DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE
Abstract
:1. Introduction
2. Results and Discussion
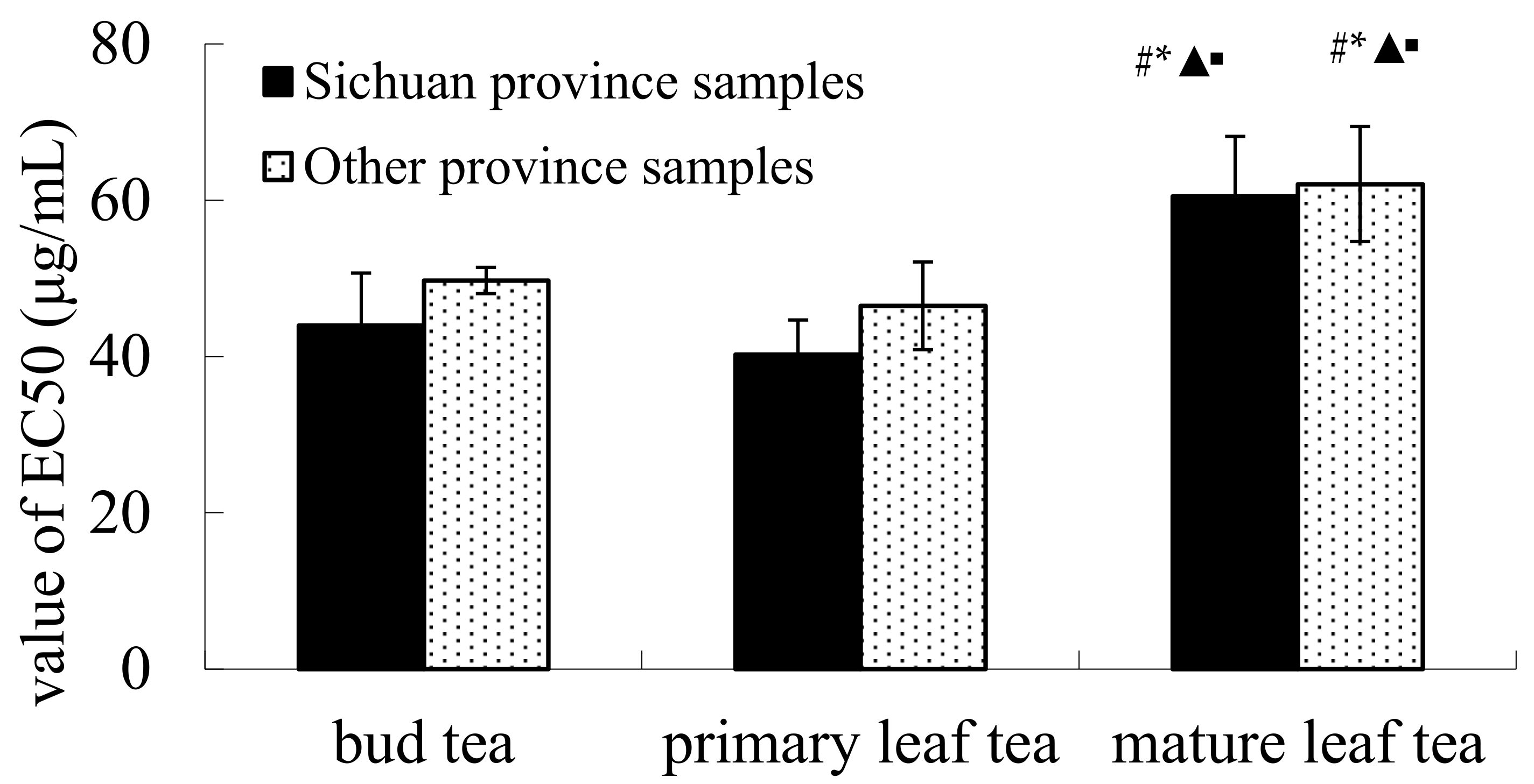
2.1. DPPH Radical Scavenging Activity
2.2. Antibacterial Test
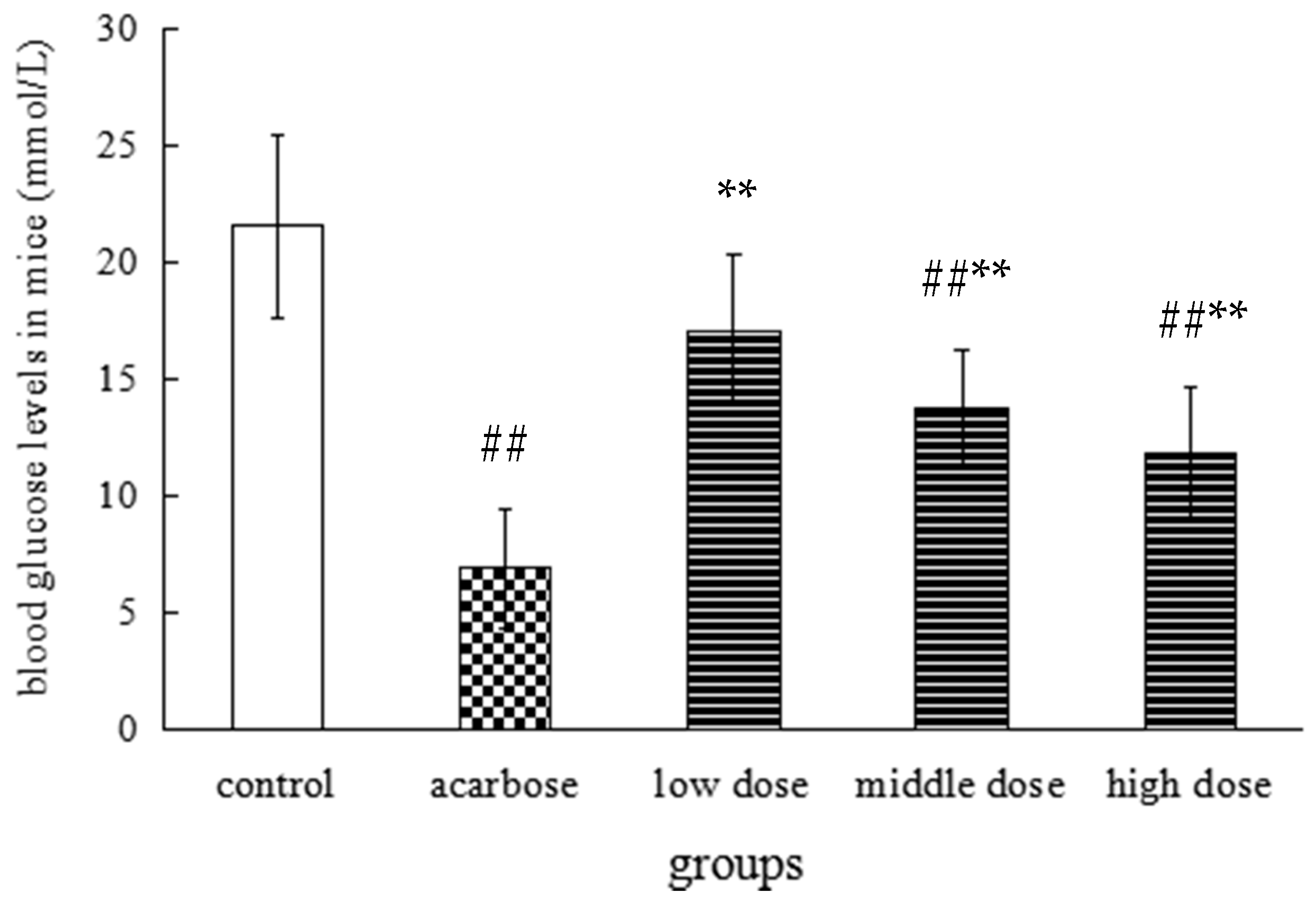
2.3. The Effect on the Level of Postprandial Blood Glucose in Mice
2.4. α-Glucosidase Inhibitory Effect In Vitro
2.5. Quantitative Analysis
2.5.1. Optimization of the Extraction Method
2.5.2. Validation of Method
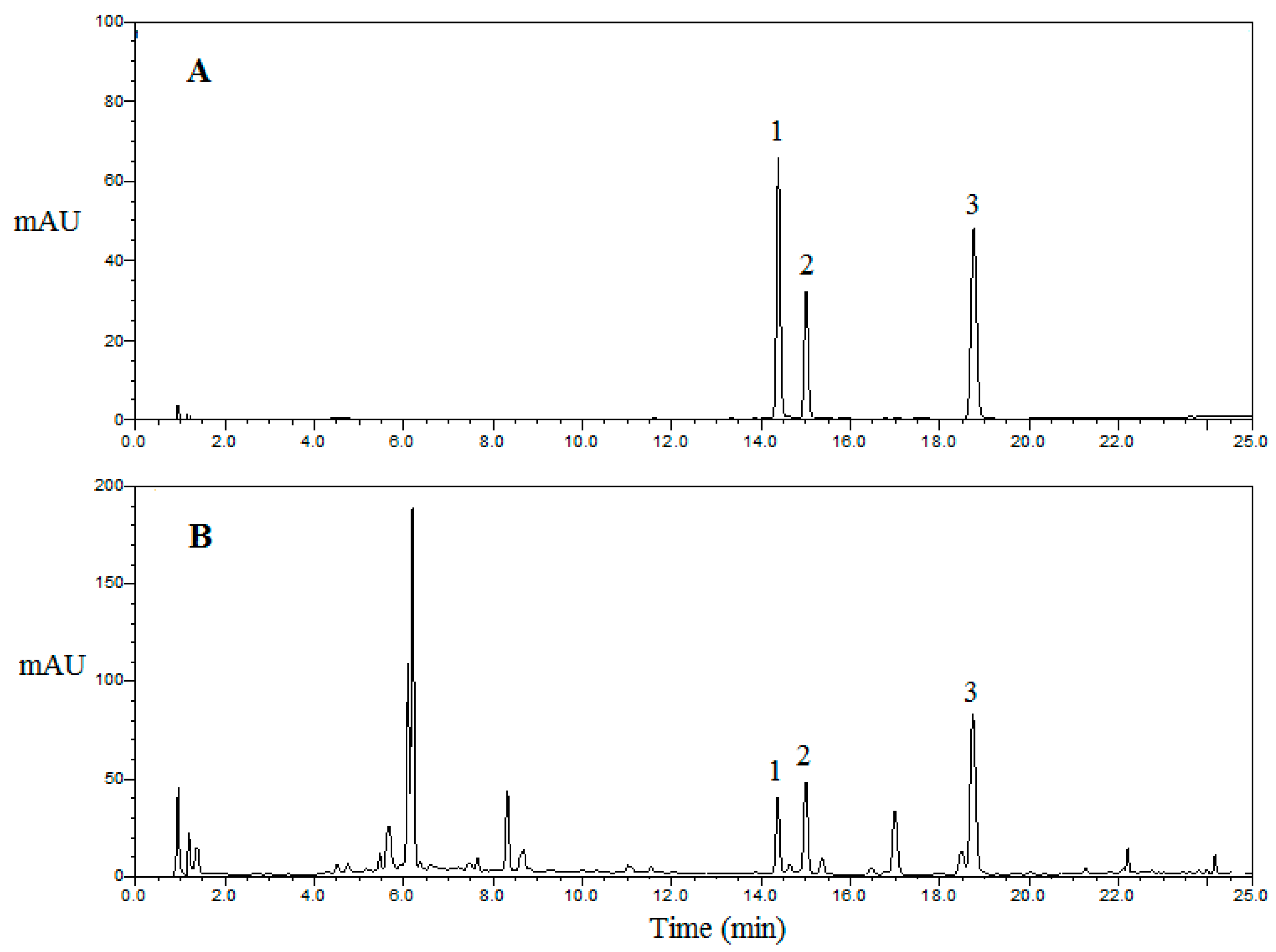
2.5.3. Quantitative Analysis of Samples
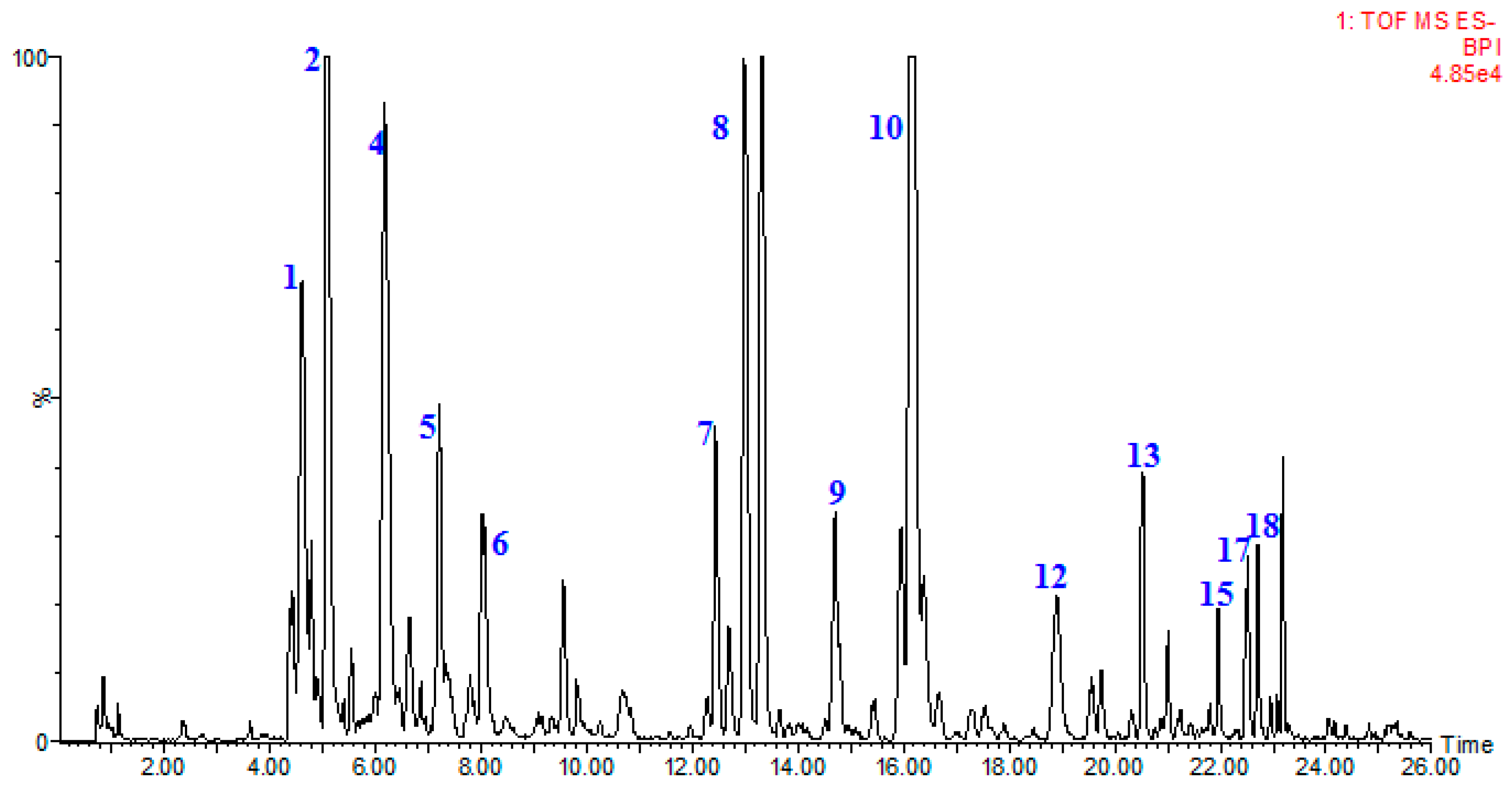
2.6. Identification of Flavonoids
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Plant Materials
3.3. Bacterial Species
3.4. Samples Preparation
3.5. Bioactivity Assay
3.5.1. DPPH Radical Scavenging Assay
3.5.2. Antibacterial Assay
3.5.3. Blood Glucose Levels in Mice
3.5.4. α-Glucosidase Inhibition Assasy
3.5.5. Statistical Analysis
3.6. Equipment and Chromatographic Conditions
3.6.1. UPLC-DAD Analysis Conditions
3.6.2. UPLC-ESI-QTOF-MS Analysis Conditions
3.7. Method Validation
3.7.1. Linearity, Limits of Detection and Limits of Quantification
3.7.2. Accuracy, Precision and Repeatability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Aoshima, H.; Hirata, S.; Ayabe, S. Antioxidative and anti-hydrogen peroxide activities of various herbal teas. Food Chem. 2007, 103, 617–622. [Google Scholar] [CrossRef]
- Hector, H.F.K.; Felipe, M.A.d.S.; Fábio, C.G.; Antonia, Q.L.D.S.; Afonso, D.L.D.S. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L.f.) by UPLC-ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar]
- Chen, L.X.; Hu, D.J.; Lam, S.C.; Ge, L.; Wu, D.; Zhao, J.; Long, Z.R.; Yang, W.J.; Fan, B.; Li, S.P. Comparison of antioxidant activities of different parts from snow chrysanthemum (Coreopsis tinctoria Nutt.) and identification of their natural antioxidants using high performance liquid chromatography coupled with diode array detection and mass spectrometry and 2,2′-azinobis(3-ethylbenzthiazoline-sulfonic acid)diammonium salt-based assay. J. Chromatogr. A 2016, 1428, 134–142. [Google Scholar] [PubMed]
- Jia, X.J.; Ding, C.B.; Yuan, S.; Zhang, Z.W.; Chen, Y.E.; Du, L.; Yuan, M. Extraction, purification and characterization of polysaccharides from Hawk tea. Carbohydr. Polym. 2014, 99, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Li, J.; Zou, Y.H.; Cheng, W.M.; Lu, C.; Zhang, L.; Ge, J.F.; Huang, C.; Jin, Y.; Lv, X.W.; et al. Preventive effects of total flavonoids of Litsea coreana leve on hepatic steatosis in rats fed with high fat diet. J. Ethnopharmacol. 