Natriuretic Peptides: The Case of Prostate Cancer
Abstract
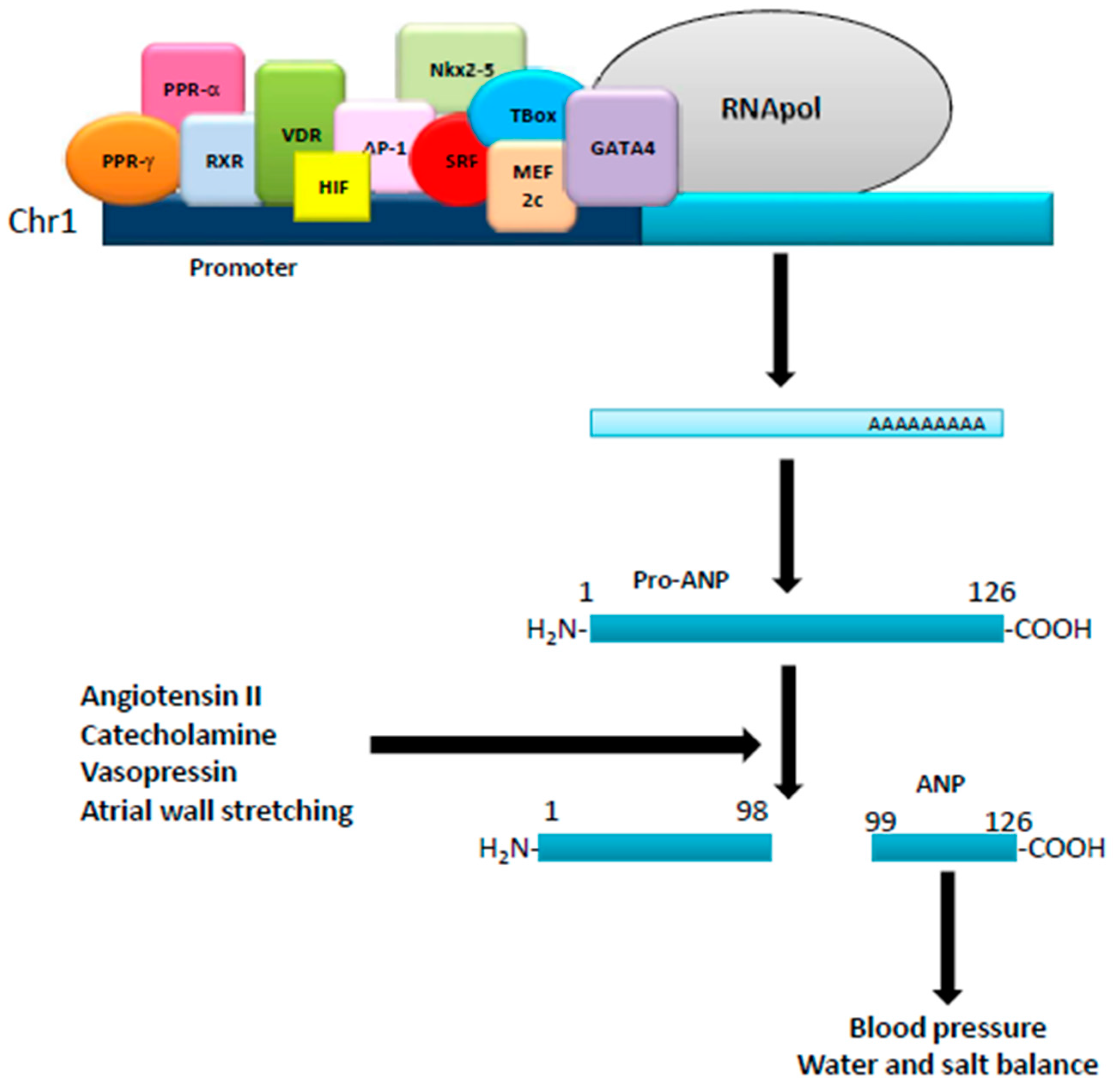
:1. Natriuretic Peptides: Background
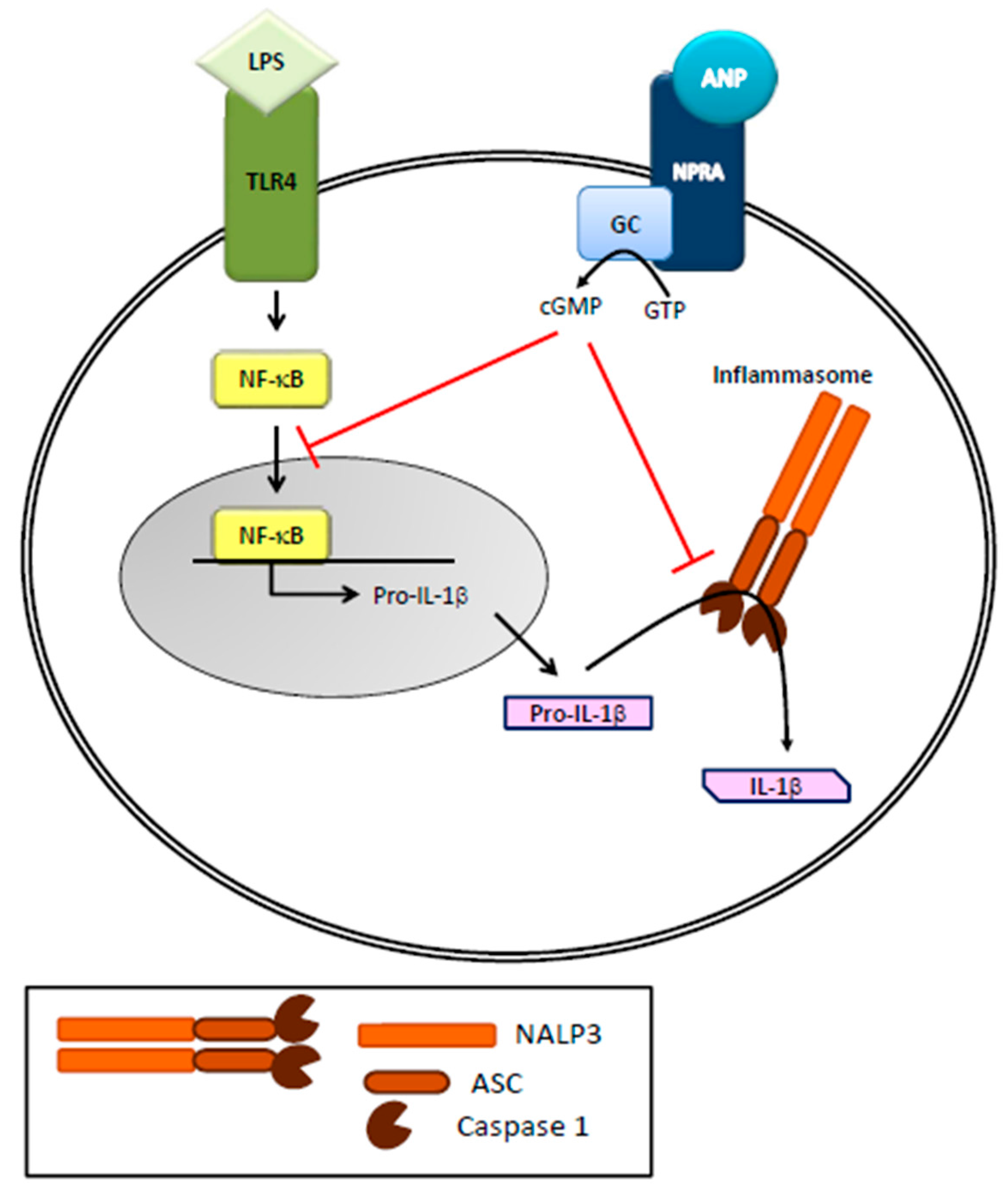
2. Natriuretic Peptides and the Immune System
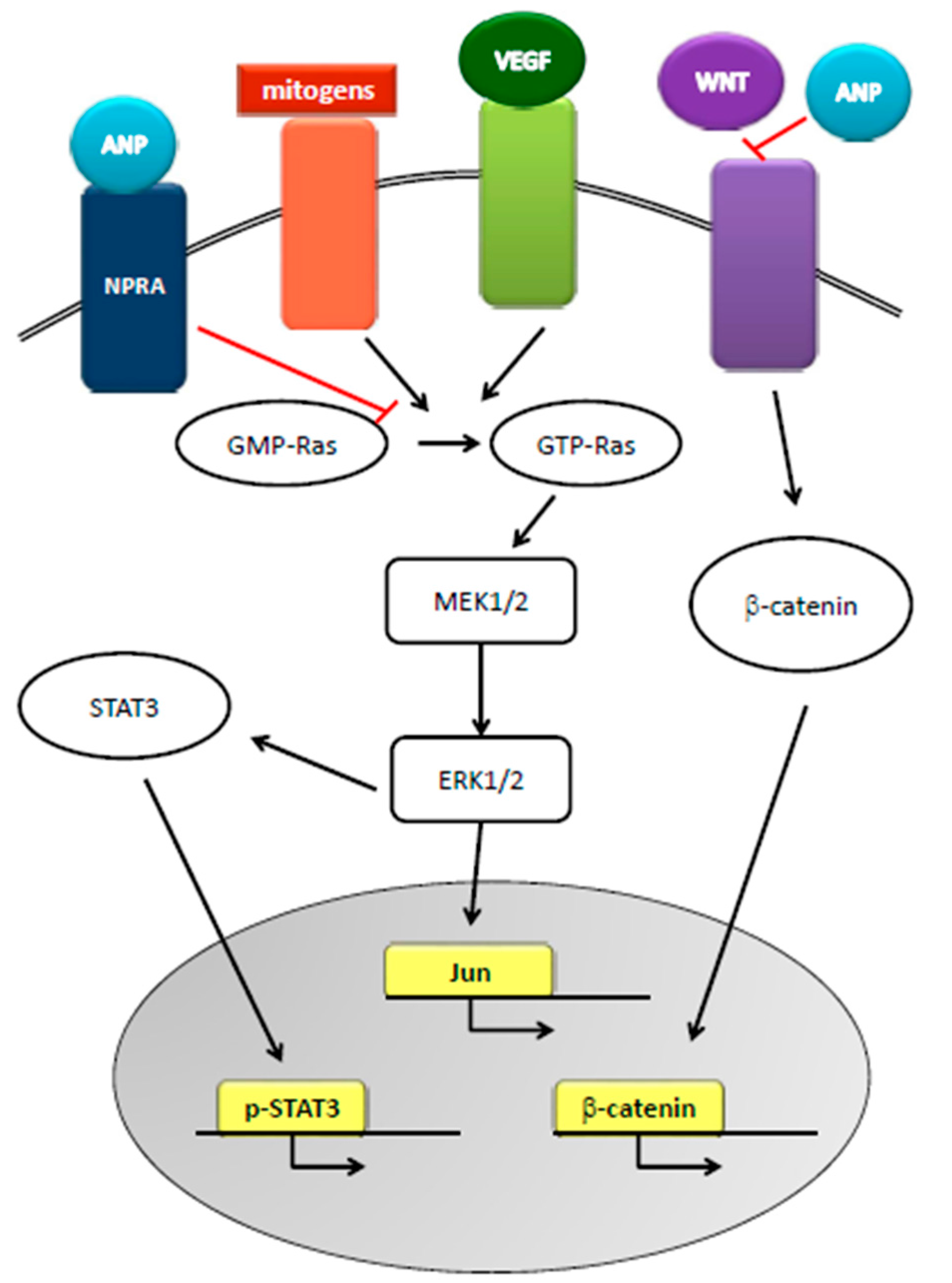
3. Natriuretic Peptides and Cancer
4. Prostate Cancer and Inflammation
5. Natriuretic Peptides and Prostate Cancer
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Santhekadur, P.K.; Kumar, D.P.; Seneshaw, M.; Mirshahi, F.; Sanyal, A.J. The multifaceted role of natriuretic peptides in metabolic syndrome. Biomed. Pharmacother. 2017, 92, 826–835. [Google Scholar] [CrossRef] [PubMed]
- De Vito, P. Atrial natriuretic peptide: An old hormone or a new cytokine? Peptides 2014, 58, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Cherian, D.; Verghese, P.P.; Jacob, J.J. Physiology and clinical significance of natriuretic hormones. Indian J. Endocrinol. Metab. 2013, 17, 83–90. [Google Scholar] [PubMed]
- Gardner, D.G.; Chen, S.; Glenn, D.J.; Grigsby, C.L. Molecular biology of the natriuretic peptide system: Implications for physiology and hypertension. Hypertension 2007, 49, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Nakamura, K.; Gardner, D.G. 1,25-dihydroxyvitamin D inhibits human ANP gene promoter activity. Regul. Pept. 2005, 128, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Buglioni, A.; Burnett, J.C., Jr. A gut-heart connection in cardiometabolic regulation. Nat. Med. 2013, 19, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Xia, X.; Xuan, Q.; Huang, B.Y.; Liu, S.Y.; Zhang, D.D.; Jiang, G.M.; Xu, Y.; Qin, Y.H. Neutral endopeptidase and natriuretic peptide receptors participate in the regulation of C-type natriuretic peptide expression in renal interstitial fibrosis. J. Recept. Signal Transduct. Res. 2017, 37, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R. Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol. Ther. 2011, 130, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Bellezza, I.; Collodel, G.; Fredholm, B.B. Promiscuous coupling and involvement of protein kinase C and extracellular signal-regulated kinase 1/2 in the adenosine A1 receptor signaling in mammalian spermatozoa. Biochem. Pharmacol. 2008, 75, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; de Sterke, A.; Willmes, D.M.; Spranger, J.; Jordan, J.; Birkenfeld, A.L. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol. Ther. 2014, 144, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.M. The role of atrial natriuretic peptide in immune system. Peptides 2005, 26, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Huang, X.; Zhang, K.; Jiang, H.; Hu, X. Anti-inflammatory effect of B-type natriuretic peptide postconditioning during myocardial ischemia-reperfusion: Involvement of PI3K/Akt signaling pathway. Inflammation 2014, 37, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Spannella, F.; Giulietti, F.; Balietti, P.; Cocci, G.; Bordicchia, M. Cardiac Natriuretic Peptides, Hypertension and Cardiovascular Risk. High Blood Press. Cardiovasc. Prev. 2017, 24, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Wu, C.; Hamid, T.; Arora, G.; Agha, O.; Allen, K.; Tainsh, R.E.; Hu, D.; Ryan, R.A.; Domian, I.J.; et al. Acute Metabolic Influences on the Natriuretic Peptide System in Humans. J. Am. Coll. Cardiol. 