New Methods of Esterification of Nanodiamonds in Fighting Breast Cancer—A Density Functional Theory Approach
Abstract
:1. Introduction
2. Materials and Methods
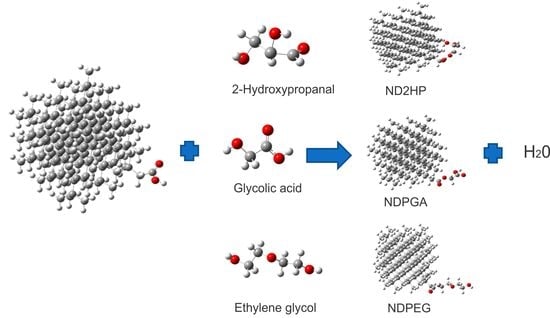
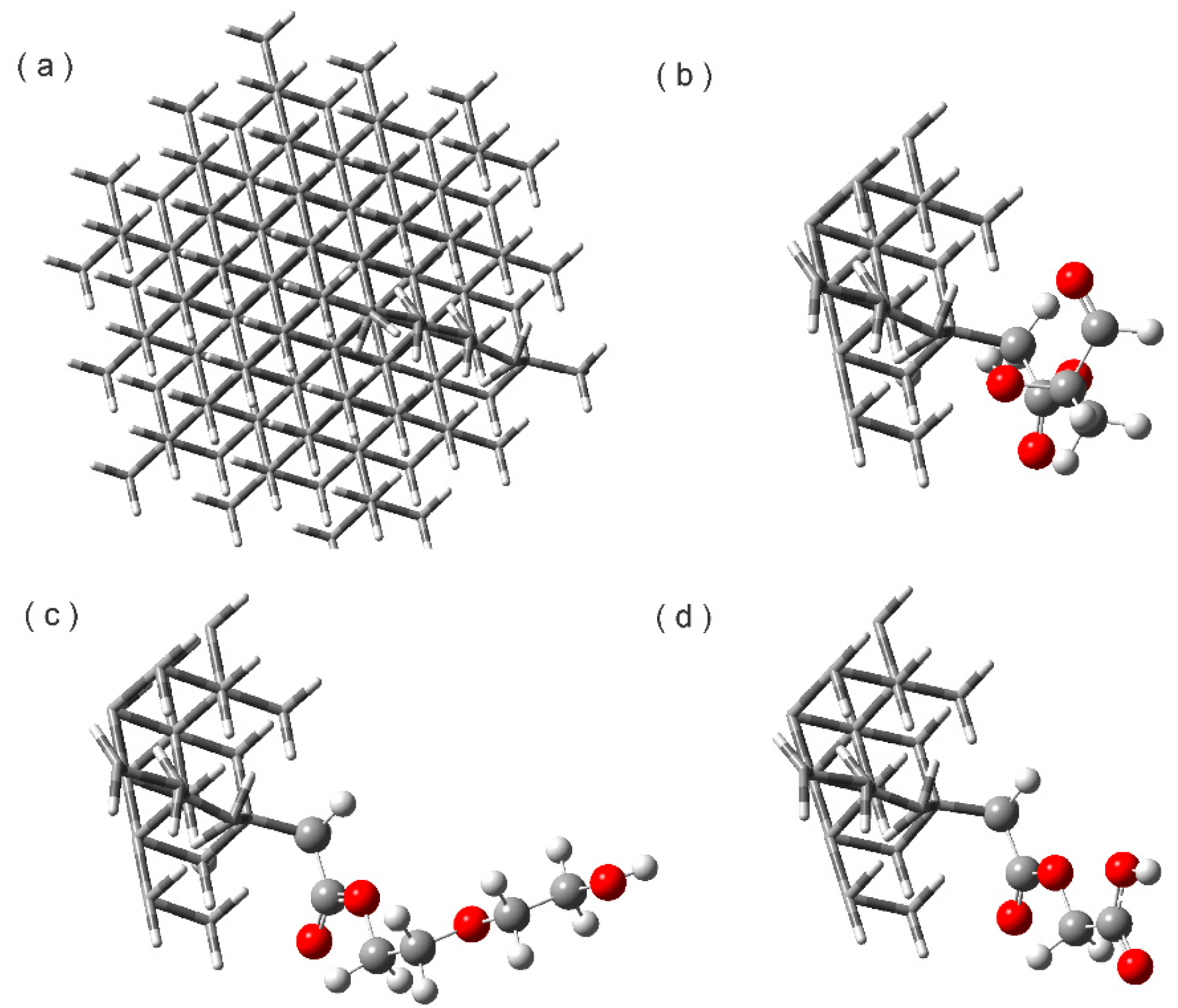
2.1 Esterified Nanodiamond (NDE)
2.2. Esterified Nanodiamond Complexes (NDE-TAM)
2.3. Reactivity Parameters
2.4. Molecular Polar Surface Area
3. Results and Discussion
3.1. Construction of the Proposed Systems
3.2. Reactivity Parameters of the NDE
3.3. Construction of the NDE-TAM Complex
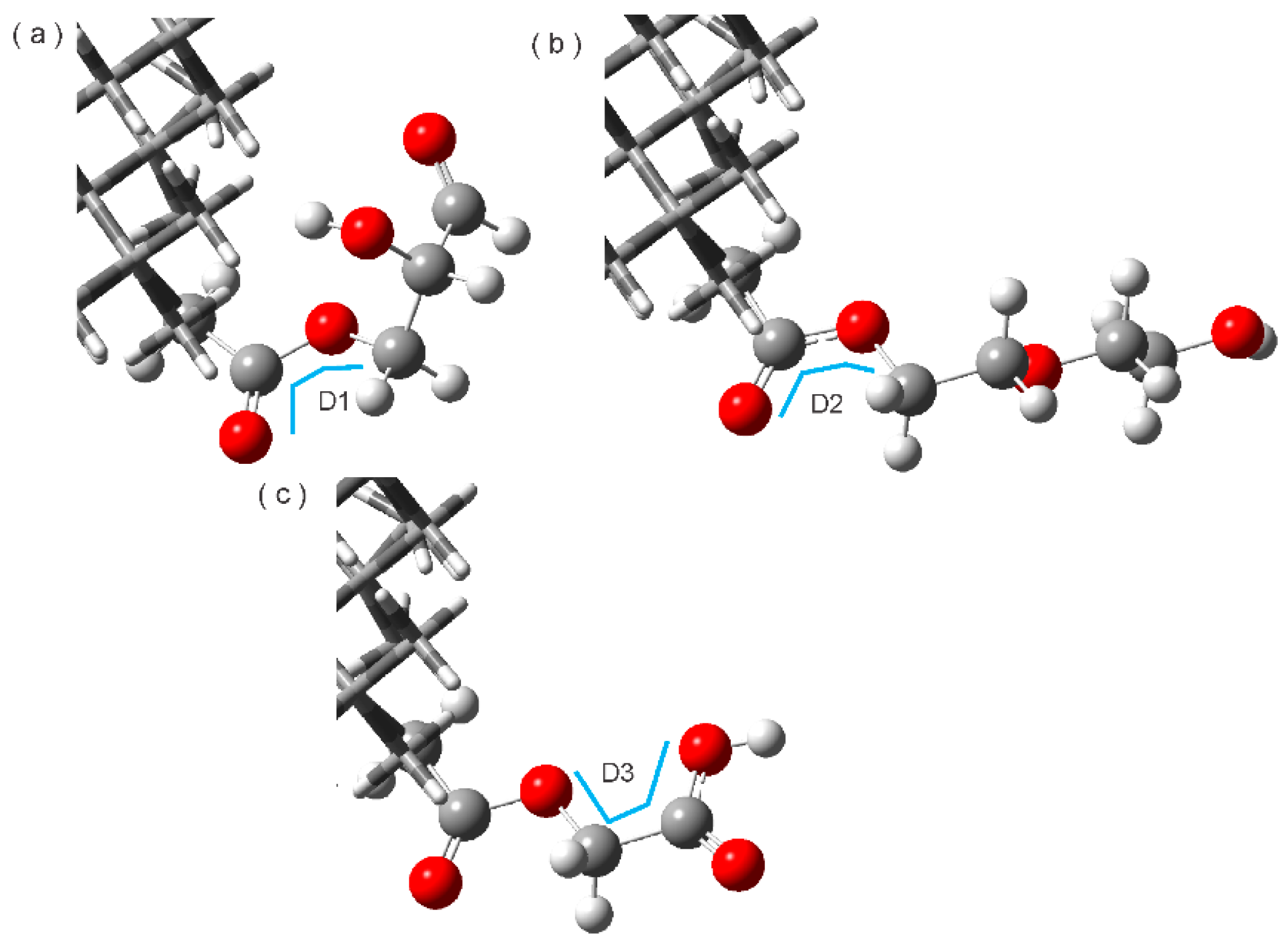
3.4. Hydrogen Bonds
3.5. Chemical Reactivity Descriptors of NDE-TAM
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.; Chen, X.; Yang, X.; Gao, W.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; et al. A nanomedicine based combination therapy based on qlpvm peptide functionalized liposomal tamoxifen and doxorubicin against luminal a breast cancer. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Altmeyer, C.; Karam, T.K.; Khalil, N.M.; Mainardes, R.M. Tamoxifen-loaded poly(l-lactide) nanoparticles: Development, characterization and in vitro evaluation of cytotoxicity. Mater. Sci. Eng. C 2016, 60, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Wakade, C.; De Sevilla, L.; Brann, D.W. Selective estrogen receptor modulators (serms) enhance neurogenesis and spine density following focal cerebral ischemia. J. Steroid Biochem. Mol. Biol. 2015, 146, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Lello, S.; Sblendorio, I.; Viapiana, O.; Fracassi, E.; Adami, S.; Gatti, D. Profile of bazedoxifene/conjugated estrogens for the treatment of estrogen deficiency symptoms and osteoporosis in women at risk of fracture. Drug Des. Dev. Ther. 2013, 7, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.P.; Lin, C.L.; Lin, Y.N.; Ma, W.C.; Hung, D.Z.; Kao, C.H. Tamoxifen treatment and the reduced risk of hyperlipidemia in asian patients with breast cancer: A population-based cohort study. Clin. Breast Cancer 2015, 15, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Man, H.B.; Ho, D. Nanodiamonds as platforms for biology and medicine. J. Lab. Autom. 