2009, 121, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Lu, Y.X.; Wang, L.Y.; Li, D.D.; Zhang, Q.; Zhang, L.Z.; Yang, F.; Li, J. Study on the mechanism of action of total flavonoids of Litsea coreana for reducing blood glucose level in rat with type 2 diabetes mellitus. Chin. J. Integr. Tradit. West. Med. 2010, 30, 617–621. [Google Scholar]
- Ye, M.; Liu, D.; Zhang, R.; Yang, L.; Wang, J. Effect of hawk tea (Litsea coreana L.) on the numbers of lactic acid bacteria and flavour compounds of yoghurt. Int. Dairy J. 2012, 23, 68–71. [Google Scholar] [CrossRef]
- Yuan, M.; Jia, X.J.; Ding, C.B.; Yuan, S.; Zhang, Z.W.; Chen, Y.E. Comparative studies on bioactive constituents in Hawk tea infusions with different maturity degree and their antioxidant activities. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.J.; Dong, L.H.; Yang, Y.; Yuan, S.; Zhang, Z.W.; Yuan, M. Preliminary structural characterization and antioxidant activities of polysaccharides extracted from Hawk tea (Litsea coreana var. Lanuginosa). Carbohydr. Polym. 2013, 95, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, D.; Yan, X.W.; Wang, J.W.; Yao, L.; Tan, L.H.; Zhao, S.P.; Li, N.; Cao, W.G. Comparative Evaluation of the Chemical Composition, Antioxidant and Antimicrobial Activities of the Volatile Oils of Hawk Tea from Six Botanical Origins. Chem. Biodivers. 2016, 13, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Qian, Z.M.; Li, X.X.; Li, D.Q.; Huang, W.H.; Zhao, J.; Li, S.Q. Free radical scavenging activity of Eagle tea and their flavonoids. Acta Pharm. Sin. B 2012, 3, 246–249. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Deetae, P.; Parichanon, P.; Trakunleewatthana, P.; Chanseetis, C.; Lertsiri, S. Antioxidant and anti-glycation properties of Thai herbal teas in comparison with conventional teas. Food Chem. 2012, 133, 953–959. [Google Scholar] [CrossRef]
- Morita, M.; Naito, Y.J.; Yoshikawa, T.; Niki, E. Antioxidant capacity of blueberry extracts: Peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants. J. Berry Res. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Ji, H.F.; Zhang, L.W.; Zhang, L.; Wang, Q.; Zhang, H.R. Microwave-assisted extraction and antibacterial bioactivity of total flavonoids from Litsea coreana L. J. Henan Inst. Sci. Technol. 2011, 39, 27–31. [Google Scholar]