2016, 67, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H.; Yanai, H.; Kake, M.; Noda, M.; Ezaki, O. The association between daily physical activity and plasma B-type natriuretic peptide in patients with glucose intolerance: A cross-sectional study. BMJ Open 2015, 5, e006276. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, G.F.; Rodrigues, F.L.; Carneiro, F.S. Are the innate and adaptive immune systems setting hypertension on fire? Pharmacol. Res. 2017, 117, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Mezzasoma, L.; Antognelli, C.; Talesa, V.N. Atrial natriuretic peptide down-regulates LPS/ATP-mediated IL-1β release by inhibiting NF-kB, NLRP3 inflammasome and caspase-1 activation in THP-1 cells. Immunol. Res. 2016, 64, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Burnett, J.C., Jr. Atrial Natriuretic Peptide- Old But New Therapeutic in Cardiovascular Diseases. Circ. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Casserly, B.P.; Sears, E.H.; Gartman, E.J. The role of natriuretic peptides in inflammation and immunity. Recent Pat. Inflamm. Allergy Drug Discov. 2010, 4, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Mezzasoma, L.; Cagini, L.; Antognelli, C.; Puma, F.; Pacifico, E.; Talesa, V.N. TNF-α regulates natriuretic peptides and aquaporins in human bronchial epithelial cells BEAS-2B. Mediat. Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A.K.; Vollmar, A.M. The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann. Rheum Dis. 2001, 60, 68–70. [Google Scholar]
- Morikis, V.A.; Radecke, C.; Jiang, Y.; Heinrich, V.; Curry, F.R.; Simon, S.I. Atrial natriuretic peptide down-regulates neutrophil recruitment on inflamed endothelium by reducing cell deformability and resistance to detachment force. Biorheology 2015, 52, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Grottelli, S.; Costanzi, E.; Scarpelli, P.; Pigna, E.; Morozzi, G.; Mezzasoma, L.; Peirce, M.J.; Moresi, V.; Adamo, S.; et al. Peroxynitrite Activates the NLRP3 Inflammasome Cascade in SOD1(G93A) Mouse Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Menu, P.; Vince, J.E. The NALP3 inflammasome in health and diseases: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A. Gout: Will the “King of Diseases” be the first rheumatic disease to be cured? BMC Med. 2016, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, J.; Li, M.; Yang, Y.; Sun, K.; Wang, J. ANP-NPRA signaling pathway-a potential therapeutic target for the treatment of malignancy. Crit. Rev. Eukaryot. Gene Expr. 2013, 23, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.Z.; Nascimento, A.M.; Medeiros, A.R.S.; Caliman, I.F.; Dalpiaz, P.L.M.; Firmes, L.B.; Sousa, G.J.; Oliveira, P.W.C.; Andrade, T.U.; Reis, A.M.; et al. The selective estrogen receptor modulators (SERMs) raloxifene and tamoxifen improve ANP levels and decrease nuclear translocation of NF-kB in estrogen-deficient rats. Pharmacol. Rep. 2017, 9, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Gopi, V.; Subramanian, V.; Manivasagam, S.; Vellaichamy, E. Angiotensin II down-regulates natriuretic peptide receptor-A expression and guanylyl cyclase activity in H9c2 (2–1) cardiac myoblast cells: Role of ROS and NF-κB. Mol. Cell. Biochem. 