Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions
Abstract
1. Introduction
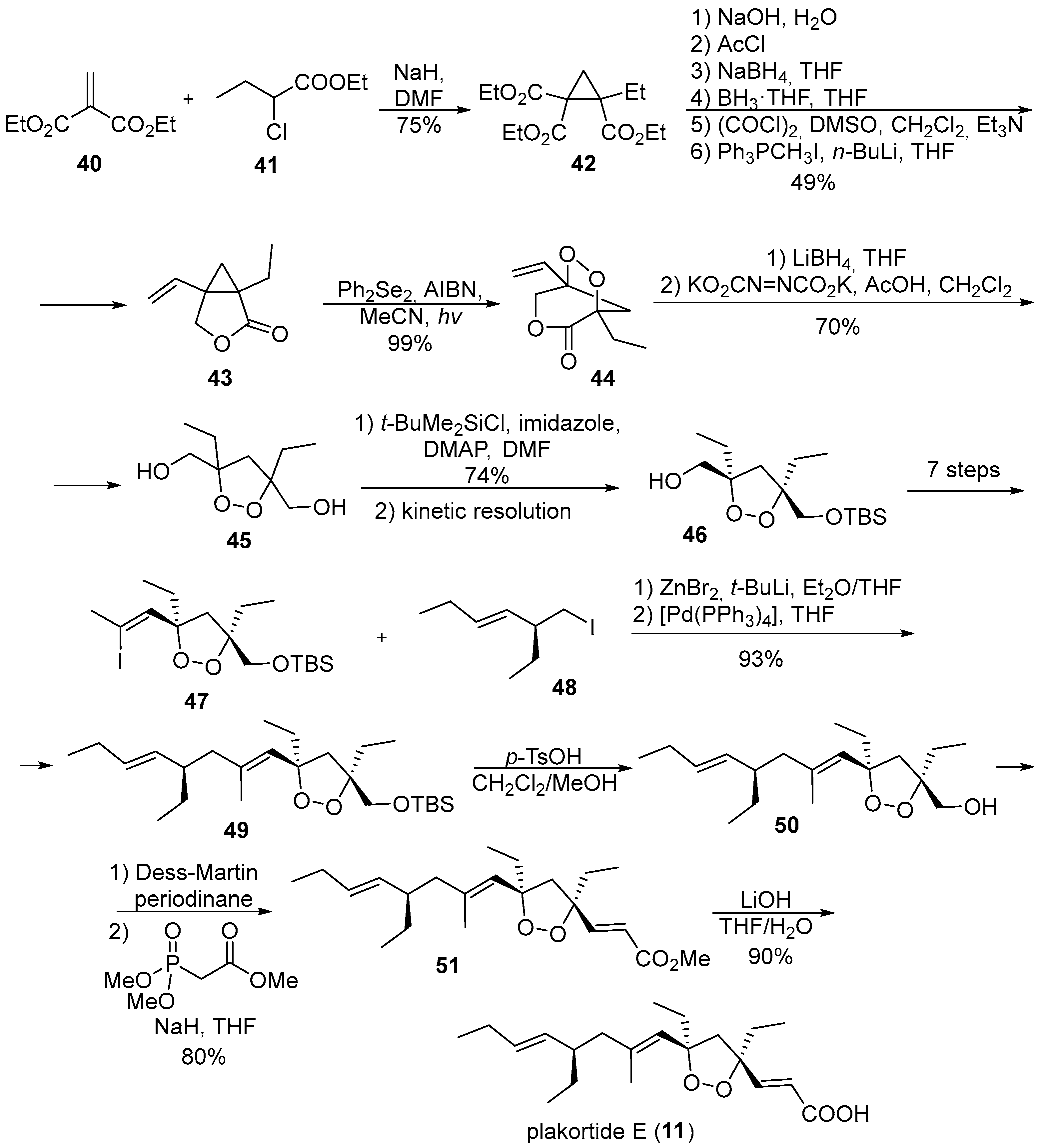
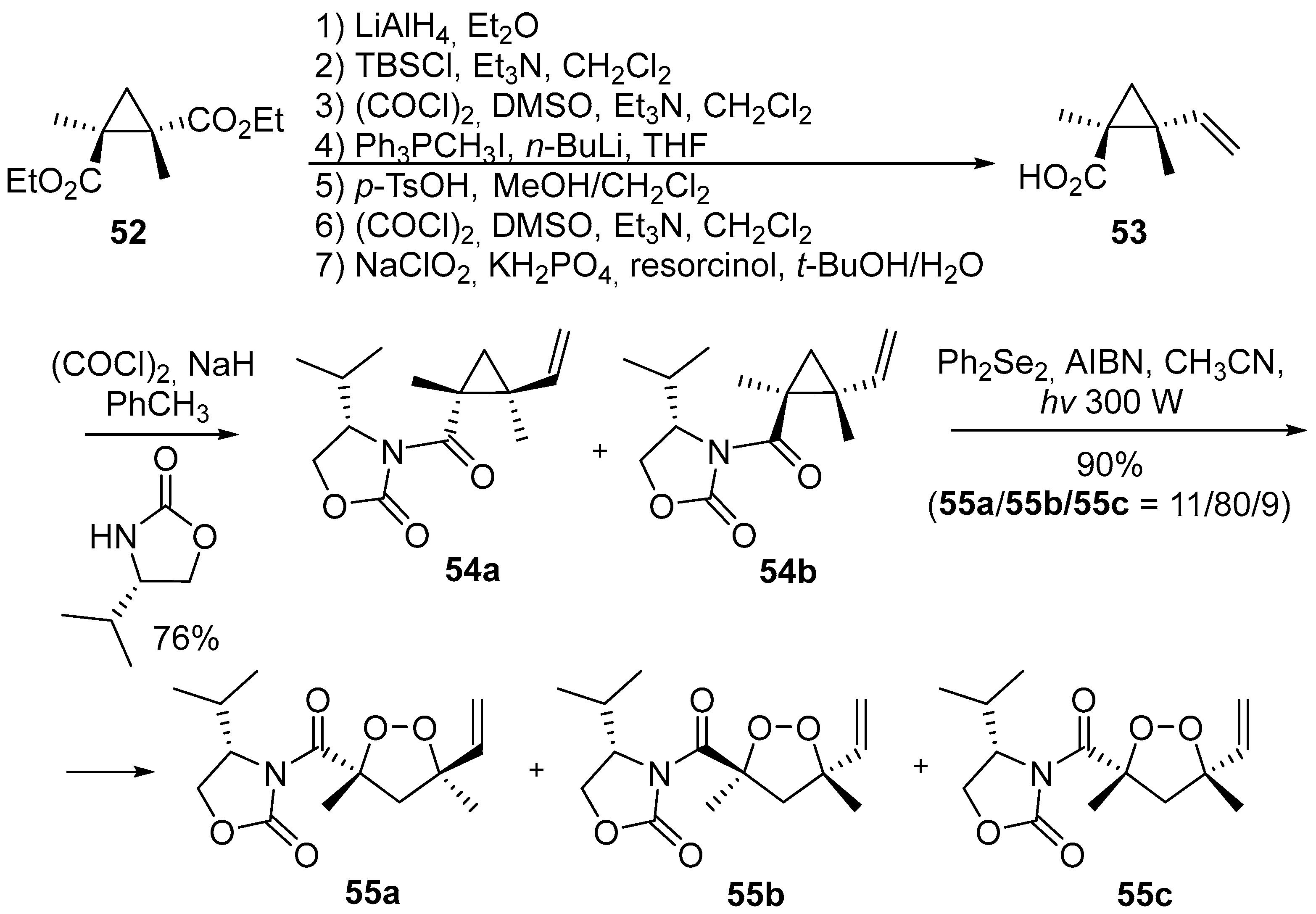
2. 1,2-Dioxolanes
3. 1,2,4-Trioxolanes (Ozonides)
4. 1,2-Dioxanes
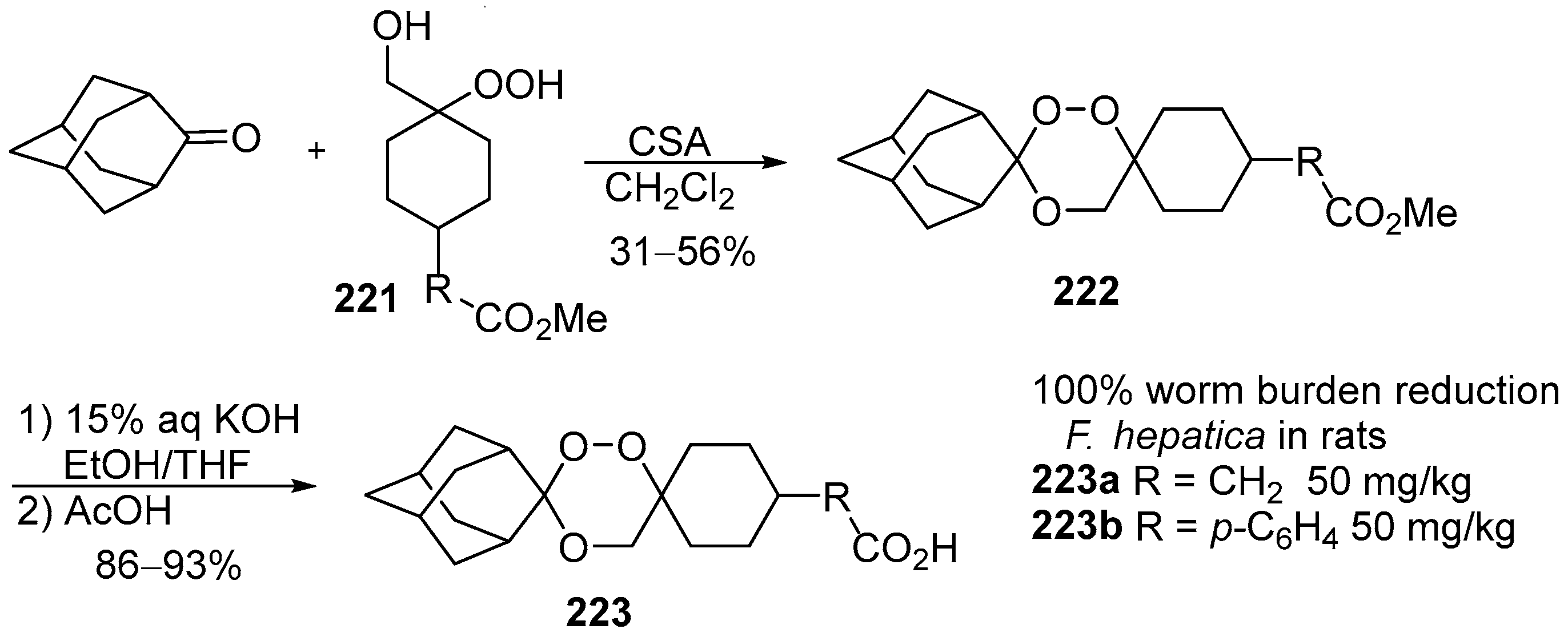
5. 1,2-Dioxenes
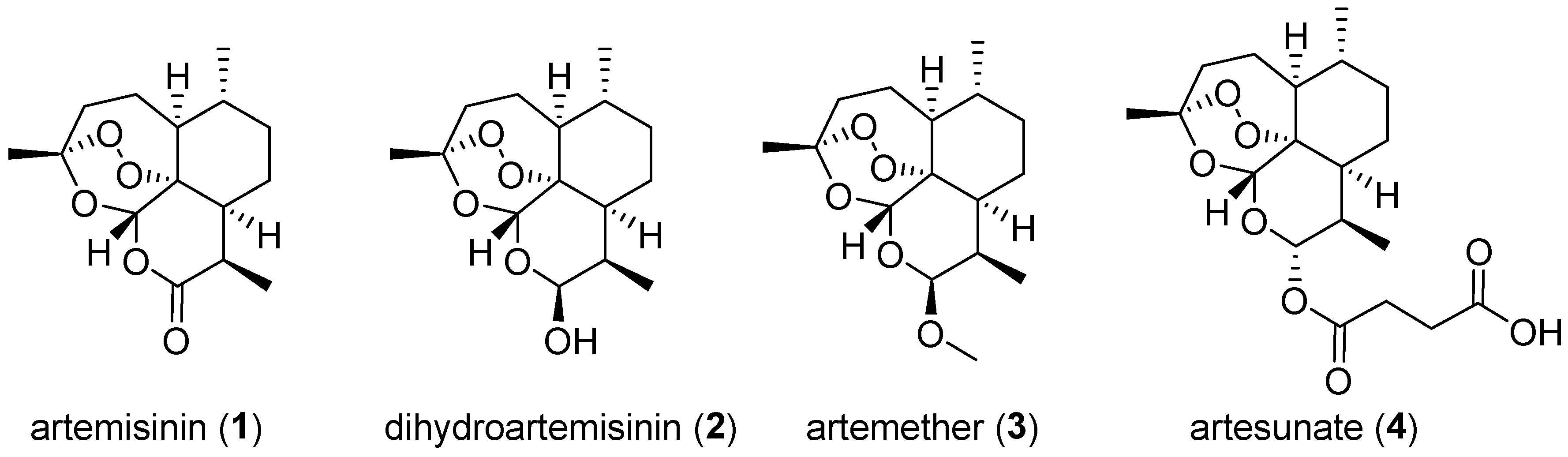
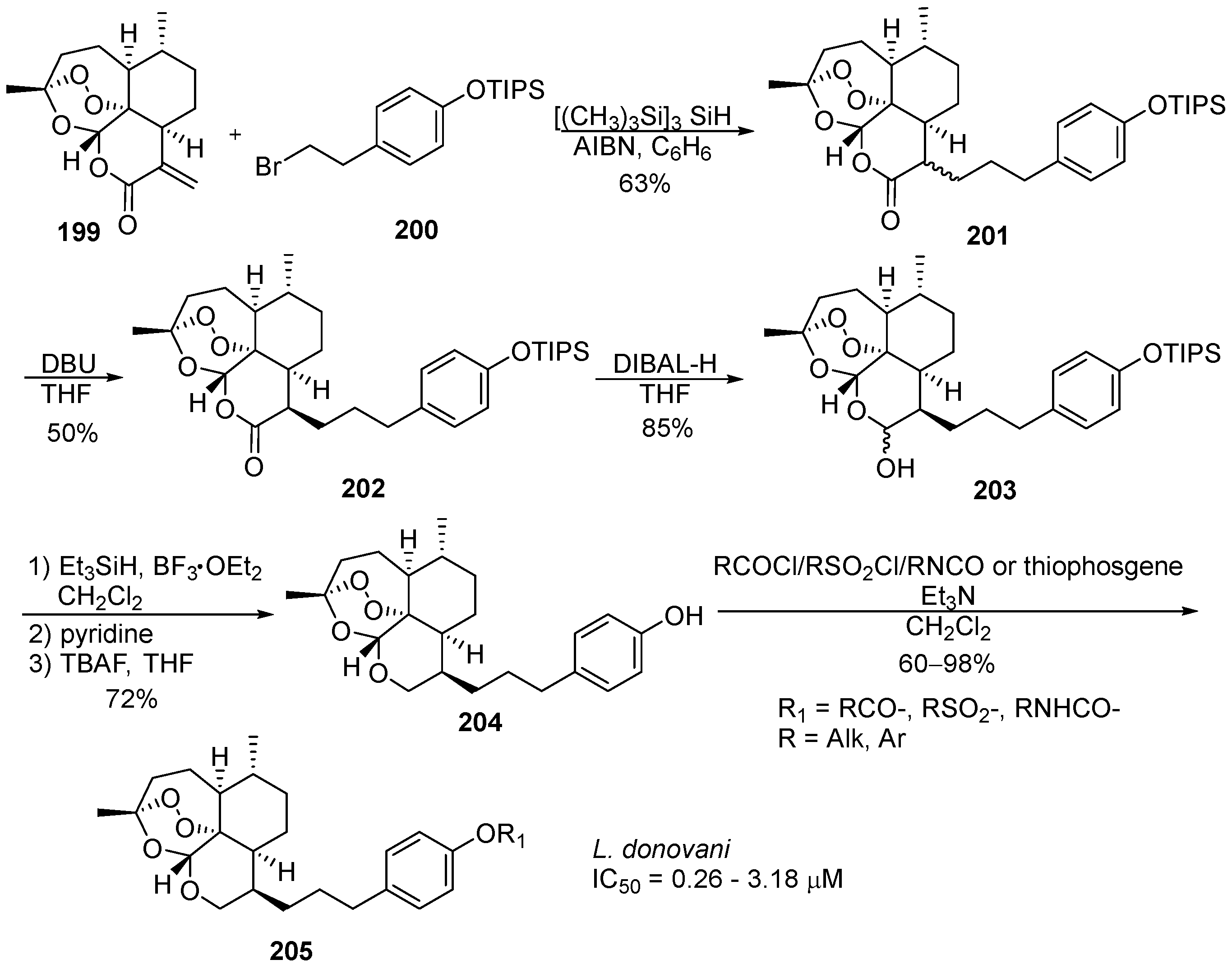
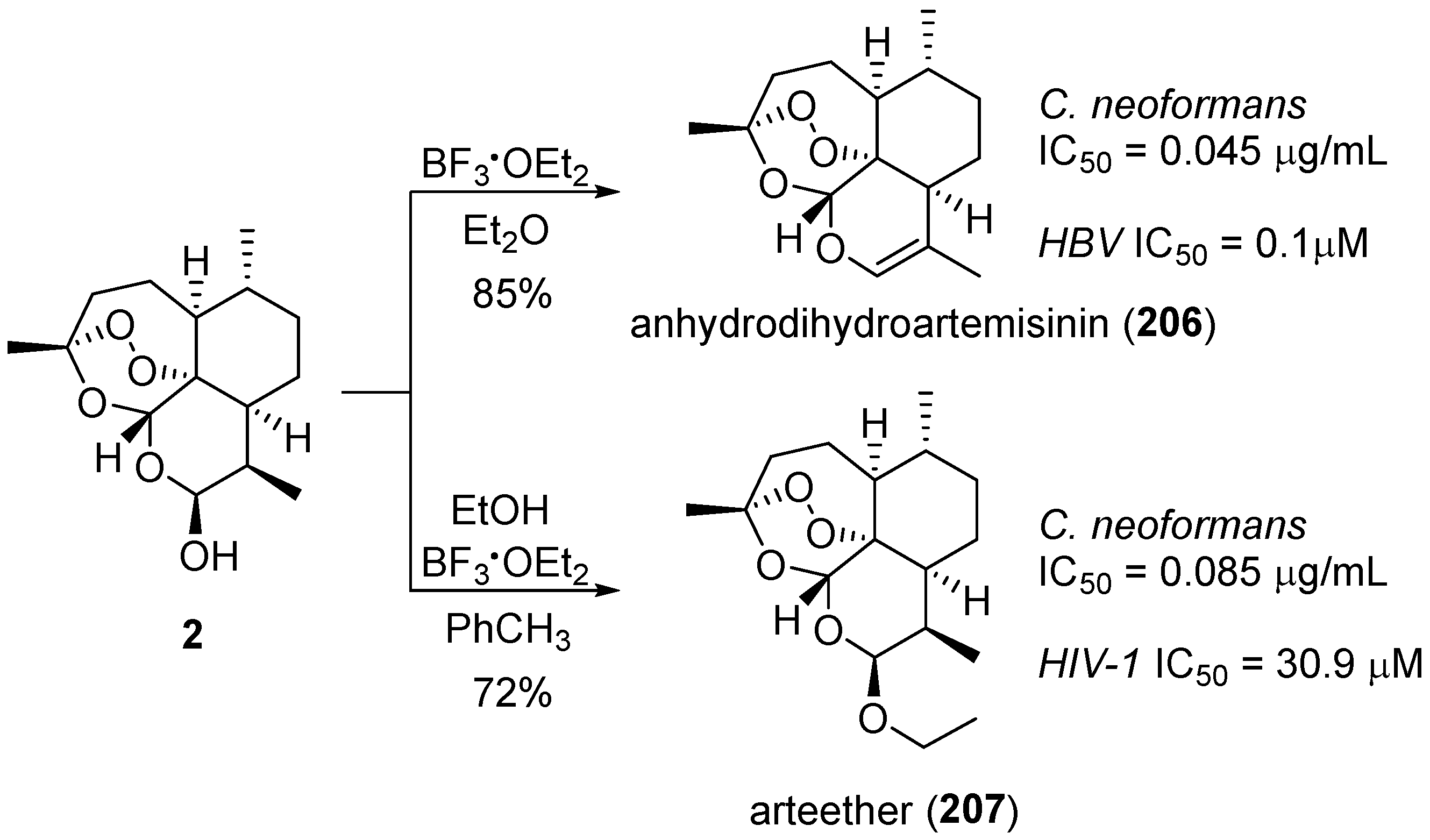
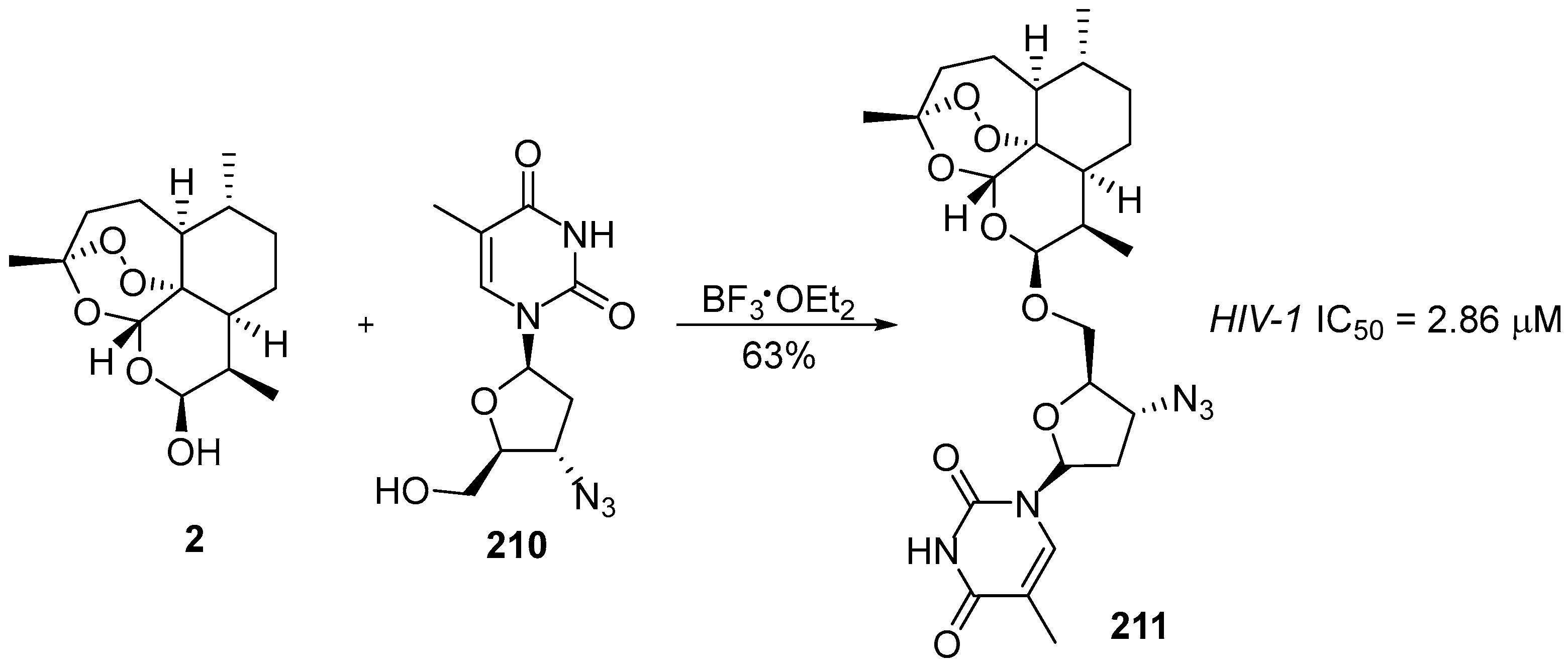
6. 1,2,4-Trioxanes
7. 1,2,4,5-Tetraoxanes
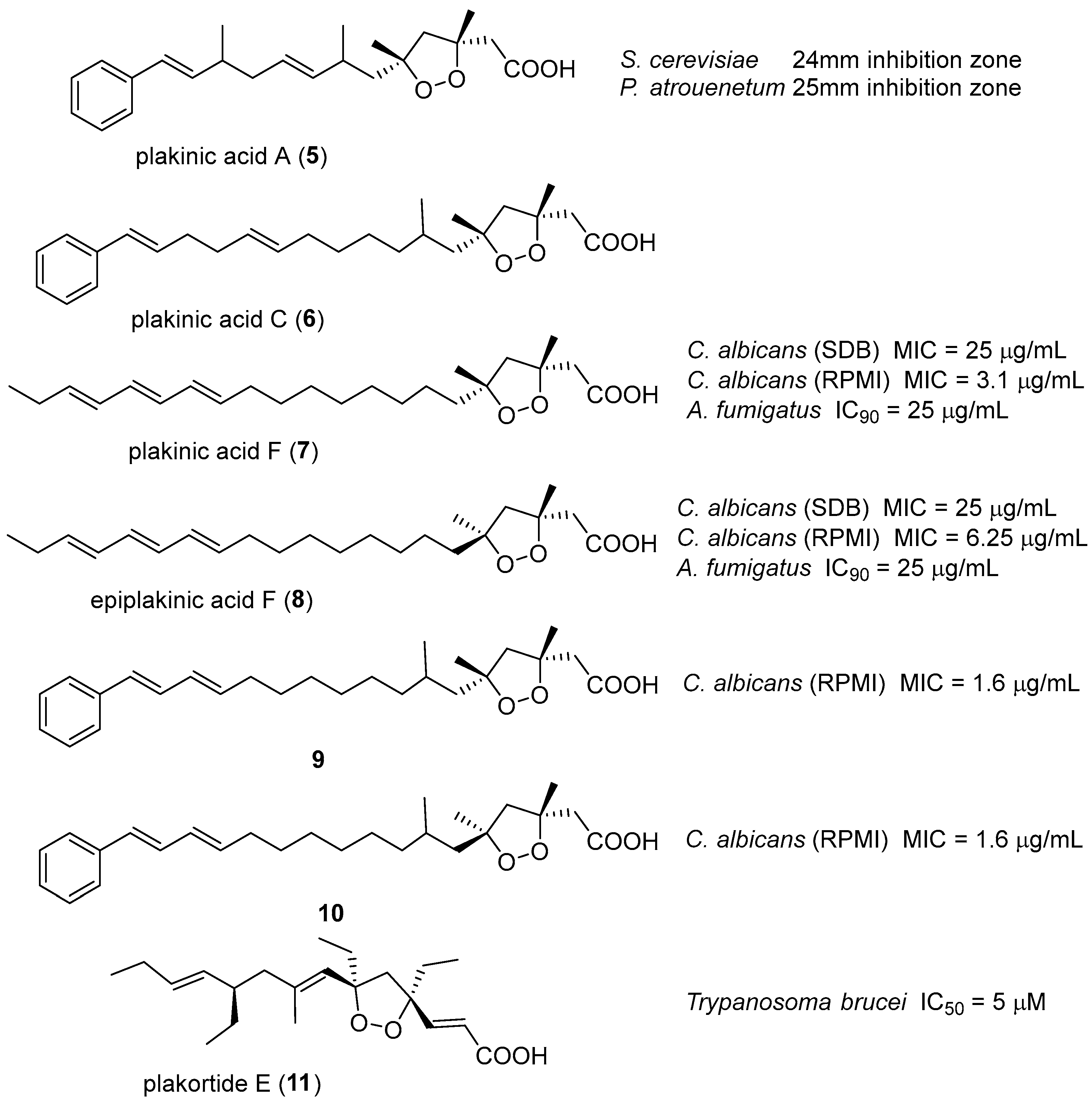
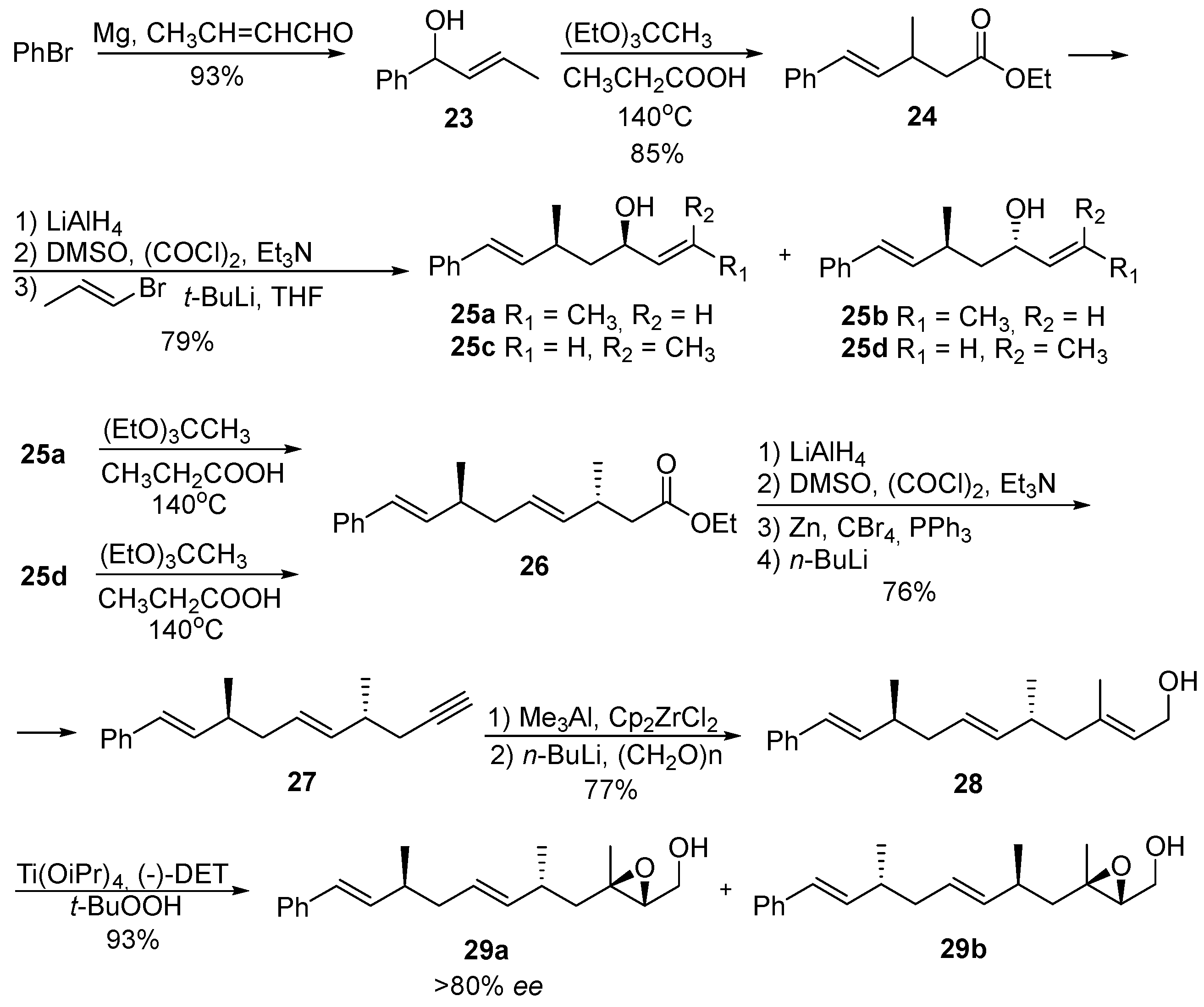
8. Acyclic Peroxides
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patnaik, P. Peroxides, organic. In A Comprehensive Guide to the Hazardous Properties of Chemical Substances; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 719–740. [Google Scholar]
- Liebman, J.F.; Greer, A. The Chemistry of Peroxides; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 3. [Google Scholar]
- Klapötke, T.M.; Wloka, T. Peroxide explosives. In Patai’s Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Denisov, E.T.; Denisova, T.G.; Pokidova, T.S. Diacyl peroxides, peroxy esters, polyatomic, and organometallic peroxides. In Handbook of Free Radical Initiators; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 129–282. [Google Scholar]
- Gaylord, N.G.; Mandal, B.M.; Martan, M. Peroxide-induced polymerization of norbornene. J. Polym. Sci. Polym. Lett. Ed. 1976, 14, 555–559. [Google Scholar] [CrossRef]
- Emami, S.H.; Salovey, R.; Hogen-Esch, T.E. Peroxide-mediated crosslinking of poly(ethylene oxide). J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3021–3026. [Google Scholar] [CrossRef]
- Russell, K.E. Free radical graft polymerization and copolymerization at higher temperatures. Prog. Polym. Sci. 2002, 27, 1007–1038. [Google Scholar] [CrossRef]
- Adam, W. Peroxide Chemistry: Mechanistic and Preparative Aspects of Oxygen Transfer; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Mishra, M.; Yagci, Y. Handbook of Vinyl Polymers: Radical Polymerization, Process, and Technology, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; p. 784. [Google Scholar]
- Ukuku, D.O.; Bari, L.; Kawamoto, S. Hydrogen peroxide. In Decontamination of Fresh and Minimally Processed Produce; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 197–214. [Google Scholar]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Chassot, A.L.C.; Poisl, M.I.P.; Samuel, S.M.W. In vivo and in vitro evaluation of the efficacy of a peracetic acid-based disinfectant for decontamination of acrylic resins. Braz. Dent. J. 2006, 17, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Baldry, M.G.C.; French, M.S. Disinfection of sewage effluent with peracetic acid. Water Sci. Technol. 1989, 21, 203–206. [Google Scholar]
- Alvaro, J.E.; Moreno, S.; Dianez, F.; Santos, M.; Carrasco, G.; Urrestarazu, M. Effects of peracetic acid disinfectant on the postharvest of some fresh vegetables. J. Food Eng. 2009, 95, 11–15. [Google Scholar] [CrossRef]
- McDonnell, G. The use of hydrogen peroxide for disinfection and sterilization applications. In Patai’s Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Omidbakhsh, N. A new peroxide-based flexible endoscope-compatible high-level disinfectant. Am. J. Infect. Control 2006, 34, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Pehkonen, S.O. Peracids in water treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1–39. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, Y.; Zhang, A. Discovery of antimalarial drug artemisinin and beyond. In Case Studies in Modern Drug Discovery and Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 227–256. [Google Scholar]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Hien, T.T.; Nosten, F.H. A brief history of qinghaosu. Trends Parasitol. 2015, 31, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Feng, Q.; Jacob, M.R.; Avula, B.; Mask, M.M.; Baerson, S.R.; Tripathi, S.K.; Mohammed, R.; Hamann, M.T.; Khan, I.A.; et al. The marine sponge-derived polyketide endoperoxide plakortide f acid mediates its antifungal activity by interfering with calcium homeostasis. Antimicrob. Agents Chemother. 2011, 55, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.Y.; Ni, M.Y.; Zhong, Y.R.; Li, L.N.; Cui, S.L.; Zhang, M.Q.; Wang, X.Z.; Liang, X.T. Studies on the constituents of artemisia annua l. (author’s transl). Yao Xue Xue Bao 1981, 16, 366–370. [Google Scholar] [PubMed]
- Miller, L.H.; Su, X. Artemisinin: Discovery from the Chinese herbal garden. Cell 2011, 146, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Dechy-Cabaret, O.; Cazelles, J.; Meunier, B. From mechanistic studies on artemisinin derivatives to new modular antimalarial drugs. Acc. Chem. Res. 2002, 35, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.H. Drug discovery for malaria: A very challenging and timely endeavor. Curr. Opin. Chem. Biol. 2007, 11, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Capela, R.; Oliveira, R.; Gonçalves, L.M.; Domingos, A.; Gut, J.; Rosenthal, P.J.; Lopes, F.; Moreira, R. Artemisinin-dipeptidyl vinyl sulfone hybrid molecules: Design, synthesis and preliminary sar for antiplasmodial activity and falcipain-2 inhibition. Bioorg. Med. Chem. Lett. 2009, 19, 3229–3232. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Mercer, A.E.; Stocks, P.A.; La Pensée, L.J.I.; Cosstick, R.; Park, B.K.; Kennedy, M.E.; Piantanida, I.; Ward, S.A.; Davies, J.; et al. Antitumour and antimalarial activity of artemisinin–acridine hybrids. Bioorg. Med. Chem. Lett. 2009, 19, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Qinghaosu (artemisinin): The price of success. Science 2008, 320, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Zhang, S.-Q.; Kandhari, S.P.; Mukherjee, P.; Chittiboyina, A.; Avery, M.A.; Avery, B.A. In vitro erythrocytic uptake studies of artemisinin and selected derivatives using lc–ms and 2d-qsar analysis of uptake in parasitized erythrocytes. Bioorg. Med. Chem. 2009, 17, 5325–5331. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Wittlin, S.; Rottmann, M.; Wenzler, T.; Kaiser, M.; Brun, R. Antiparasitic agents: New drugs on the horizon. Curr. Opin. Pharmacol. 2012, 12, 562–566. [Google Scholar] [CrossRef] [PubMed]
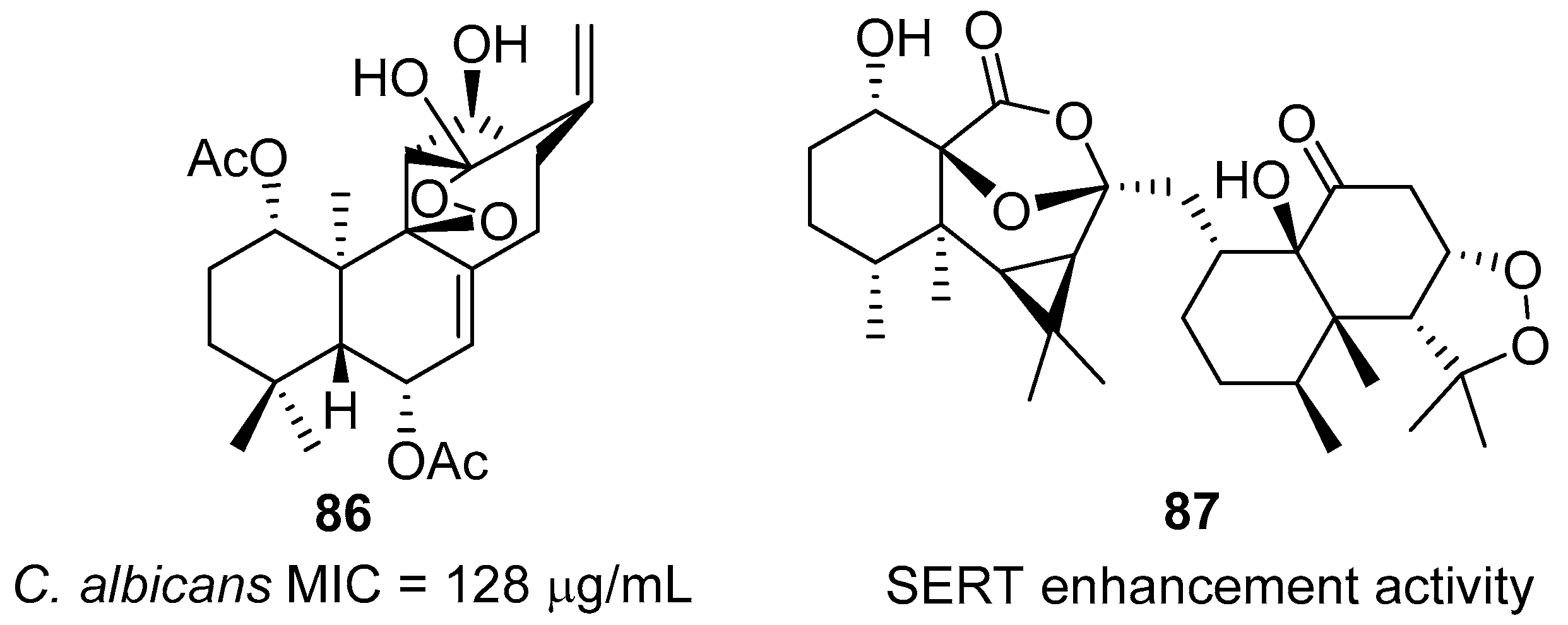
