NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [67Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice
Abstract
1. Introduction
2. Results
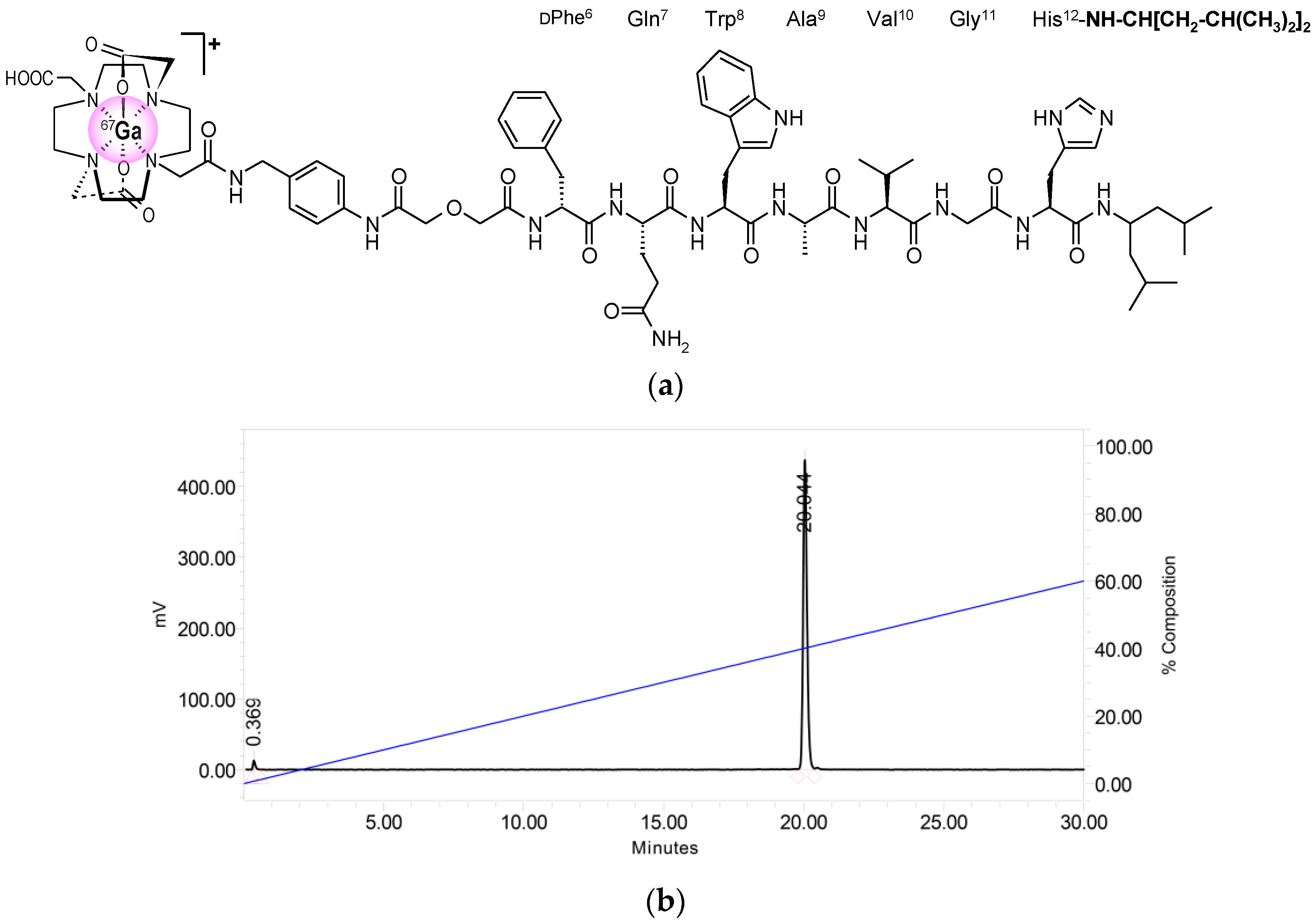
2.1. Peptides and Radioligands
2.2. In Vitro Assays in T-47D Cells
2.2.1. Affinity of NeoBOMB1 and [natGa]NeoBOMB1 for the GRPR
2.2.2. Time-Dependent Internalization of [67Ga]NeoBOMB1 in T-47D Cells
2.3. In Vivo Evaluation of [67Ga]NeoBOMB1
2.3.1. Stability of [67Ga]NeoBOMB1 in Healthy Mice
2.3.2. Biodistribution of [67Ga]NeoBOMB1 in Mice Bearing Human T-47D Xenografts
3. Discussion
4. Materials and Methods
4.1. Peptides and Radioligands
4.1.1. Preparation and Quality Control of [67Ga]NeoBOMB1
4.1.2. Preparation of [125I-Tyr4]BBN
4.1.3. Preparation of [natGa]NeoBOMB1
4.2. In Vitro Assays
4.2.1. Cell Lines and Culture
4.2.2. Competition Binding Assay in T-47D Cells
4.2.3. Internalization Assay in T-47D Cells
4.3. Animal Studies
4.3.1. In Vivo Stability Tests
4.3.2. Induction of T-47D Xenografts in SCID Mice
4.3.3. Biodistribution in T-47D Xenograft-Bearing SCID Mice
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Maecke, H.R. Radiopharmaceutical development of radiolabelled peptides. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl. 1), 11–30. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled peptides: Valuable tools for the detection and treatment of cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Kwekkeboom, D.J.; de Jong, M. Radiopeptides for imaging and therapy: A radiant future. J. Nucl. Med. 2015, 56, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[d-Tyr6,beta-Ala11,Phe13,Nle14]bombesin(6–14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar] [PubMed]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar] [PubMed]
- Körner, M.; Waser, B.; Rehmann, R.; Reubi, J.C. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate 2014, 74, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Muntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2012, 72, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Halmos, G.; Wittliff, J.L.; Schally, A.V. Characterization of bombesin/gastrin-releasing peptide receptors in human breast cancer and their relationship to steroid receptor expression. Cancer Res. 1995, 55, 280–287. [Google Scholar] [PubMed]
- Gugger, M.; Reubi, J.C. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am. J. Pathol. 1999, 155, 2067–2076. [Google Scholar] [CrossRef]
- Reubi, C.; Gugger, M.; Waser, B. Co-expressed peptide receptors in breast cancer as a molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nock, B.A. From bench to bed: New gastrin-releasing peptide receptor-directed radioligands and their use in prostate cancer. PET Clin. 2017, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Martens, J.W.; Sieuwerts, A.M.; van Deurzen, C.H.; Koelewijn, S.J.; de Blois, E.; Maina, T.; Nock, B.A.; Brunel, L.; Fehrentz, J.A.; et al. In Vitro and in vivo application of radiolabeled gastrin releasing peptide receptor ligands in breast cancer. J. Nucl. Med. 2015, 56, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Verzijlbergen, J.F.; De Jong, M. Review: Receptor targeted nuclear imaging of breast cancer. Int. J. Mol. Sci. 2017, 18, 260. [Google Scholar] [CrossRef] [PubMed]