2015, 409, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Yang, Y.; Yan, Y.; Li, J.; Qu, J.; Wang, J. NPR-A: A Therapeutic Target in Inflammation and Cancer. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Vellaichamy, E. Atrial natriuretic peptide (ANP) inhibits DMBA/croton oil induced skin tumor growth by modulating NF-κB, MMPs, and infiltrating mast cells in swiss albino mice. Eur. J. Pharmacol. 2014, 740, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Collier, P.; Voon, V.; Ledwidge, M.; McDonald, K.; Watson, C.; Baugh, J. Attenuation of monocyte chemotaxis-a novel anti-inflammatory mechanism of action for the cardio-protective hormone B-type natriuretic peptide. J. Cardiovasc. Transl. Res. 2013, 4, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Nagai-Okatani, C.; Kangawa, K.; Minamino, N. Three molecular forms of atrial natriuretic peptides: Quantitative analysis and biological characterization. J. Pept. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mezzasoma, L.; Antognelli, C.; Talesa, V.N. A Novel Role for Brain Natriuretic Peptide: Inhibition of IL-1β Secretion via Downregulation of NF-κB/Erk 1/2 and NALP3/ASC/Caspase-1 Activation in Human THP-1 Monocyte. Mediat. Inflamm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gentili, A.; Frangione, M.R.; Albini, E.; Vacca, C.; Ricci, M.A.; De Vuono, S.; Boni, M.; Rondelli, F.; Rotelli, L.; Lupattelli, G.; et al. Modulation of natriuretic peptide receptors in human adipose tissue: Molecular mechanisms behind the “natriuretic handicap” in morbidly obese patients. Transl. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bando, S.; Soeki, T.; Matsuura, T.; Tobiume, T.; Ise, T.; Kusunose, K.; Yamaguchi, K.; Yagi, S.; Fukuda, D.; Iwase, T.; et al. Plasma brain natriuretic peptide levels are elevated in patients with cancer. PLoS ONE 2017, 12, e0178607. [Google Scholar] [CrossRef] [PubMed]
- Furrer, R.; Eisele, P.S.; Schmidt, A.; Beer, M.; Handschin, C. Paracrine cross-talk between skeletal muscle and macrophages in exercise by PGC-1α-controlled BNP. Sci. Rep. 2017, 7, 40789. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Luo, C.Q.; Li, X. Systemic inflammatory response syndrome following burns is mediated by brain natriuretic peptide/natriuretic peptide A receptor-induced shock factor 1 signaling pathway. Clin. Exp. Pharmacol. Physiol. 2016, 43, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xia, H.Y.; Zhang, T.; Qi, L.C.; Zhang, B.Y.; Cui, R.; Chen, X.; Zhao, Y.R.; Li, X.Q. Protective effect of lyophilized recombinant human brain natriuretic peptide on renal ischemia/reperfusion injury in mice. Genet. Mol. Res. 2015, 14, 13300–13311. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.M.; Critchley, W.R.; Puchalka, C.M.; Williams, S.G.; Yonan, N.; Fildes, J.E. Brain natriuretic peptide induces CD8+ T cell death via a caspase 3 associated pathway-implications following heart transplantation. Transpl. Immunol. 