- Dembitsky, V.M. Bioactive peroxides as potential therapeutic agents. Eur. J. Med. Chem. 2008, 43, 223–251. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Ebel, R.; Wray, V.; Müller, W.E.G.; Edrada-Ebel, R.; Proksch, P. Diacarperoxides, norterpene cyclic peroxides from the sponge diacarnus megaspinorhabdosa. J. Nat. Prod. 2008, 71, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Liu, J.-K. Peroxy natural products. Nat. Prod. Bioprospect. 2013, 3, 161–206. [Google Scholar] [CrossRef]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus talaromyces flavus isolated from the mangrove plant sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R.; Jefford, C.W.; Posner, G.H.; Avery, M.A.; Peters, W. Second-generation antimalarial endoperoxides. Parasitol. Today 1996, 12, 79–82. [Google Scholar] [CrossRef]
- Ploypradith, P. Development of artemisinin and its structurally simplified trioxane derivatives as antimalarial drugs. Acta Trop. 2004, 89, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Dong, Y.; Vennerstrom, J.L. Synthetic peroxides as antimalarials. Med. Res. Rev. 2004, 24, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Jefford, C.W. New developments in synthetic peroxidic drugs as artemisinin mimics. Drug Discov. Today 2007, 12, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Opsenica, D.M.; Šolaja, B.A. Antimalarial peroxides. J. Serb. Chem. Soc. 2009, 74, 1155–1193. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Natural peroxy anticancer agents. Mini Rev. Med. Chem. 2007, 7, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kim, H.; Lee, K.; Park, M. Naturally occurring peroxides with biological activities. Mini Rev. Med. Chem. 2003, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, D.; Goswami, A.; Pratim Saikia, P.; Barua, N.C.; Rao, P.G. Artemisinin and its derivatives: A novel class of anti-malarial and anti-cancer agents. Chem. Soc. Rev. 2010, 39, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Artemisinin, promising lead natural product for various drug developments. Mini Rev. Med. Chem. 2007, 7, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Pandey-Rai, S. Updates on artemisinin: An insight to mode of actions and strategies for enhanced global production. Protoplasma 2016, 253, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, F.; Wang, H. Immunomodulation of artemisinin and its derivatives. Sci. Bull. 2016, 61, 1399–1406. [Google Scholar] [CrossRef]
- Utzinger, J.; Shuhua, X.; N’Goran, E.K.; Bergquist, R.; Tanner, M. The potential of artemether for the control of schistosomiasis. Int. J. Parasitol. 2001, 31, 1549–1562. [Google Scholar] [CrossRef]
- Utzinger, J.; Xiao, S.; Keiser, J.; Chen, M.; Zheng, J.; Tanner, M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr. Med. Chem. 2001, 8, 1841–1860. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J. Food-borne trematodiasis: Current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol. 2007, 23, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.G.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, K.M.; Avery, M.A. Progress in the development of peroxide-based anti-parasitic agents. Drug Discov. Today 2009, 14, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Panic, G.; Duthaler, U.; Speich, B.; Keiser, J. Repurposing drugs for the treatment and control of helminth infections. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.-P.; Chen, K.-P. Anti-hbv agents derived from botanical origin. Fitoterapia 2013, 84, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International union of pharmacology committee on receptor nomenclature and drug classification. Xxxviii. Update on terms and symbols in quantitative pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate ec50/ic50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ann Casteel, D. Peroxy natural products. Nat. Prod. Rep. 1999, 16, 55–73. [Google Scholar] [CrossRef]
- Phillipson, D.W.; Rinehart, K.L. Antifungal peroxide-containing acids from two caribbean sponges. J. Am. Chem. Soc. 1983, 105, 7735–7736. [Google Scholar] [CrossRef]
- Chen, Y.; Killday, K.B.; McCarthy, P.J.; Schimoler, R.; Chilson, K.; Selitrennikoff, C.; Pomponi, S.A.; Wright, A.E. Three new peroxides from the sponge plakinastrella species. J. Nat. Prod. 2001, 64, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McCarthy, P.J.; Harmody, D.K.; Schimoler-O’Rourke, R.; Chilson, K.; Selitrennikoff, C.; Pomponi, S.A.; Wright, A.E. New bioactive peroxides from marine sponges of the family plakiniidae. J. Nat. Prod. 2002, 65, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Oli, S.; Abdelmohsen, U.R.; Hentschel, U.; Schirmeister, T. Identification of plakortide e from the caribbean sponge plakortis halichondroides as a trypanocidal protease inhibitor using bioactivity-guided fractionation. Mar. Drugs 2014, 12, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Bloodworth, A.J.; Bothwell, B.D.; Collins, A.N.; Maidwell, N.L. A short synthesis of naturally occurring and other analogues of plakinic acids that contain the 1,2-dioxolane group. Tetrahedron Lett. 1996, 37, 1885–1888. [Google Scholar] [CrossRef]
- Dussault, P.H.; Liu, X. Lewis acid-mediated displacements of alkoxydioxolanes: Synthesis of a 1,2-dioxolane natural product. Org. Lett. 1999, 1, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Trullinger, T.K.; Liu, X.; Dussault, P.H. Asymmetric synthesis of 1,2-dioxolane-3-acetic acids: Synthesis and configurational assignment of plakinic acid A. J. Org. Chem. 2006, 71, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Tian, X.-Y.; Li, Z.-W.; Peng, X.-S.; Wong, H.N.C. Total synthesis of plakortide e and biomimetic synthesis of plakortone B. Chemistry 2011, 17, 5874–5880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wong, H.N.C. Synthetic studies toward plakortide E: Application of the feldman oxygenation to synthesis of highly substituted 1,2-dioxolanes. Tetrahedron 2007, 63, 6296–6305. [Google Scholar] [CrossRef]
- Tian, X.-Y.; Han, J.-W.; Zhao, Q.; Wong, H.N.C. Asymmetric synthesis of 3,3,5,5-tetrasubstituted 1,2-dioxolanes: Total synthesis of epiplakinic acid f. Org. Biomol. Chem. 2014, 12, 3686–3700. [Google Scholar] [CrossRef] [PubMed]
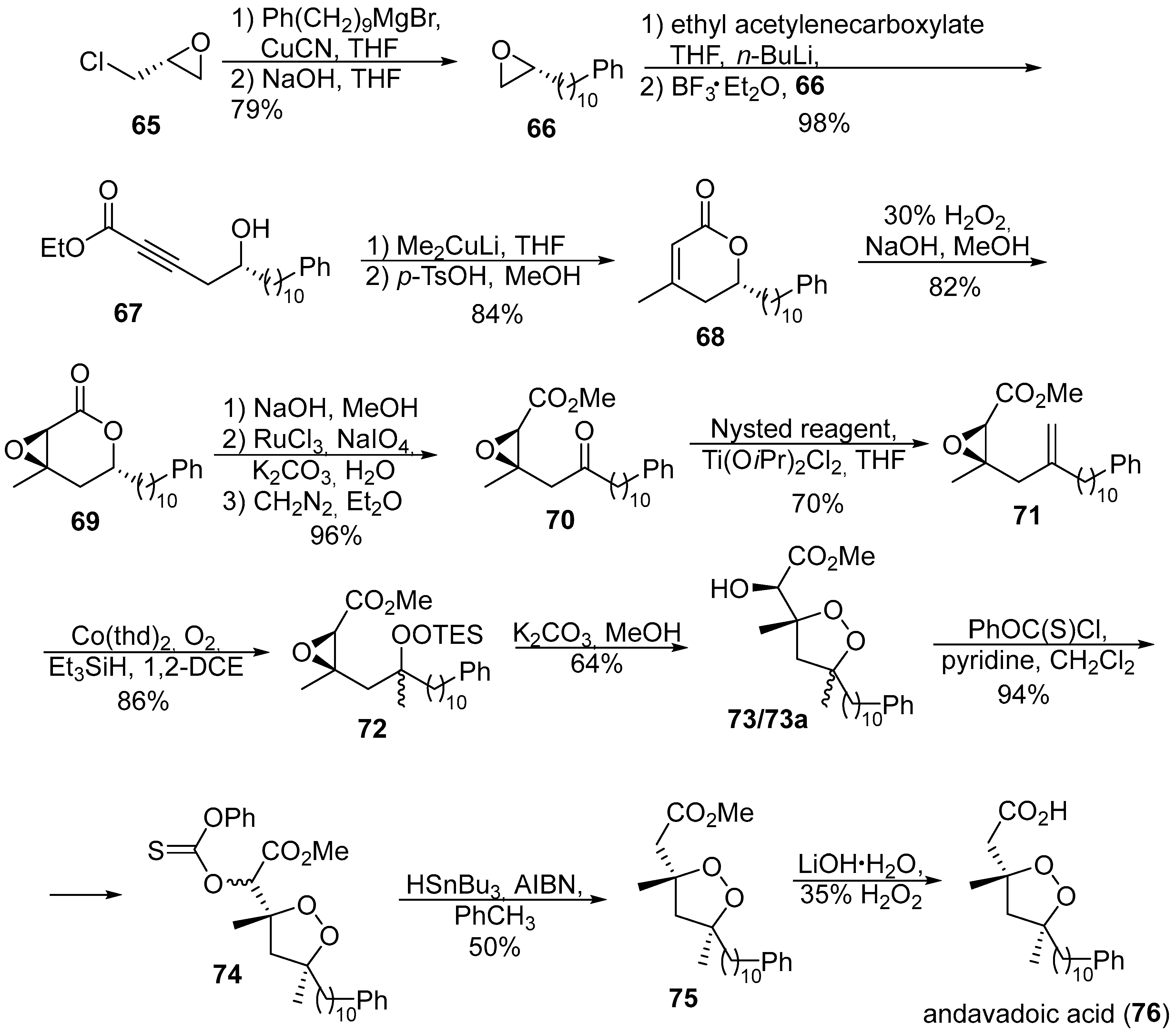
- Barnych, B.; Fenet, B.; Vatèle, J.-M. Asymmetric synthesis of andavadoic acid via base-catalyzed 5-exo-tet cyclization of a β-hydroperoxy epoxide. Tetrahedron 2013, 69, 334–340. [Google Scholar] [CrossRef]