- Morgat, C.; MacGrogan, G.; Brouste, V.; Velasco, V.; Sevenet, N.; Bonnefoi, H.; Fernandez, P.; Debled, M.; Hindie, E. Expression of gastrin-releasing peptide receptor in breast cancer and its association with pathologic, biologic, and clinical parameters: A study of 1432 primary tumors. J. Nucl. Med. 2017, 58, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nock, B.A.; Kulkarni, H.; Singh, A.; Baum, R.P. Theranostic prospects of gastrin-releasing peptide receptor-radioantagonists in oncology. PET Clin. 2017, 12, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Giarika, A.; Kulkarni, H.R.; Klette, I.; Singh, A.; Krenning, E.P.; de Jong, M.; Maina, T.; et al. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist NeoBOMB1: Preclinical and first clinical results. J. Nucl. Med. 2017, 58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Bakker, I.L.; de Blois, E.; Doeswijk, G.N.; Konijnenberg, M.W.; Orlandi, F.; Barbato, D.; Tedesco, M.; Maina, T.; Nock, B.A.; et al. 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J. Nucl. Med. 2017, 58, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Heimbrook, D.C.; Saari, W.S.; Balishin, N.L.; Fisher, T.W.; Friedman, A.; Kiefer, D.M.; Rotberg, N.S.; Wallen, J.W.; Oliff, A. Gastrin releasing peptide antagonists with improved potency and stability. J. Med. Chem. 1991, 34, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Keydar, I.; Chen, L.; Karby, S.; Weiss, F.R.; Delarea, J.; Radu, M.; Chaitcik, S.; Brenner, H.J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur. J. Cancer 1979, 15, 659–670. [Google Scholar] [CrossRef]
- Giacchetti, S.; Gauville, C.; de Cremoux, P.; Bertin, L.; Berthon, P.; Abita, J.P.; Cuttitta, F.; Calvo, F. Characterization, in some human breast cancer cell lines, of gastrin-releasing peptide-like receptors which are absent in normal breast epithelial cells. Int. J. Cancer 1990, 46, 293–298. [Google Scholar] [CrossRef] [PubMed]
- De Castiglione, R.; Gozzini, L. Bombesin receptor antagonists. Crit. Rev. Oncol. Hematol. 1996, 24, 117–151. [Google Scholar] [CrossRef]
- Heinrich, E.; Schally, A.V.; Buchholz, S.; Rick, F.G.; Halmos, G.; Mile, M.; Groot, K.; Hohla, F.; Zarandi, M.; Varga, J.L. Dose-dependent growth inhibition in vivo of PC-3 prostate cancer with a reduction in tumoral growth factors after therapy with GHRH antagonist MZ-J-7-138. Prostate 2008, 68, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Lamharzi, N.; Schally, A.V.; Halmos, G.; Szepeshazi, K.; Groot, K.; Cai, R.Z. Inhibition of growth of MDA-MB-231 human breast cancer xenografts in nude mice by bombesin/gastrin-releasing peptide (GRP) antagonists RC-3940-II and RC-3095. Eur. J. Cancer 1998, 34, 710–717. [Google Scholar] [CrossRef]
- Davis, T.P.; Crowell, S.; Taylor, J.; Clark, D.L.; Coy, D.; Staley, J.; Moody, T.W. Metabolic stability and tumor inhibition of bombesin/GRP receptor antagonists. Peptides 1992, 13, 401–407. [Google Scholar] [CrossRef]
