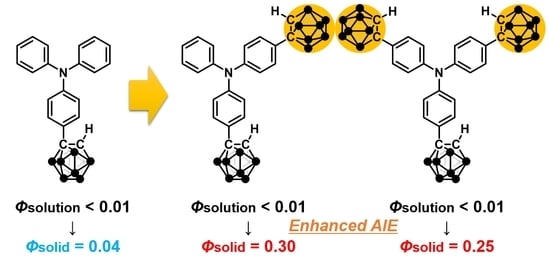
Enhancement of Aggregation-Induced Emission by Introducing Multiple o-Carborane Substitutions into Triphenylamine
Abstract
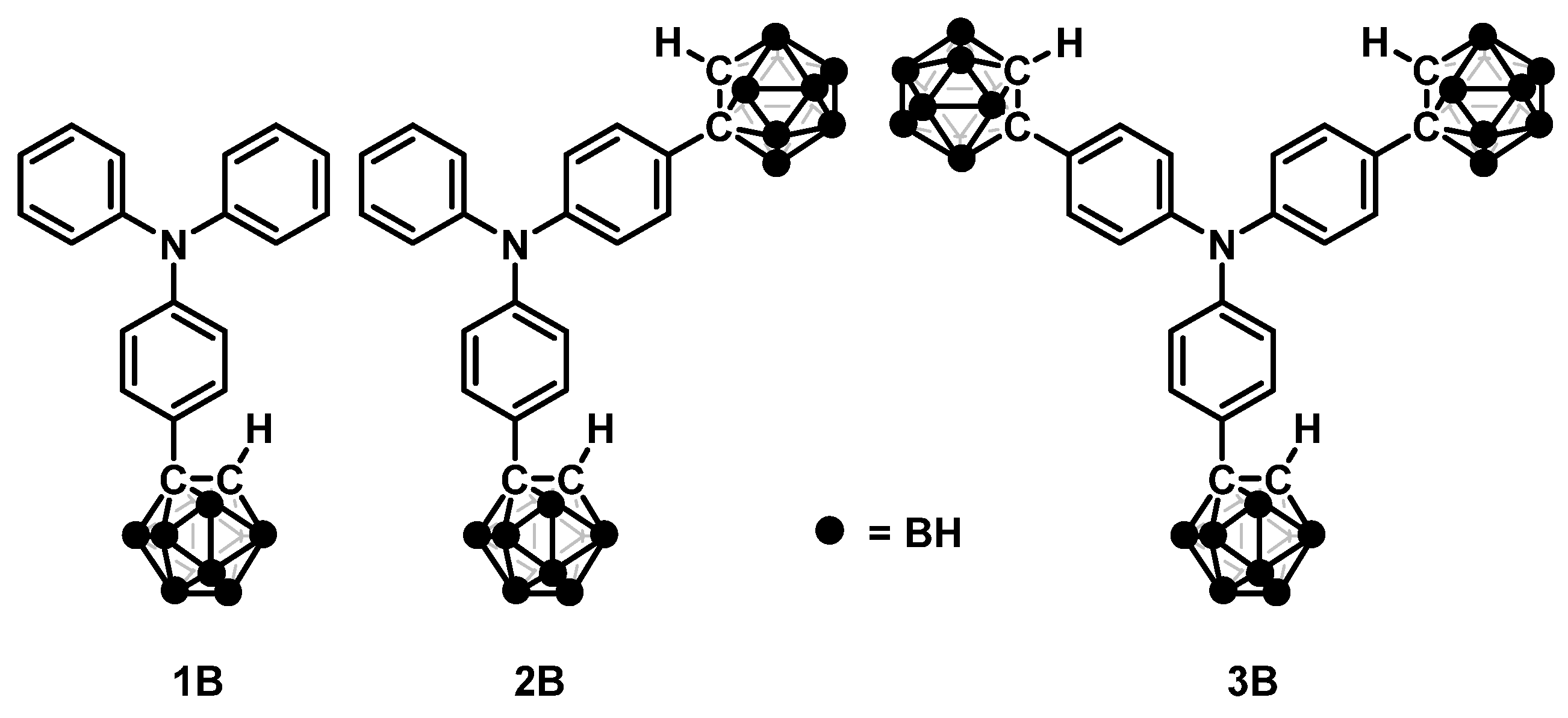
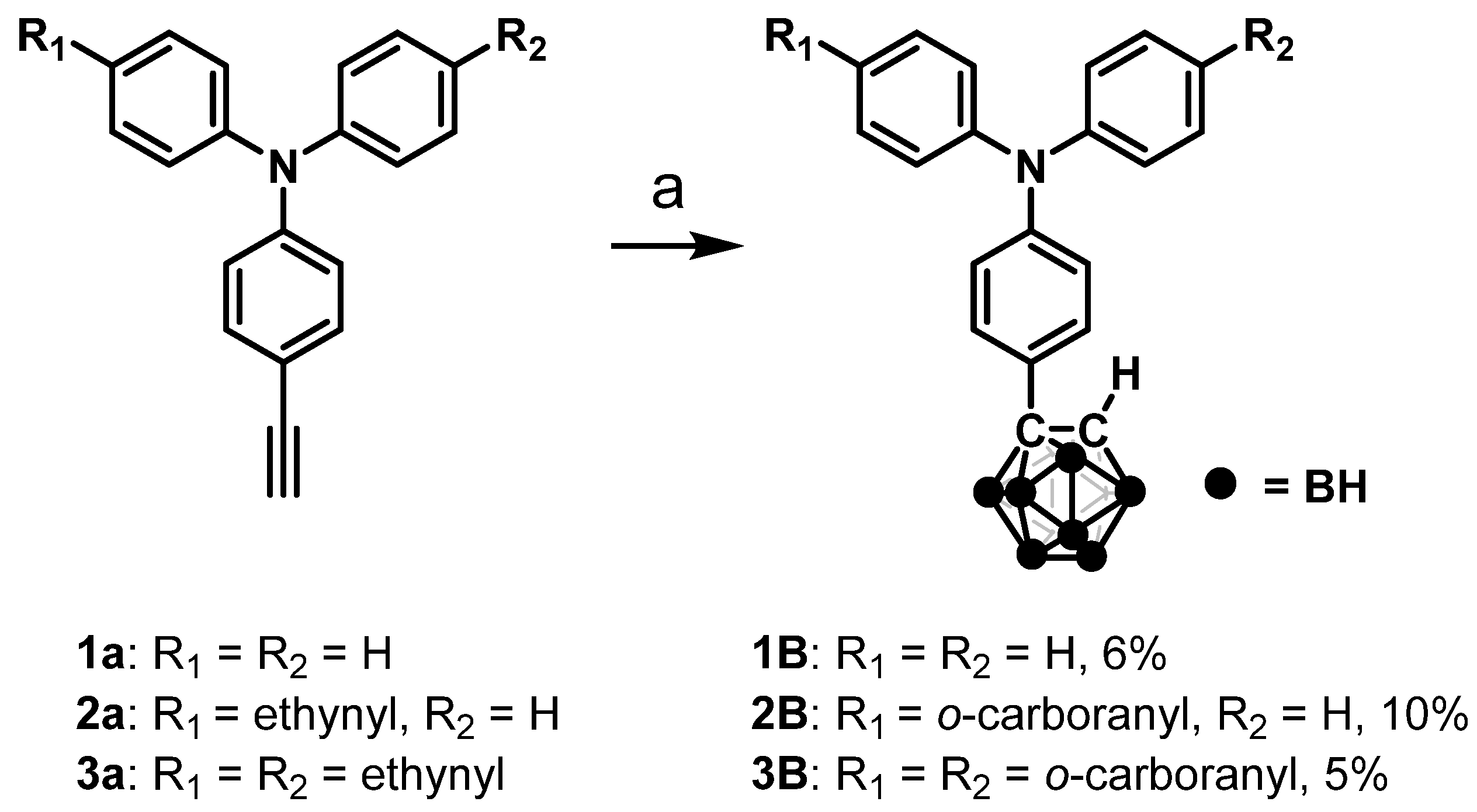
1. Introduction
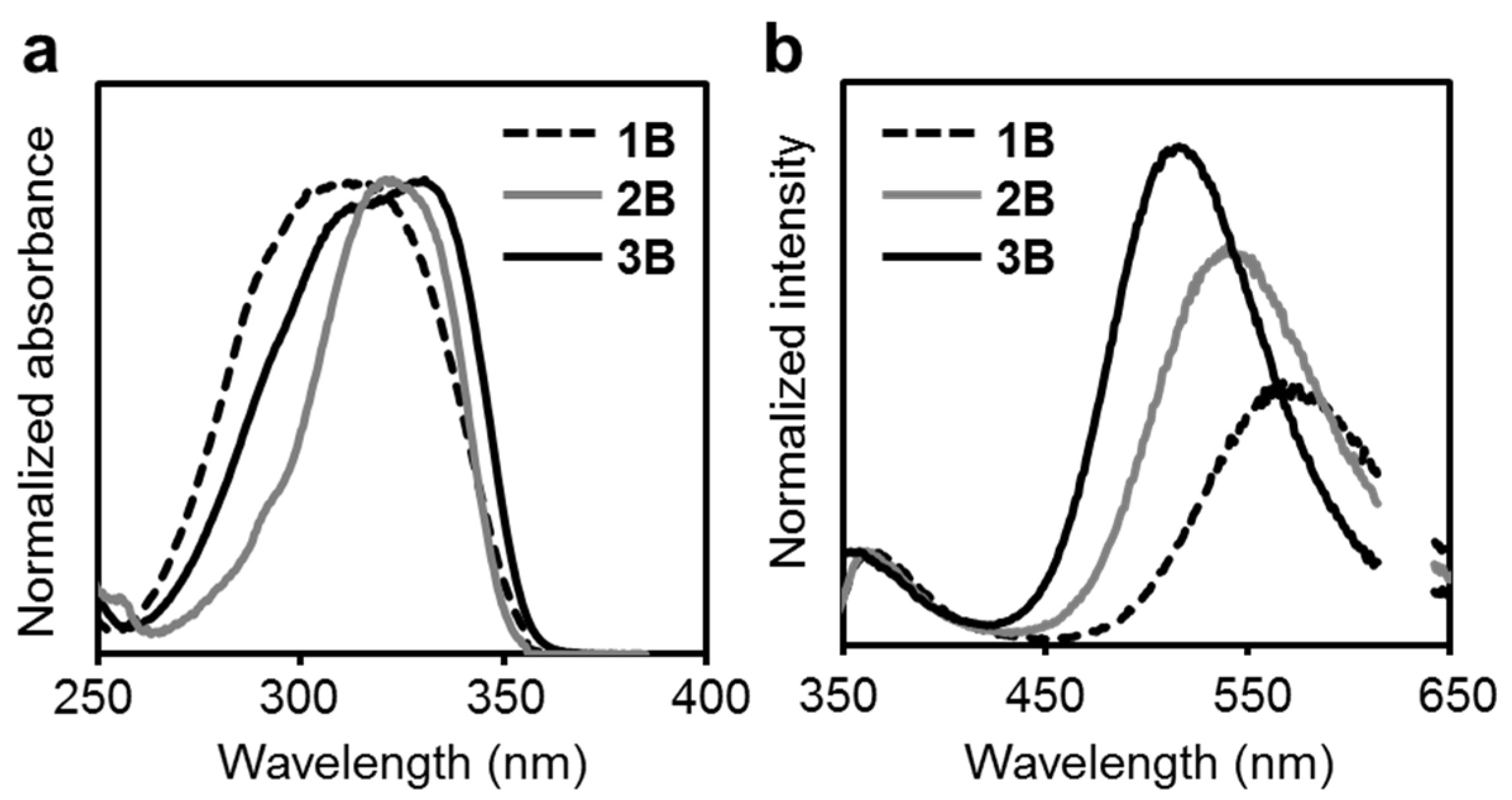
2. Results and Discussion
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chujo, Y.; Tanaka, K. New polymeric materials based on element-blocks. Bull. Chem. Soc. Jpn. 2015, 88, 633–643. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Tanaka, K.; Chujo, Y. Recent progress in the development of advanced element-block materials. Polym. J. 2017. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Recent progress of optical functional nanomaterials based on organoboron complexes with β-diketonate, ketoiminate and diiminate. NPG Asia Mater. 2015, 7, e223. [Google Scholar] [CrossRef]
- Bregadze, V.I. Dicarba-closo-dodecaboranes C2B10H12 and their derivatives. Chem. Rev. 1992, 92, 209–223. [Google Scholar] [CrossRef]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.; Terrés, M.; Ferrer-Ugalde, A.; Biani, F.F.D.; Teixidor, F. Electrochemistry of boron compounds. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef] [PubMed]
- Grimes, R.N. Carboranes, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 301–540. [Google Scholar]
- Li, X.; Yan, H.; Zhao, Q. Carboranes as a tool to tune phosphorescence. Chem. Eur. J. 2016, 22, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef] [PubMed]
- Böhling, L.; Brockhinke, A.; Kahlert, J.; Weber, L.; Harder, R.A.; Yufit, D.S.; Howard, J.A.K.; MacBride, J.A.H.; Fox, M.A. Substituent effects on the fluorescence properties of ortho-carboranes: Unusual emission behaviour in C-(2′-pyridyl)-ortho-carboranes. Eur. J. Inorg. Chem. 2016, 403–412. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Halama, J.