A Network Pharmacology-Based Study on the Hepatoprotective Effect of Fructus Schisandrae
Abstract
1. Introduction
2. Results and Discussion
2.1. Potential Biological Effect of Fructus Schisandrae on Liver Disease Predicted by Network Pharmacological Analysis
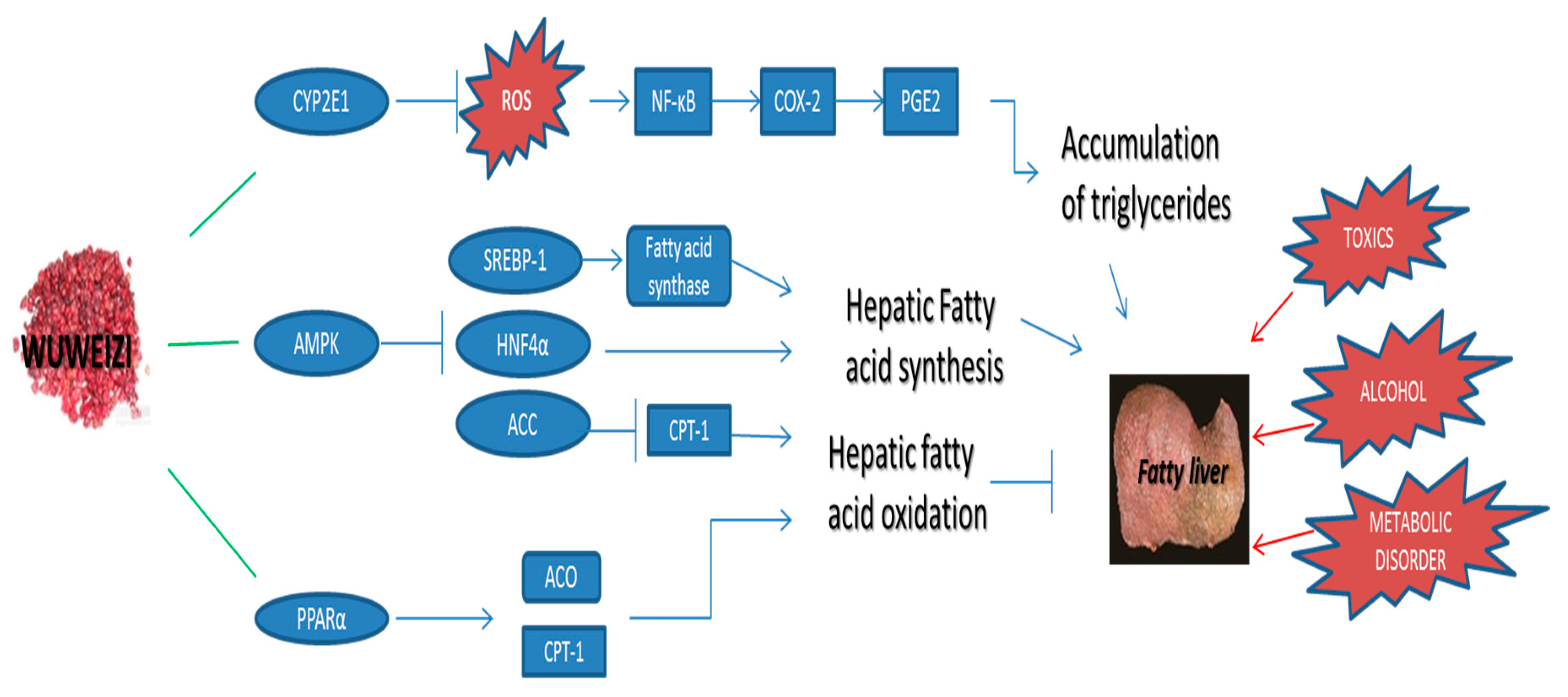
2.2. Potential Hepatoprotective Molecular Mechanism of Wuweizi
3. Materials and Methods
3.1. Database Construction and ADME Screening of Wuweizi Ingredients
3.2. Preparation of Potential Targets and Ligand Structures
3.3. Molecular Docking and Network Building
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Melo, A.P.S.; Franca, E.B.; Malta, D.C.; Garcia, L.P.; Mooney, M.; Naghavi, M. Mortality due to cirrhosis, liver cancer, and disorders attributed to alcohol use: Global burden of disease in Brazil, 1990 and 2015. Rev. Bras. Epidemiol. 2017, 20, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Rush, B.; Walley, K.R.; Celi, L.A.; Rajoriya, N.; Brahmania, M. Palliative care access for hospitalized patients with end stage liver disease across the United States. Hepatology 2017, 66, S372–S373. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Tan, H.Y.; Wang, N.; Tsao, S.W.; Feng, Y. Current status of herbal medicines in chronic liver disease therapy: The biological effects, molecular targets and future prospects. Int. J. Mol. Sci. 2015, 16, 28705–28745. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Hu, J.P.; Sheng, L.; Li, Y. Effects of schisandra chinensis (Wuweizi) constituents on the activity of hepatic microsomal CYP450 isozymes in rats detected by using a cocktail probe substrates method. Yao Xue Xue Bao 2011, 46, 922–927. [Google Scholar] [PubMed]
- Kim, J.W.; Ku, S.K.; Kim, K.Y.; Kim, S.G.; Han, M.H.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. Schisandrae fructus supplementation ameliorates sciatic neurectomy-induced muscle atrophy in mice. Oxid. Med. Cell. Longev. 2015, 2015, 872428. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.S.; Yu, Z.L.; Pan, S.Y.; Jia, Z.H.; Wang, X.Y.; Zhang, Y.; Zhu, P.L.; Wang, X.J.; Ko, K.M. A comparative study between wuweizi seed and its post-ethanol extraction residue in normal and hypercholesterolemic mice. Lipids Health Dis. 2015, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.T. Biochemical and pharmacologic effects of wuweizi (Schizandra chinensis (Turcz.) Baill.) and its chemical contents on animal livers. Sheng Li Ke Xue Jin Zhan 1988, 19, 197–203. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, D.; Zhang, M. Inhibition mechanism of compound ethanol extracts from wuweizi (Fructus Schisandrae Chinensis) on renal interstitial fibrosis in diabetic nephropathy model mice. J. Tradit. Chin. Med. 2012, 32, 669–673. [Google Scholar] [CrossRef]