- Maina, T.; Bergsma, H.; Kulkarni, H.R.; Mueller, D.; Charalambidis, D.; Krenning, E.P.; Nock, B.A.; de Jong, M.; Baum, R.P. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, E.; Jambor, I.; Kemppainen, J.; Lehtio, K.; Gronroos, T.J.; Kuisma, A.; Luoto, P.; Sipila, H.J.; Tolvanen, T.; Alanen, K.; et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Mansi, R.; Grosu, A.L.; Schultze-Seemann, W.; Dumont-Walter, R.A.; Meyer, P.T.; Maecke, H.R.; Reubi, J.C.; Weber, W.A. Positron emission tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist—From mice to men. Theranostics 2014, 4, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Popp, I.; Christian Rischke, H.; Drendel, V.; Grosu, A.L.; Bartholoma, M.; Weber, W.A.; Mansi, R.; Wetterauer, U.; Schultze-Seemann, W.; et al. Diagnosis of recurrent prostate cancer with PET/CT imaging using the gastrin-releasing peptide receptor antagonist 68Ga-RM2: Preliminary results in patients with negative or inconclusive [18F]Fluoroethylcholine-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Bakker, I.L.; Fröberg, A.C.; Busstra, M.; van Leenders, G.J.L.H.; de Blois, E.; Schoots, I.; Veenland, J.; Maina, T.; van Weerden, W.M.; Nock, B.A.; et al. PET imaging of therapy-naïve primary prostate cancer patients using the GRPr-targeting ligand Sarabesin 3. Eur. Urol. Suppl. 2016, 15, e567. [Google Scholar] [CrossRef]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Iagaru, A. Will GRPR compete with PSMA as a target in prostate cancer? J. Nucl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Stoykow, C.; Erbes, T.; Maecke, H.R.; Bulla, S.; Bartholoma, M.; Mayer, S.; Drendel, V.; Bronsert, P.; Werner, M.; Gitsch, G.; et al. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist 68Ga-RM2 and PET. Theranostics 2016, 6, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.J.; Andrews, R.; Rogers, B.E. MicroPET imaging of breast cancer using radiolabeled bombesin analogs targeting the gastrin-releasing peptide receptor. Breast Cancer Res. Treat. 2007, 101, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Prasanphanich, A.F.; Retzloff, L.; Lane, S.R.; Nanda, P.K.; Sieckman, G.L.; Rold, T.L.; Ma, L.; Figueroa, S.D.; Sublett, S.V.; Hoffman, T.J.; et al. In vitro and in vivo analysis of [64Cu-NO2A-8-Aoc-BBN(7–14)NH2]: A site-directed radiopharmaceutical for positron-emission tomography imaging of T-47D human breast cancer tumors. Nucl. Med. Biol. 2009, 36, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To serve and protect”: Enzyme inhibitors as radiopeptide escorts promote tumor targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.E.; Metcalfe, E.; Arunachalam, T.; Chen, J.; Eaton, S.M.; Feng, W.; Fan, H.; Raju, N.; Cagnolini, A.; Lantry, L.E.; et al. In vitro and in vivo metabolism of Lu-AMBA, a GRP-receptor binding compound, and the synthesis and characterization of its metabolites. Bioconjug. Chem. 2009, 20, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Kaloudi, A.; Valverde, I.E.; Mindt, T.L.; Nock, B.A. Amide-to-triazole switch vs. in vivo NEP-inhibition approaches to promote radiopeptide targeting of GRPR-positive tumors. Nucl. Med. Biol. 2017, 52, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Konijnenberg, M.; Nonnekens, J.; de Blois, E.; Hoeben, S.; de Ridder, C.; Brunel, L.; Fehrentz, J.A.; Martinez, J.; van Gent, D.C.; et al. In vivo stabilization of a gastrin-releasing peptide receptor antagonist enhances PET imaging and radionuclide therapy of prostate cancer in preclinical studies. Theranostics 2016, 6, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Vigna, S.R.; Giraud, A.S.; Reeve, J.R., Jr.; Walsh, J.H. Biological activity of oxidized and reduced iodinated bombesins. Peptides 1988, 9, 923–926. [Google Scholar] [CrossRef]
