3), 41.03 (C-1, C-3 or C-3a, C-6a), 35.46 (C-4, C-6), 30.29 (C-2).
3.16. Synthesis of the Ethylene Ketal of the Unsymmetric Ketone 6a
The same reaction conditions: 6a (0.93 g, 2.37 mmol); TLC (VI, Rf5a = 0.69, Rf11 = 0.83). The crude product (0.73 g) was purified similarly, resulting 605 mg (81.2%) of pure starting ketone 6a (IR and NMR as for compound 6a).
3.17. Synthesis of Symmetrical Ketone 14 by Deprotection of the Benzoate Groups of 5a
To a soln. of bisbenzoate ketone 5a (0.4 g, 1.02 mmol) in methanol (30 mL), a solution of MeONa (0.065 g/mL) (1 mL) was added and stirred at r.t. for 3 h, monitoring the reaction by TLC (VII, Rf14 = 0.18). The base was neutralized with methanol-washed Dowex 50 × 4, the filtrate was concentrated and the crude product was purified by LPC (eluent, ethyl acetate–hexanes, 5:1), resulting a pure fraction of 165 mg (86.8%) of (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6,6a-1,3-bis(hydroxymethyl)-hexahydropentalen-5-one (14) as an oil, 1H-NMR (DMSO-d6, δ ppm, J Hz): 3.55 (dd, 2H, 10.7, 5.7, H-7, H-8), 3.27 (dd, 2H, 10.7, 7.4, H-7, H-8), 2.81–2.70 (m, 2H, H-3a, H-6a), 2.22–2.12 (m, 2H, H-1, H-3), 2.15 (dd, 2H, 18.5, 5.9, H-4, H-6), 2.07 (dd, 2H, 18.5, 9.5, H-4, H-6), 1.66 (dt, 1H, 12.5, 5.0, H-2), 1.11 (q, 1H, 12.5, H-2), 13C-NMR (DMSO-d6, δ ppm): 220.02 (C=O), 61.90 (2C, C-7, C-8), 42.92 (2C, C-1, C-3), 41.02 (2C, C-3a, C-6a), 38.36 (2C, C-4, C-6), 30.07 (C-2).
3.18. Synthesis of (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6,6a-Hexahydro-1H-spiro[pentalene-4,4'-[1,3]dioxolane]-1,3-diyl)dimethanol (13)
Ethylene ketal 11 (0.49 g, 1.12 mmol) was hydrolyzed as described above for compound 5a: TLC (VII, Rf11 = 0.81, Rf13 = 0.20); LPC (ethyl acetate-hexane, 5:1); 235 mg (91.2%) of pure compound 13 were obtained as an oil, 1-H-NMR (CDCl3, δ ppm, J Hz): 3.91 (m, 4H, OCH2CH2O), 3.59 (d, 4H, 7.4, H-7, H-8), 3.17 (br s, 2H, exchangeable with D2O, OH), 2.75–2.68 (m, 2H, H-3a, H-6a), 2.20–2.10 (m, 2H, H-1, H-3), 1.79 (dt, 1H, 12.4, 5.4, H-2), 1.72 (dd, 2H, 14.6, 8.5, H-4, H-6), 1.55 (dd, 2H, 14.6, 7.7, H-4, H-6), 1.05 (q, 1H, 12.4, H-2), 13C-NMR (CDCl3, δ ppm): 118.02 (C-5), 64.63, 63.95 (OCH2CH2O), 63.30 (2C, C-7, C-8), 44.32 (C-1, C-3), 40.73 (C-3a, 6a), 34.84 (2C, C-4, C-6), 30.02 (C-2).