- Scott, J.J.; Oh, D.-C.; Yuceer, M.C.; Klepzig, K.D.; Clardy, J.; Currie, C.R. Bacterial protection of beetle-fungus mutualism. Science 2008, 322, 63. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-C.; Scott, J.J.; Currie, C.R.; Clardy, J. Mycangimycin, a polyene peroxide from a mutualist streptomyces sp. Org. Lett. 2009, 11, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Ferrié, L.; Figadère, B. Synthesis of 3,5-disubstituted-1,2-dioxolanes: Access to analogues of mycangimycin and some rearrangement products. Tetrahedron Lett. 2016, 57, 5286–5289. [Google Scholar] [CrossRef]
- Qu, J.-B.; Zhu, R.-L.; Zhang, Y.-L.; Guo, H.-F.; Wang, X.-N.; Xie, C.-F.; Yu, W.-T.; Ji, M.; Lou, H.-X. Ent-kaurane diterpenoids from the liverwort jungermannia atrobrunnea. J. Nat. Prod. 2008, 71, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-H.; Chen, Y.-P.; Ying, S.-S.; Zhang, P.; Xu, Y.-T.; Gao, X.-M.; Zhu, Y. Dinardokanshones A and B, two unique sesquiterpene dimers from the roots and rhizomes of nardostachys chinensis. Tetrahedron Lett. 2015, 56, 5851–5854. [Google Scholar] [CrossRef]
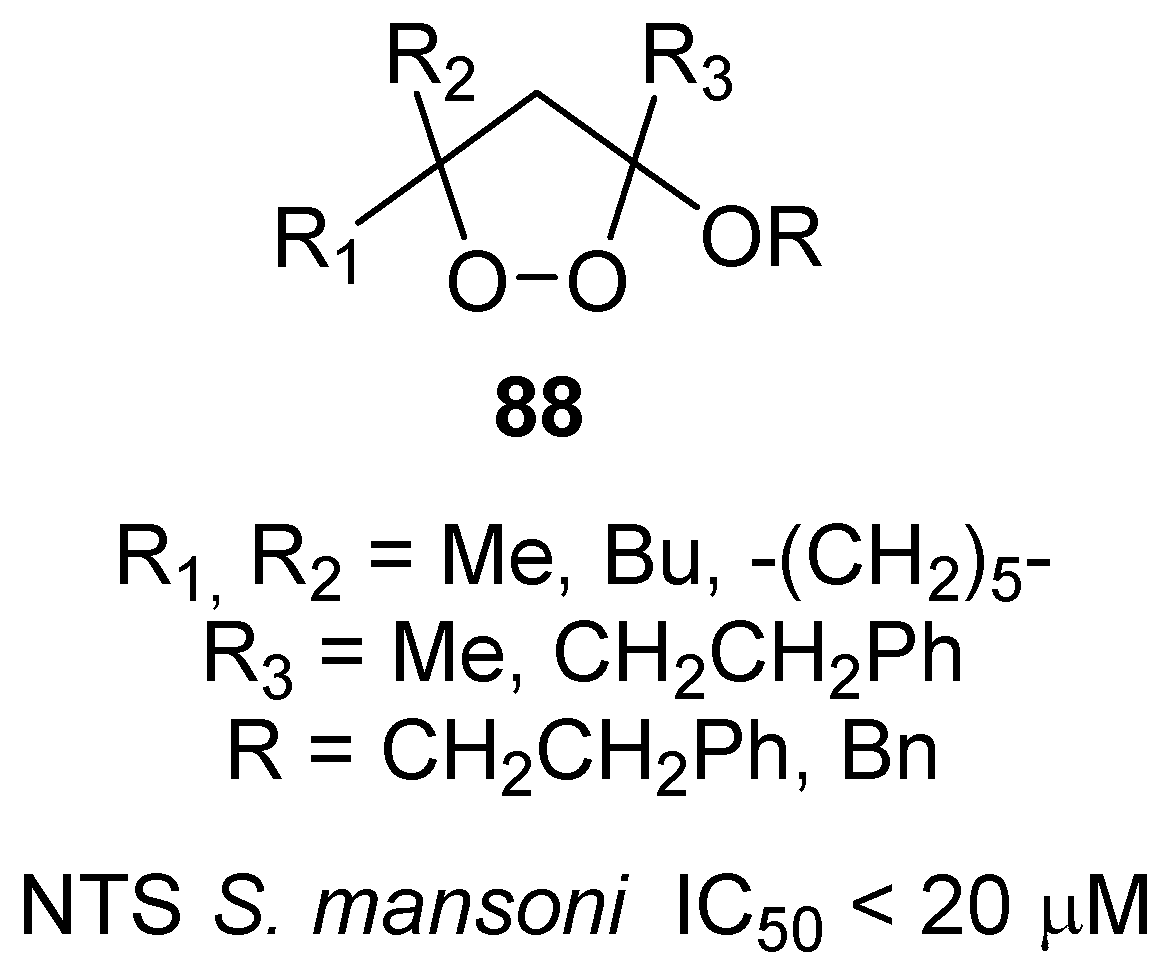
- Ingram, K.; Schiaffo, C.E.; Sittiwong, W.; Benner, E.; Dussault, P.H.; Keiser, J. In vitro and in vivo activity of 3-alkoxy-1,2-dioxolanes against schistosoma mansoni. J. Antimicrob. Chemother. 2012, 67, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Schiaffo, C.E.; Rottman, M.; Wittlin, S.; Dussault, P.H. 3-Alkoxy-1,2-dioxolanes: Synthesis and evaluation as potential antimalarial agents. ACS Med. Chem. Lett. 2011, 2, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Ingram, K.; Yaremenko, I.A.; Krylov, I.B.; Hofer, L.; Terent’ev, A.O.; Keiser, J. Identification of antischistosomal leads by evaluating bridged 1,2,4,5-tetraoxanes, alphaperoxides, and tricyclic monoperoxides. J. Med. Chem. 2012, 55, 8700–8711. [Google Scholar] [CrossRef] [PubMed]
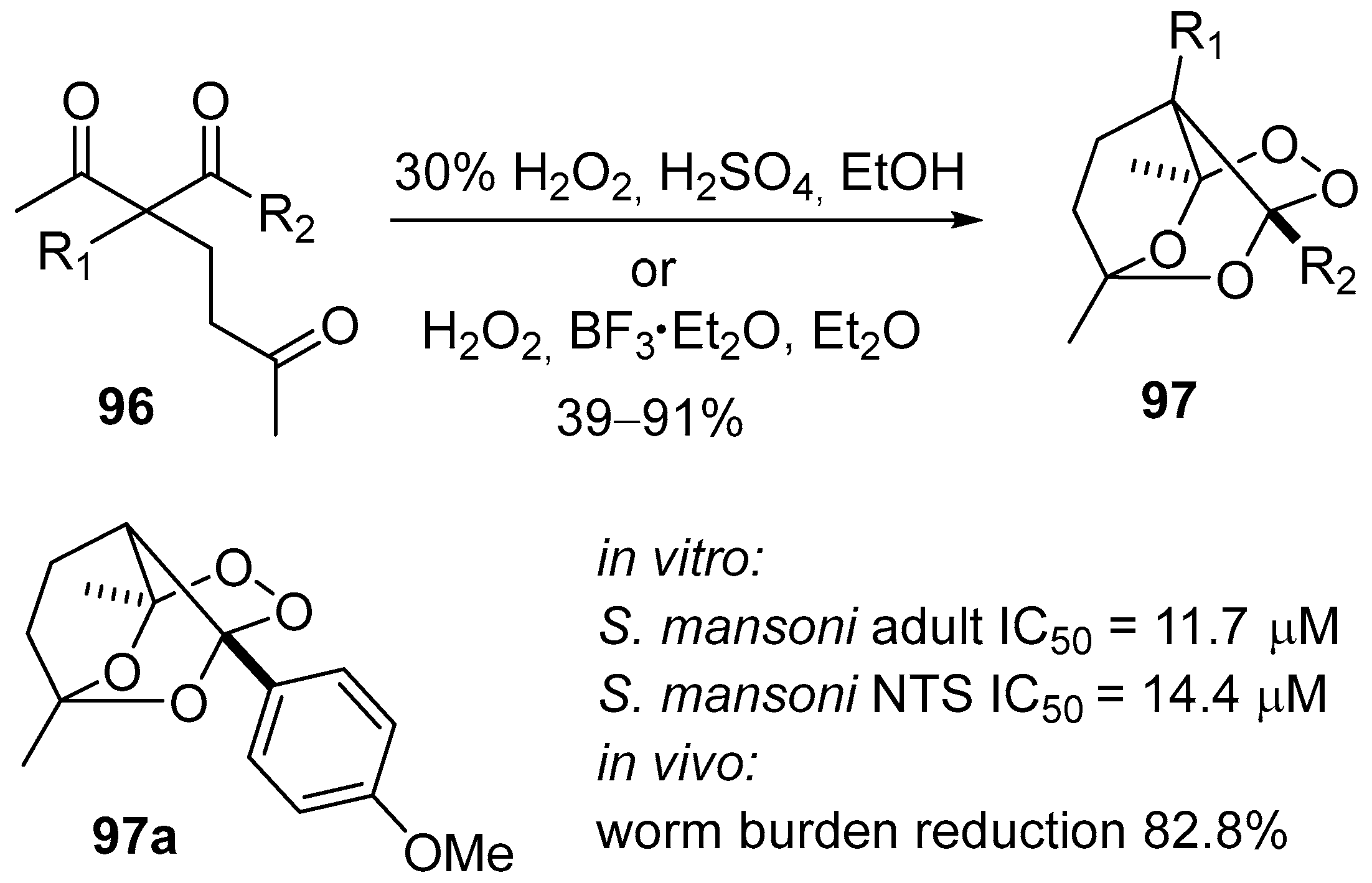
- Terent’ev, A.O.; Yaremenko, I.A.; Chernyshev, V.V.; Dembitsky, V.M.; Nikishin, G.I. Selective synthesis of cyclic peroxides from triketones and H2O2. J. Org. Chem. 2012, 77, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Yaremenko, I.A.; Vil’, V.A.; Dembitsky, V.M.; Nikishin, G.I. Boron trifluoride as an efficient catalyst for the selective synthesis of tricyclic monoperoxides from β,δ-triketones and H2O2. Synthesis 2013, 45, 246–250. [Google Scholar] [CrossRef]
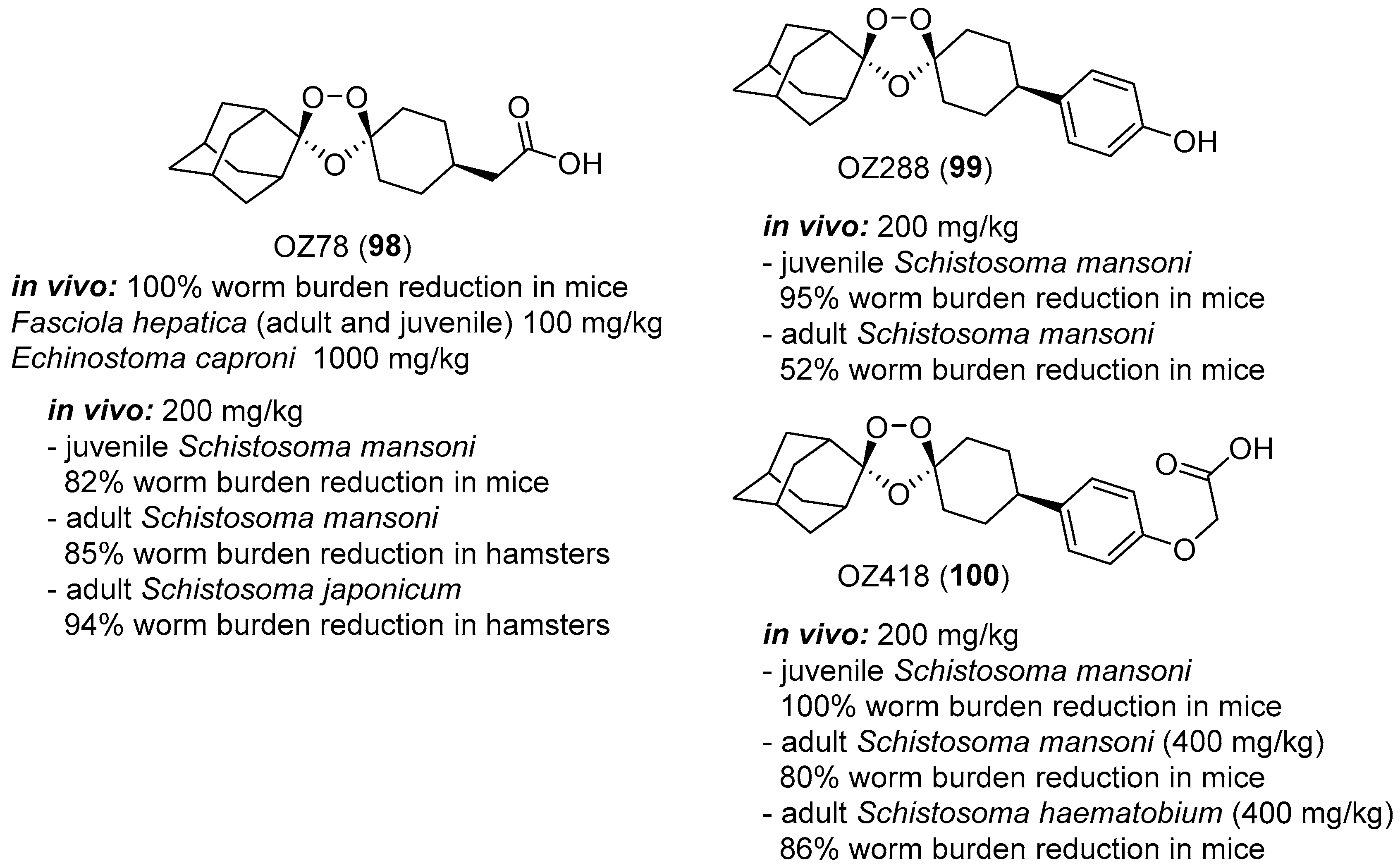
- Keiser, J.; Utzinger, J.; Tanner, M.; Dong, Y.; Vennerstrom, J.L. The synthetic peroxide OZ78 is effective against echinostoma caproni and fasciola hepatica. J. Antimicrob. Chemother. 2006, 58, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J.; Vennerstrom, J.L.; Dong, Y.; Brennan, G.; Fairweather, I. Activity of artemether and OZ78 against triclabendazole-resistant fasciola hepatica. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Kirchhofer, C.; Haschke, M.; Huwyler, J.; Dong, Y.; Vennerstrom, J.L.; Vanhoff, K.; Kaminsky, R.; Malikides, N. Efficacy, safety and pharmacokinetics of 1,2,4-trioxolane OZ78 against an experimental infection with fasciola hepatica in sheep. Vet. Parasitol. 2010, 173, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Meister, I.; Duthaler, U.; Huwyler, J.; Rinaldi, L.; Bosco, A.; Cringoli, G.; Keiser, J. Efficacy and pharmacokinetics of OZ78 and MT04 against a natural infection with fasciola hepatica in sheep. Vet. Parasitol. 2013, 198, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Vargas, M.; Dong, Y.; Zhou, L.; Wang, X.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. Structure-activity relationship of an ozonide carboxylic acid (OZ78) against fasciola hepatica. J. Med. Chem. 2010, 53, 4223–4233. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-H.; Keiser, J.; Chollet, J.; Utzinger, J.; Dong, Y.; Endriss, Y.; Vennerstrom, J.L.; Tanner, M. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 2007, 51, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-H.; Mei, J.-Y.; Jiao, P.-Y. Schistosoma japonicum-infected hamsters (mesocricetus auratus) used as a model in experimental chemotherapy with praziquantel, artemether, and OZ compounds. Parasitol. Res. 2011, 108, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-H.; Xue, J.; Mei, J.-Y.; Jiao, P.-Y. Effectiveness of synthetic trioxolane OZ78 against schistosoma japonicum in mice and rabbits. Parasitol. Res. 2012, 110, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Ingram, K.; Vargas, M.; Chollet, J.; Wang, X.; Dong, Y.; Vennerstrom, J.L. In vivo activity of aryl ozonides against schistosoma species. Antimicrob. Agents Chemother. 2012, 56, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Griesbaum, K.; Övez, B.; Huh, T.S.; Dong, Y. Ozonolyses of o-methyloximes in the presence of acid derivatives: A new access to substituted ozonides. Liebigs Ann. 1995, 1995, 1571–1574. [Google Scholar] [CrossRef]
- Griesbaum, K.; Liu, X.; Kassiaris, A.; Scherer, M. Ozonolyses of o-alkylated ketoximes in the presence of carbonyl groups: A facile access to ozonides. Liebigs Ann. 1997, 1997, 1381–1390. [Google Scholar] [CrossRef]
- Tang, Y.; Dong, Y.; Karle, J.M.; DiTusa, C.A.; Vennerstrom, J.L. Synthesis of tetrasubstituted ozonides by the griesbaum coozonolysis reaction: Diastereoselectivity and functional group transformations by post-ozonolysis reactions. J. Org. Chem. 2004, 69, 6470–6473. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chollet, J.; Matile, H.; Charman, S.A.; Chiu, F.C.K.; Charman, W.N.; Scorneaux, B.; Urwyler, H.; Santo Tomas, J.; Scheurer, C.; et al. Spiro and dispiro-1,2,4-trioxolanes as antimalarial peroxides: Charting a workable structure-activity relationship using simple prototypes. J. Med. Chem. 2005, 48, 4953–4961. [Google Scholar] [CrossRef] [PubMed]
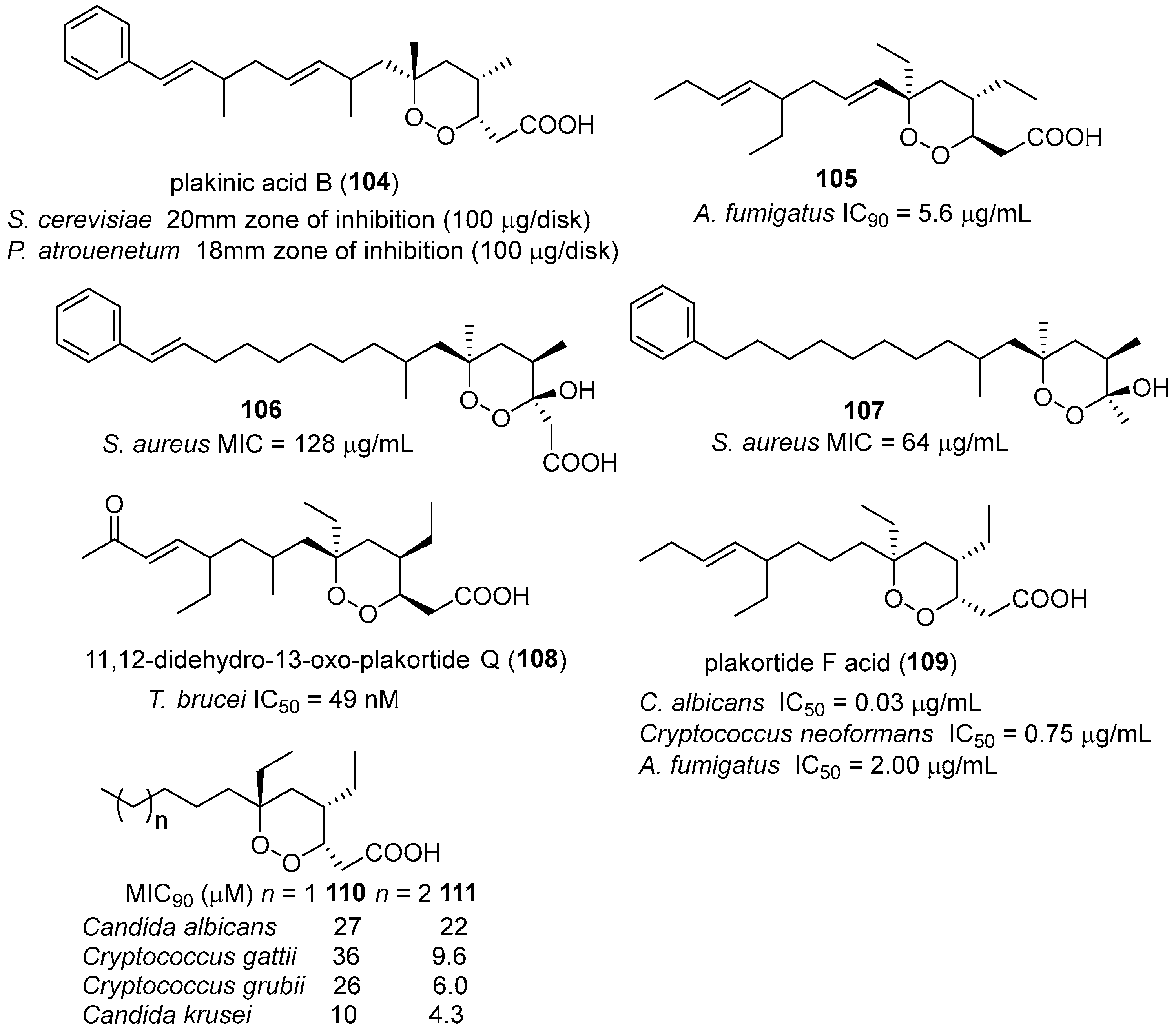
- Manzo, E.; Ciavatta, M.L.; Melck, D.; Schupp, P.; de Voogd, N.; Gavagnin, M. Aromatic cyclic peroxides and related keto-compounds from the plakortis sp. Component of a sponge consortium. J. Nat. Prod. 2009, 72, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Davis, R.A.; Sykes, M.; Avery, V.M.; Camp, D.; Quinn, R.J. Antitrypanosomal cyclic polyketide peroxides from the australian marine sponge plakortis sp. J. Nat. Prod. 2010, 73, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Jamison, M.T.; Dalisay, D.S.; Molinski, T.F. Peroxide natural products from plakortis zyggompha and the sponge association plakortis halichondrioides–xestospongia deweerdtae: Antifungal activity against cryptococcus gattii. J. Nat. Prod. 2016, 79, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kondo, K.; Kitagawa, I. Antifungal peroxyketal acids from an okinawan marine sponge of plakortis sp. Chem. Pharm. Bull. 1993, 41, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