- Han, H.Y.; Cao, A.; Wang, L.; Guo, H.J.; Zang, Y.J.; Li, Z.Z.; Zhang, X.M.; Peng, W. Huangqi Decoction Ameliorates Streptozotocin-Induced Rat Diabetic Nephropathy through Antioxidant and Regulation of the TGF-β/MAPK/PPAR-γ Signaling. Cell. Physiol. Biochem. 2017, 42, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Umpierrez, G.E. Oxidative stress and inflammation in hyperglycemic crises and resolution with insulin: Implications for the acute and chronic complications of hyperglycemia. J. Diabetes Complicat. 2012, 26, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Zhang, Q.; Fu, J.; Woods, C.G.; Hou, Y.; Corkey, B.E.; Collins, S.; Andersen, M.E. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharmacol. 2010, 244, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Guido, M.C.; Marques, A.F.; Tavares, E.R.; Tavares de Melo, M.D.; Salemi, V.M.C.; Maranhão, R.C. The Effects of Diabetes Induction on the Rat Heart: Differences in Oxidative Stress, Inflammatory Cells, and Fibrosis between Subendocardial and Interstitial Myocardial Areas. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, J.; Zhao, T.; Liu, H. Effects of intensive glycemic control in ocular complications in patients with type 2 diabetes: A meta-analysis of randomized clinical trials. Endocrine 2015, 49, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.J.; Li, T.M.; Ko, C.H.; Cheng, C.F.; Ho, T.J.; Liu, X.; Tsang, H.Y.; Lin, T.H.; Liao, C.C.; Li, J.P.; et al. Effects of Chinese herbal medicines on the occurrence of diabetic retinopathy in type 2 diabetes patients and protection of ARPE-19 retina cells by inhibiting oxidative stress. Oncotarget 2017. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.M.; Weerarathna, T.P.; Fonseka, C.L.; Vidanagamage, A.S. Targeting postprandial blood sugar over fasting blood sugar: A clinic based comparative study. Diabetes Metab. Syndr. 2017, 11, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.J.; Bailey, C.J. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Dai, Y.; Villani, T.; Giurleo, D.; Simon, J.E.; Wu, Q. Synthesis of novel flavonoid alkaloids as α-glucosidase inhibitors. Bioorg. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Zhong, Y.Y.; Yang, Y.; Wang, X.Y. Inhibitory effect of ethanol extract from Hawk tea on α-glucosidase. J. Xiamen Univ. (Nat. Sci.) 2016, 55, 842–846. [Google Scholar]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y.F. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Liu, M.Y.; Ruan, J.Y. Metabolomics analysis reveals the metabolic and functional roles of flavonoids in light-sensitive tea leaves. BMC Plant Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromatogr. A 2003, 1018, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Cheng, W.M.; Li, J. Analyse on the chemical constituents from flavonoids of Litsea coreana L. Acta Univ. Med. Anhui 2008, 43, 65–67. [Google Scholar]