2012, 18, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Chen, M.; Lam, R.; Xu, X.; Osawa, E.; Ho, D. Polymer-functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 2009, 3, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Badea, I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int. J. Nanomed. 2013, 8, 203–220. [Google Scholar]
- Mostofizadeh, A.; Li, Y.; Song, B.; Huang, Y. Synthesis, properties, and applications of low-dimensional carbon-related nanomaterials. J. Nanomater. 2011, 2011, 16. [Google Scholar] [CrossRef]
- Lim, D.G.; Prim, R.E.; Kim, K.H.; Kang, E.; Park, K.; Jeong, S.H. Combinatorial nanodiamond in pharmaceutical and biomedical applications. Int. J. Pharm. 2016, 514, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Duan, X.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Nanodiamonds-mediated doxorubicin nuclear delivery to inhibit lung metastasis of breast cancer. Biomaterials 2013, 34, 9648–9656. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.; Calderón, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kobayashi, Y.; Kaneko, N. Conformational studies of dl-lactaldehyde by 1h-nmr, raman and i.r. Spectroscopy. Spectrochim. Acta Part A Mol. Spectrosc. 1983, 39, 569–572. [Google Scholar] [CrossRef]
- Frazza, E.; Schmitt, E. A new absorbable suture. J. Biomed. Mater. Res. 1971, 5, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.; Organ, G.; Vacanti, J.; Langer, R. Design and fabrication of biodegradable polymer devices to engineer tubular tissues. Cell Transplant. 1993, 3, 203–210. [Google Scholar] [CrossRef]
- Mooney, D.J.; Mazzoni, C.L.; Breuer, C.; McNamara, K.; Hern, D.; Vacanti, J.P.; Langer, R. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials 1996, 17, 115–124. [Google Scholar] [CrossRef]
- Chung, P.H.; Perevedentseva, E.; Tu, J.S.; Chang, C.C.; Cheng, C.L. Spectroscopic study of bio-functionalized nanodiamonds. Diam. Relat. Mater. 2006, 15, 622–625. [Google Scholar] [CrossRef]