3.19. Deprotection of Benzoate Groups of Compound 6a by Transesterification
The benzoate groups of the compound 6a (0.41 g, 1.04 mmol) were hydrolyzed by transesterification, as described above (TLC, VII, Rf12 = 0.24, less active to dinitrophenylhydrazine); to give 185 mg (quantitative yield) of (±)-(2aα,2a1α,4α,4aα,6aα)-4-(hydroxymethyl)octahydro-6aH-pentaleno[1,6-bc]furan-6a-ol (15) as a cyclic hemiketal. The compound was crystallized from ethyl acetate, m.p. 106–108 °C, IR: 3442, 3353, 3250, 2952, 2913, 2878, 2858, 1462, 1428, 1315, 1078, 1056, 1024, 981, 898, 790 cm−1, 1H-NMR (DMSO-d6, δ ppm, J Hz): 5.75 (s, 1H, OH-4, exchangeable with D2O), 4.29 (t, 1H, 4.7, OH-8, exchangeable with D2O,), 3.82 (dd, 1H, 8.5, 4.8, H-7), 3.53 (d, 1H, 8.5, H-7), 3.28 (d, 2H, 7.3, H-8), 2.61 (t, 1H, 8.6, H-3a), 2.45–2.60 (m, 2H, H-6a. H-3), 1.96 (m, 1H, 6.6, H-1), 1.86 (dd, 1H, 12.4, 6.5, H-5), 1.80 (m, 1H, 12.6, H-2), 1.54 (dt, 1H, 13.3, 6.5, H-6), 1.43 (dt, 1H, 13.3, 6.9, H-5), 1.25 (m, 1H, 12.4, 10.2, 6.0, H-6), 1.01 (td, 1H, 12.6, 8.9, H-2), 13C-NMR (DMSO-d6, δ ppm): 115.00 (C-4), 71.74 (C-7 ), 61.38 (C-8), 58.90 (C-3a), 47.16 (C-1), 44.14 (C-6a or C-3), 43.72 (C-3 or C-6a), 38.44 (C-5), 33.37 (C-2), 24.02 (C-6). 1H-NMR (CDCl3, δ ppm, J ppm): 4.05 (dd, 1H, 8.8, 5.1, H-7), 3.75 (d, 1H, 8.8, H-7), 3.58 (d, 2H, 7.3, H-8), 2.86 (t, 1H, 9.0, H-3a), 2.77–2.66 (m, 2H, H-3, H-6a), 2.15 (m, 1H, H-1), 2.05 (dd, 1H, 6.3, 12.4, H-6), 1.93 (ddd, 1H, 12.6, 8.4, 6.1, H-2), 1.78 (td, 1H, 12.5, 6.8, H-5), 1.59 (dt, 1H, 12.5, 7.4, H-5), 1.43 (ddt, 1H, 11.7, 12.4, H-6), 1.20 (dt, 1H, 12.6, 9.6, H-2), 13C-NMR (CDCl3, δ ppm): 115.00 (C-5), 71.44 (C-7), 61.38 (C-8), 58.90 (C-3a), 47.16 (C-1), 44.14 (C-6a or C-3), 43.72 (C-3 or C-6a), 38.44 (C-5), 33.72 (C-2), 24.02 (C-6). The molecular structure was confirmed by X-ray crystallography (Figure 7).
4. Conclusions
Hydroboration-oxidation of 2α,4α-dimethanol-1β,5β-bicyclo[3.3.0]oct-6-en dibenzoate (1) gave the alcohols 2 (symmetric) and 3 (unsymmetric) in ~60% yield, together with the monobenzoate diol 4a (37%), resulting from by the reduction of the closer benzoate by the intermediate alkylborane. Alkene 7 and dialdehyde 10 gave only the triols 8 and 9 in ~1:1 ratio, in >73% yield. By increasing the reaction time and the reaction temperature, the isomerization of alkylboranes favors the un-symmetrical triol 9. The mechanism of reduction of the closer ester to 4-alkylborane and the isomerization of alkylboranes toward the unsymmetrical regioisomer were also presented. The oxidation of the secondary alcohols cleanly gave the corresponding ketones 5 and 6 and the deprotection of the benzoate groups gave the symmetrical ketone 14, and the cyclic hemiketal 15, all in high yields. The ethylene ketals of the symmetrical ketones 11 and 13 were also obtained. In conclusion, different symmetric and un-symmetric ketones and protected ketone compounds were synthesized. They are potentially useful key intermediate for the synthesis of new carbacyclin and/or isocarbacyclin analogues with different structural radicals. The structure of the compounds was established by NMR spectroscopy and confirmed by X-ray crystallography.