- Pan, S.Y.; Dong, H.; Zhao, X.Y.; Xiang, C.J.; Fang, H.Y.; Fong, W.F.; Yu, Z.L.; Ko, K.M. Schisandrin B from Schisandra chinensis reduces hepatic lipid contents in hypercholesterolaemic mice. J. Pharm. Pharmacol. 2008, 60, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Leung, H.Y.; Siu, A.H.; Poon, M.K.; Ko, K.M. Schisandrin B decreases the sensitivity of mitochondria to calcium ion-induced permeability transition and protects against carbon tetrachloride toxicity in mouse livers. Biol. Pharm. Bull. 2007, 30, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Wang, N.; Tan, H.Y.; Cheung, F.; Feng, Y. A biomedical investigation of the hepatoprotective effect of radix salviae miltiorrhizae and network pharmacology-based prediction of the active compounds and molecular targets. Int. J. Mol. Sci. 2017, 18, 620. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Tan, H.Y.; Cheung, F.; Wang, N.; Huang, J.; Feng, Y. A network-based pharmacology study of the herb-induced liver injury potential of traditional hepatoprotective Chinese herbal medicines. Molecules 2017, 22, 632. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Chen, Z. Trypsin inhibitor screening in traditional Chinese medicine by using an immobilized enzyme microreactor in capillary and molecular docking study. J. Sep. Sci. 2017, 40, 3168–3174. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, J.; Dai, Q.; Zhang, H.; Pang, W.; Hu, J. Prediction of anti-tumor chemical probes of a traditional Chinese medicine formula by HPLC fingerprinting combined with molecular docking. Eur. J. Med. Chem. 2014, 83, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Karthick, V.; Ramanathan, K. Virtual screening for oseltamivir-resistant a (H5N1) influenza neuraminidase from traditional Chinese medicine database: A combined molecular docking with molecular dynamics approach. Springerplus 2013, 2, 115. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.N.; Li, S.X.; Zhai, K.F.; Kou, J.P.; Yu, B.Y. Network pharmacology-based prediction and verification of the molecular targets and pathways for schisandrin against cerebrovascular disease. Chin. J. Nat. Med. 2014, 12, 251–258. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, L.; Wang, W.; Zhang, Z.; Yang, H. Study about target-network of anti-cerebral infarction neuropathy based on theory of neurovascular unit and network pharmacology. Zhongguo Zhong Yao Za Zhi 2012, 37, 138–141. [Google Scholar] [PubMed]
- Chen, Q.; Wu, Y.J.; Cheng, N.N.; Li, Y.L.; Wang, Y.M. Dual effects of extract of Schisandra chinensis Baill on rat hepatic CYP3A. Yao Xue Xue Bao 2010, 45, 1194–1198. [Google Scholar] [PubMed]