- Ho, D.; Wang, C.-H.K.; Chow, E.K.-H. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci. Adv. 2015, 1, e1500439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, W.X.; Li, Q.N.; Li, Y.G.; Li, Y.F.; Zhang, X.Y.; Huang, Q. Effects of serum proteins on intracellular uptake and cytotoxicity of carbon nanoparticles. Carbon 2009, 47, 1351–1358. [Google Scholar] [CrossRef]
- Zhu, Y.; Ran, T.C.; Li, Y.G.; Guo, J.X.; Li, W.X. Dependence of the cytotoxicity of multiwalled carbon nanotubes on the culture medium. Nanotechnology 2006, 17, 4668–4674. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Tong, Y.L.; Cao, R.X.; Tian, Z.M.; Yang, B.S.; Yang, P. In vivo enhancement of anticancer therapy using bare or chemotherapeutic drug-bearing nanodiamond particles. Int. J. Nanomed. 2014, 4, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Gueorguiev, G.K.; Broitman, E.; Furlan, A.; Stafström, S.; Hultman, L. Dangling bond energetics in carbon nitride and phosphorus carbide thin films with fullerene-like and amorphous structure. Chem. Phys. Lett. 2009, 482, 110–113. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Czigány, Z.; Furlan, A.; Stafström, S.; Hultman, L. Intercalation of p atoms in fullerene-like cpx. Chem. Phys. Lett. 2011, 501, 400–403. [Google Scholar] [CrossRef]
- Gueorguiev, G.; Pacheco, J. Shapes of cagelike metal carbide clusters: First-principles calculations. Phys. Rev. B 2003, 68, 241401. [Google Scholar] [CrossRef]
- Landeros-Martinez, L.-L.; Chavez-Flores, D.; Orrantia-Borunda, E.; Flores-Holguin, N. Construction of a Nanodiamond Tamoxifen Complex as a Breast Cancer Drug Delivery Vehicle. J. Nanomater. 2016, 2016, 9. [Google Scholar] [CrossRef]
- Oliva, M.; Safont, V.S.; Andrés, J.; Castillo, R.; Moliner, V. Understanding the mechanism of the addition of organomagnesium reagents to 2-hydroxypropanal: An ab initio molecular orbital analysis. Int. J. Quantum Chem. 1997, 65, 719–728. [Google Scholar] [CrossRef]
- Safont, V.S.; Moliner, V.; Oliva, M.; Castillo, R.; Andrés, J.; González, F.; Carda, M. A theoretical study of addition of organomagnesium reagents to chiral α-alkoxy carbonyl compounds. J. Org. Chem. 1996, 61, 3467–3475. [Google Scholar] [CrossRef]