- Yu, J.P.; Gu, L.Q. The chemical constituent of laoying Tea from Guizhou. J. Plant Resour. Environ. 2001, 10, 61–62. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-González, M.; Cancho-Grande, B.; Boso, S.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Evolution of flavonoids in Mouratón berries taken from both bunch halves. Food Chem. 2013, 138, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.; Tattini, M.; Jay-Allemand, C. New evidences for the functional roles of secondary metabolites in plant-environment interactions. Environ. Exp. Bot. 2015, 196, 1–5. [Google Scholar]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Liu, G.Q.; Dong, J.; Wang, H.; Wan, L.R.; Duan, Y.S.; Chen, S.Z. ESI fragmentation studies of four tea catechins. Chem. J. Chin. Univ. 2009, 8, 1566–1570. [Google Scholar]
- Hümmer, W.; Schreier, P. Analysis of proanthocyanidins. Mol. Nutr. Food Res. 2008, 12, 1381–1398. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Yu, J.N.; Li, B.L.; Wen, Y.; Liu, R.; Tan, J. Separation and identification of A-type and B-type dimers of procyanidins from Peanut Skin by RP-HPLC-ESI-MS/MS. Food Sci. 2013, 23, 142–146. [Google Scholar]
- Fraser, K.; Collette, V.; Hancock, K.R. Characterization of proanthocyanidins from seeds of perennial ryegrass (Lolium perenne L.) and tall fescue (Festuca arundinacea) by liquid chromatography-Mass spectrometry. J. Agric. Food Chem. 2016, 35, 6676–6684. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, R.; Tang, Y.N. LC-MS/MS analysis of eight kinds of flavonoids. Chin. Pharm. 2014, 17, 1292–1297. [Google Scholar]
- Tan, L.H.; Zhang, D.; Wang, G.; Yu, B.; Zhao, S.P.; Wang, J.W.; Yao, L.; Cao, W.G. Comparative analyses of flavonoids compositions and antioxidant activities of Hawk tea from six botanical origins. Ind. Crop. Prod. 2016, 80, 123–130. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.L.; Zhang, Y.L.; Tang, M.F.; Tang, W.J.; Li, J. Chemical constituents from Litsea Coreana L. Acta Univ. Med. Anhui 2014, 4, 479–483. [Google Scholar]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G. Inhibitory effects of polyphenols from water chestnut (Trapa japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated alpha-glucosidase and alpha-amylase inhibition. J. Food Sci. 2010, 75, 97–102. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the S10–S27 are available from the authors. |