- Jiang, Y.; Fan, X.; Wang, Y.; Tan, H.; Chen, P.; Zeng, H.; Huang, M.; Bi, H. Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem. Biol. Interact. 2015, 231, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Maemura, S.; Sudo, K.; Kase, Y.; Arai, I.; Ohkura, Y.; Funo, S.; Fujii, Y.; Aburada, M.; Hosoya, E. Effects of gomisin a, a lignan component of schizandra fruits, on experimental liver injuries and liver microsomal drug-metabolizing enzymes. Nihon Yakurigaku Zasshi 1986, 87, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Kim, J.H.; Kim, J.H.; Jung, M.H. Protective effects of gomisin n against hepatic steatosis through AMPK activation. Biochem. Biophys. Res. Commun. 2017, 482, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Funo, S.; Iizuka, A.; Kase, Y.; Arai, I.; Ohkura, Y.; Sudo, K.; Kiuchi, N.; Yoshida, C.; Maeda, S.; et al. Pharmacological studies on schizandra fruits. III. Effects of wuweizisu C, a lignan component of schizandra fruits, on experimental liver injuries in rats. Nihon Yakurigaku Zasshi 1985, 85, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y.; Tohkin, M.; Hikino, H.; Ikeya, Y.; Taguchi, H. Mechanism of antihepatotoxic activity of wuweizisu C and gomisin A1. Planta Med. 1985, 51, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Rigamonti, C.; Vidali, M.; Albano, E. CYP2E1 autoantibodies in liver diseases. Redox Biol. 2014, 3, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Yang, F.; Li, B.; Zhang, B.; Guan, L.; Sheng, J.; Ye, Y.; Wang, Z.; Li, P.; Xu, L.; et al. Meta-analysis of CYP2E1 polymorphisms in liver carcinogenesis. Dig. Liver Dis. 2017, 49, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.D.; Cao, Z.H.; Li, X.F.; Kang, X.L.; Xue, Y.; Li, Y.L.; Zhang, D.; Liu, X.Y.; Xue, Y.Z. Effect of ammonium pyrrolidine dithiocarbamate (PDTC) on NF-kB activation and CYP2E1 content of rats with immunological liver injury. Pharm. Biol. 2014, 52, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, G.; Gao, L.; Shi, L.; Qi, Y.; Lv, X.; Jin, Y. Roles of CYP2E1 in 1,2-dichloroethane-induced liver damage in mice. Environ. Toxicol. 2016, 31, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Shirato, T.; Homma, T.; Lee, J.; Kurahashi, T.; Fujii, J. Oxidative stress caused by a sod1 deficiency ameliorates thioacetamide-triggered cell death via CYP2E1 inhibition but stimulates liver steatosis. Arch. Toxicol. 2017, 91, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Perwitasari, D.A.; Irham, L.M.; Darmawan, E.; Mulyani, U.A.; Atthobari, J. CYP2E1 polymorphism, acetylator profiles and drug-induced liver injury incidence of indonesian tuberculosis patients. Indian J. Tuberc. 