- Williams, B.Y.; Schonbrunn, A. Bombesin receptors in a human duodenal tumor cell line: Binding properties and function. Cancer Res. 1994, 54, 818–824. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds NeoBOMB1 and [natGa]NeoBOMB1 are not available from the authors. |


| Tissue/Dose | 10 pmol 1 | 200 pmol 2 | 40 nmol 3 |
|---|---|---|---|
| Blood | 0.53 ± 0.04 | 0.42 ± 0.06 | 0.46 ± 0.03 |
| Liver | 3.37 ± 0.19 | 6.50 ± 0.34 | 9.67 ± 0.75 |
| Heart | 0.26 ± 0.01 | 0.34 ± 0.04 | 0.38 ± 0.02 |
| Kidneys | 3.40 ± 0.45 | 2.76 ± 0.26 | 3.95 ± 0.94 |
| Stomach | 4.36 ± 0.56 | 2.38 ± 0.13 | 0.42 ± 0.10 |
| Intestines | 16.71 ± 2.18 | 15.13 ± 1.52 | 11.08 ± 2.71 |
| Spleen | 1.02 ± 0.09 | 0.70 ± 0.09 | 0.79 ± 0.11 |
| Muscle | 0.10 ± 0.03 | 0.12 ± 0.01 | 0.16 ± 0.06 |
| Lungs | 0.50 ± 0.06 | 0.43 ± 0.06 | 0.42 ± 0.02 |
| Femur | 0.58 ± 0.06 | 0.28 ± 0.01 | 0.31 ± 0.02 |
| Pancreas | 206.29 ± 17.35 | 42.46 ± 1.31 | 1.17 ± 0.11 |
| Tumor | 9.52 ± 2.15 | 7.79 ± 1.54 | 0.64 ± 0.10 |
| Tissue/Time | 1 h | 4 h | 24 h |
|---|---|---|---|
| Blood | 5.78 ± 0.29 | 0.52 ± 0.03 | 0.15 ± 0.01 |
| Liver | 25.66 ± 1.97 | 7.05 ± 0.31 | 2.26 ± 0.18 |
| Heart | 1.94 ± 0.09 | 0.52 ± 0.04 | 0.22 ± 0.04 |
| Kidneys | 7.33 ± 0.47 | 3.10 ± 0.75 | 2.15 ± 0.42 |
| Stomach | 5.89 ± 1.02 | 2.66 ± 0.16 | 2.07 ± 0.43 |
| Intestines | 10.62 ± 0.87 | 18.52 ± 2.12 | 2.31 ± 0.34 |
| Spleen | 1.71 ± 0.50 | 0.89 ± 0.12 | 0.46 ± 0.08 |
| Muscle | 0.58 ± 0.11 | 0.17 ± 0.00 | 0.13 ± 0.01 |
| Lungs | 2.88 ± 0.20 | 0.47 ± 0.04 | 0.15 ± 0.02 |
| Femur | 0.73 ± 0.11 | 0.37 ± 0.02 | 0.24 ± 0.08 |
| Pancreas | 32.45 ± 0.78 | 36.86 ± 3.58 | 21.33 ± 2.77 |
| Tumor | 7.40 ± 0.68 | 8.67 ± 2.88 | 7.89 ± 1.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaloudi, A.; Lymperis, E.; Giarika, A.; Dalm, S.; Orlandi, F.; Barbato, D.; Tedesco, M.; Maina, T.; De Jong, M.; Nock, B.A. NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [67Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice. Molecules 2017, 22, 1950. https://doi.org/10.3390/molecules22111950
Kaloudi A, Lymperis E, Giarika A, Dalm S, Orlandi F, Barbato D, Tedesco M, Maina T, De Jong M, Nock BA. NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [67Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice. Molecules. 2017; 22(11):1950. https://doi.org/10.3390/molecules22111950
Chicago/Turabian StyleKaloudi, Aikaterini, Emmanouil Lymperis, Athina Giarika, Simone Dalm, Francesca Orlandi, Donato Barbato, Mattia Tedesco, Theodosia Maina, Marion De Jong, and Berthold A. Nock. 2017. "NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [67Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice" Molecules 22, no. 11: 1950. https://doi.org/10.3390/molecules22111950
APA StyleKaloudi, A., Lymperis, E., Giarika, A., Dalm, S., Orlandi, F., Barbato, D., Tedesco, M., Maina, T., De Jong, M., & Nock, B. A. (2017). NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [67Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice. Molecules, 22(11), 1950. https://doi.org/10.3390/molecules22111950