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Nervi, C.; Harder, R.A.; et al. C,C′-Bis(benzodiazaborolyl)dicarba-closo-dodecaboranes: Synthesis, structures, photophysics and electrochemistry. Dalton Trans. 2013, 42, 10982–10996. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, J.; Böhling, L.; Brockhinke, A.; Stammler, H.-G.; Neumann, B.; Rendina, L.M.; Low, P.J.; Weber, L.; Fox, M.A. Syntheses and reductions of C-dimesitylboryl-1,2-dicarba-closo-dodecaboranes. Dalton Trans. 2015, 44, 9766–9781. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Lee, J.H.; Hwang, H.; Lee, K.M.; Park, M.H. Novel dimeric o-carboranyl triarylborane: Intriguing ratiometric color-tunable sensor via aggregation-induced emission by fluoride anions. Organometallics 2016, 35, 1771–1777. [Google Scholar] [CrossRef]
- Eo, M.; Park, M.H.; Kim, T.; Do, Y.; Lee, M.H. Polynorbornene copolymers with pendent o-carborane and carbazole groups: Novel side-chain donor-acceptor copolymers for turn-on sensing of nucleophilic anions. Polymer 2013, 54, 6321–6328. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.; Lee, K.M.; Lee, Y.S.; Lee, M.H. Phosphorescence color tuning of cyclometalated iridium complexes by o-carborane substitution. Inorg. Chem. 2013, 52, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Davis, A.R.; Were, M.; Coughlin, E.B.; Carter, K.R. Carborane-containing poly(fluorene): Response to solvent vapors and amines. ACS Appl. Mater. Interfaces 2011, 3, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.; Leong, P.; Li, Z.; Hu, R.; Shi, C.; Zhang, K.Y.; Yan, H.; Zhao, Q. A carborane-triggered metastable charge transfer state leading to spontaneous recovery of mechanochromic luminescence. Chem. Commun. 