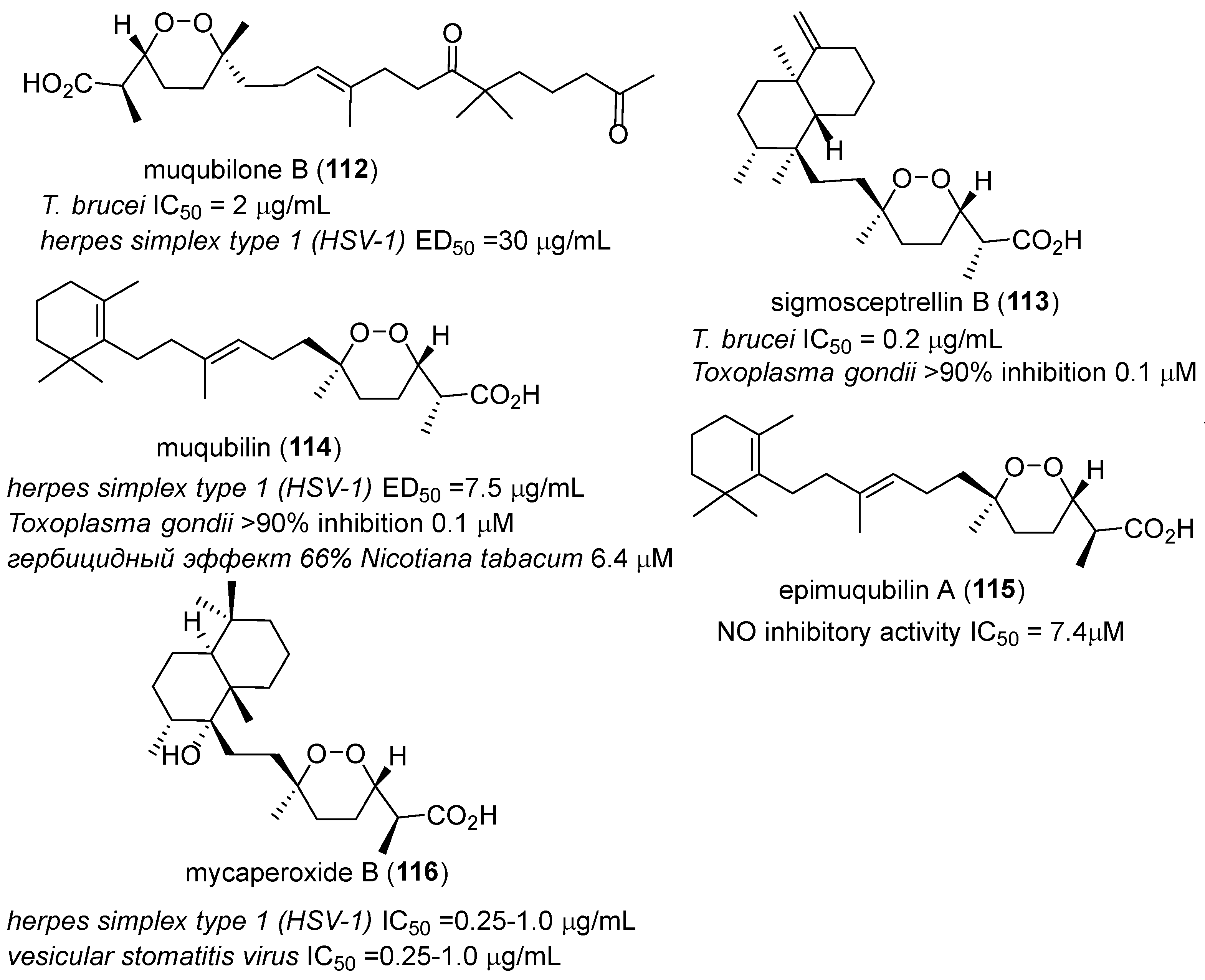
- Rubio, B.K.; Tenney, K.; Ang, K.-H.; Abdulla, M.; Arkin, M.; McKerrow, J.H.; Crews, P. The marine sponge diacarnus bismarckensis as a source of peroxiterpene inhibitors of trypanosoma brucei, the causative agent of sleeping sickness. J. Nat. Prod. 2009, 72, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Kashman, Y.; Rotem, M. Muqubilin, a new c24-isoprenoid from a marine sponge. Tetrahedron Lett. 1979, 20, 1707–1708. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Hamann, M.T.; Hashish, N.E.; Shier, W.T.; Kelly, M.; Khan, A.A. Antimalarial, antiviral, and antitoxoplasmosis norsesterterpene peroxide acids from the red sea sponge diacarnus erythraeanus. J. Nat. Prod. 2001, 64, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Shen, X.; El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Bobzin, S.; Huesing, J.; Camp, R.; et al. Marine natural products as prototype agrochemical agents. J. Agric. Food Chem. 2003, 51, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Cheenpracha, S.; Park, E.-J.; Rostama, B.; Pezzuto, J.M.; Chang, L.C. Inhibition of nitric oxide (no) production in lipopolysaccharide (lps)-activated murine macrophage raw 264.7 cells by the norsesterterpene peroxide, epimuqubilin A. Mar. Drugs 2010, 8, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Higa, T.; Suwanborirux, K.; Kokpol, U.; Bernardinelli, G.; Jefford, C.W. Bioactive norsesterterpene 1,2-dioxanes from a thai sponge, mycale sp. J. Org. Chem. 1993, 58, 2999–3002. [Google Scholar] [CrossRef]
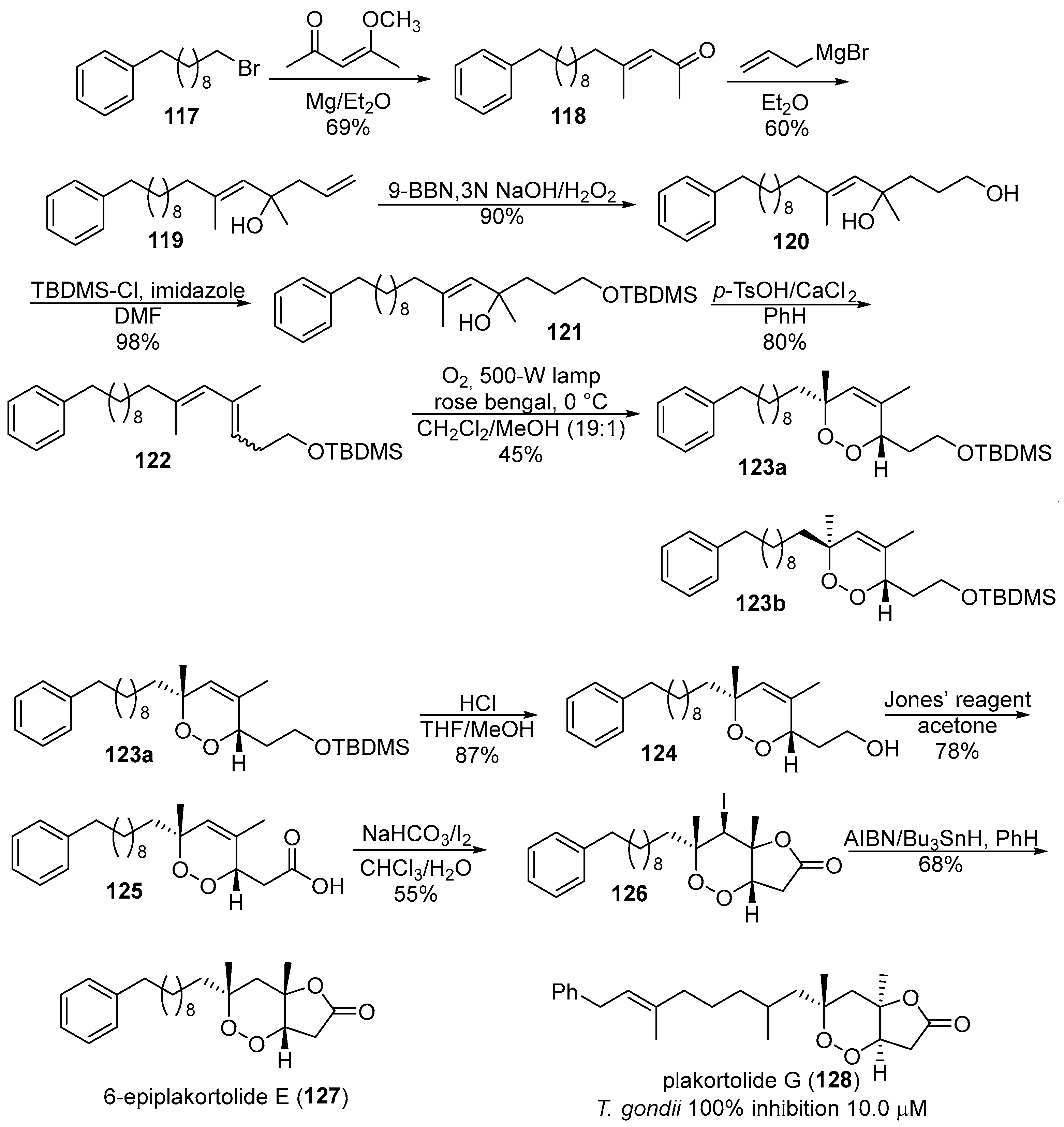
- Jung, M.; Ham, J.; Song, J. First total synthesis of natural 6-epiplakortolide e. Org. Lett. 2002, 4, 2763–2765. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.L.; Dickerson, A.; Khan, A.A.; Kondru, R.K.; Beratan, D.N.; Wipf, P.; Kelly, M.; Hamann, M.T. New peroxylactones from the Jamaican sponge plakinastrella onkodes, with inhibitory activity against the aids opportunistic parasitic infection toxoplasma gondii. Tetrahedron 2001, 57, 1483–1487. [Google Scholar] [CrossRef]
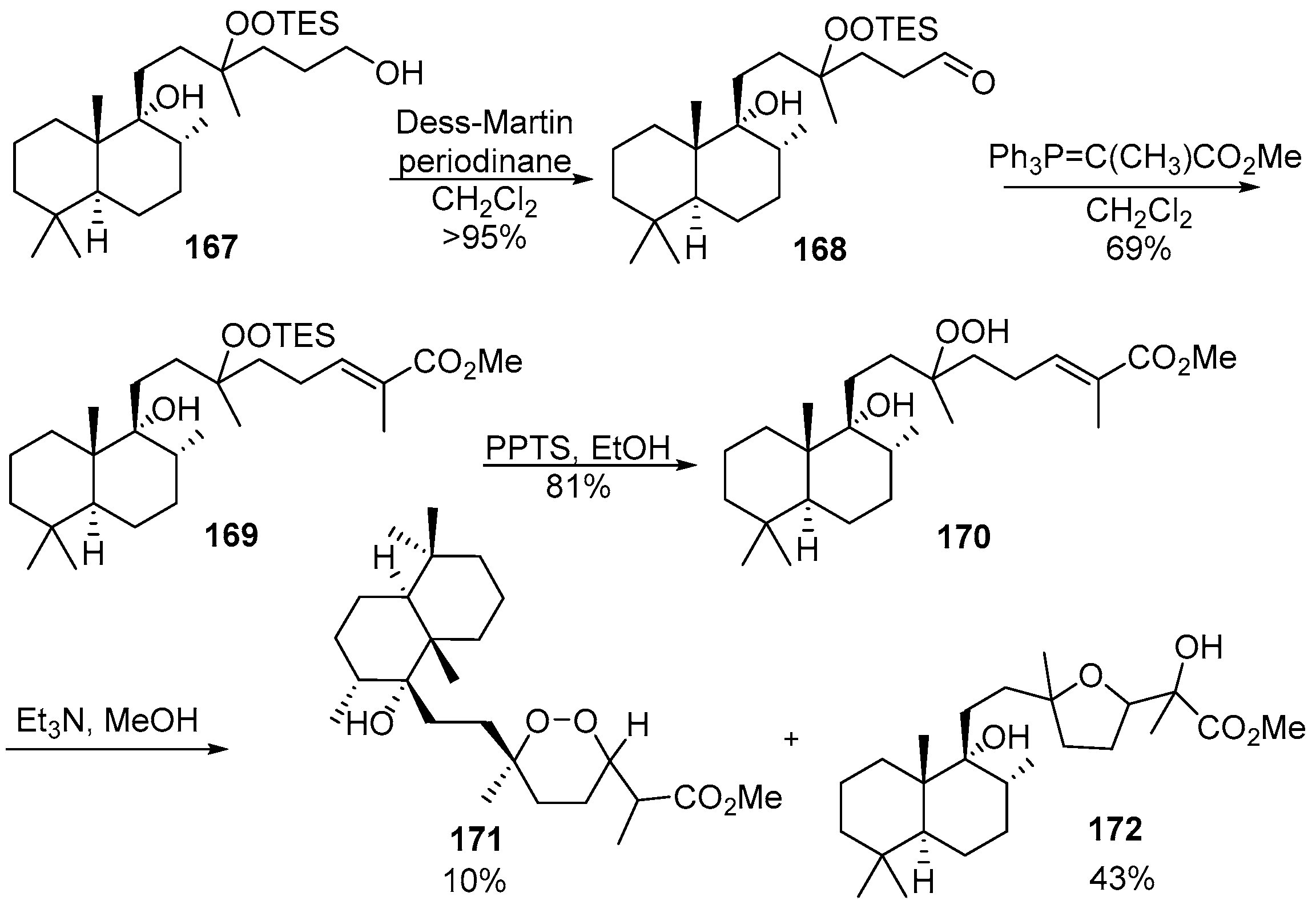
- Xu, C.; Schwartz, C.; Raible, J.; Dussault, P.H. Asymmetric synthesis of 1,2-dioxanes: Approaches to the peroxyplakoric acids. Tetrahedron 2009, 65, 9680–9685. [Google Scholar] [CrossRef] [PubMed]
- Gemma, S.; Gabellieri, E.; Sanna Coccone, S.; Martí, F.; Taglialatela-Scafati, O.; Novellino, E.; Campiani, G.; Butini, S. Synthesis of dihydroplakortin, 6-epi-dihydroplakortin, and their c10-desethyl analogues. J. Org. Chem. 2010, 75, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
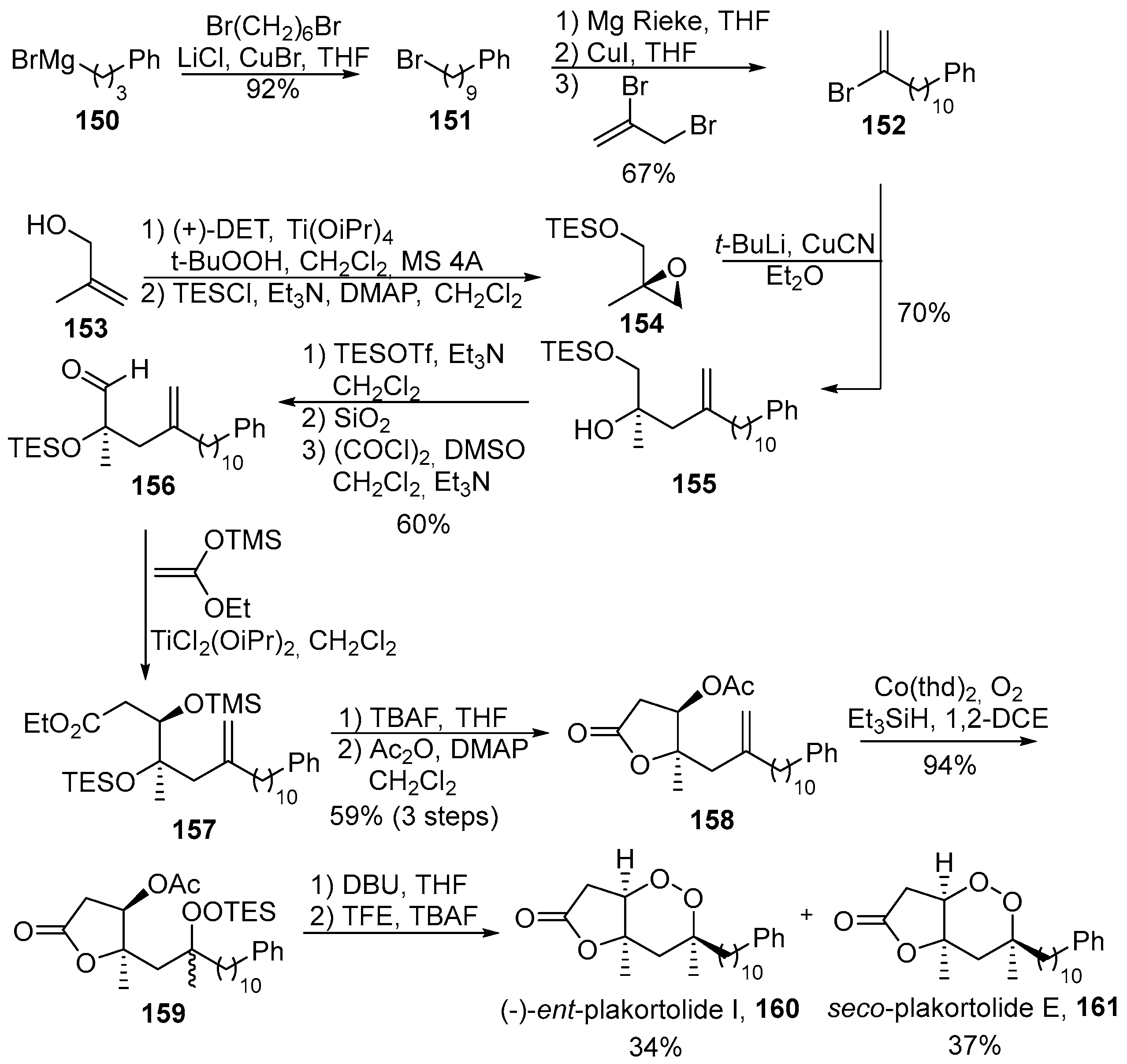
- Barnych, B.; Vatèle, J.-M. Total synthesis of seco-plakortolide E and (−)-ent-plakortolide I: Absolute configurational revision of natural plakortolide I. Org. Lett. 2012, 14, 564–567. [Google Scholar] [CrossRef] [PubMed]
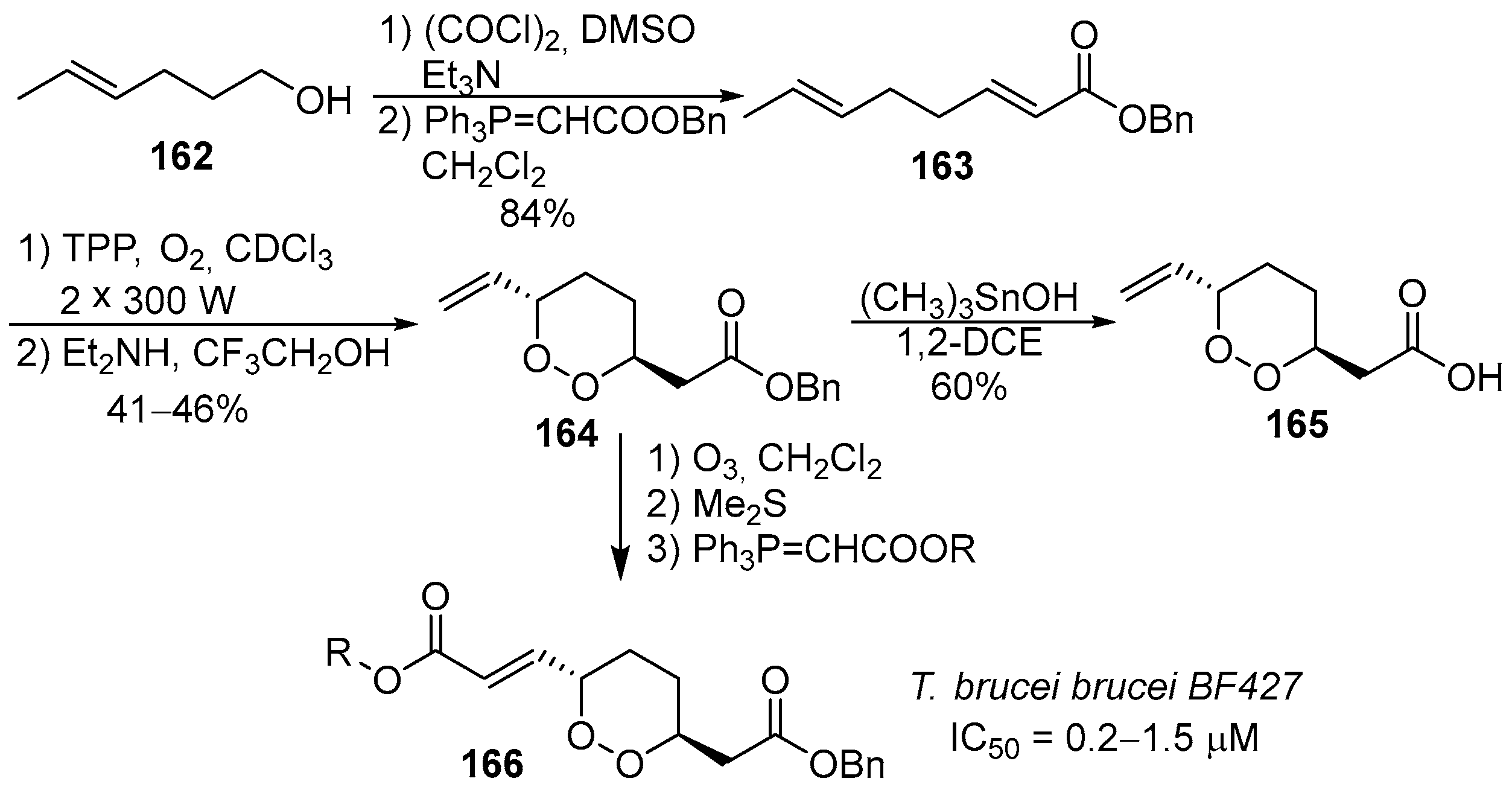
- Holla, H.; Labaied, M.; Pham, N.; Jenkins, I.D.; Stuart, K.; Quinn, R.J. Synthesis of antitrypanosomal 1,2-dioxane derivatives based on a natural product scaffold. Bioorg. Med. Chem. Lett. 2011, 21, 4793–4797. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.P.; Pye, R.J.; Brown, G.D.; Harwood, L.M. Towards the total synthesis of mycaperoxide b: Probing biosynthetic rationale. Eur. J. Org. Chem. 2012, 2012, 1209–1216. [Google Scholar] [CrossRef]
- Kiuchi, F.; Itano, Y.; Uchiyama, N.; Honda, G.; Tsubouchi, A.; Nakajima-Shimada, J.; Aoki, T. Monoterpene hydroperoxides with trypanocidal activity from chenopodium ambrosioides. J. Nat. Prod. 2002, 65, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Montalvo, A.M.; Almanonni, S.; Scull, R.; Miranda, M.; Abreu, J. Activity of the essential oil from Chenopodium ambrosioides grown in Cuba against Leishmania amazonensis. Chemotherapy 2006, 52, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Wallach, O. Zur kenntnis der terpene und der ätherischen öle. Justus Liebigs Ann. Chem. 1912, 392, 49–75. [Google Scholar] [CrossRef]
- Nelson, E.K. The composition of oil of chenopodium from various sources. J. Am. Chem. Soc. 1920, 42, 1204–1208. [Google Scholar] [CrossRef]
- Smillie, W.G.; Pessôa, S.B. A study of the anthelmintic properties of the constituents of the oil of chenopodium. J. Pharmacol. Exp. Ther. 1924, 24, 359–370. [Google Scholar]
- Bodendorf, K. Über ungesättigte peroxyde. Zugleich ein beitrag zur kenntnis der autoxydationsvorgänge. Arch. Pharm. 1933, 271, 1–35. [Google Scholar] [CrossRef]
- Beckett, A.H.; Donbrow, M.; Jolliffe, G.O. Ascaridole studies: Part iii. The purification and characterisation of ascaridole. J. Pharm. Pharmacol. 1955, 7, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Beckett, A.H.; Jolliffe, G.O. A note on the determination of ascaridole in oil of chenopodium. J. Pharm. Pharmacol. 1955, 7, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Schenck, G.O.; Ziegler, K. Die synthese des ascaridols. Naturwissenschaften 1944, 32, 157. [Google Scholar]
- Pape, M. Industrial applications of photochemistry. Pure Appl. Chem. 1975, 41, 535–558. [Google Scholar] [CrossRef]
- Aubry, J.-M.; Bouttemy, S. Preparative oxidation of organic compounds in microemulsions with singlet oxygen generated chemically by the sodium molybdate/hydrogen peroxide system1. J. Am. Chem. Soc. 1997, 119, 5286–5294. [Google Scholar] [CrossRef]
- Paré, P.W.; Zajicek, J.; Ferracini, V.L.; Melo, I.S. Antifungal terpenoids from chenopodium ambrosioides. Biochem. Syst. Ecol. 1993, 21, 649–653. [Google Scholar] [CrossRef]
- MacDonald, D.; VanCrey, K.; Harrison, P.; Rangachari, P.K.; Rosenfeld, J.; Warren, C.; Sorger, G. Ascaridole-less infusions of chenopodium ambrosioides contain a nematocide(s) that is(are) not toxic to mammalian smooth muscle. J. Ethnopharmacol. 2004, 92, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Gunasekera, M.; Gunawardana, G.P.; McCarthy, P.; Burres, N. Two new bioactive cyclic peroxides from the marine sponge plakortis angulospiculatus. J. Nat. Prod. 1990, 53, 669–674. [Google Scholar] [CrossRef]