| Sample | S. aureus | B. subtilis | B. cereus | E. coli | P. aeruginosa | P. vulgaris |
|---|---|---|---|---|---|---|
| Bud tea | 3.48 ± 1.89 | 3.69 ± 1.81 | 4.56 ± 1.96 | 4.99 ± 2.78 | 4.78 ± 2.42 | 10.86 ± 5.79 |
| Primary leaf tea | 3.48 ± 0.86 | 3.26 ± 0.98 | 6.95 ± 1.72 | 3.91 ± 1.69 | 6.73 ± 2.20 | 6.52 ± 1.96 # |
| Mature leaf tea | 5.65 ± 2.06 # * | 5.43 ± 2.35 | 5.87 ± 2.39 | 6.08 ± 3.96 | 5.21 ± 2.59 | 4.78 ± 1.72 ## |
| Groups | α-Glucosidase Inhibitory Effect (%) | ||
|---|---|---|---|
| Low Concentrations (0.25 mg/mL) | Middle Concentrations (0.5 mg/mL) | High Concentrations (1 mg/mL) | |
| Acarbose | 51.71 ± 2.17 | 65.43 ± 3.50 | 73.94 ± 4.15 |
| ME | 72.19 ± 4.21 ** | 81.56 ± 3.11 ** | 88.23 ± 2.99 ** |
| PE | 19.56 ± 3.47 **, ## | 41.24 ± 2.77 **, ## | 54.87 ± 3.01 **, ## |
| EA | 81.16 ± 4.33 ** | 86.37 ± 3.89 ** | 92.17 ± 3.67 ** |
| n-BuOH | 80.92 ± 2.08 ** | 84.16 ± 3.68 ** | 91.29 ± 3.43 ** |
| WF | 56.23 ± 4.26 ## | 68.94 ± 3.54 ## | 77.86 ± 2.91 ## |
| Analytes | Linear Regression Equation of 3 Flavonoids | LOD (μg/mL) | LOQ (μg/mL) | Recovery (%) Mean ± SD | ||
|---|---|---|---|---|---|---|
| Regressive Equation | r2 | Test Range (μg/mL) | ||||
| Hyperoside | Y = 35.125X − 1.3227 | 0.9991 | 1.72–880.00 | 0.218 | 0.656 | 98.2 ± 2.35 |
| Isoquercitrin | Y = 48.820X − 1.0970 | 0.9993 | 1.24–639.00 | 0.308 | 0.922 | 101.3 ± 1.78 |
| Astragalin | Y = 12.930X − 0.6021 | 0.9984 | 0.36–360.00 | 0.086 | 0.257 | 103.1 ± 2.66 |
| Sample | Kinds | Hyperoside (mg/g) | Isoquercitrin (mg/g) | Astragalin (mg/g) | Total Content (mg/g) |
|---|---|---|---|---|---|
| S1 | bud tea | 1.62 ± 0.02 | 3.24 ± 0.11 | 1.18 ± 0.02 | 6.04 |
| S2 | bud tea | 2.54 ± 0.04 | 5.81 ± 0.08 | 2.23 ± 0.04 | 10.58 |
| S3 | bud tea | 0.81 ± 0.01 | 1.51 ± 0.02 | 0.56 ± 0.02 | 2.88 |
| S4 | bud tea | 0.68 ± 0.02 | 1.49 ± 0.02 | 0.61 ± 0.003 | 2.78 |
| S5 | bud tea | 0.95 ± 0.03 | 2.24 ± 0.04 | 0.45 ± 0.02 | 3.64 |
| S6 | bud tea | 1.21 ± 0.01 | 4.39 ± 0.04 | 2.87 ± 0.02 | 8.47 |
| S7 | bud tea | 0.75 ± 0.03 | 1.96 ± 0.01 | 0.78 ± 0.01 | 3.49 |
| S8 | bud tea | 0.35 ± 0.002 | 1.62 ± 0.01 | 0.41 ± 0.02 | 2.38 |
| S9 | bud tea | 0.41 ± 0.01 | 0.92 ± 0.02 | 0.43 ± 0.01 | 1.76 |
| S10 | primary leaf tea | 2.19 ± 0.06 | 6.73 ± 0.17 | 5.01 ± 0.18 | 13.93 |
| S11 | primary leaf tea | 5.54 ± 0.11 | 15.55 ± 0.24 | 8.57 ± 0.09 | 29.66 |
| S12 | primary leaf tea | 2.34 ± 0.04 | 5.56 ± 0.16 | 2.57 ± 0.08 | 10.47 |
| S13 | primary leaf tea | 3.89 ± 0.03 | 8.87 ± 0.01 | 3.01 ± 0.12 | 15.77 |