2016, 63, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Tyburski, J.B.; Wang, Y.W.; Strawn, S.; Moon, B.H.; Kallakury, B.V.; Gonzalez, F.J.; Fornace, A.J., Jr. Modulation of fatty acid and bile acid metabolism by peroxisome proliferator-activated receptor α protects against alcoholic liver disease. Alcohol. Clin. Exp. Res. 2014, 38, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.D.; Shah, Y.M.; Matsubara, T.; Krausz, K.W.; Gonzalez, F.J. Peroxisome proliferator-activated receptor α induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 2012, 56, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhong, H.; Yin, Y.; Jiang, Z. Genistein has beneficial effects on hepatic steatosis in high fat-high sucrose diet-treated rats. Biomed. Pharmacother. 2017, 91, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.; Menezes-Garcia, Z.; Mario, E.G.; Delpuerto, H.L.; Martins, A.S.; Botion, L.M. Increased expression of oxidative enzymes in adipose tissue following pparalpha-activation. Metabolism 2014, 63, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.J.; Song, Y.; Jang, S.H.; Ko, Y.G.; Kang, S.N.; Chung, B.Y.; Kim, H.D.; Kim, G.S.; Cho, J.H. Schisandra chinensis prevents alcohol-induced fatty liver disease in rats. J. Med. Food 2014, 17, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Wang, Y.M.; Wang, J.F.; Xue, Y.; Li, Z.J.; Nagao, K.; Yanagita, T.; Xue, C.H. Dietary saponins of sea cucumber alleviate orotic acid-induced fatty liver in rats via PPARα and SREBP-1c signaling. Lipids Health Dis. 2010, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhou, D.L.; Wei, X.H.; Zhong, R.Y.; Xu, J.; Sun, L. Astragaloside IV attenuates free fatty acid-induced ER stress and lipid accumulation in hepatocytes via AMPK activation. Acta Pharmacol. Sin. 2017, 38, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Kwon, S.W.; Jung, B.H.; Oh, S.H.; Lee, B.H. Role of the AMPK/SREBP-1 pathway in the development of orotic acid-induced fatty liver. J. Lipid Res. 2011, 52, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Cao, C.; Chen, F.; Li, Q.; Bi, X.; Sun, Y.; Zhan, Z. Electroacupuncture attenuates hepatic lipid accumulation via AMP-activated protein kinase (AMPK) activation in obese rats. Acupunct. Med. 2016, 34, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Song, C.Y.; Shi, J.; Zeng, X.; Zhang, Y.; Xie, W.F.; Chen, Y.X. Sophocarpine alleviates hepatocyte steatosis through activating AMPK signaling pathway. Toxicol. In Vitro 2013, 27, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhai, R.; Ma, C.; Wei, M. The anti-growth and anti-metastasis effects of schisandrin B on hepatocarcinoma cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Chiu, P.Y.; Leung, H.Y.; Ko, K.M. Cytochrome P450-catalysed reactive oxygen species production mediates the (−) schisandrin b-induced glutathione and heat shock responses in AML12 hepatocytes. Cell Biol. Int. 2012, 36, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, Y.; Wang, J.; Zhang, J.; Hou, T. ADME evaluation in drug discovery. 9. Prediction of oral bioavailability in humans based on molecular properties and structural fingerprints. Mol. Pharm. 