- Yao, G.; Steliou, K. Synthetic studies toward bioactive cyclic peroxides from the marine sponge plakortis angulospiculatus. Org. Lett. 2002, 4, 485–488. [Google Scholar] [CrossRef] [PubMed]
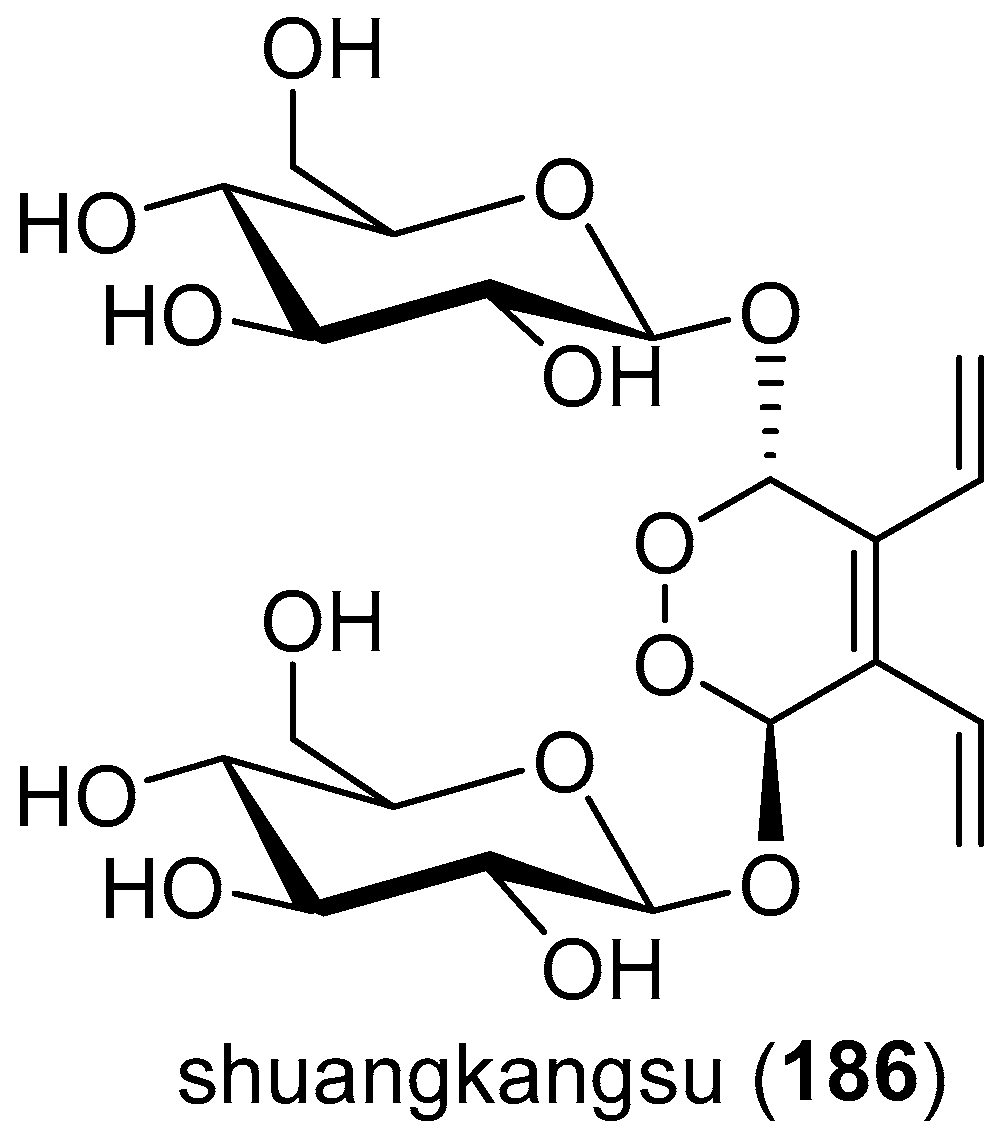
- Yu, D.-Q.; Chen, R.-Y.; Huang, L.-J.; Xie, F.-Z.; Ming, D.-S.; Zhou, K.; Li, H.-Y.; Tong, K.-M. The structure and absolute configuration of shuangkangsu: A novel natural cyclic peroxide from Lonicera japonica (Thunb.). J. Asian Nat. Prod. Res. 2008, 10, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S. HPLC and NMR studies of phenoxazone alkaloids from pycnoporus cinnabarinus. Nat. Prod. Commun. 2009, 4, 489–498. [Google Scholar] [PubMed]
- Wang, F.-W. Bioactive metabolites from Guignardia sp., an endophytic fungus residing in undaria pinnatifida. Chin. J. Nat. Med. 2012, 10, 72–76. [Google Scholar] [CrossRef] [PubMed]
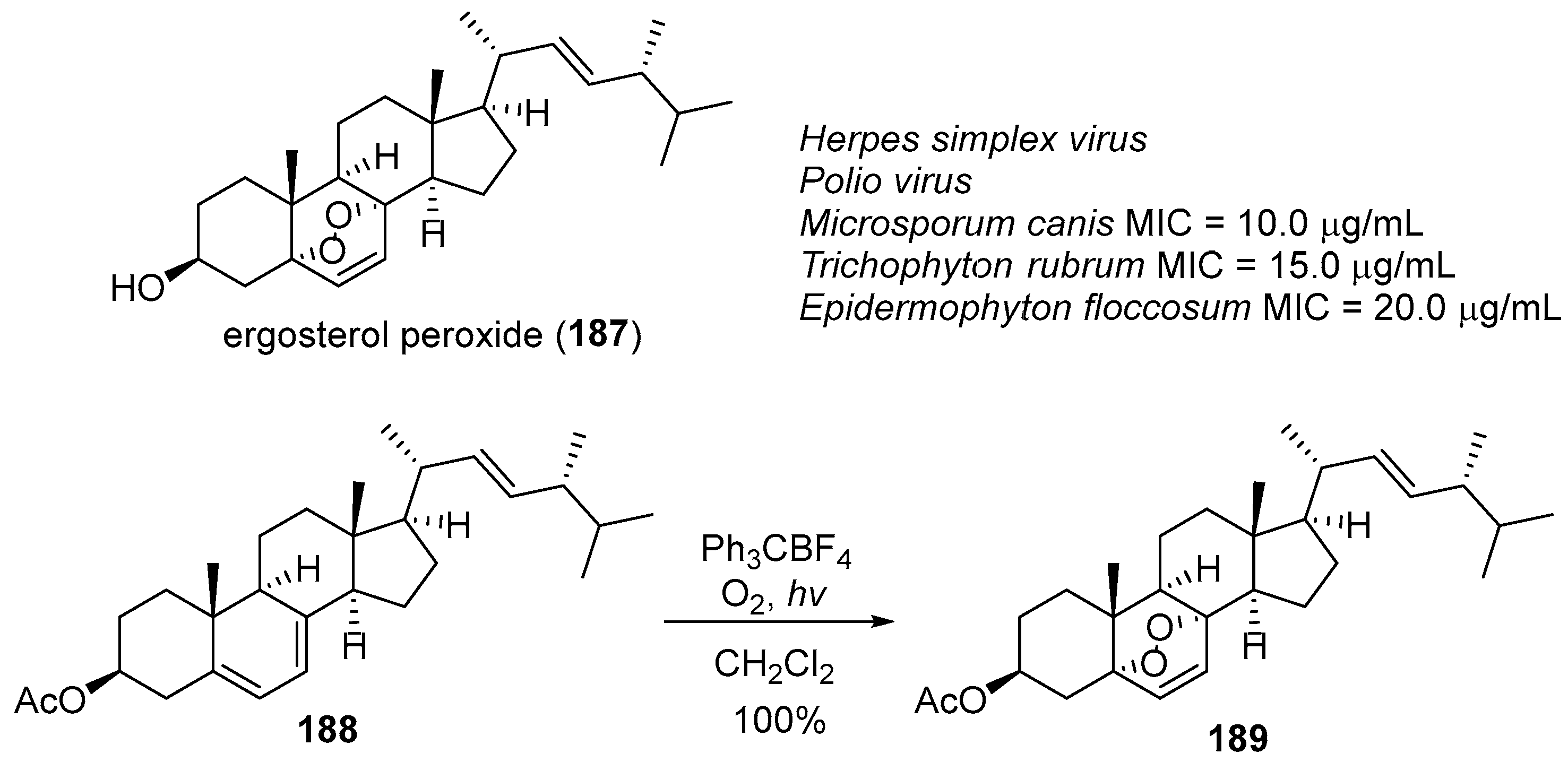
- Barton, D.H.R.; Leclerc, G.; Magnus, P.D.; Menzies, I.D. An unusual synthesis of ergosterol acetate peroxide. J. Chem. Soc. Chem. Commun. 1972, 447–449. [Google Scholar] [CrossRef]
- Jia, M.; Zhao, R.; Xu, B.; Yan, W.; Chu, F.; Gu, H.; Xie, T.; Xiang, H.; Ren, J.; Chen, D.; et al. Synthesis and biological activity evaluation of novel peroxo-bridged derivatives as potential anti-hepatitis b virus agents. MedChemComm 2017, 8, 148–151. [Google Scholar] [CrossRef]
- Macreadie, P.; Avery, T.; Greatrex, B.; Taylor, D.; Macreadie, I. Novel endoperoxides: Synthesis and activity against candida species. Bioorg. Med. Chem. Lett. 2006, 16, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Avery, T.D.; Macreadie, P.I.; Greatrex, B.W.; Robinson, T.V.; Taylor, D.K.; Macreadie, I.G. Design of endoperoxides with anti-candida activity. Bioorg. Med. Chem. 2007, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Macreadie, I.G.; Avery, T.D.; Robinson, T.V.; Macreadie, P.; Barraclough, M.; Taylor, D.K.; Tiekink, E.R.T. Design of 1,2-dioxines with anti-candida activity: Aromatic substituted 1,2-dioxines. Tetrahedron 2008, 64, 1225–1232. [Google Scholar] [CrossRef]
- Vil’, V.; Yaremenko, I.; Ilovaisky, A.; Terent’ev, A. Synthetic strategies for peroxide ring construction in artemisinin. Molecules 2017, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Kopetzki, D.; Lévesque, F.; Seeberger, P.H. A continuous-flow process for the synthesis of artemisinin. Chemistry 2013, 19, 5450–5456. [Google Scholar] [CrossRef] [PubMed]
- Vonwiller, S.C.; Warner, J.A.; Mann, S.T.; Haynes, R.K. Copper(ii) trifluoromethanesulfonate-induced cleavage oxygenation of allylic hydroperoxides derived from qinghao acid in the synthesis of qinghaosu derivatives: Evidence for the intermediacy of enols. J. Am. Chem. Soc. 1995, 117, 11098–11105. [Google Scholar] [CrossRef]
- Yang, D.M.; Liew, F.Y. Effects of qinghaosu (artemisinin) and its derivatives on experimental cutaneous leishmaniasis. Parasitology 1993, 106, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Bandyopadhyay, S.; Dutta, A.; Mandal, G.; Ganguly, S.; Saha, P.; Chatterjee, M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in leishmania donovani promastigotes. J. Med. Microbiol. 2007, 56, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Mishina, Y.V.; Krishna, S.; Haynes, R.K.; Meade, J.C. Artemisinins inhibit trypanosoma cruzi and trypanosoma brucei rhodesiense in vitro growth. Antimicrob. Agents Chemother. 2007, 51, 1852–1854. [Google Scholar] [CrossRef] [PubMed]
- Ke, O.Y.; Krug, E.C.; Marr, J.J.; Berens, R.L. Inhibition of growth of toxoplasma gondii by qinghaosu and derivatives. Antimicrob. Agents Chemother. 1990, 34, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Upadhyay, S.K.; Hassan, W.; Madan, T.; Sirdeshmukh, R.; Sundaram, C.S.; Gade, W.N.; Basir, S.F.; Singh, Y.; Sarma, P.U. Transcriptomic and proteomic profile of aspergillus fumigatus on exposure to artemisinin. Mycopathologia 2011, 172, 331. [Google Scholar] [CrossRef] [PubMed]
- Buragohain, P.; Surineni, N.; Barua, N.C.; Bhuyan, P.D.; Boruah, P.; Borah, J.C.; Laisharm, S.; Moirangthem, D.S. Synthesis of a novel series of fluoroarene derivatives of artemisinin as potent antifungal and anticancer agent. Bioorg. Med. Chem. Lett. 2015, 25, 3338–3341. [Google Scholar] [CrossRef] [PubMed]
- Oguariri, R.M.; Adelsberger, J.W.; Baseler, M.W.; Imamichi, T. Evaluation of the effect of pyrimethamine, an anti-malarial drug, on hiv-1 replication. Virus Res. 2010, 153, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Paeshuyse, J.; Coelmont, L.; Vliegen, I.; Hemel, J.V.; Vandenkerckhove, J.; Peys, E.; Sas, B.; Clercq, E.D.; Neyts, J. Hemin potentiates the anti-hepatitis c virus activity of the antimalarial drug artemisinin. Biochem. Biophys. Res. Commun. 2006, 348, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Serrano, M.A.; Vallejo, M.; Efferth, T.; Alvarez, M.; Marin, J.J.G. Antiviral effect of artemisinin from artemisia annua against a model member of the flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med. 2006, 72, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Arbab, A.H.; Al-Dosari, M.S.; Al-Rehaily, A.J. Antiviral natural products against chronic hepatitis b: Recent developments. Curr. Pharm. Des. 2016, 22, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Chen, H.-G.; He, H.-B.; Hou, X.-Y.; Ellis, M.; McManus, D.P. A double-blind field trial on the effects of artemether on schistosoma japonicum infection in a highly endemic focus in southern china. Acta Trop. 2005, 96, 184–190. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.F.; Johnston, R.C.; Halferty, L.; Hanna, R.E.B.; Brennan, G.P.; Fairweather, I. A comparative study on the impact of two artemisinin derivatives, artemether and artesunate, on the female reproductive system of fasciola hepatica. Vet. Parasitol. 2015, 211, 182–194. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.F.; Johnston, R.C.; Halferty, L.; Hanna, R.E.B.; Brennan, G.P.; Fairweather, I. Disruption of spermatogenesis in the liver fluke, fasciola hepatica by two artemisinin derivatives, artemether and artesunate. J. Helminthol. 2017, 91, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Sayed, H.; El-Ghanam, M.; Sabry, H.; Anani, S.; El-Wakeel, A.; Hatz, C.; Utzinger, J.; El-Din, S.S.; El-Maadawy, W.; et al. Efficacy and safety of artemether in the treatment of chronic fascioliasis in egypt: Exploratory phase-2 trials. PLoS Negl. Trop. Dis. 2011, 5, e1285. [Google Scholar] [CrossRef] [PubMed]