2012, 26, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.F.; Tang, T.T.; Dong, W.Y.; Li, Y.Y.; Xia, N.; Zhang, W.C.; Zhou, S.F.; Yuan, J.; Liao, M.Y.; Li, J.J.; et al. Defective circulating CD4+ LAP+ regulatory T cells in patients with dilated cardiomyopathy. J. Leukoc. Biol. 2015, 97, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Wang, D.; Sullivan, R.; Fan, T.H.; Gladysheva, I.P.; Reed, G.L. Depressed Corin Levels Indicate Early Systolic Dysfunction Before Increases of Atrial Natriuretic Peptide/B-Type Natriuretic Peptide and Heart Failure Development. Hypertension 2016, 67, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Vesely, D.L. Cardiac and renal hormones: Anticancer effects in vitro and in vivo. J. Investig. Med. 2009, 57, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Vesely, D.L. New anticancer agents: Hormones made within the heart. Anticancer Res. 2012, 32, 2515–2521. [Google Scholar] [PubMed]
- Vesely, D.L. Family of peptides synthesized in the human body have anticancer effects. Anticancer Res. 2014, 34, 1459–1466. [Google Scholar] [PubMed]
- Vesely, D.L. Heart Peptide Hormones: Adjunct and Primary Treatments of Cancer. Anticancer Res. 2016, 36, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Pierimarchi, P. Atrial natriuretic peptide: A magic bullet for cancer therapy targeting Wnt signaling and cellular pH regulators. Curr Med. Chem. 2014, 21, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Raulji, P.; Mohapatra, S.S.; Patel, R.; Hellermann, G.; Kong, X.; Vera, P.L.; Meyer-Siegler, K.L.; Coppola, D.; Mohapatra, S. Natriuretic peptide receptor a as a novel target for prostate cancer. Mol. Cancer 2011, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Skelton, W.P., 4th; Pi, G.E.; Vesely, D.L. Four cardiac hormones cause death of human cancer cells but not of healthy cells. Anticancer Res. 2011, 31, 395–402. [Google Scholar] [PubMed]
- Popat, J.; Rivero, A.; Pratap, P.; Guglin, M. What is causing extremely elevated amino terminal brain natriuretic peptide in cancer patients? Congest. Heart Fail. 2013, 19, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, F.; Noris, A.; Beretta, L.; Mortini, P.; Gemma, M. Serum B-Type Natriuretic Peptide is Affected by Neoplastic Edema in Patients with a Brain Tumor. World Neurosurg. 2016, 85, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Tuñón, J.; Higueras, J.; Tarín, N.; Cristóbal, C.; Lorenzo, Ó.; Blanco-Colio, L.; Martín-Ventura, J.L.; Huelmos, A.; Alonso, J.; Aceña, Á.; et al. N-Terminal Pro-Brain Natriuretic Peptide Is Associated with a Future Diagnosis of Cancer in Patients with Coronary Artery Disease. PLoS ONE 2015, 10, e0126741. [Google Scholar]
- Rignault-Clerc, S.; Bielmann, C.; Liaudet, L.; Waeber, B.; Feihl, F.; Rosenblatt-Velin, N. Natriuretic Peptide Receptor B modulates the proliferation of the cardiac cells expressing the Stem Cell Antigen-1. Sci. Rep. 2017, 7, 41936. [Google Scholar] [CrossRef] [PubMed]
- Andreu, A.; Guglin, M. Exaggerated NT-proBNP production in patients with hematologic malignancies: A case series. Congest. Heart Fail. 2012, 18, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Vincent Rajkumar, S.; Merlini, G.; Kumar, S.; Gertz, M.A.; Palladini, G.; Lacy, M.Q.; Hayman, S.R.; Leung, N.; et al. N-terminal fragment of the type-B natriuretic peptide (NT-proBNP) contributes to a simple new frailty score in patients with newly diagnosed multiple myeloma. Am. J. Hematol. 