2011, 8, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Saghir, S.A. Determination of ADME and bioavailability following intravenous, oral, and dermal routes of exposure. Curr. Protoc. Toxicol. 2009. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Li, Y.; Li, X.; Tao, W.; Li, B.; Wang, Y.; Yang, L. Systems pharmacology uncovers Janus functions of botanical drugs: Activation of host defense system and inhibition of influenza virus replication. Integr. Biol. 2013, 5, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Araujo, F.; Barrias, C.C.; Granja, P.L.; Sarmento, B. Dissecting stromal-epithelial interactions in a 3D in vitro cellularized intestinal model for permeability studies. Biomaterials 2015, 56, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, F.; Lu, H.; Zhan, Y.; Zhang, M.; Guo, W.; Ding, G. Schisantherin A protects against liver ischemia-reperfusion injury via inhibition of mitogen-activated protein kinase pathway. Int. Immunopharmacol. 2017, 47, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhou, K.; Li, D.; Xie, X.; Jun, F.; Wang, J. Schisantherin A suppresses interleukin-1β-induced inflammation in human chondrocytes via inhibition of Nf-kB and MAPKs activation. Eur. J. Pharmacol. 2016, 780, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiang, L.; Liu, Y.; Venkatraman, P.; Chong, L.; Cho, J.; Bonilla, S.; Jin, Z.B.; Pang, C.P.; Ko, K.M.; et al. A naturally-derived compound Schisandrin B enhanced light sensation in the pde6c zebrafish model of retinal degeneration. PLoS ONE 2016, 11, e0149663. [Google Scholar]
- El-Saadi, M.W.; Williams-Hart, T.; Salvatore, B.A.; Mahdavian, E. Use of in-silico assays to characterize the ADMET profile and identify potential therapeutic targets of fusarochromanone, a novel anti-cancer agent. In Silico Pharmacol. 2015, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, L.; Zhu, Y.; Qiu, S.; Wang, T.; Zhang, L. Ligand and structure-based approaches for the identification of peptide deformylase inhibitors as antibacterial drugs. Int. J. Mol. Sci. 2016, 17, 1141. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.; Derksen, S.; Radestock, S.; Morchen, F. Maximum common binding modes (MCBM): Consensus docking scoring using multiple ligand information and interaction fingerprints. J. Chem. Inf. Model. 2008, 48, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, Y.; Wang, S. Comparative evaluation of 11 scoring functions for molecular docking. J. Med. Chem. 2003, 46, 2287–2303. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
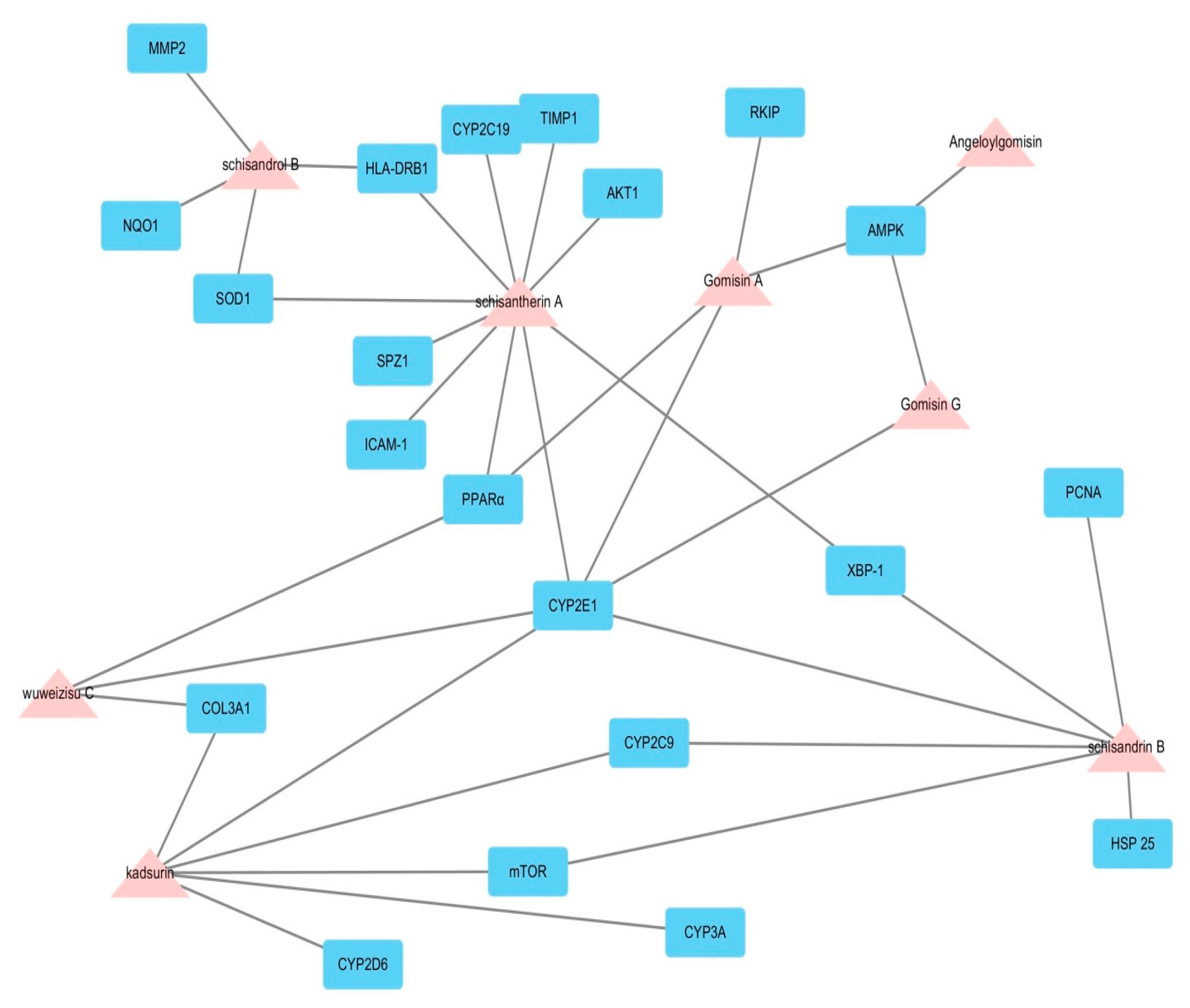
| Target Gene | Target Protein | Roles in Hepatoprotective Effects |
|---|---|---|
| HLA-DRB1 | major histocompatibility complex, class II, DQ beta 1 | Anti-virus |
| IFNL3 | interferon lambda 3 | Anti-virus |