- Jones-Brando, L.; D’Angelo, J.; Posner, G.H.; Yolken, R. In vitro inhibition of toxoplasma gondii by four new derivatives of artemisinin. Antimicrob. Agents Chemother. 2006, 50, 4206–4208. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Saadat, F.; Di Paola, R.; Mirshafiey, A. Artemether: A new therapeutic strategy in experimental rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2005, 27, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Tang, W.; Shi, L.P.; Wan, J.; Zhou, R.; Ni, J.; Fu, Y.F.; Yang, Y.F.; Li, Y.; Zuo, J.P. Investigation of the immunosuppressive activity of artemether on t-cell activation and proliferation. Br. J. Pharmacol. 2007, 150, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Vonwiller, S.C. Extraction of artemisinin and artemisinic acid: Preparation of artemether and new analogues. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 23–26. [Google Scholar] [CrossRef]
- Stringham, R.W.; Teager, D.S. Streamlined process for the conversion of artemisinin to artemether. Org. Process Res. Dev. 2012, 16, 764–768. [Google Scholar] [CrossRef]
- Gilmore, K.; Kopetzki, D.; Lee, J.W.; Horvath, Z.; McQuade, D.T.; Seidel-Morgenstern, A.; Seeberger, P.H. Continuous synthesis of artemisinin-derived medicines. Chem. Commun. 2014, 50, 12652–12655. [Google Scholar] [CrossRef] [PubMed]
- Yaseneva, P.; Plaza, D.; Fan, X.; Loponov, K.; Lapkin, A. Synthesis of the antimalarial API artemether in a flow reactor. Catal. Today 2015, 239, 90–96. [Google Scholar] [CrossRef]
- Li, S.; Wu, L.; Liu, Z.; Hu, L.; Xu, P.; Xuan, Y.; Liu, Y.; Liu, X.; Fan, J. Studies on prophylactic effect of artesunate on schistosomiasis japonica. Chin. Med. J. 1996, 109, 848–853. [Google Scholar] [PubMed]
- Liu, R.; Dong, H.-F.; Guo, Y.; Zhao, Q.-P.; Jiang, M.-S. Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: A systematic review and meta-analysis. Parasites Vectors 2011, 4, 201. [Google Scholar] [CrossRef] [PubMed]
- Hien, T.T.; Truong, N.T.; Minh, N.H.; Dat, H.D.; Dung, N.T.; Hue, N.T.; Dung, T.K.; Tuan, P.Q.; Campbell, J.I.; Farrar, J.J.; et al. A randomized controlled pilot study of artesunate versus triclabendazole for human fascioliasis in central Vietnam. Am. J. Trop. Med. Hyg. 2008, 78, 388–392. [Google Scholar] [PubMed]
- Romero, M.R.; Efferth, T.; Serrano, M.A.; Castaño, B.; Macias, R.I.R.; Briz, O.; Marin, J.J.G. Effect of artemisinin/artesunate as inhibitors of hepatitis b virus production in an “in vitro” replicative system. Antivir. Res. 2005, 68, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Xiao, X.; Peng, F.; Li, M.; Gong, G. Artesunate, an anti-malarial drug, has a potential to inhibit hcv replication. Virus Genes 2016, 52, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Auerochs, S.; Korn, K.; Marschall, M. Sensitivity of human herpesvirus 6 and other human herpesviruses to the broad-spectrum antiinfective drug artesunate. J. Clin. Virol. 2009, 46, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hakacova, N.; Klingel, K.; Kandolf, R.; Engdahl, E.; Fogdell-Hahn, A.; Higgins, T. First therapeutic use of artesunate in treatment of human herpesvirus 6b myocarditis in a child. J. Clin. Virol. 2013, 57, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Marschall, M.; Wang, X.; Huong, S.-M.; Hauber, I.; Olbrich, A.; Kronschnabl, M.; Stamminger, T.; Huang, E.-S. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. 2002, 80, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Flobinus, A.; Taudon, N.; Desbordes, M.; Labrosse, B.; Simon, F.; Mazeron, M.-C.; Schnepf, N. Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J. Antimicrob. Chemother. 2014, 69, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.-H.; Ou, Y.-Y.; Guo, M.-M.; Dai, X.; Zhou, D.-Z.; Chen, R. Human embryonic lung fibroblasts treated with artesunate exhibit reduced rates of proliferation and human cytomegalovirus infection in vitro. J. Thorac. Dis. 2015, 7, 1151–1157. [Google Scholar] [PubMed]
- Chou, S.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir. Res. 2011, 92, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.B.; Kannoth, M.M.; Tekwani, B.L.; Gut, J.; Rosenthal, P.J.; Avery, M.A. A new library of c-16 modified artemisinin analogs and evaluation of their anti-parasitic activities. Combinatorial Chem. High Throughput Screen. 2006, 9, 729–741. [Google Scholar] [CrossRef]
- D’Angelo, J.G.; Bordón, C.; Posner, G.H.; Yolken, R.; Jones-Brando, L. Artemisinin derivatives inhibit toxoplasma gondii in vitro at multiple steps in the lytic cycle. J. Antimicrob. Chemother. 2009, 63, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.M.; Ross, S.A.; Jacob, M.; ElSohly, M.A. Antifungal activity of artemisinin derivatives. J. Nat. Prod. 2005, 68, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.J.; Lee, M.; Klayman, D.L. Antimalarial activity of new water-soluble dihydroartemisinin derivatives. 2. Stereospecificity of the ether side chain. J. Med. Chem. 1989, 32, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Brossi, A.; Venugopalan, B.; Dominguez Gerpe, L.; Yeh, H.J.C.; Flippen-Anderson, J.L.; Buchs, P.; Luo, X.D.; Milhous, W.; Peters, W. Arteether, a new antimalarial drug: Synthesis and antimalarial properties. J. Med. Chem. 1988, 31, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Schinazi, R.F. Synthesis and in vitro anti-human immunodeficiency virus activity of artemisinin (qinghaosu)-related trioxanes. Bioorg. Med. Chem. Lett. 1994, 4, 931–934. [Google Scholar] [CrossRef]
- Blazquez, A.G.; Fernandez-Dolon, M.; Sanchez-Vicente, L.; Maestre, A.D.; Gomez-San Miguel, A.B.; Alvarez, M.; Serrano, M.A.; Jansen, H.; Efferth, T.; Marin, J.J.G.; et al. Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg. Med. Chem. 2013, 21, 4432–4441. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Bustos, D.A.; ElSohly, H.N.; McChesney, J.D. A concise and stereoselective synthesis of (+)-12-n-butyldeoxoartemisinin. Synlett 1990, 1990, 743–744. [Google Scholar] [CrossRef]
- Aminake, M.N.; Mahajan, A.; Kumar, V.; Hans, R.; Wiesner, L.; Taylor, D.; de Kock, C.; Grobler, A.; Smith, P.J.; Kirschner, M.; et al. Synthesis and evaluation of hybrid drugs for a potential HIV/AIDS-malaria combination therapy. Bioorg. Med. Chem. 2012, 20, 5277–5289. [Google Scholar] [CrossRef] [PubMed]