2016, 52, 12494–12497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Luo, Y.; Xu, Y.; Tian, L.; Li, M.; He, R.; Shen, W. The electronic structures and photophysical properties of platinum complexes with C^N^N ligands: The influence of the carborane substituent. Dalton Trans. 2015, 44, 18130–18137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tang, X.; Yu, Q.; Lv, W.; Yan, H.; Zhao, Q.; Huang, W. Tuning the optical properties of 2-thienylpyridyl iridium complexes through carboranes and anions. Chem. Eur. J. 2015, 21, 4721–4730. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Kim, S.-Y.; Cho, M.; Han, W.-S.; Son, H.-J.; Cho, D.W.; Kang, S.O. Aggregation-induced emission of diarylamino-π-carborane triads: Effects of charge transfer and π-conjugation. Phys. Chem. Chem. Phys. 2016, 19, 9702–9708. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Cho, Y.-J.; Jin, G.F.; Han, W.-S.; Son, H.-J.; Cho, D.W.; Kang, S.O. Intriguing emission properties of triphenylamine-carborane systems. Phys. Chem. Chem. Phys. 2015, 17, 15679–15682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, P.; Wang, T.; Moxey, G.J.; Cifuentes, M.P.; Zhang, C.; Humphrey, M.G. Blue-shifted emission and enhanced quantum efficiency via p-bridge elongation in carbazole–carborane dyads. Phys. Chem. Chem. Phys. 2016, 18, 15719–15726. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Wee, K.-R.; Cho, Y.-J.; Kang, S.O. Carborane dyads for photoinduced electron transfer: Photophysical studies on carbazole and phenyl-o-carborane molecular assemblies. Chem. Eur. J. 2014, 20, 5953–5960. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Highly-efficient solid-state emissions of the anthracene-o-carborane dyads with various substituents and their thermochromic luminescent properties. J. Mater. Chem. C 2017, 4, 10047–10054. [Google Scholar] [CrossRef]