Supplementary Materials
The Supplementary Material associated with this article (1H-NMR and 13C-NMR spectra for compounds, crystallography data in Table S1 for compounds 8, 3, 5 and 15) are available online. Also CIF files and cifreport for compounds: 8 (shI_3623-CoTa.cif), 3 (shI_3624-CoTa.cif), 5 (shI_3625-CoTa.cif) and 15 (shI_1265-CoTa.cif) are available as Supplementary material.
Acknowledgments
We thank the Romanian Ministry for Research and Innovations for funds to cover the cost of publishing the article in open access.
Author Contributions
Constantin I. Tănase and Florea G. Cocu conceived and designed the experiments, performed the experiments, and analyzed the data; Constantin I. Tănase and Constantin Drăghici wrote the paper; Miron Teodor Căproiu, Constantin Drăghici did the physico-chemical studies of the synthesized compounds; Sergiu Shova did the X-ray analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collins, P.W.; Djuric, S.W. Synthesis of Therapeutically Useful Prostaglandin and Prostacyclin Analogs. Chem. Rev. 1993, 93, 1533–1564. [Google Scholar] [CrossRef]
- Skuballa, W.; Raduchel, B.; Vorbrueggen, H. Prostacyclin and Its Stable Analogue Iloprost; Griglewski, R.J., Stock, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Skuballa, W.; Vorbrueggen, H. A New Route to 6a-Carbacyclins–Synthesis of a Stable, Biologically Potent Prostacyclin Analogue. Angew. Chem. Int. Ed. Engl. 1981, 20, 1046–1048. [Google Scholar] [CrossRef]
- Skuballa, W.; Schillinger, E.; Sturzebecher, C.S.; Vorbrueggen, H. Synthesis of a new chemically and metabolically stable prostacyclin analogue with high and long-lasting oral activity. J. Med. Chem. 1986, 29, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Constantini, V.; Giampietri, A.; Allegrucci, M.; Agnelli, G.; Nenci, G.G.; Fioretti, M.C. Mechanisms of the antimetastatic activity of stable prostacyclin analogues: Modulation of host immunocompetence. Adv. Prostaglandin Thromboxane Leukot. Res. 1991, 21B, 917–920. [Google Scholar]
- Djuric, S.W.; Miyano, M.; Clare, M.; Rydzewski, R.M. A stereocontrolled synthesis of a novel prostacyclin analog “allene-carbacyclin”. Application of molecular mechanics calculations to organic synthesis. Tetrahedron Lett. 1987, 28, 299–302. [Google Scholar] [CrossRef]
- Kanoyama, T.; Kimura, Y.; Iseki, K.; Hayashi, Y.; Tamao, Y.; Misogami, S. Antithrombotic effects of KP-10614, a novel and stable prostacyclin (PGI2) analog. J. Pharmacol. Exp. Ther. 1990, 255, 1210–1217. [Google Scholar]
- Berdhend, R.A.; Braish, T.F.; Jakubowski, J.A.; Fuchs, R.L. Triply-convergent synthesis of a homochiral 3,3-dimethyl, 15-cyclohexyl prostacyclin analog. Bioorg. Med. Chem. Lett. 1991, 1, 649–652. [Google Scholar]
- Wiechers, J.W.; Herder, R.E.; Drenth, B.F.H.; DeZeeuw, R.A. Percutaneous absorption, disposition, metabolism, and excretion of 14C-labelled Cyoctol in humans after a single dermal application. Int. J. Pharm. 1990, 65, 77–85. [Google Scholar] [CrossRef]
- Nishida, M.; Iseki, K.; Shibasaki, M.; Ikegami, S. A total synthesis of (+)-hirsutic acid. Chem. Pharm. Bull. 1990, 38, 3230–3237. [Google Scholar] [CrossRef]