- Slade, D.; Galal, A.M.; Gul, W.; Radwan, M.M.; Ahmed, S.A.; Khan, S.I.; Tekwani, B.L.; Jacob, M.R.; Ross, S.A.; ElSohly, M.A. Antiprotozoal, anticancer and antimicrobial activities of dihydroartemisinin acetal dimers and monomers. Bioorg. Med. Chem. 2009, 17, 7949–7957. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Paik, I.-H.; Sur, S.; McRiner, A.J.; Borstnik, K.; Xie, S.; Shapiro, T.A. Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high stability and efficacy. J. Med. Chem. 2003, 46, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Arav-Boger, R.; He, R.; Chiou, C.-J.; Liu, J.; Woodard, L.; Rosenthal, A.; Jones-Brando, L.; Forman, M.; Posner, G. Artemisinin-derived dimers have greatly improved anti-cytomegalovirus activity compared to artemisinin monomers. PLoS ONE 2010, 5, e10370. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; He, R.; Kapoor, A.; Forman, M.; Mazzone, J.R.; Posner, G.H.; Arav-Boger, R. Inhibition of human cytomegalovirus replication by artemisinins: Effects mediated through cell cycle modulation. Antimicrob. Agents Chemother. 2015, 59, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.S.; Chen, X.; Liu, J.O.; West, D.C.; Hergenrother, P.J.; Shapiro, T.A.; Posner, G.H. Malaria-infected mice are cured by a single oral dose of new dimeric trioxane sulfones which are also selectively and powerfully cytotoxic to cancer cells. J. Med. Chem. 2009, 52, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Alagbala, A.A.; McRiner, A.J.; Borstnik, K.; Labonte, T.; Chang, W.; D’Angelo, J.G.; Posner, G.H.; Foster, B.A. Biological mechanisms of action of novel c-10 non-acetal trioxane dimers in prostate cancer cell lines. J. Med. Chem. 2006, 49, 7836–7842. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Mott, B.T.; Rosenthal, A.S.; Genna, D.T.; Posner, G.H.; Arav-Boger, R. An artemisinin-derived dimer has highly potent anti-cytomegalovirus (CMV) and anti-cancer activities. PLoS ONE 2011, 6, e24334. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Park, K.; Cai, H.; Kapoor, A.; Forman, M.; Mott, B.; Posner, G.H.; Arav-Boger, R. Artemisinin-derived dimer diphenyl phosphate is an irreversible inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 2012, 56, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Forman, M.; Mott, B.T.; Venkatadri, R.; Posner, G.H.; Arav-Boger, R. Unique and highly selective anticytomegalovirus activities of artemisinin-derived dimer diphenyl phosphate stem from combination of dimer unit and a diphenyl phosphate moiety. Antimicrob. Agents Chemother. 2013, 57, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Mott, B.T.; He, R.; Chen, X.; Fox, J.M.; Civin, C.I.; Arav-Boger, R.; Posner, G.H. Artemisinin-derived dimer phosphate esters as potent anti-cytomegalovirus (anti-cmv) and anti-cancer agents: A structure-activity study. Bioorg. Med. Chem. 2013, 21, 3702–3707. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.; Fröhlich, T.; Gruber, L.; Hutterer, C.; Marschall, M.; Voigtländer, C.; Friedrich, O.; Kappes, B.; Efferth, T.; Tsogoeva, S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg. Med. Chem. 2015, 23, 5452–5458. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, C.; Niemann, I.; Milbradt, J.; Fröhlich, T.; Reiter, C.; Kadioglu, O.; Bahsi, H.; Zeitträger, I.; Wagner, S.; Einsiedel, J.; et al. The broad-spectrum antiinfective drug artesunate interferes with the canonical nuclear factor kappa b (Nf-κB) pathway by targeting RelA/p65. Antivir. Res. 2015, 124, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.; Fröhlich, T.; Zeino, M.; Marschall, M.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Hampel, F.; Efferth, T.; et al. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur. J. Med. Chem. 2015, 97, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Obeid, S.; Alen, J.; Nguyen, V.H.; Pham, V.C.; Meuleman, P.; Pannecouque, C.; Le, T.N.; Neyts, J.; Dehaen, W.; Paeshuyse, J. Artemisinin analogues as potent inhibitors of in vitro hepatitis c virus replication. PLoS ONE 2013, 8, e81783. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Q.; Vargas, M.; Dong, Y.; Sriraghavan, K.; Keiser, J.; Vennerstrom, J.L. The activity of dispiro peroxides against fasciola hepatica. Bioorg. Med. Chem. Lett. 2011, 21, 5320–5323. [Google Scholar] [CrossRef] [PubMed]
- Amewu, R.; Stachulski, A.V.; Ward, S.A.; Berry, N.G.; Bray, P.G.; Davies, J.; Labat, G.; Vivas, L.; O’Neill, P.M. Design and synthesis of orally active dispiro 1,2,4,5-tetraoxanes; synthetic antimalarials with superior activity to artemisinin. Organ. Biomol. Chem. 2006, 4, 4431–4436. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.-W.; Qiu, S.-J.; Zhao, Y.; Sun, H.; Qiao, C.; Xia, C.-M. Praziquantel derivatives exhibit activity against both juvenile and adult schistosoma japonicum. Bioorg. Med. Chem. Lett. 2012, 22, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-J.; Boissier, J.; Chen, J.-L.; Yao, H.; Yang, S.; Rognon, A.; Qiao, C. Design, synthesis and biological evaluation of praziquantel and endoperoxide conjugates as antischistosomal agents. Future Med. Chem. 2015, 7, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N.; Yaremenko, I.A.; Krylov, I.B.; Terent’ev, A.O.; Keiser, J. Elucidation of the in vitro and in vivo activities of bridged 1,2,4-trioxolanes, bridged 1,2,4,5-tetraoxanes, tricyclic monoperoxides, silyl peroxides, and hydroxylamine derivatives against schistosoma mansoni. Bioorg. Med. Chem. 2015, 23, 5175–5181. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Borisov, D.A.; Chernyshev, V.V.; Nikishin, G.I. Facile and selective procedure for the synthesis of bridged 1,2,4,5-tetraoxanes; strong acids as cosolvents and catalysts for addition of hydrogen peroxide to β-diketones. J. Org. Chem. 2009, 74, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Yaremenko, I.A.; Vil, V.A.; Moiseev, I.K.; Kon’kov, S.A.; Dembitsky, V.M.; Levitsky, D.O.; Nikishin, G.I. Phosphomolybdic and phosphotungstic acids as efficient catalysts for the synthesis of bridged 1,2,4,5-tetraoxanes from [small beta]-diketones and hydrogen peroxide. Org. Biomol. Chem. 2013, 11, 2613–2623. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Wu, X.-F.; Gong, J.-L.; Qi, X. A powerful combination: Recent achievements on using tbai and tbhp as oxidation system. Org. Biomol. Chem. 2014, 12, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Saiz, A.I.; Manrique, G.D.; Fritz, R. Determination of benzoyl peroxide and benzoic acid levels by hplc during wheat flour bleaching process. J. Agric. Food Chem. 2001, 49, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Abe-Onishi, Y.; Yomota, C.; Sugimoto, N.; Kubota, H.; Tanamoto, K. Determination of benzoyl peroxide and benzoic acid in wheat flour by high-performance liquid chromatography and its identification by high-performance liquid chromatography–mass spectrometry. J. Chromatogr. A 2004, 1040, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Liu, H.; Gao, Y.; Luan, F. Determination of benzoyl peroxide, as benzoic acid, in wheat flour by capillary electrophoresis compared with hplc. J. Sci. Food Agric. 2012, 92, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, J.A. Benzoyl peroxide. Acta Derm.-Venereol. Suppl. 1980, (Suppl. 89), 57–63. [Google Scholar]