- Talebian, E.; Talebian, M. A comparative dft study on the differences between normal modes of polyethylene and polyethylene glycol via b3lyp hamiltonian and the hartree-fock method in multiple bases. Optik-Int. J. Light Electron Opt. 2014, 125, 228–231. [Google Scholar] [CrossRef]
- Kimani, S.; Ghosh, G.; Ghogare, A.; Rudshteyn, B.; Bartusik, D.; Hasan, T.; Greer, A. Synthesis and characterization of mono-, di-, and tri-poly(ethylene glycol) chlorin e6 conjugates for the photokilling of human ovarian cancer cells. J. Org. Chem. 2012, 77, 10638–10647. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, J.; Mannfors, B.; Pietilä, L.O. Studies on aliphatic polyesters. Part ii. Ab initio, density functional and force field studies of model molecules with two carboxyl groups. J. Mol. Struct. THEOCHEM 2000, 531, 359–374. [Google Scholar] [CrossRef]
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four m06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* basis set for third-row atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods v: Modification of nddo approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Yamaguchi, K.; Shinohara, I.; Ito, F.; Yoshitake, Y.; Harano, K. Role of edge-to-face interaction between aromatic rings in clathrate formation of 1-benzoyl-2-hydroxyindoline derivatives with benzene. X-ray crystal and pm6 analyses of the interaction. Tetrahedron 2011, 67, 7400–7405. [Google Scholar] [CrossRef]
- Temelso, B.; Alser, K.A.; Gauthier, A.; Palmer, A.K.; Shields, G.C. Structural analysis of α-fetoprotein (afp)-like peptides with anti-breast-cancer properties. J. Phys. Chem. B 2014, 118, 4514–4526. [Google Scholar] [CrossRef] [PubMed]
- Benghodbane, S.; Khatmi, D. A theoretical study on the inclusion complexation of doxycycline with crysmeb. C. R. Chim. 2012, 15, 371–377. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. In Proceedings of the National Academy of Sciences of the United States of America, Washington, DC, USA, 15 November, 1986; pp. 8440–8441. [Google Scholar]