- Mizuno, H.; Domon, K.; Masuya, K.; Tanino, K.; Kuwajima, I. Total Synthesis of (−)-Coriolin. J. Org. Chem. 1999, 64, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Kim, B.G. A Facile Construction of the Quadranoid Skeleton: Application to the Total Synthesis of (±)-Suberosenone. Org. Lett. 2000, 2, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Westermann, J.; Hare, M.; Nickish, K. E- or Z-Selective Wittig reactions in the synthesis of the carbocyclic Iloprost. Tetrahedron Lett. 1992, 33, 8055–8056. [Google Scholar] [CrossRef]
- Shibasaki, M.; Fukasawa, H.; Ikegami, S. Regiocontrolled Conversion of α,β-unsaturated ketones to olefins via allylsilanes: Synthesis of dl-9(0)-methano-Δ6(9α)-PGI1. Tetrahedron Lett. 1983, 24, 3497–3500. [Google Scholar] [CrossRef]
- Hashimoto, S.; Suzuki, A.; Shimoda, T.; Miyazaki, Y.; Ikegami, S. A controlled synthesis of 9α-methyl- and 9α-cyanoisocarbacyclins. Chem. Lett. 1992, 21, 1835–1838. [Google Scholar] [CrossRef]
- Ikegami, S.; Hashimoto, S.; Kurozumi, S. Preparation of Bicyclo[3.3.0]octane Compounds as Intermediates for Isocarbacyclins. Japanese Patent 01 68, 333 [8968333], 14 March 1989. [Google Scholar]
- Shinoda, M.; Iseki, K.; Oguri, T.; Hayasi, Y.; Yamada, S.-I.; Shibasaki, M. A convenient synthesis of β-alkynylpropionic acids from β-propiolactones. Synthesis of 4,4,5,5-tetrahydro-9(O)-methano-Δ6(9α)-PGI1. Tetrahedron Lett. 1986, 27, 87–90. [Google Scholar] [CrossRef]
- Bannai, K.; Tanaka, T.; Okamura, N.; Hazato, A.; Sugiura, S.; Manabe, K.; Tomimori, K.; Kato, Y.; Kurozumi, S.; Noyori, R. Syntheses of isocarbacyclin by highly regioselective alkylation of allylic alcohols. Tetrahedron 1990, 46, 6689–6704. [Google Scholar] [CrossRef]
- Tanaka, T.; Bannai, K.; Hazato, A.; Koga, M.; Kurozumi, S.; Kato, Y. Short synthesis of isocarbacyclin by regioselective SN2′ alkylation of bicyclic allylic esters with zinc-copper reagents. Tetrahedron 1991, 47, 1861–1876. [Google Scholar] [CrossRef]
- Gais, H.-J.; Horst, H. NeuesVerfahren zur Herstellung von Optisch Aktiven Bicyclo[3.3.0]octan-Derivaten. German Patent 3,909,325 A1, 17 March 1989. [Google Scholar]
- Skuballa, W.; Stuerzebecher, C.S.; Thierauch, K.H. 20-Alkyl-13,14,18,18,19,19-hexadehydro-3-oxa-Isocarbacycline Derivatives and Their Pharmaceutical Use. German Patent 3,909,329 A1, 20 September 1990. [Google Scholar]
- Deicke, P.; Klar, U.; Vorbruggen, H. Synthesis of chemically stable 9-substituted carbacyclin derivatives and their biological use. Bioorg. Med. Chem. 1992, 2, 439–444. [Google Scholar] [CrossRef]
- Iseki, K.; Kanayama, T.; Hayashi, Y.; Shibasaki, M. Synthesis of a New Chemically Stable Prostacyclin Analogue with High and Long-Lasting Activity. Chem. Pharm. Bull. 1990, 38, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Hazato, A.; Hanajima, N.; Makino, Y.; Takeda, T.; Koyama, T. Dermatologic Preparation Composition Containing Prostacyclin as Active Ingredient. U.S. Patent 5,530,027, 25 June 1996. [Google Scholar]