- Nishino, K.; Yamamoto, H.; Tanaka, K.; Chujo, Y. Development of solid-state emissive materials based on multi-functional o-carborane-pyrene dyads. Org. Lett. 2016, 18, 4064–4067. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Luminescence color tuning of stable luminescent solid materials from blue to NIR based on bis-o-carborane-substituted oligoacenes. Chem. Asian J. 2017, 12, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Furue, R.; Nishimoto, T.; Park, I.S.; Lee, J.; Yasuda, T. Aggregation-induced delayed fluorescence based on donor/acceptor-tethered Janus carborane triads: Unique photophysical properties of nondoped OLEDs. Angew. Chem. Int. Ed. 2016, 55, 7171–7175. [Google Scholar] [CrossRef] [PubMed]
- Wee, K.-R.; Cho, Y.-J.; Jeong, S.; Kwon, S.; Lee, J.-D.; Suh, I.-H.; Kang, S.O. Carborane-based optoelectronically active organic molecules: Wide band gap host materials for blue phosphorescence. J. Am. Chem. Soc. 2012, 134, 17982–17990. [Google Scholar] [CrossRef] [PubMed]
- Inagi, S.; Hosoi, K.; Kubo, T.; Shida, N.; Fuchigami, T. o-Carborane-triphenylamine dyad: Studies on its acceptor-donor behavior toward dual redox mediator. Electrochemistry 2013, 81, 368–370. [Google Scholar] [CrossRef]
- Son, M.R.; Cho, Y.-J.; Kim, S.-Y.; Son, H.-J.; Cho, D.W.; Kang, S.O. Direct observation of the photoinduced electron transfer processes of bis(4-arylphenylamino benzo)-ortho-carborane using transient absorption spectroscopic measurements. Phys. Chem. Chem. Phys. 2017, 19, 24485–24492. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.; Leong, P.; Guo, S.; Yan, H.; Lu, C.; Zhao, Q. Highly emissive organic single-molecule white emitters by engineering o-carborane-based luminophores. Angew. Chem. Int. Ed. 2017, 56, 11370–11374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, J.; Wu, X.; Jia, D.; Tong, F. Color-tuning aggregation-induced emission of o-carborane-bis(1,3,5-triaryl-2-pyrazoline) triads: Preparation and investigation of the photophysics. Dyes Pigment. 2018, 148, 180–188. [Google Scholar] [CrossRef]