| HLA-DRB5 | major histocompatibility complex, class II, DQ beta 5 | Anti-virus |
| IL6 | Interleukin 6 | Anti-virus |
| NAT2 | N-acetyltransferase 2 | Reduce antituberculosis drug-induced liver injury |
| STAT3 | signal transducer and activator of transcription 3 | Regulate hepatic cell growth and apoptosis |
| CYP2C9 | Cytochrome P450 2C9 | Regulate drug metabolism |
| CYP2D6 | Cytochrome P450 2D6 | Regulate drug metabolism |
| CYP3A | Cytochrome P450 3A | Regulate drug metabolism |
| ITPA | inosine triphosphate pyrophosphatase | Regulate drug metabolism |
| UGT1A1 | UDP glucuronosyltransferase family 1 member A1 | Regulate drug metabolism |
| UGT1A3 | UDP glucuronosyltransferase family 1 member A3 | Regulate drug metabolism |
| CYP2E1 | Cytochrome P450 2E1 | Regulate drug metabolism |
| HSPA1L | Heat shock 70 kDa protein 1L | Regulate drug metabolism |
| CYP2C19 | Cytochrome P450 C19 | Regulate drug metabolism |
| PEMT | phosphatidylethanolamine N-methyltransferase | Regulate drug metabolism |
| PNPLA3 | Patatin-like phospholipase domain-containing protein 3 | Regulate fatty metabolism |
| AKT1 | v-akt murine thymoma viral oncogene homolog 1 | Regulate lipid metabolism |
| CYP2B6 | cytochrome P450, family 2, subfamily B, polypeptide 6 | Oxidizes a variety of structurally unrelated compounds, including steroids, fatty acids |
| CYP1B1 | cytochrome P450, family 1, subfamily B, polypeptide 1 | Oxidizes a variety of structurally unrelated compounds, including steroids, fatty acids |
| MMP2 | matrix metallopeptidase 2 | Tissue repair and induce interstitial fibrosis |
| PPARα | peroxisome proliferator-activated receptor alpha | Key regulator of lipid metabolism |
| NFKBIA | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha | On cellular stimulation by immune and proinflammatory responses |
| AHSA1 | activator of heat shock 90kDa protein ATPase homolog 1 | Involve in Grb2-p38 MAPK signaling pathway in fibrosis |
| NQO1 | NAD(P)H dehydrogenase, quinone 1 | Involve in alcohol detoxification pathways |
| HMOX1 | heme oxygenase (decycling) 1 | Alleviate liver inflammation and reduced oxidative stress |
| ICAM-1 | intercellular adhesion molecule 1 | Mediate adhesive interaction in fibrosis process |
| MAPK1 | mitogen-activated protein kinase 1 | Regulate cytoskeletal rearrangements in fibrosis process |
| PRKCB | protein kinase C, beta | Regulate oxidative stress-induced cell damage |
| ACTA2 | actin, alpha 2, smooth muscle, aorta | Involve in myofibroblast cell motility during wound healing in liver |
| SPZ1 | spermatogenic leucine zipper 1 | The transcriptional factors of liver fatty acid binding protein |
| COL1A1 | collagen, type I, alpha 1 | Transcriptional repressor of the collagen |
| BCL2 | B-cell CLL/lymphoma 2 | Regulate the response to mitochondrial damage and related oxidative damage |