- Tănase, C.I.; Cocu, F.G.; Drăghici, C.; Căproiu, M.T. 1,2,3,3a,4,6a-Hexahydro-1,3-pentalenedimethanol, mono and bis OH-protected derivatives, useful intermediates for fine organic synthesis. Rev. Roum. Chim. 2008, 53, 195–202. [Google Scholar]
- Tănase, C.I.; Cocu, F.G.; Căproiu, M.T.; Drăghici, C. “Pseudocarbacyclins”, Carbacyclin Type Compounds with non-Conventional Positions of the Structural Elements. Rev. Chim. 2001, 2, 11–13. (In English) [Google Scholar]
- Tănase, C.I.; Cocu, F.G.; Drăghici, C.; Căproiu, M.T. “Pseudocarbacyclin” Derivatives and a Procedure for Their Obtaining. RO Patent 116896 B, 30 July 2001. [Google Scholar]
- Tănase, C.I.; Cocu, F.G.; Drăghici, C.; Căproiu, M.T. Useful intermediates for fine organic synthesis by hydroxylation of 1,2,3,3a,4,6a-hexahydro-1,3-pentalenedimethanol and bis OH-protected derivatives. Rev. Roum. Chim. 2008, 53, 189–193. [Google Scholar]
- Tănase, C.I.; Drăghici, C.; Căproiu, M.T.; Shova, S.; Cojocaru, A.; Munteanu, C.V.A. MCPB treatment of (±)-(1α,3α,3aβ,6aβ)-1,2,3,3a,4,6a-hexahydro-1,3-pentalenedimethanol and its O-acyl-protected derivatives; X-ray crystallography. Tetrahedron 2015, 71, 4154–4162. [Google Scholar] [CrossRef]
- Tănase, C.I.; Drăghici, C.; Shova, S.; Maganu, M.; Cojocaru, A.; Munteanu, C.V.A.; Cocu, F. Regioselective reactions on a 1,3-disubstituted dihydroxymethyl or dicarboxyl hexahydropentalene skeleton. Tetrahedron 2015, 71, 6852–6859. [Google Scholar] [CrossRef]
- Narayama, C.; Periasamy, M. Acetoxyborohydride: A Simple Selective Hydroborating Agent. Tetrahedron Lett. 1985, 26, 1757–1760. [Google Scholar] [CrossRef]
- Brown, H.C.; Keblys, K. Hydroboration XXII. The reaction of unsaturated esters with diborane and disiamylborane. J. Am. Chem. Soc. 1964, 86, 1795–1801. [Google Scholar] [CrossRef]
- Brown, H.C.; Zweifel, G. Organic Synthesis via Boranes; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Brown, H.C.; Zweifel, G. Organoboranes. V. The Thermal Cyclization of Dialkylboranes. A Convenient Synthesis of 2,4,4-Trimethyl-1,5-pentanediol and Related 1,5-Diols. J. Am. Chem. Soc. 1966, 88, 1433–1437. [Google Scholar] [CrossRef]
- Brown, H.C.; Zweifel, G. Hydroboration of Terpenes. II. The Hydroboration of α- and β-Pinene-The Absolute Configuration of the Dialkylborane from the Hydroboration of α-Pinene. J. Am. Chem. Soc. 1964, 86, 393–397. [Google Scholar]
- Brown, H.C.; Bhatt, M.V. Organoboranes. IV. The Displacement Reaction with Organoboranes Derived from the Hydroboration of Branched-Chain Olefins. A Contrathermodynamic Isomerization of Olefins. J. Am. Chem. Soc. 1966, 88, 1440–1443. [Google Scholar] [CrossRef]
- Wilder, P., Jr.; Portis, A.R.; Wright, G.W.; Shepherd, J.M. Oxymercuration-demercuration and hydroboration-oxidation of endo-dicyclopentadiene (endo-tricyclo[5.2.1.02,6]deca-3,8-diene). J. Org. Chem. 1974, 39, 1636–1641. [Google Scholar] [CrossRef]
- CrysAlis CCD and CrysAlis RED, version 1.171.36.32; Oxford Diffraction Ltd.: Yarnton, Oxfordshire, UK, 2003.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXS. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).