- Ferrer-Ugalde, A.; Cabrera-González, J.; Juárez-Pérez, E.J.; Teixidor, F.; Pérez-Inestrosa, E.; Montenegro, J.M.; Sillanpää, R.; Haukka, M.; Núñez, R. Carborane-stilbene dyads: The influence of substituents and cluster isomers on photoluminescence properties. Dalton Trans. 2017, 46, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, T.; Zhang, C.; Humphrey, M.G. Efficient crystallization-induced emission in fluorenyl-tethered carboranes. Phys. Chem. Chem. Phys. 2017, 19, 12928–12935. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yin, Y.; Yan, H.; Lu, C. Aggregation-induced emission characteristics of o-carborane-functionalized tetraphenylethylene luminogens: The influence of carborane cages on photoluminescence. Chem. Asian J. 2017, 12, 2207–2210. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nishino, K.; Ito, S.; Yamane, H.; Suenaga, K.; Hashimoto, K.; Chujo, Y. Development of the Solid-State Emissive o-Carborane and Theoretical Investigation for Mechanism of Aggregation-Induced Emission Behaviors of Organoboron “Element-Blocks”. Faraday Discuss. 2017, 196, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Hashimoto, K.; Tanaka, K.; Morisaki, Y.; Chujo, Y. Synthesis and properties of highly-rigid conjugation system based on bi(benzo[b]thiophene)-fused o-carborane. Tetrahedron Lett. 2016, 57, 2025–2028. [Google Scholar] [CrossRef]
- Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Electron-donating abilities and luminescent properties of tolane-substituted nido-carboranes. New J. Chem. 2017, 15, 10550–10554. [Google Scholar] [CrossRef]
- Nishino, K.; Yamamoto, H.; Tanaka, K.; Chujo, Y. Solid-state themochromic luminescence via twisted intramolecular charge transfer and excimer formation of the carborane-pyrene dyad with an ethynyl spacer. Asian J. Org. Chem. 2017. [Google Scholar] [CrossRef]