2016, 91, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Mylin, A.K.; Goetze, J.P.; Heickendorff, L.; Ahlberg, L.; Dahl, I.M.; Abildgaard, N.; Gimsing, P.; Nordic Myeloma Study Group. N-terminal pro-C-type natriuretic peptide in serum associated with bone destruction in patients with multiple myeloma. Biomark. Med. 2015, 9, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nojiri, T.; Hino, J.; Hosoda, H.; Miura, K.; Shintani, Y.; Inoue, M.; Zenitani, M.; et al. C-type natriuretic peptide ameliorates pulmonary fibrosis by acting on lung fibroblasts in mice. Respir. Res. 2016, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Zenitani, M.; Nojiri, T.; Uehara, S.; Miura, K.; Hosoda, H.; Kimura, T.; Nakahata, K.; Miyazato, M.; Okuyama, H.; Kangawa, K. C-type natriuretic peptide in combination with sildenafil attenuates proliferation of rhabdomyosarcoma cells. Cancer Med. 2016, 5, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Ge, D.; Cunningham, D.M.; Huang, F.; Ma, L.; Burris, T.P.; You, Z. Targeting Th17-IL-17 Pathway in Prevention of Micro-Invasive Prostate Cancer in a Mouse Model. Prostate 2017, 77, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Tanday, S. Guanylin hormone loss could trigger colon cancer. Lancet Oncol. 2014, 15, e537. [Google Scholar] [CrossRef]
- Mallela, J.; Ravi, S.; Jean Louis, F.; Mulaney, B.; Cheung, M.; Sree Garapati, U.; Chinnasamy, V.; Wang, C.; Nagaraj, S.; Mohapatra, S.S.; et al. Natriuretic peptide receptor A signaling regulates stem cell recruitment and angiogenesis: A model to study linkage between inflammation and tumorigenesis. Stem Cells 2013, 31, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Associazione italiana registri tumori (AIRTUM). 2015. Available online: http://www.registri-tumori.it/cms/ (accessed on 15 March 2017).
- Strope, J.D.; Price, D.K.; Figg, W.D. Building a hit list for the fight against metastatic castration resistant prostate cancer. Cancer Biol. Ther. 2016, 17, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Uhlman, M.A.; Bing, M.T.; Lubaroff, D.M. Prostate cancer vaccines in combination with additional treatment modalities. Immunol. Res. 2014, 59, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Scarpelli, P.; Pizzo, S.V.; Grottelli, S.; Costanzi, E.; Minelli, A. ROS-independent Nrf2 activation in prostate cancer. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Autio, K.; Ryan, C.J.; Mulders, P.; Shore, N.; Kheoh, T.; Fizazi, K.; Logothetis, C.J.; Rathkopf, D.; Smith, M.R.; et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: Patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013, 14, 1193–1199. [Google Scholar] [CrossRef]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Sigala, S.; Sandhu, S.; Bonetta, A.; Cappelletti, M.R.; Zanotti, L.; Bottini, A.; Sternberg, C.N.; Fox, S.B.; Generali, D. Role of the novel generation of androgen receptor pathway targeted 111 agents in the management of castration-resistant prostate cancer: A literature based meta-analysis of 112 randomized trials. Eur. J. Cancer 2016, 61, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z. Targeting the androgen receptor in prostate cancer. Expert Opin. Pharmacother. 2014, 15, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.S.; Amin, M.A.; Li, X.; Kalyana-Sundaram, S.; Veeneman, B.A.; Wang, L.; Ghosh, A.; Aslam, A.; et al. Inflammation-Induced Oxidative Stress Mediates Gene Fusion Formation in Prostate Cancer. Cell Rep. 2016, 17, 2620–2631. [Google Scholar] [CrossRef] [PubMed]