- Putz, M.V.; Russo, N.; Sicilia, E. Atomic radii scale and related size properties from density functional electronegativity formulation. J. Phys. Chem. A 2003, 107, 5461–5465. [Google Scholar] [CrossRef]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801–3807. [Google Scholar] [CrossRef]
- Robert, G.; Parr, Y.W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Cheminformatics, M. Bratislava. Slovak Republic Home Page. Available online: http://www.molinspiration.com/services/properties.html (accessed on 20 February 2017).
- Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Ho, D.N. Applications in Biology and Nanoscale Medicine; Springer: New York, NY, USA, 2010; Available online: https://link.springer.com/content/pdf/10.1007/978-1-4419-0531-4.pdf (accessed on 12 October 2017).
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nano 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, S.; Varga, M.; Ledinsky, M.; Jirasek, V.; Artemenko, A.; Kozak, H.; Ondic, L.; Skakalova, V.; Argentero, G.; Pennycook, T.; et al. Size and purity control of hpht nanodiamonds down to 1 nm. J. Phys. Chem. C 2015, 119, 27708–27720. [Google Scholar] [CrossRef] [PubMed]
- Pichot, V.; Bonnot, K.; Piazzon, N.; Schaefer, M.; Comet, M.; Spitzer, D. Deposition of detonation nanodiamonds by langmuir-blodgett technique. Diam. Relat. Mater. 2010, 19, 479–483. [Google Scholar] [CrossRef]
- Marc, C.; Vincent, P.; Benny, S.; Fabienne, B.; Denis, S. Detonation nanodiamonds for doping kevlar. J. Nanosci. Nanotechnol. 2010, 10, 4286–4292. [Google Scholar]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Rios-Gutierrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P. Polar Surface Area; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; Available online: https://books.google.com.mx/books?hl=es&lr=&id=mZ6rNWu_xnEC&oi=fnd&pg=PA111&dq=56.%09Ertl,+P.+Polar+surface+area,+Wiley-VCH+Verlag+GmbH+%26+Co.+KGaA,+Weinheim,+Germany,+2008%3B+&ots=Y3GriSxqX6&sig=0QfavQZavPqrzpE2-p7qhOL9IfY#v=onepage&q&f=false (acessed on 12 October 2017).