- You, D.K.; Lee, J.H.; Choi, B.H.; Hwang, H.; Lee, M.H.; Lee, K.M.; Par, M.H. Effects of multi-carborane substitution on the photophysical and electron-accepting properties of o-carboranylbenzene compounds. Eur. J. Inorg. Chem. 2017, 2017, 2496–2503. [Google Scholar] [CrossRef]
- Zhu, L.; Lv, W.; Liu, S.; Yan, H.; Zhao, Q.; Huang, W. Carborane enhanced two-photon absorption of tribranched fluorophores for fluorescence microscopy imaging. Chem. Commun. 2013, 49, 10638–10640. [Google Scholar] [CrossRef] [PubMed]
- Nicoud, J.-F.; Bolze, F.; Sun, X.-H.; Hayek, A.; Baldeck, P. Boron-containing two-photon-absorbing chromophores. 3. One- and two-photon photophysical properties of p-carborane-containing fluorescent bioprobes. Inorg. Chem. 2011, 50, 4272–4278. [Google Scholar] [CrossRef] [PubMed]
- Uebe, M.; Ito, A.; Kameoka, Y.; Sato, T.; Tanaka, K. Fluorescence enhancement of non-fluorescent triphenylamine: A recipe to utilize carborane cluster substituents. Chem. Phys. Lett. 2015, 633, 190–194. [Google Scholar] [CrossRef]
- Kameoka, Y.; Uebe, M.; Ito, A.; Sato, T.; Tanaka, K. Fluorescent triphenylamine derivative: Theoretical design based on reduced vibronic coupling. Chem. Phys. Lett. 2014, 615, 44–49. [Google Scholar] [CrossRef]
- Rajavelu, K.; Rajakumar, P.; Sudip, M.; Kothandaraman, R. Synthesis, photophysical, electrochemical, and DSSC application of novel donor-acceptor triazole bridged dendrimers with a triphenylamine core and benzoheterazole as a surface unit. New J. Chem. 2016, 40, 10246–10258. [Google Scholar] [CrossRef]
- Tydlitát, J.; Achelle, S.; Rodríguez-López, J.; Pytela, O.; Mikýsek, T.; Cabon, N.; Guen, F.R.; Miklík, D.; Růžičková, Z.; Bureš, F. Photophysical properties of acid-responsive triphenylamine derivatives bearing pyridine fragments: Towards white light emission. Dyes Pigment. 2017, 146, 467–478. [Google Scholar] [CrossRef]