| S14 | primary leaf tea | 4.15 ± 0.11 | 8.13 ± 0.07 | 3.12 ± 0.09 | 15.40 |
| S15 | primary leaf tea | 3.05 ± 0.02 | 9.19 ± 0.11 | 6.82 ± 0.12 | 19.06 |
| S16 | primary leaf tea | 1.69 ± 0.04 | 7.51 ± 0.07 | 3.95 ± 0.03 | 13.15 |
| S17 | primary leaf tea | 2.09 ± 0.03 | 6.55 ± 0.11 | 4.83 ± 0.08 | 13.47 |
| S18 | primary leaf tea | 6.48 ± 0.08 | 11.39 ± 0.14 | 7.80 ± 0.11 | 25.67 |
| S19 | mature leaf tea | 1.28 ± 0.09 | 5.61 ± 0.12 | 3.47 ± 0.14 | 10.36 |
| S20 | mature leaf tea | 0.75 ± 0.006 | 3.98 ± 0.03 | 2.21 ± 0.04 | 6.94 |
| S21 | mature leaf tea | 0.66 ± 0.005 | 3.12 ± 0.02 | 2.82 ± 0.04 | 6.60 |
| S22 | mature leaf tea | 0.67 ± 0.01 | 1.19 ± 0.03 | 0.74 ± 0.02 | 2.60 |
| S23 | mature leaf tea | 0.67 ± 0.02 | 1.16 ± 0.01 | 0.31 ± 0.01 | 2.14 |
| S24 | mature leaf tea | 0.72 ± 0.009 | 1.46 ± 0.02 | 0.86 ± 0.01 | 3.04 |
| S25 | mature leaf tea | 0.71 ± 0.01 | 1.52 ± 0.03 | 0.67 ± 0.01 | 2.90 |
| S26 | mature leaf tea | 0.37 ± 0.01 | 1.09 ± 0.06 | 0.32 ± 0.009 | 1.78 |
| S27 | mature leaf tea | 0.32 ± 0.009 | 0.67 ± 0.02 | 0.34 ± 0.01 | 1.33 |
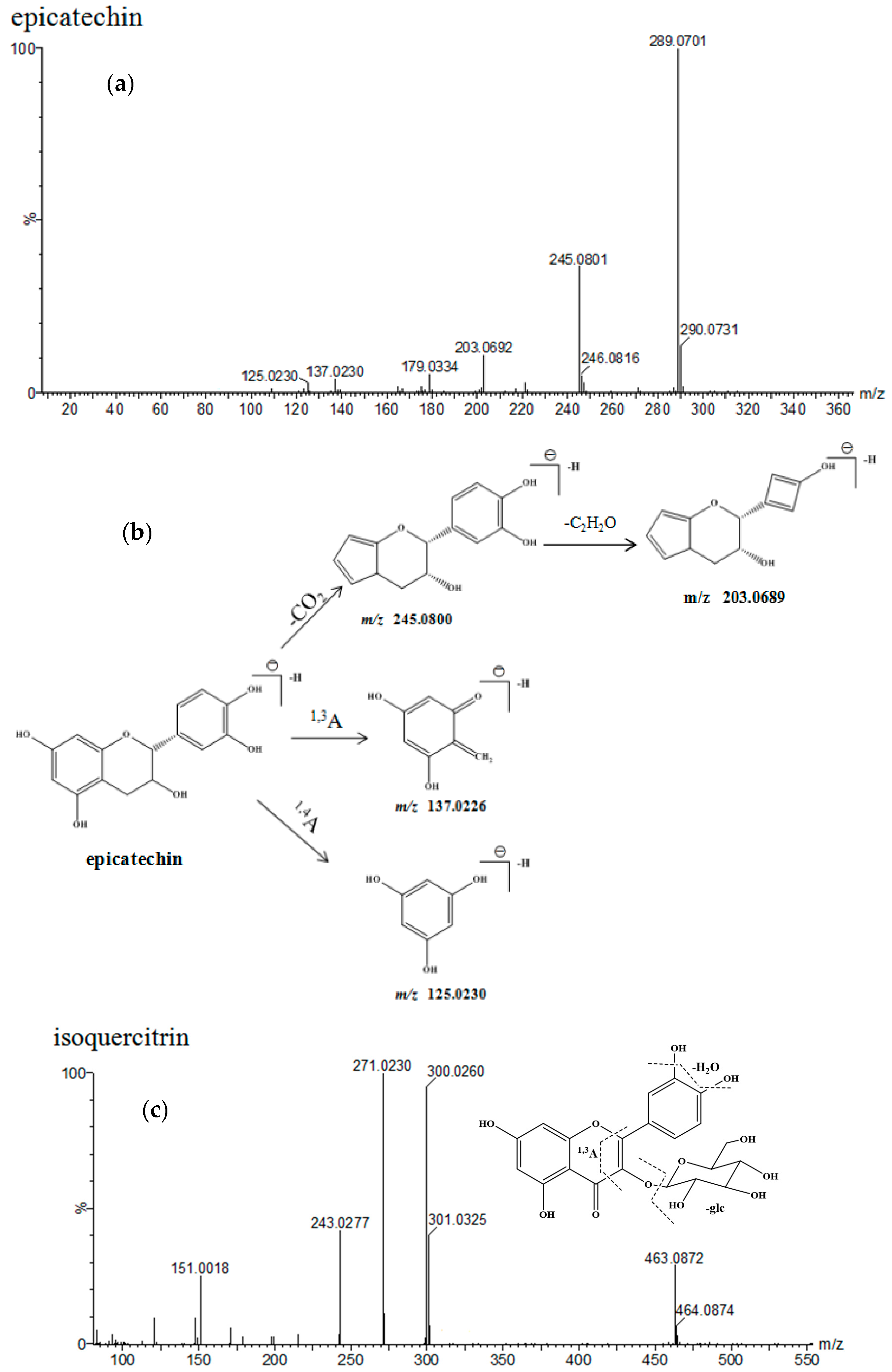
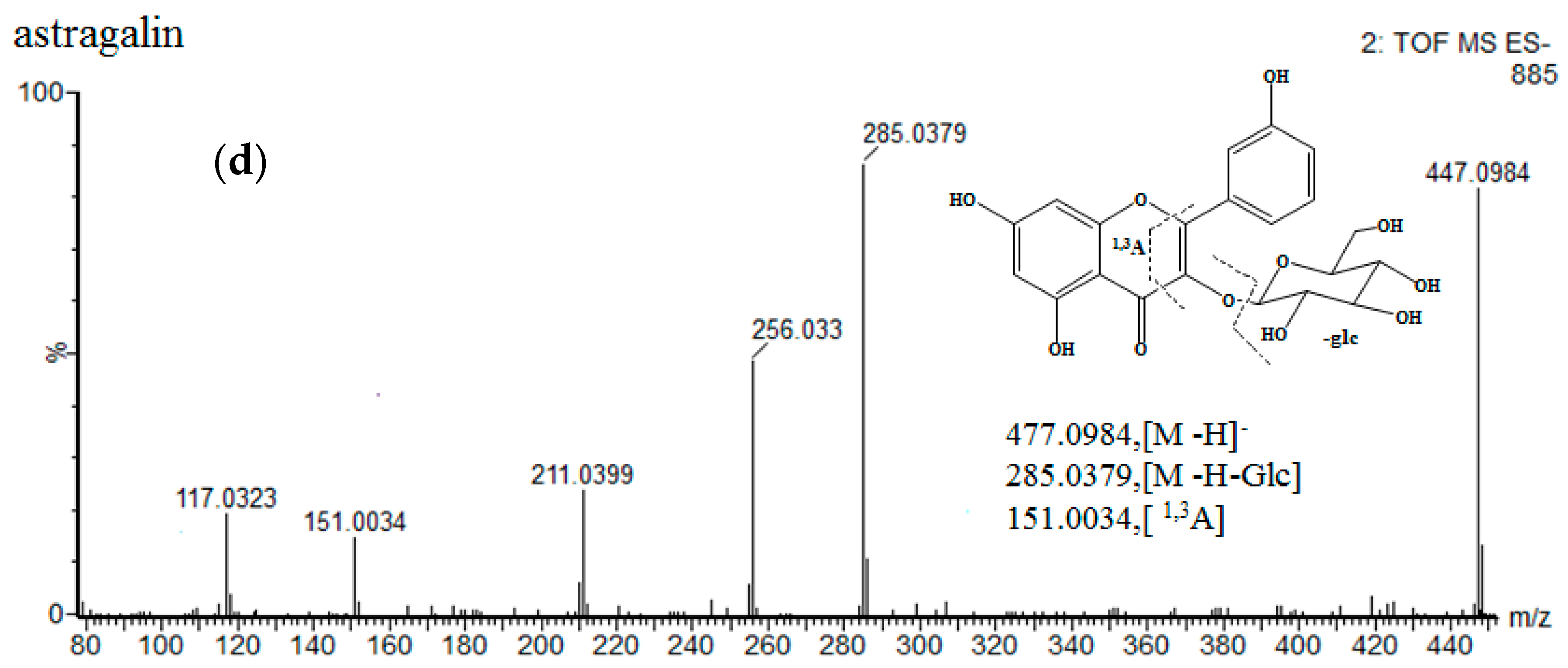
| No. | Identification | Rt. (min) | UV λmax (nm) | Formula | Quasi-Molecular ES- | (ES-)MSE Ions (m/z) | ||
|---|---|---|---|---|---|---|---|---|
| Calc. Mass | Measured Mass | Error (ppm) | ||||||
| 1 | epicatechin-(4-8)-epicatechin | 4.63 | 203 279 | C30H26O12 | 577.1346 | 577.1352 [M − H]− | 1 | 425.0876, 407.0754, 289.0692, 245.0478 |
| 2 | catechin | 5.09 | 203 278 | C15H14O6 | 289.0712 | 289.0711 [M − H]− | −0.7 | 245.0816, 203.0692, 137.0236 |
| 3 | epicatechin-(4-6)-epicatechin | 5.82 | 201 279 | C30H26O12 | 577.1346 | 577.1354 [M − H]− | 1.4 | 425.0876, 407.0779, 289.0707, 245.0478 |
| 4 | epiafzelechin-(4-8)-epicatechin | 6.19 | 198 278 | C30H26O11 | 561.1397 | 561.1401 [M − H]−− | 0.7 | 425.0872, 407.0774, 289.0714, 271.0599 |
| 5 | epicatechin | 7.21 | 202 278 | C15H14O6 | 289.0712 | 289.0717 [M − H]− | 1.7 | 245.0802, 203.0689, 137.0156 |
| 6 | epiafzelechin-epiafzelechin-epicatechin | 8.03 | 196 278 | C45H38O16 | 833.2082 | 833.2103 [M − H]− | 2.5 | 561.1407, 543.1275, 407.0939, 289.0724, 271.0651 |
| 7 | hyperoside | 12.45 | 254 354 | C21H20O12 | 463.0877 | 463.0877 [M − H]− | −0.4 | 301.0302, 151.0022 |
| 8 | isoquercitrin | 13.02 | 255 353 | C21H20O12 | 463.0877 | 463.0878 [M − H]− | 0.2 | 301.0323, 151.0018 |
| 9 | quercitrin | 14.70 | 264 347 | C21H20O11 | 447.0927 | 447.0929 [M − H]− | 0.4 | 301.0318, 151.0016 |
| 10 | astragalin | 16.21 | 264 346 | C21H20O11 | 447.0927 | 447.0936 [M − H]− | 2 | 285.0398, 151.0018 |