- Popovics, P.; Schally, A.V.; Salgueiro, L.; Kovacs, K.; Rick, F.G. Antagonists of growth hormone-releasing hormone inhibit proliferation induced by inflammation in prostatic epithelial cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.K.; Daugherty, S.E.; Liao, L.M.; Freedman, N.D.; Abnet, C.C.; Pfeiffer, R.; Cook, M.B. Do Aspirin and Other NSAIDs Confer a Survival Benefit in Men Diagnosed with Prostate Cancer? A Pooled Analysis of NIH-AARP and PLCO Cohorts. Cancer Prev. Res. (Phila.) 2017. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.D.; Chen, Y.H.; Xu, S.; Zhang, C.; Wang, D.M.; Wang, H.; Chen, L.; Zhang, Z.H.; Xia, M.Z.; Xu, D.X.; et al. Low vitamin D status is associated with inflammation in patients with prostate cancer. Oncotarget 2017, 8, 22076–22085. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Kazma, R.; Mefford, J.A.; Cheng, I.; Plummer, S.J.; Levin, A.M.; Rybicki, B.A.; Casey, G.; Witte, J.S. Association of the innate immunity and inflammation pathway with advanced prostate cancer risk. PLoS ONE 2012, 7, e51680. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Candore, G.; Lio, D.; Carruba, G. Prostate cancer: From the pathophysiologic implications of some genetic risk factors to translation in personalized cancer treatments. Cancer Gene Ther. 2014, 21, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Bellezza, I.; Tucci, A.; Conte, C.; Bracarda, S.; Culig, Z. 2-chloroadenosine modulates PAR-1 and IL-23 expression and enhances docetaxel effects on PC3 cells. Prostate 2008, 68, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Bellezza, I.; Tucci, A.; Rambotti, M.G.; Conte, C.; Culig, Z. Differential involvement of reactive oxygen species and nucleoside transporters in cytotoxicity induced by two adenosine analogues in human prostate cancer cells. Prostate 2009, 69, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Bellezza, I.; Conte, C.; Culig, Z. Oxidative stress-related aging: A role for prostate cancer? Biochim. Biophys. Acta 2009, 1795, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Mezzasoma, L.; Mearini, E.; Talesa, V.N. Glyoxalase 1–419C > A variant is associated with oxidative stress: Implications in prostate cancer progression. PLoS ONE 2013, 8, e74014. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Mezzasoma, L.; Fettucciari, K.; Mearini, E.; Talesa, V.N. Role of glyoxalase I in the proliferation and apoptosis control of human LNCaP and PC3 prostate cancer cells. Prostate 2013, 73, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Mezzasoma, L.; Fettucciari, K.; Talesa, V.N. A novel mechanism of methylglyoxal cytotoxicity in prostate cancer cells. Int. J. Biochem. Cell Biol. 2013, 45, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Iyengar, N.M.; Zhou, X.K.; Giri, D.D.; Falcone, D.J.; Wang, H.; Williams, S.; Krasne, M.D.; Yaghnam, I.; Kunzel, B.; et al. Periprostatic adipose inflammation is associated with high-grade prostate cancer. Prostate Cancer Prostatic. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Dorsey, T.H.; Tang, W.; Jordan, S.V.; Loffredo, C.A.; Ambs, S. Aspirin Use Reduces the Risk of Aggressive Prostate Cancer and Disease Recurrence in African-American Men. Cancer Epidemiol. Biomark. Prev. 2017, 26, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Li, J.; Yadav, S.S.; Tewari, A.K. Recent insights into NF-κB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014, 114, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Taverna, G.; Pedretti, E.; Di Caro, G.; Borroni, E.M.; Marchesi, F.; Grizzi, F. Inflammation and prostate cancer: Friends or foe? Inflamm. Res. 2015, 64, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Rohena-Rivera, K.; Sánchez-Vázquez, M.M.; Aponte-Colón, D.A.; Forestier-Román, I.S.; Quintero-Aguiló, M.E.; Martínez-Ferrer, M. IL-15 regulates migration, invasion, angiogenesis and genes associated with lipid metabolism and inflammation in prostate cancer. PLoS ONE 2017, 12, e0172786. [Google Scholar] [CrossRef] [PubMed]
- Strasner, A.; Karin, M. Immune Infiltration and Prostate Cancer. Front. Oncol. 2015, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Karan, D.; Dubey, S. From Inflammation to Prostate Cancer: The Role of Inflammasomes. Adv. Urol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Livas, L.; Kyprianou, N. Inflammation in prostate cancer progression and therapeutic targeting. Transl. Androl. Urol. 2015, 4, 455–463. [Google Scholar] [PubMed]
- Nakai, Y.; Nonomura, N. Inflammation and prostate carcinogenesis. Int. J. Urol. 2013, 20, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, P.F.; Brustmann, H.; Seklehner, S.; Riedl, C.R. Chronic asymptomatic inflammation of the prostate type IV and carcinoma of the prostate: Is there a correlation? Scand. J. Urol. 2013, 47, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.; Lucia, M.S.; Thompson, I.M., Jr.; Goodman, P.J.; Tangen, C.M.; Kristal, A.R.; Parnes, H.L.; Hoque, A.; Lippman, S.M.; Sutcliffe, S.; et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 847–856. [Google Scholar]
- Moreira, D.M.; Nickel, J.C.; Gerber, L.; Muller, R.L.; Andriole, G.L.; Castro-Santamaria, R.; Freedland, S.J. Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: Results from the REDUCE study. Cancer 2014, 120, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yli-Hemminki, T.H.; Laurila, M.; Auvinen, A.; Määttänen, L.; Huhtala, H.; Tammela, T.L.; Kujala, P.M. Histological inflammation and risk of subsequent prostate cancer among men with initially elevated serum prostate-specific antigen (PSA) concentration in the Finnish prostate cancer screening trial. BJU Int. 2013, 112, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Fernandez-Gomez, J.; Sacristán, R.; Fernandez-Garcia, B.; Lobo, B.; Gonzalez-Suarez, J.; Quintas, A.; Escaf, S.; Vizoso, F.J. Stromal factors involved in human prostate cancer development, progression and castration resistance. J. Cancer Res. Clin. Oncol. 2017, 143, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.D.; Goldstein, A.S. Functional evidence that progenitor cells near sites of inflammation are precursors for aggressive prostate cancer. Mol. Cell. Oncol. 2017, 4, e1279723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Deng, R.; Wang, Y.; Zhang, H.; Dou, J.; Li, L.; Du, Y.; Chen, R.; Cheng, J.; Yu, J. Twist1/Dnmt3a and miR186 establish a regulatory circuit that controls inflammation-associated prostate cancer progression. Oncogenesis 2017, 6, e315. [Google Scholar] [CrossRef] [PubMed]