- Cvejn, D.; Michail, E.; Seintis, K.; Klikar, M.; Pytela, O.; Mikysek, T.; Almonasy, N.; Ludwig, M.; Giannetas, V.; Fakis, M.; et al. Solvent and branching effect on the two-photon absorption properties of push-pull triphenylamine derivatives. RSC Adv. 2016, 6, 12819–12828. [Google Scholar] [CrossRef]
- Hrobárik, P.; Hrobáriková, V.; Sigmundová, I.; Zahradník, P.; Fakis, M.; Polyzos, I.; Persephonis, P. Benzothiazoles with tunable electron-withdrawing strength and reverse polarity: A route to triphenylamine-based chromophores with enhanced two-photon absorption. J. Org. Chem. 2011, 76, 8726–8736. [Google Scholar] [CrossRef] [PubMed]
- Romain, M.; Tondelier, D.; Jeannin, O.; Geffroy, B.; Rault-Berthelot, J.; Poriel, C. Properties modulation of organic semi-conductors based on a donor-spiro-acceptor (D-spiro-A) molecular design: New host materials for efficient sky-blue PhOLEDs. J. Mater. Chem. C 2015, 3, 9701–9714. [Google Scholar] [CrossRef]
- Naito, H.; Nishino, K.; Morisaki, Y.; Tanaka, K.; Chujo, Y. Solid-state emission of the anthracene-o-carborane dyad via twisted-intramolecular charge transfer in the crystalline state. Angew. Chem. Int. Ed. 2017, 56, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Takamine, H.; Tanaka, K.; Chujo, Y. Design of bond-cleavage-induced intramolecular charge transfer emission with dibenzoboroles and their application to ratiometric sensors for discriminating chain lengths of alkanes. Mater. Chem. Front. 2017, 1, 2368–2375. [Google Scholar] [CrossRef]
- Weber, L.; Kahlert, J.; Brockhinke, R.; Böhling, L.; Brockhinke, A.; Stammler, J.-G.; Neumann, B.; Harder, R.A.; Fox, M.A. Luminescence properties of C-diazaborolyl-ortho-carboranes as donor-acceptor systems. Chem. Eur. J. 2012, 18, 8347–8357. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