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 1. Prediction of intestinal absorption. J. Pharm. Sci. 1999, 88, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.B.F.a.Æ. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian, Inc.: Pittsburgh, PA, USA, 1996; p. 171. [Google Scholar]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In structural Chemistry and Biology; Oxford: New York, NY, USA, 2001. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
Sample Availability: Exclusively the virtual samples are available from the authors. |



| Molecules | N (eV) | ω (eV) |
|---|---|---|
| Nanodiamond (ND) | 6.18 | 0.81 |
| 2-Hydroxypropanal (2HP) | 1.87 | 2.82 |
| Polyethylene glycol PEG | 1.91 | 4.28 |
| Polyglicolic acid (PGA) | 1.78 | 5.96 |
| NDEsterified | EA (eV) | IP (eV) | η (eV) | χ = −μ (eV) | ω (eV) |
|---|---|---|---|---|---|
| ND2HP | 0.53/0.50 | 2.73/2.99 | 1.11/1.25 | 1.64/1.74 | 1.21/1.22 |
| NDPEG | −0.25/−0.35 | 2.71/2.96 | 1.48/1.66 | 1.23/1.30 | 0.51/0.51 |
| NDPGA | 0.07/−0.11 | 2.72/2.99 | 1.32/1.55 | 1.39/1.44 | 0.73/0.67 |
| Complex | Interaction Type | Bond Lengths A----B (Å) | Bond Angles (A-H----B) (°) | Type |
|---|---|---|---|---|
| ND2HP-TAM | C-H----O | 3.38 | 164.48 | Weak |
| C-H----O | 3.38 | 110.75 | Weak | |
| C-H----N | 4.69 | 149.21 | Weak | |
| C-H----O | 4.2 | 124.87 | Weak | |
| NDPEG-TAM | C-H----O | 4.2 | 89.35 | Weak |
| O-H----C | 4.01 | 132.79 | Weak | |
| C-H----O | 3.31 | 158.41 | Weak | |
| C-H----N | 3.96 | 129.22 | Weak | |
| NDPGA-TAM | C-H----O | 3.12 | 138.53 | Weak |
| C-H----O=C | 3.57 | 91.74 | Weak | |
| C-H----O=C | 4.07 | 92.05 | Weak |
| Complex | EA (eV) | IP (eV) | η (eV) | χ = −μ (eV) | ω (eV) |
|---|---|---|---|---|---|
| ND2HP-TAM | 0.75/0.64 | 2.84/3.08 | 1.05/1.22 | 1.79/1.86 | 1.53/1.42 |
| NDPEG-TAM | 0.66/0.35 | 2.75/3.00 | 1.05/1.33 | 1.72/1.67 | 1.39/1.05 |
| NDPGA-TAM | 0.70/0.41 | 2.73/2.98 | 1.02/1.29 | 1.72/1.69 | 1.45/1.11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landeros-Martinez, L.-L.; Glossman-Mitnik, D.; Orrantia-Borunda, E.; Flores-Holguín, N. New Methods of Esterification of Nanodiamonds in Fighting Breast Cancer—A Density Functional Theory Approach. Molecules 2017, 22, 1740. https://doi.org/10.3390/molecules22101740
Landeros-Martinez L-L, Glossman-Mitnik D, Orrantia-Borunda E, Flores-Holguín N. New Methods of Esterification of Nanodiamonds in Fighting Breast Cancer—A Density Functional Theory Approach. Molecules. 2017; 22(10):1740. https://doi.org/10.3390/molecules22101740
Chicago/Turabian StyleLanderos-Martinez, Linda-Lucila, Daniel Glossman-Mitnik, Erasmo Orrantia-Borunda, and Norma Flores-Holguín. 2017. "New Methods of Esterification of Nanodiamonds in Fighting Breast Cancer—A Density Functional Theory Approach" Molecules 22, no. 10: 1740. https://doi.org/10.3390/molecules22101740
APA StyleLanderos-Martinez, L.-L., Glossman-Mitnik, D., Orrantia-Borunda, E., & Flores-Holguín, N. (2017). New Methods of Esterification of Nanodiamonds in Fighting Breast Cancer—A Density Functional Theory Approach. Molecules, 22(10), 1740. https://doi.org/10.3390/molecules22101740