| CCND1 | cyclin D1 | Functions as a mediator of β-catenin during hepatocarcinogenesis |
| RKIP | Raf kinase inhibitor protein | Regulate carbon tetrachloride-induced apoptotic hepatic cell death |
| HERC5 | HECT and RLD domain containing E3 ubiquitin protein ligase 5 | Acts as a positive regulator of innate antiviral response in liver cells |
| CDKN1A | cyclin-dependent kinase inhibitor 1A | Regulate hepatic cell cycle in hepatocarcinogenesis |
| mTOR | mammalian target of rapamycin | Regulate hepatic cell autophagy |
| EIF6 | eukaryotic translation initiation factor 6 | Regulate hepatocarcinogenesis by mediating cellular response to DNA damage. |
| CASP3 | caspase 3 | Apoptosis inhibitory protein in hepatocarcinogenesis |
| COL7A1 | collagen, type VII, alpha 1 | Regulate fibrosis by impacts on extracellular matrix (ECM) proteins such as type IV collagen |
| COL3A1 | collagen, type III, alpha 1 | Regulate fibrosis by impacts on extracellular matrix (ECM) proteins such as type IV collagen |
| HSP 25 | 25 kilodalton heat shock proteins | Protect cells from oxidation stress |
| TGFB1 | transforming growth factor, beta 1 | Regulate liver cancer cells proliferation |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | Tissue repair and induce interstitial fibrosis |
| SOD1 | superoxide dismutase 1 | Destroys radicals that are normally produced within the cells, such as oxidants |
| RELA | v-rel reticuloendotheliosis viral oncogene homolog A | Involve in hepatic inflammation |
| PCNA | Proliferating Cell Nuclear Antigen | Inhibit HCC cell proliferation |
| XBP-1 | X-box-binding protein-1 | Protection against endoplasmic reticulum (ER) stress |
| AMPK | AMP-activated protein kinase | Regulate hepatic fatty acid oxidation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Zhang, Y.; Li, S.; Tan, H.Y.; Wang, N.; Mu, S.; Hao, X.; Feng, Y. A Network Pharmacology-Based Study on the Hepatoprotective Effect of Fructus Schisandrae. Molecules 2017, 22, 1617. https://doi.org/10.3390/molecules22101617
Hong M, Zhang Y, Li S, Tan HY, Wang N, Mu S, Hao X, Feng Y. A Network Pharmacology-Based Study on the Hepatoprotective Effect of Fructus Schisandrae. Molecules. 2017; 22(10):1617. https://doi.org/10.3390/molecules22101617
Chicago/Turabian StyleHong, Ming, Yongsheng Zhang, Sha Li, Hor Yue Tan, Ning Wang, Shuzhen Mu, Xiaojiang Hao, and Yibin Feng. 2017. "A Network Pharmacology-Based Study on the Hepatoprotective Effect of Fructus Schisandrae" Molecules 22, no. 10: 1617. https://doi.org/10.3390/molecules22101617
APA StyleHong, M., Zhang, Y., Li, S., Tan, H. Y., Wang, N., Mu, S., Hao, X., & Feng, Y. (2017). A Network Pharmacology-Based Study on the Hepatoprotective Effect of Fructus Schisandrae. Molecules, 22(10), 1617. https://doi.org/10.3390/molecules22101617