| 11 | quercetin-3-O-α-l-rhamnoside | 16.37 | 255 348 | C21H20O11 | 447.0927 | 447.0911 [M − H]− | −3.6 | 301.0337, 151.0027 |
| 12 | dihydrokaempferol-3-O-β-d-glucopyranoside | 18.62 | 199 | C21H22O11 | 449.1084 | 449.1073 [M − H]− | −2.4 | 287.0551, 151.0028 |
| 13 | kaempferol-3-O-α-l-rhamnoside | 20.51 | 212 264 | C21H20O10 | 431.0978 | 431.0983 [M − H]− | 1.2 | 285.0404, 151.0017 |
| 14 | dihydrokaempferol | 21.72 | 199 287 | C15H12O6 | 287.0556. | 287.0543 [M − H]− | −4.5 | 151.0017, 135.0436 |
| 15 | quercetin-3-O-β-d-rutinose | 21.95 | 255 248 | C30H26O14 | 609.1244 | 609.1246 [M − H]− | 0.2 | 463.0860, 301.0325, 151.0017 |
| 16 | quercetin | 22.24 | 255 347 | C15H10O7 | 301.0348 | 301.0330 [M − H]− | −1.8 | 285.0398, 151.0018 |
| 17 | kaempferol-3-O-β-d-(6-O-trans-p-coumaroyl)-glucopyranoside | 22.51 | 266 313 | C30H26O13 | 593.1295 | 593.1310 [M − H]− | 2.5 | 447.0961, 285.0400, 151.0016 |
| 18 | kaempferol-3-O-β-d-(6-O-trans-p-coumaroyl)-mannoside | 22.69 | 266 312 | C30H26O13 | 593.1295 | 593.1310 [M − H]− | 2.5 | 447.0961, 285.0400, 151.0016 |
| 19 | dihydrokaempferol-3-O-β-d-(6-O-trans-p-coumaroyl)-glucopyranoside | 23.49 | 266 313 | C30H28O13 | 595.1452 | 595.1467 [M − H]− | 0.8 | 449.1109, 287.0541, 151.0020 |
| 20 | dihydrokaempferol-3-O-β-d-(6-O-trans-p-coumaroyl)-mannoside | 24.31 | 266 313 | C30H28O13 | 595.1452 | 595.1467 [M − H]− | 0.8 | 449.1109, 287.0541, 151.0020 |
| Samples | Region | Kinds | Harvesting Time |
|---|---|---|---|
| S1 | Pingtou Village, Meiluo Town, Shimian County, Sichuan province | Bud tea | March 2014 |
| S2 | Shanquan Village, Meiluo Town, Shimian County, Sichuan province | Bud tea | March 2014 |
| S3 | Liuhe Village, Cheling Town, Mingshan County, Sichuan province | Bud tea | March 2014 |
| S4 | Chapingli Village, Chaba Town, Qingchuan County, Sichuan province | Bud tea | March 2014 |
| S5 | Xinshi Village, Wawushan Town, Hongya County, Sichuan province | Bud tea | March 2014 |
| S6 | Anlezhai Village, Anle Town, Jiuzhaigou County, Sichuan province | Bud tea | March 2014 |
| S7 | Chunxiao Village, Nanling Town, Wushan County, Chongqing | Bud tea | March 2014 |
| S8 | Longfeng Village, Xinglong Town, Meitan County, Guizhou province | Bud tea | March 2014 |
| S9 | Hong Village, Fangtang Town, Ningguo County, Anhui province | Bud tea | March 2014 |
| S10 | Pingtou Village, Meiluo Town, Shimian County, Sichuan province | Primary leaf tea | May 2014 |
| S11 | Shanquan Village, Meiluo Town, Shimian County, Sichuan province | Primary leaf tea | May 2014 |