- Cimadamore, A.; Scarpelli, M.; Piva, F.; Massari, F.; Gasparrini, S.; Doria, A.; Cheng, L.; Lopez-Beltran, A.; Montironi, R. Activity of chemokines in prostate and renal tumors and their potential role as future therapeutic targets. Future Oncol. 2017, 13, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, J.; Frey, L.; Gang, X.; Wu, K.; Liu, Q.; Lilly, M.; Wu, J. Prostate-specific IL-6 transgene autonomously induce prostate neoplasm through amplifying inflammation in the prostate and peri-prostatic adipose tissue. J. Hematol. Oncol. 2017, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.T.; Son, D.J.; Lee, C.K.; Yoon, D.Y.; Lee, D.H.; Park, M.H. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 2017, 174, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Colón, L.; Cajigas-Du Ross, C.K.; Basu, A.; Elix, C.; Alicea-Polanco, I.; Sanchez, T.W.; Radhakrishnan, V.; Chen, C.S.; Casiano, C.A. Targeting the stress oncoprotein LEDGF/p75 to sensitize chemoresistant prostate cancer cells to taxanes. Oncotarget. 2017, 8, 24915–24931. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, X.; Xu, W.; Behera, S.; Hellermann, G.; Kumar, A.; Lockey, R.F.; Mohapatra, S.; Mohapatra, S.S. Natriuretic peptide receptor a as a novel anticancer target. Cancer Res. 2008, 68, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T.; Chang, H.W.; Wu, M.J.; Lai, Y.T.; Wu, W.C.; Yu, W.C.; Chang, V.H. Klf10 deficiency in mice exacerbates pulmonary inflammation by increasing expression of the proinflammatory molecule NPRA. Int. J. Biochem. Cell Biol. 2016, 79, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Santos, M.; Jones, L.W.; Beckman, J.A.; Penson, D.F.; Morgans, A.K.; Moslehi, J. Cardiovascular Effects of Androgen Deprivation Therapy for the Treatment of Prostate Cancer: ABCDE Steps to Reduce Cardiovascular Disease in Patients With Prostate Cancer. Circulation 2016, 33, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Pressly, E.D.; Pierce, R.A.; Connal, L.A.; Hawker, C.J.; Liu, Y. Nanoparticle PET/CT imaging of natriuretic peptide clearance receptor in prostate cancer. Bioconj. Chem. 2013, 24, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Skelton, W.P., 4th; Skelton, M.; Vesely, D.L. Cardiac hormones are potent inhibitors of secreted frizzled-related protein-3 in human cancer cells. Exp. Ther. Med. 2013, 5, 475–478. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezzasoma, L.; Peirce, M.J.; Minelli, A.; Bellezza, I. Natriuretic Peptides: The Case of Prostate Cancer. Molecules 2017, 22, 1680. https://doi.org/10.3390/molecules22101680
Mezzasoma L, Peirce MJ, Minelli A, Bellezza I. Natriuretic Peptides: The Case of Prostate Cancer. Molecules. 2017; 22(10):1680. https://doi.org/10.3390/molecules22101680
Chicago/Turabian StyleMezzasoma, Letizia, Matthew J. Peirce, Alba Minelli, and Ilaria Bellezza. 2017. "Natriuretic Peptides: The Case of Prostate Cancer" Molecules 22, no. 10: 1680. https://doi.org/10.3390/molecules22101680
APA StyleMezzasoma, L., Peirce, M. J., Minelli, A., & Bellezza, I. (2017). Natriuretic Peptides: The Case of Prostate Cancer. Molecules, 22(10), 1680. https://doi.org/10.3390/molecules22101680