- Leyden, J.J.; Wortzman, M.; Baldwin, E.K. Antibiotic-resistant propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis 2008, 82, 417–421. [Google Scholar] [PubMed]
- Burkhart, C.G.; Burkhart, C.N.; Isham, N. Synergistic antimicrobial activity by combining an allylamine with benzoyl peroxide with expanded coverage against yeast and bacterial species. Br. J. Dermatol. 2006, 154, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, L.; Toso, S.M.; Kitay, K.; Webster, C.F. Anti-inflammatory actions of benzoyl peroxide: Effects on the generation of reactive oxygen species by leucocytes and the activity of protein kinase c and calmodulin. Br. J. Dermatol. 1994, 130, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.M.; Dreher, F.; Behnam, S.; Ford, C.; Lee, C.; Tiet, T.; Weinstein, G.D.; Maibach, H.I. ‘Keratolytic’ properties of benzoyl peroxide and retinoic acid resemble salicylic acid in man. Skin Pharmacol. Physiol. 2006, 19, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Merker, P.C. Benzoyl peroxide: A history of early research and researchers. Int. J. Dermatol. 2002, 41, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, O.M.; Mertz, P.M.; Eaglstein, W.H. Benzoyl peroxide and epidermal wound healing. Arch. Dermatol. 1983, 119, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Sagransky, M.; Yentzer, B.A.; Feldman, S.R. Benzoyl peroxide: A review of its current use in the treatment of acne vulgaris. Expert Opin. Pharmacother. 2009, 10, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, C.G.; Burkhart, C.N. The chemistry and synergy of benzoyl peroxide with clindamycin. Br. J. Dermatol. 2008, 159, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Sehgal, V.K.; Gupta, A.K.; Singh, S.P. A comparative study to evaluate the efficacy and safety of combination topical preparations in acne vulgaris. Int. J. Appl. Basic Med. Res. 2015, 5, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, C.G.; Burkhart, C.N. Treatment of acne vulgaris without antibiotics: Tertiary amine–benzoyl peroxide combination vs. Benzoyl peroxide alone (proactiv solution™). Int. J. Dermatol. 2007, 46, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Price, C.C.; Krebs, E. P-nitrobenzoyl peroxide. Org. Synth. 1943, 23, 65. [Google Scholar]
- Her, B.; Jones, A.; Wollack, J.W. A three-step synthesis of benzoyl peroxide. J. Chem. Educ. 2014, 91, 1491–1494. [Google Scholar] [CrossRef]
- La Barge, R.G.; Johnson, G.L. Preparation of Benzoyl Peroxide. U.S. Patent 3,674,858, 4 July 1972. [Google Scholar]
- Wright, J.J.K.; Merill, Y.; Puar, M.S.; McPhail, A.T. Structure of oxanthromicin (antibiotic 16-550), a novel dimeric anthrone peroxide. J. Chem. Soc. Chem. Commun. 1984, 473–474. [Google Scholar] [CrossRef]
- Patel, M.; Horan, A.C.; Gullo, V.P.; Loebenberg, D.; Marquez, J.A.; Miller, G.H.; Waltz, J.A. Oxanthromicin, a novel antibiotic from actinomadura. J. Antibiot. 1984, 37, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tukashima, A.; Kiyohara, H.; Hashimoto, T.; Asakawa, Y.; Ōmura, S.; Yamada, H. In vitro antitrypanosomal activity of plant terpenes against trypanosoma brucei. Phytochemistry 2011, 72, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Tropina, V.I.; Krivykh, O.V.; Sadchikova, N.P.; Terent’ev, A.O.; Krylov, I.B. Synthesis and antimicrobial activity of geminal bis-hydroperoxides. Pharm. Chem. J. 2010, 44, 248–250. [Google Scholar] [CrossRef]











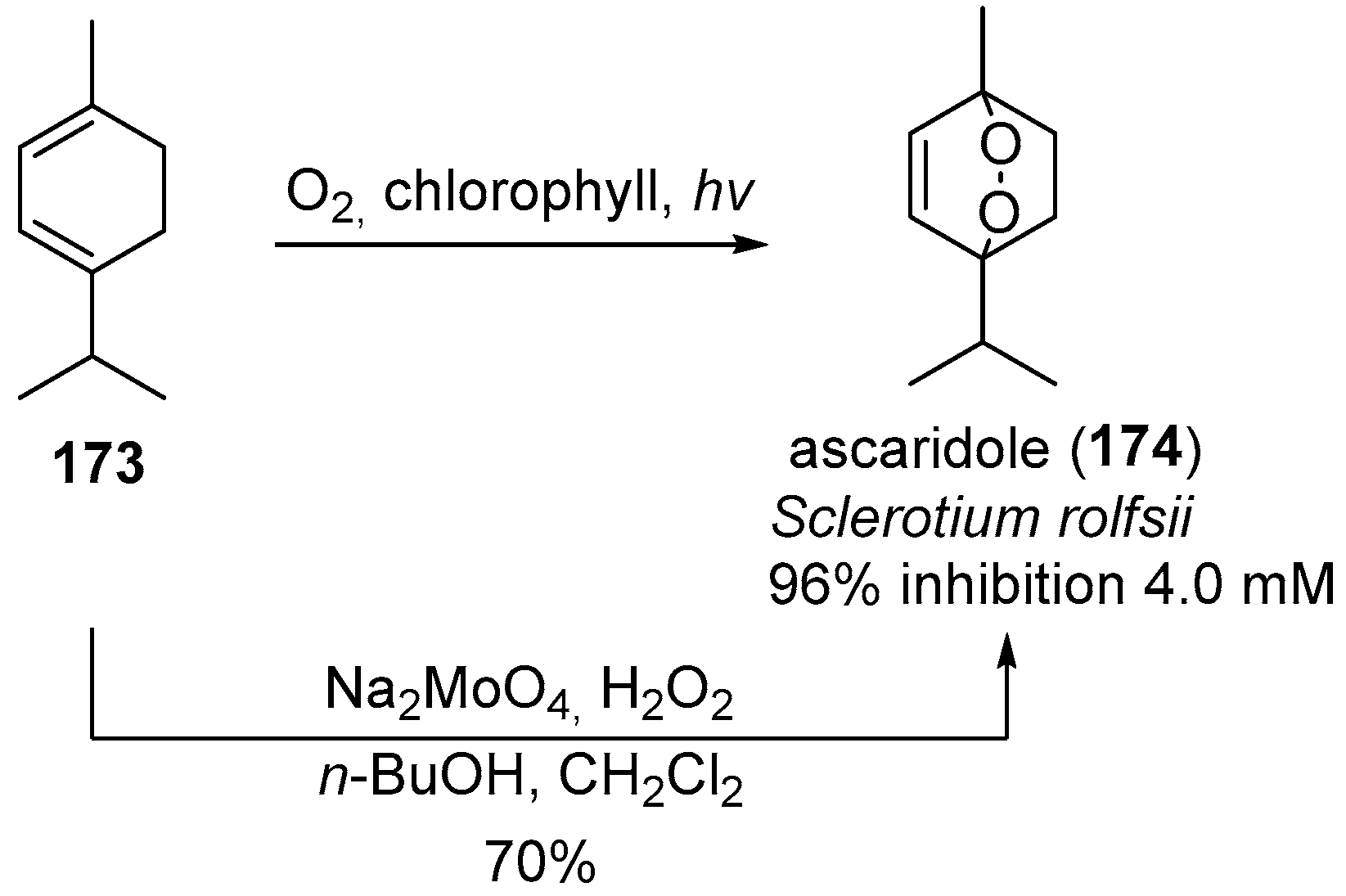
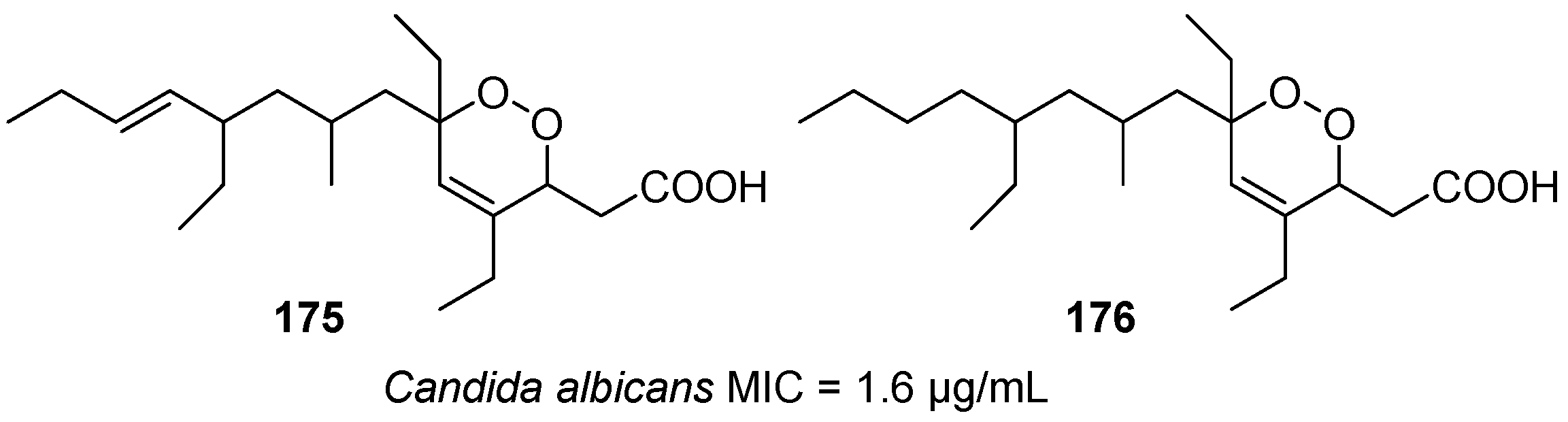

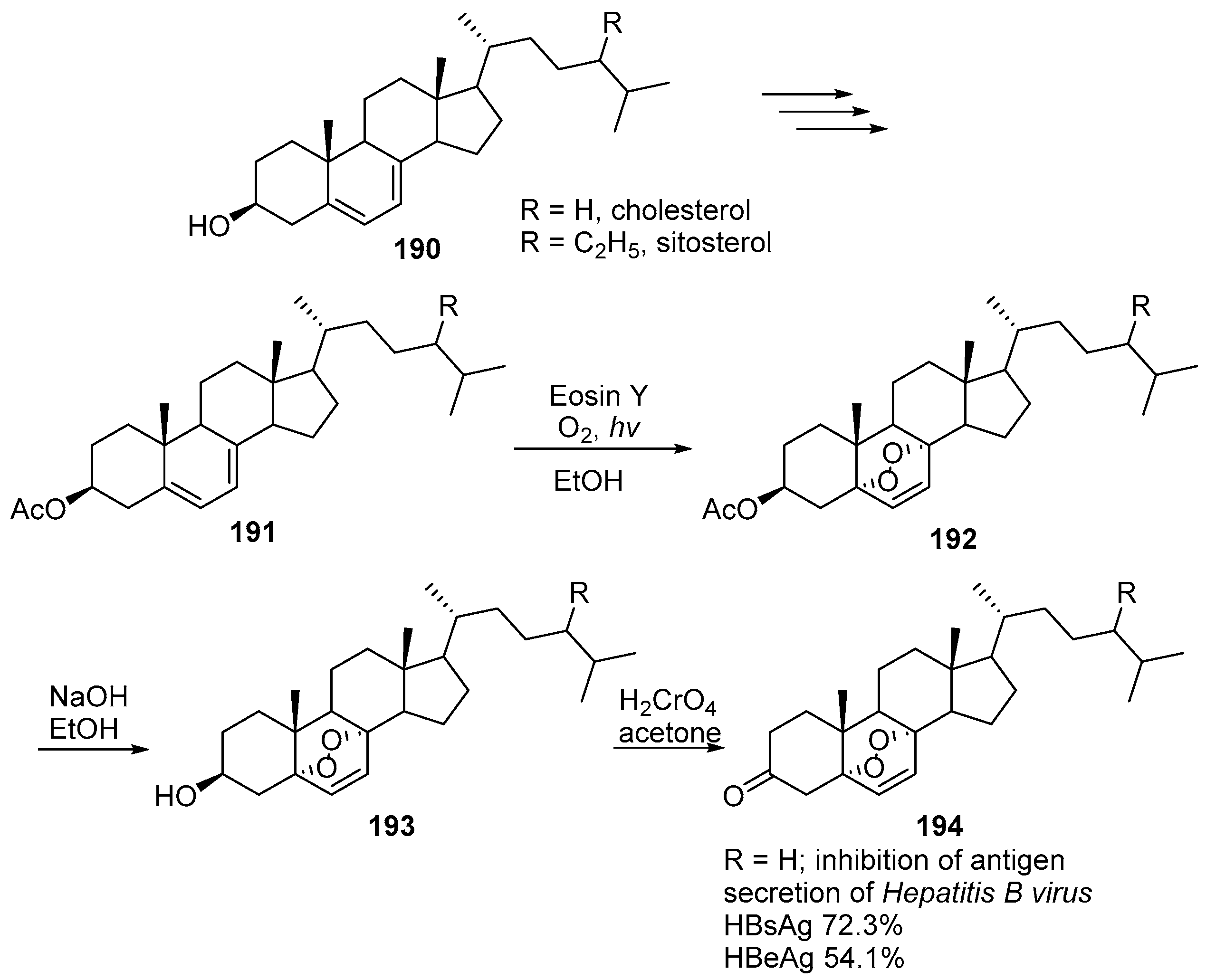
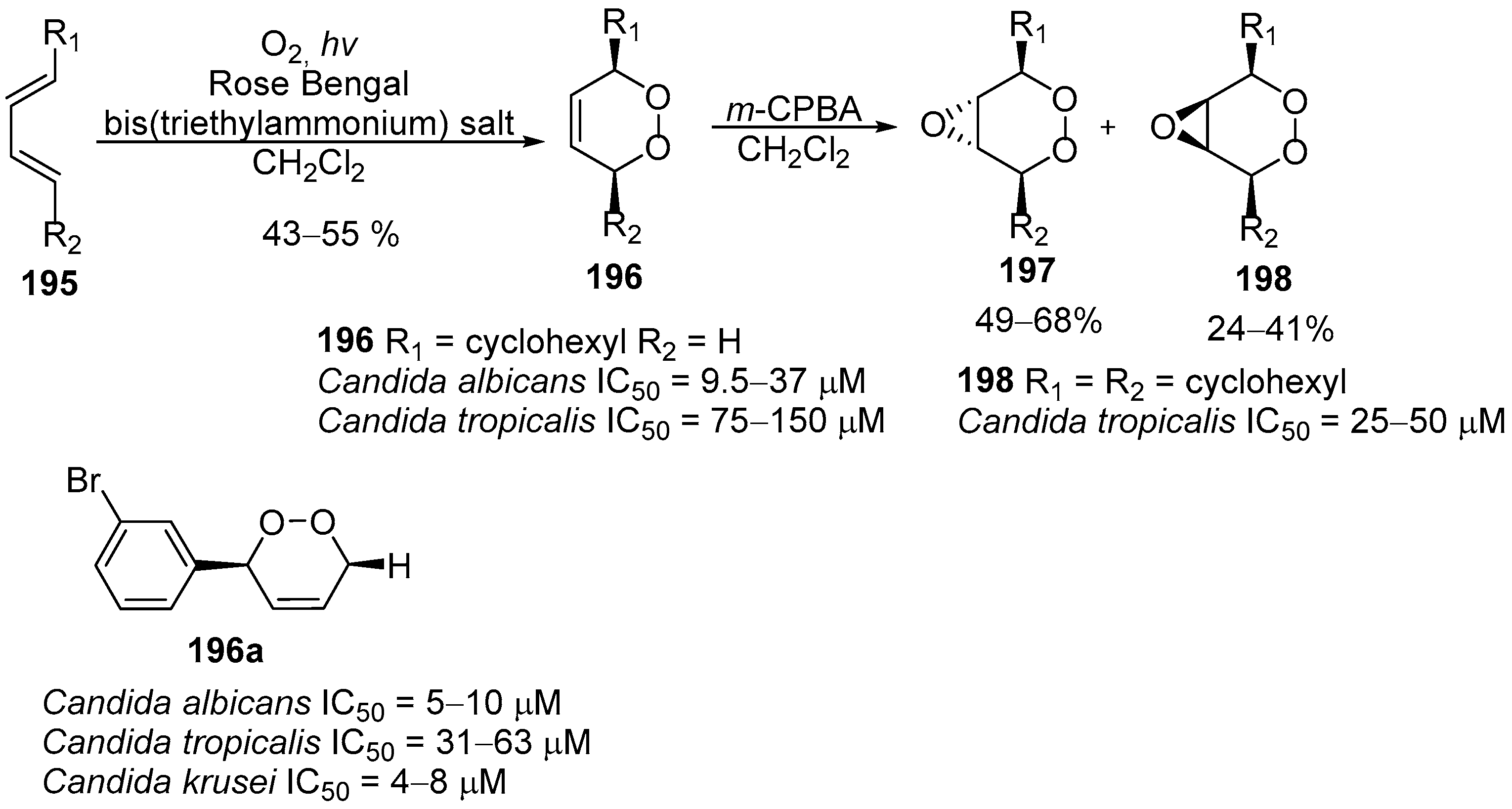
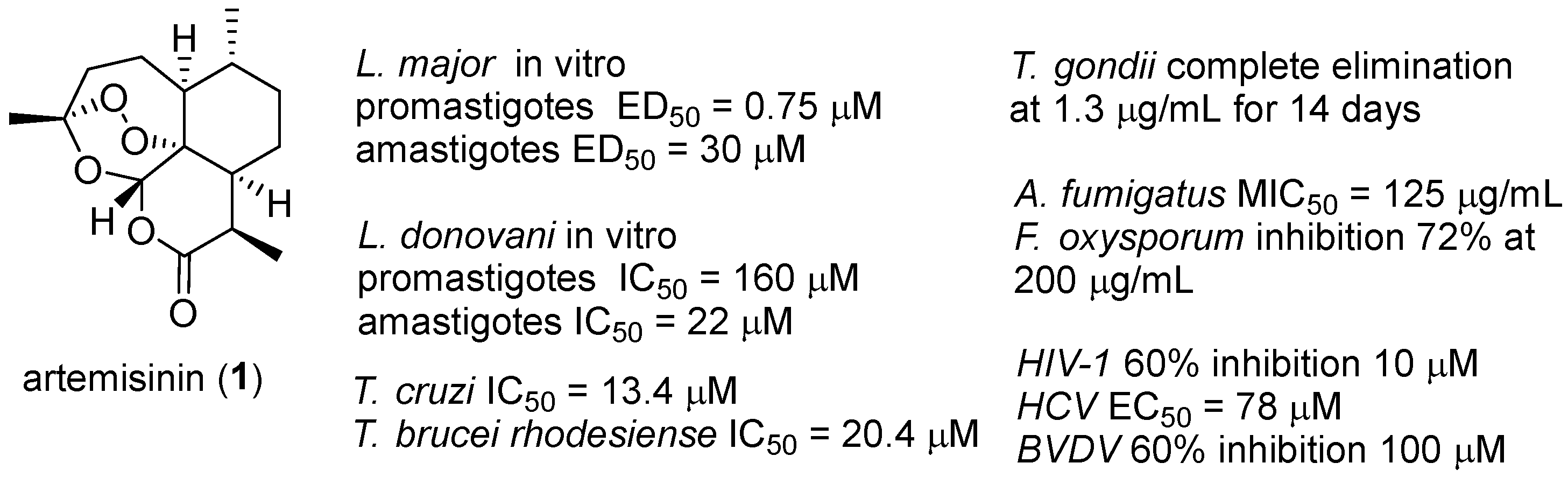
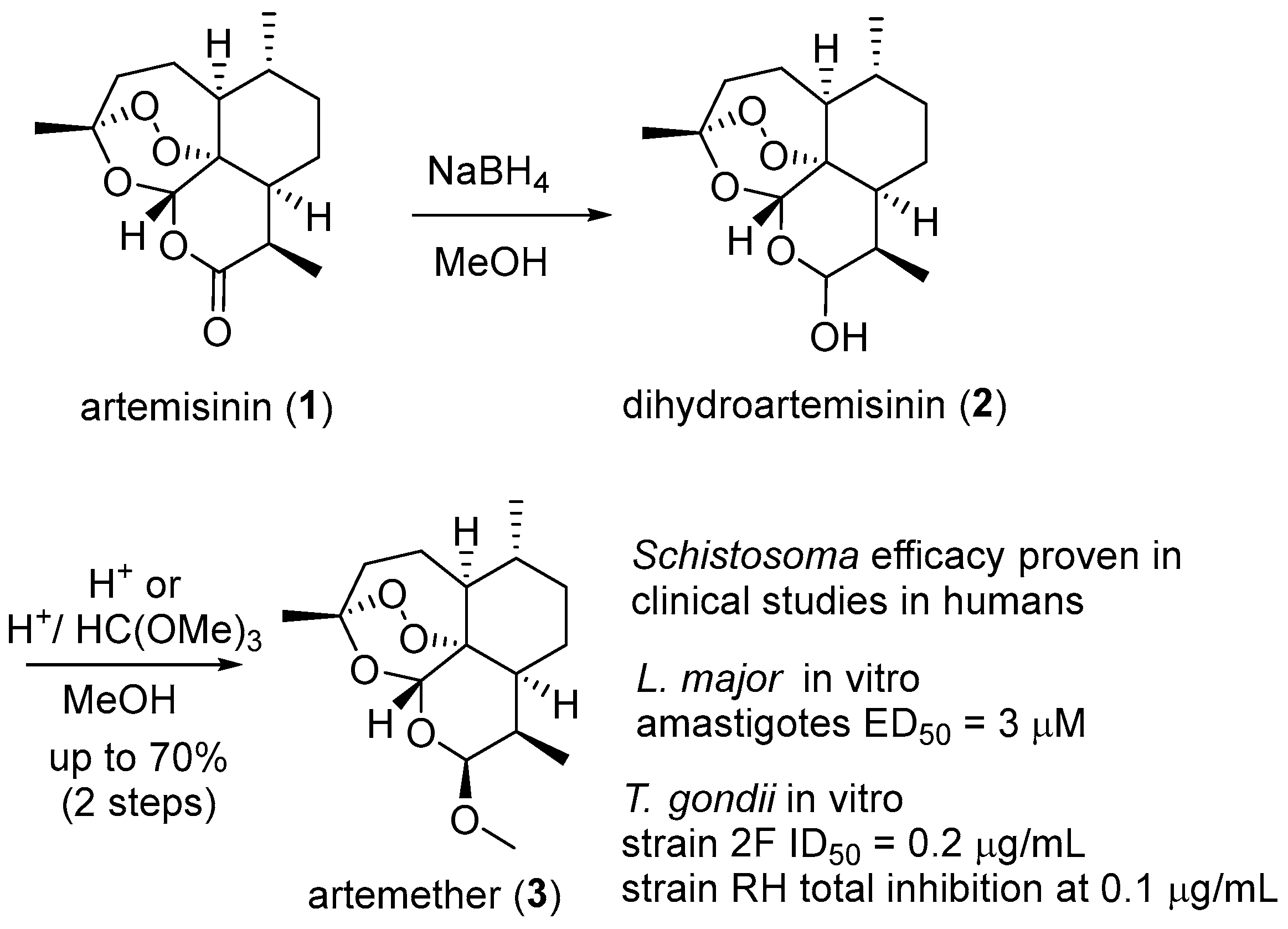



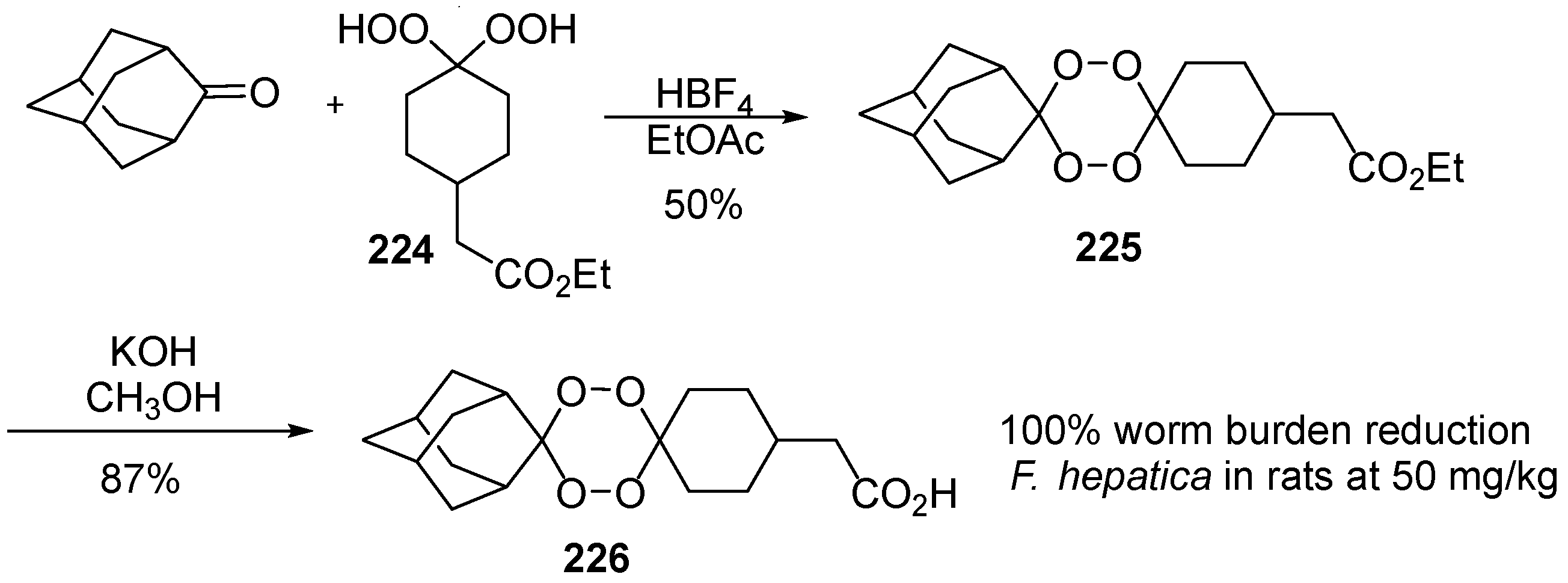


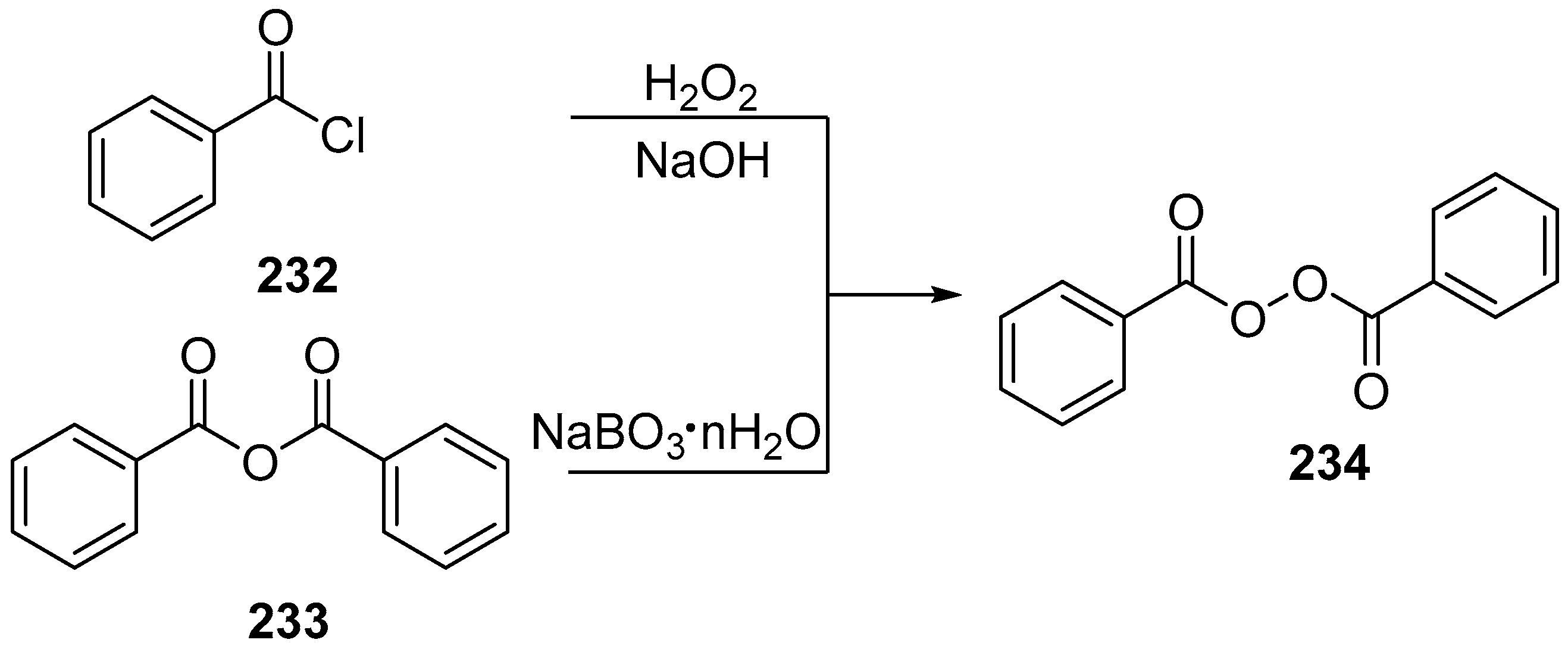
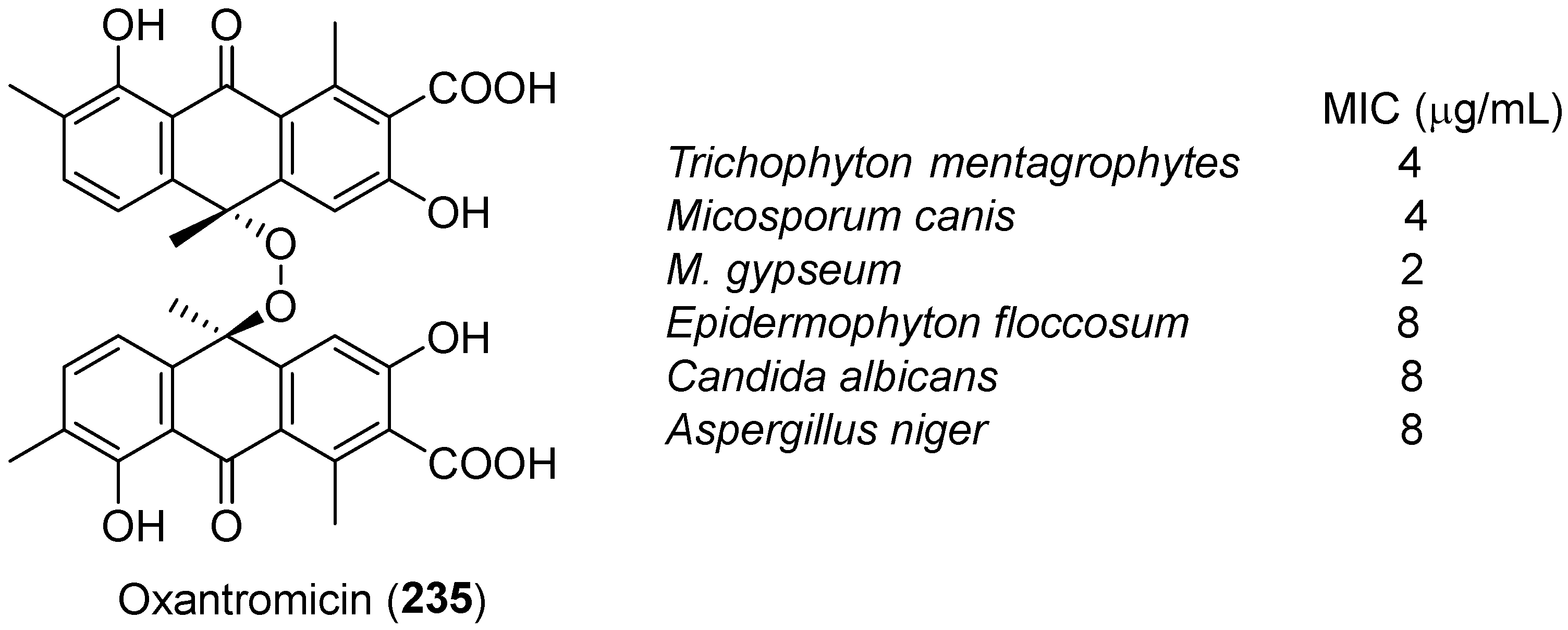

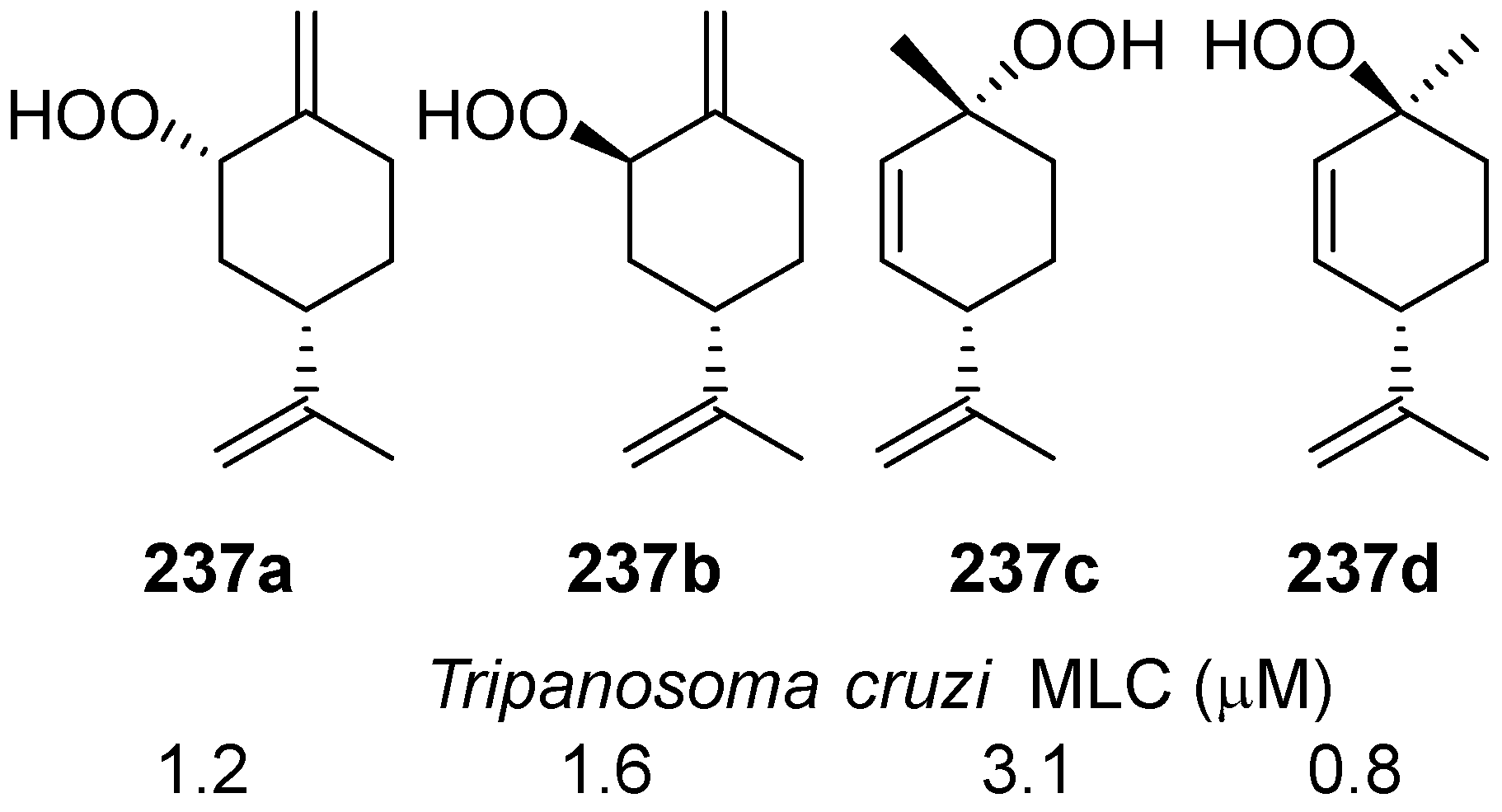
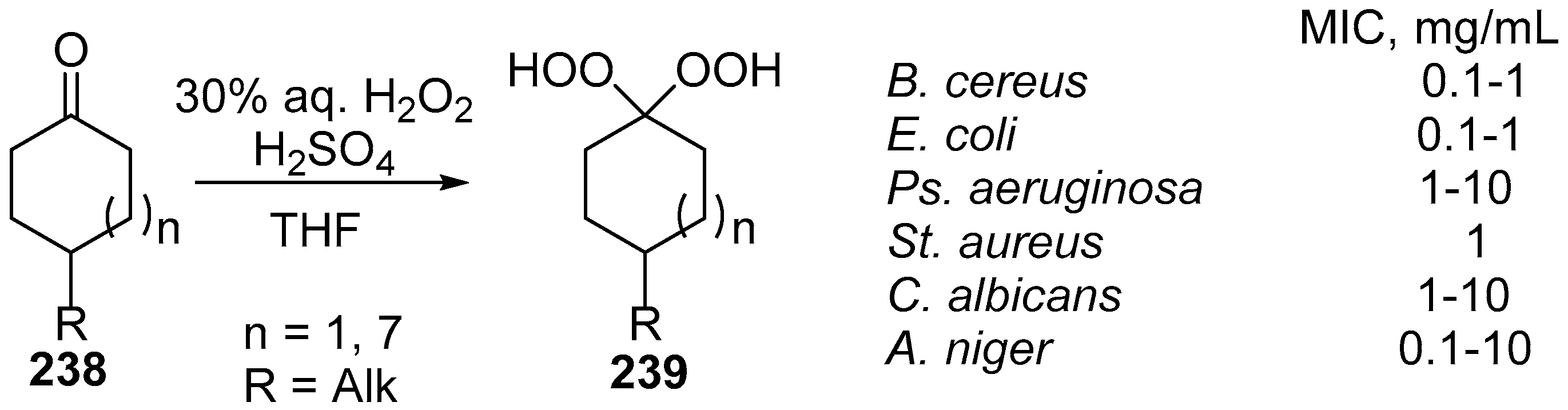
| No. | Dimer/Trimer Structure | Synthesis | Bioactivity |
|---|---|---|---|
| 1 | 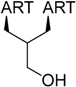 214 | from artemisinin (1) 3 steps, 67% [175] | HCMV EC50 = 0.15 µM [176,177] |
| 2 | 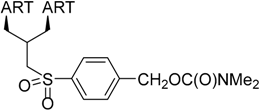 215 | from 214 3 steps, 42% [178] | HCMV EC50 = 0.06 µM [176] |
| 3 | 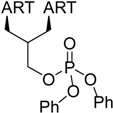 216 | from 214 1 step, 96% [179] | HCMV EC50 = 0.04 µM [180,181,182] |
| 4 | 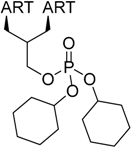 217 | from 214 1 step, 25% [183] | HCMV EC50 = 44 nM [183] |
| 5 | 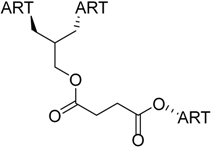 218 | from threo-214 and artesunate (4), 1 step, quantitative yield [184] | HCMV EC50 = 0.04 µM [184,185] |
| 6 | 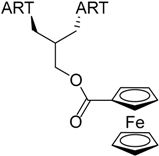 219 | from threo-214 1 step, 67% [186] | HCMV EC50 = 0.11 µM [186] |
| 7 | 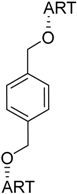 220 | not reported | HCV EC50 = 3.2 µM [187] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vil’, V.A.; Yaremenko, I.A.; Ilovaisky, A.I.; Terent’ev, A.O. Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions. Molecules 2017, 22, 1881. https://doi.org/10.3390/molecules22111881
Vil’ VA, Yaremenko IA, Ilovaisky AI, Terent’ev AO. Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions. Molecules. 2017; 22(11):1881. https://doi.org/10.3390/molecules22111881
Chicago/Turabian StyleVil’, Vera A., Ivan A. Yaremenko, Alexey I. Ilovaisky, and Alexander O. Terent’ev. 2017. "Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions" Molecules 22, no. 11: 1881. https://doi.org/10.3390/molecules22111881
APA StyleVil’, V. A., Yaremenko, I. A., Ilovaisky, A. I., & Terent’ev, A. O. (2017). Peroxides with Anthelmintic, Antiprotozoal, Fungicidal and Antiviral Bioactivity: Properties, Synthesis and Reactions. Molecules, 22(11), 1881. https://doi.org/10.3390/molecules22111881