| S12 | Liuhe Village, Cheling Town, Mingshan County, Sichuan province | Primary leaf tea | May 2014 |
| S13 | Chapingli Village, Chaba Town, Qingchuan County, Sichuan province | Primary leaf tea | May 2014 |
| S14 | Xinshi Village, Wawushan Town, Hongya County, Sichuan province | Primary leaf tea | May 2014 |
| S15 | Anlezhai Village, Anle Town, Jiuzhaigou County, Sichuan province | Primary leaf tea | May 2014 |
| S16 | Chunxiao Village, Nanling Town, Wushan County, Chongqing | Primary leaf tea | May 2014 |
| S17 | Longfeng Village, Xinglong Town, Meitan County, Guizhou province | Primary leaf tea | May 2014 |
| S18 | Hong Village, Fangtang Town, Ningguo County, Anhui province | Primary leaf tea | May 2014 |
| S19 | Pingtou Village, Meiluo Town, Shimian County, Sichuan province | Mature leaf tea | July 2014 |
| S20 | Shanquan Village, Meiluo Town, Shimian County, Sichuan province | Mature leaf tea | July 2014 |
| S21 | Liuhe Village, Cheling Town, Mingshan County, Sichuan province | Mature leaf tea | July 2014 |
| S22 | Chapingli Village, Chaba Town, Qingchuan County, Sichuan province | Mature leaf tea | July 2014 |
| S23 | Xinshi Village, Wawushan Town, Hongya County, Sichuan province | Mature leaf tea | July 2014 |
| S24 | Anlezhai Village, Anle Town, Jiuzhaigou County, Sichuan province | Mature leaf tea | July 2014 |
| S25 | Chunxiao Village, Nanling Town, Wushan County, Chongqing | Mature leaf tea | July 2014 |
| S26 | Longfeng Village, Xinglong Town, Meitan County, Guizhou province | Mature leaf tea | July 2014 |
| S27 | Hong Village, Fangtang Town, Ningguo County, Anhui province | Mature leaf tea | July 2014 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Xu, L.; Hu, H.; Yang, Y.; Zhang, X.; Peng, Y.; Xiao, P. DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE. Molecules 2017, 22, 1622. https://doi.org/10.3390/molecules22101622
Xiao X, Xu L, Hu H, Yang Y, Zhang X, Peng Y, Xiao P. DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE. Molecules. 2017; 22(10):1622. https://doi.org/10.3390/molecules22101622
Chicago/Turabian StyleXiao, Xuan, Lijia Xu, Huagang Hu, Yinjun Yang, Xinyao Zhang, Yong Peng, and Peigen Xiao. 2017. "DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE" Molecules 22, no. 10: 1622. https://doi.org/10.3390/molecules22101622
APA StyleXiao, X., Xu, L., Hu, H., Yang, Y., Zhang, X., Peng, Y., & Xiao, P. (2017). DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE. Molecules, 22(10), 1622. https://doi.org/10.3390/molecules22101622