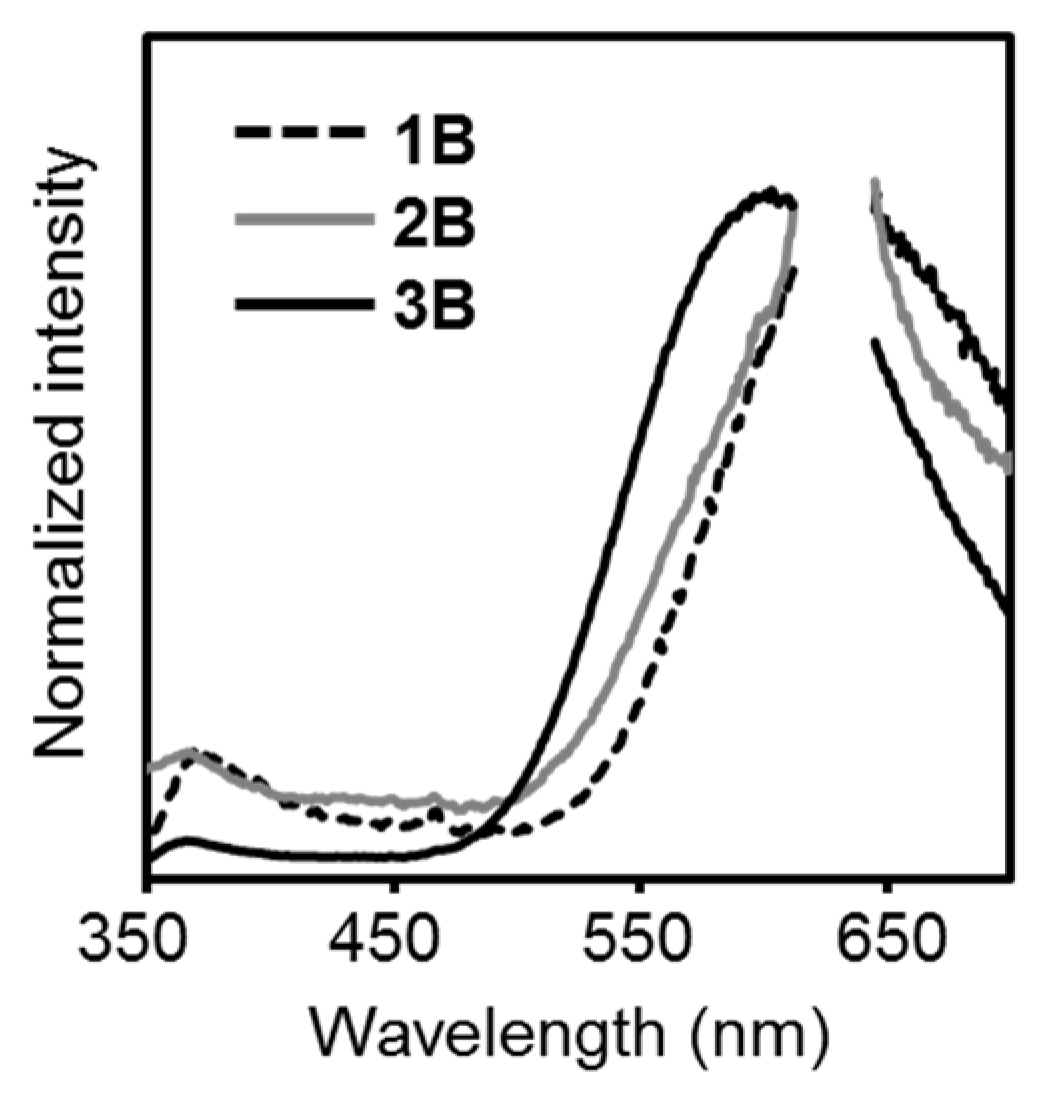
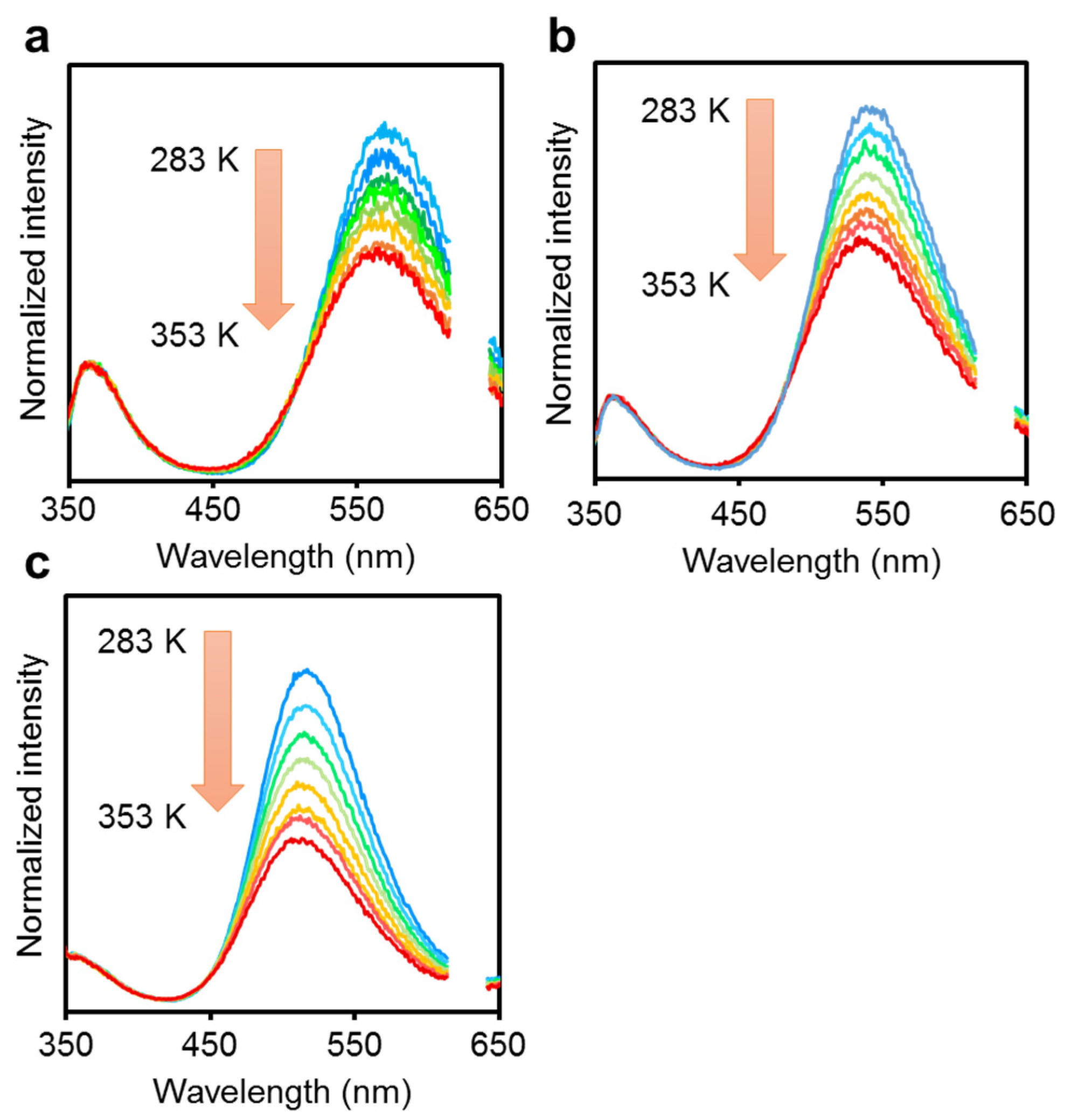
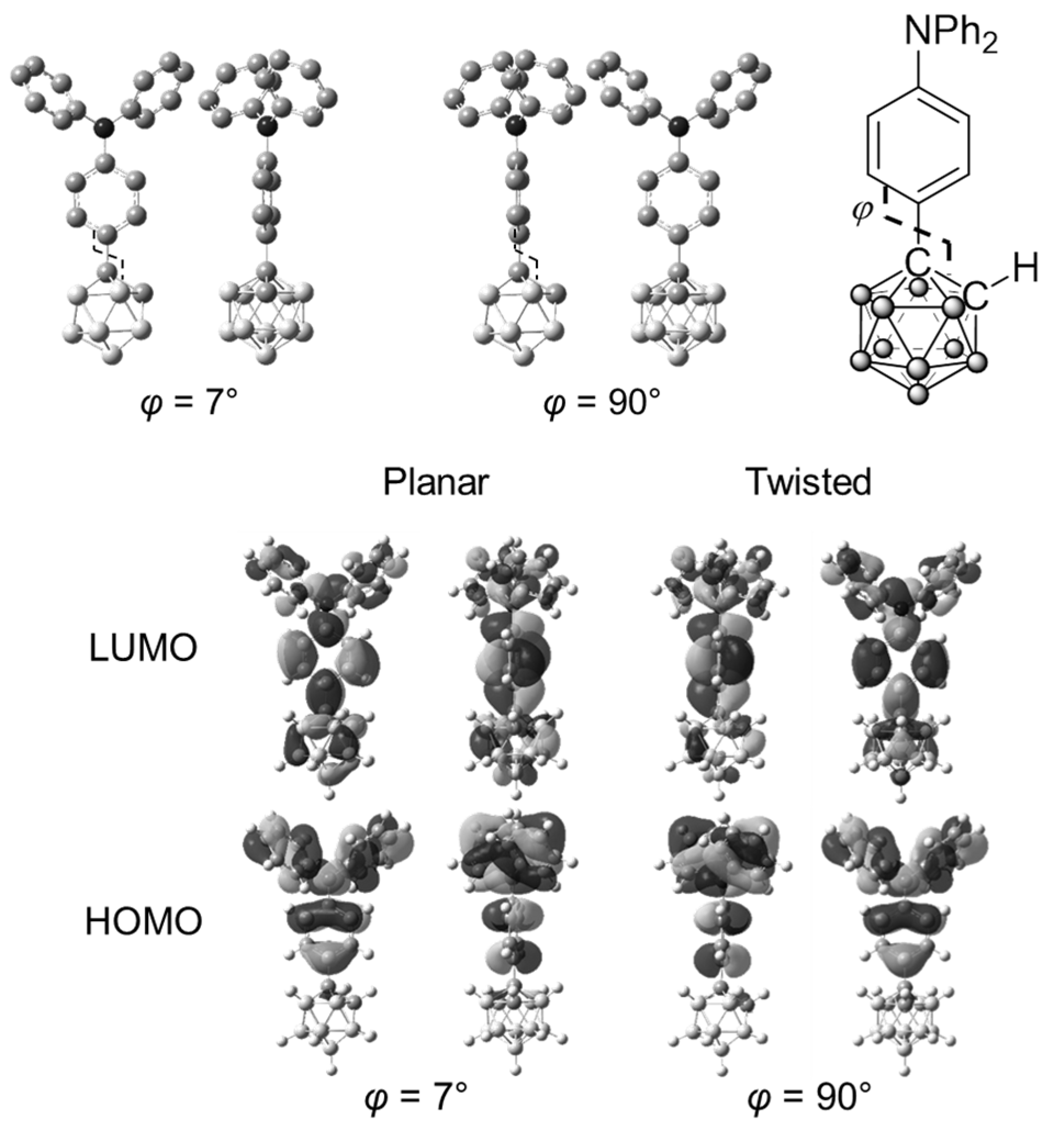
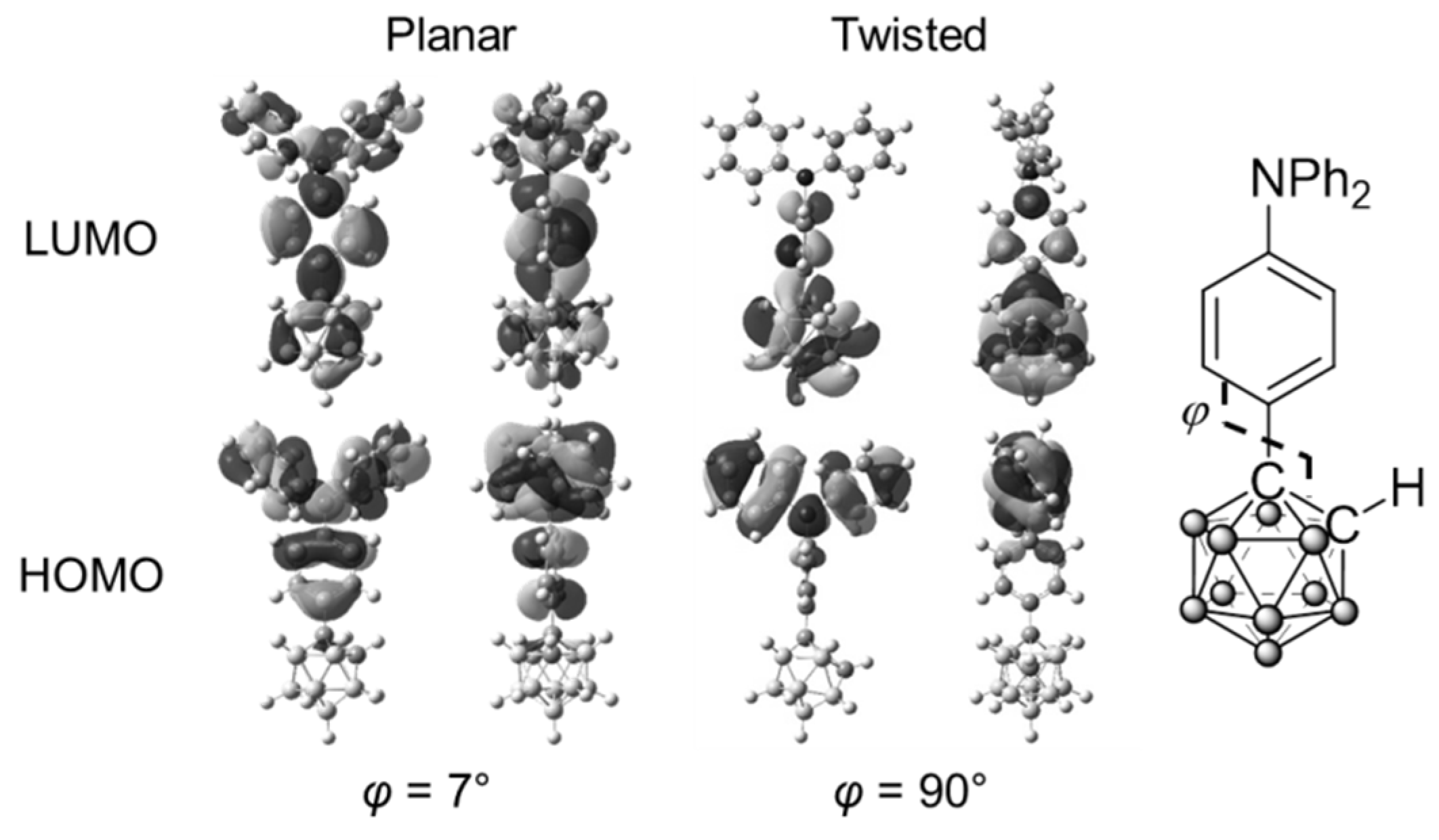
| Compound | λabs (nm) a | εmax (M−1 cm−1) | λem,rt (nm) b | λem,rt (nm) c | ΦPL b,d | λem,77K (nm) e | λem,rt (n Ea + ΔH Ea + ΔH m) f | ΦPL d,f | λem,77K (nm) f | Ea (kJ/mol) | ΔH (kJ/mol) | Ea + ΔH (kJ/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1B | 314 | 23,300 | 374, 557 | 364, 567 | <0.01 | 361, 424, 662 | 372, 635 | 0.04 | 372, 649 | 3.4 | 4.4 | 7.8 |
| 2B | 331 | 28,900 | 362, 541 | 382, 694 | <0.01 | 382, 460, 625 | 623 | 0.30 | 602 | 5.0 | 4.7 | 9.7 |
| 3B | 321 | 45,300 | 358, 516 | 379, 677 | <0.01 | 377, 452, 609 | 602 | 0.25 | 586 | 5.6 | 2.9 | 8.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishino, K.; Uemura, K.; Gon, M.; Tanaka, K.; Chujo, Y. Enhancement of Aggregation-Induced Emission by Introducing Multiple o-Carborane Substitutions into Triphenylamine. Molecules 2017, 22, 2009. https://doi.org/10.3390/molecules22112009
Nishino K, Uemura K, Gon M, Tanaka K, Chujo Y. Enhancement of Aggregation-Induced Emission by Introducing Multiple o-Carborane Substitutions into Triphenylamine. Molecules. 2017; 22(11):2009. https://doi.org/10.3390/molecules22112009
Chicago/Turabian StyleNishino, Kenta, Kyoya Uemura, Masayuki Gon, Kazuo Tanaka, and Yoshiki Chujo. 2017. "Enhancement of Aggregation-Induced Emission by Introducing Multiple o-Carborane Substitutions into Triphenylamine" Molecules 22, no. 11: 2009. https://doi.org/10.3390/molecules22112009
APA StyleNishino, K., Uemura, K., Gon, M., Tanaka, K., & Chujo, Y. (2017). Enhancement of Aggregation-Induced Emission by Introducing Multiple o-Carborane Substitutions into Triphenylamine. Molecules, 22(11), 2009. https://doi.org/10.3390/molecules22112009