Formation and Physiochemical Properties of Silver Nanoparticles with Various Exopolysaccharides of a Medicinal Fungus in Aqueous Solution
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of EPS Fractions
2.2. Molecular Characteristics of EPS Fractions
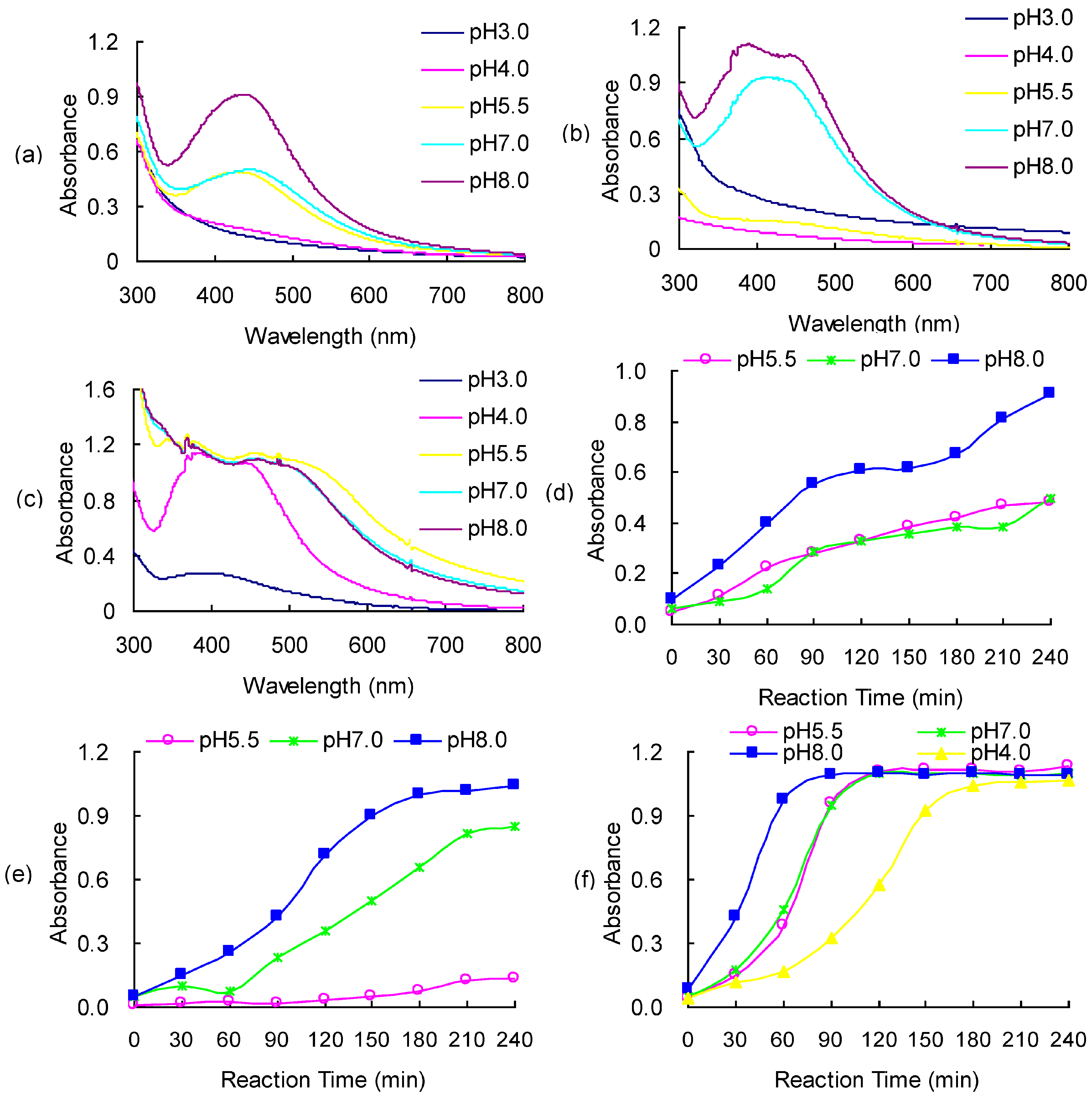
2.3. Optical Properties of AgNP Dispersions
2.4. Characteristics of EPS-AgNP Complexes
2.5. Hydrodynamic Radii of EPS-AgNP Complexes
2.6. Stability of EPS-AgNP Dispersions
2.7. Mechanisms of EPS—AgNP Complex Formation
3. Materials and Methods
3.1. Fungal Fermentation
3.2. Preparation of EPS Fractions
3.3. Composition Analysis and Characterization of EPS Fractions
3.4. Preparation of AgNPs with EPS Fractions
3.5. UV-Vis Spectrum Measurements
3.6. Transmission Electron Microscope (TEM)
3.7. Dynamic Light Scattering Measurement
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, Y.Y.; Monty, J.; Linhardt, R.J. Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z. One-pot synthesis of biocompatible superparamagnetic iron oxide nanoparticles/hydrogel based on salep: Characterization and drug delivery. Carbohydr. Polym. 2014, 101, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvi, K.; Tharnbidurai, S. Synthesis, characterization, and antimicrobial activity of chitosan-zinc oxide/polyaniline composites. Mater. Sci. Semicond. Process. 2015, 31, 573–581. [Google Scholar] [CrossRef]
- El Kadib, A.; Bousmina, M.; Brunel, D. Recent Progress in Chitosan Bio-Based Soft Nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 308–331. [Google Scholar] [CrossRef] [PubMed]
- Glisic, S.; Cakic, M.; Nikolic, G.; Danilovic, B. Synthesis, characterization and antimicrobial activity of carboxymethyl dextrane stabilized silver nanoparticles. J. Mol. Struct. 2015, 1084, 345–351. [Google Scholar] [CrossRef]
- Xu, W.; Jin, W.; Lin, L.; Zhang, C.; Li, Z.; Li, Y.; Song, R.; Li, B. Green synthesis of xanthan conformation-based silver nanoparticles: Antibacterial and catalytic application. Carbohydr. Polym. 2014, 101, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Bankura, K.; Maity, D.; Mollick, M.M.; Mondal, D.; Bhowmick, B.; Bain, M.; Chakraborty, A.; Sarkar, J.; Acharya, K.; Chattopadhyay, D. Synthesis, characterization and antimicrobial activity of dextran stabilized silver nanoparticles in aqueous medium. Carbohydr. Polym. 2012, 89, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Murawala, P.; Shiras, A.; Pokharkar, V.; Prasad, B. Gellan gum capped silver nanoparticle dispersions and hydrogels: Cytotoxicity and in vitro diffusion studies. Nanoscale 2012, 4, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, F.; Zhang, H. Facile synthesis of carboxymethyl curdlan-capped silver nanoparticles and their application in SERS. Carbohydr. Polym. 2012, 90, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Zhong, L.; Wu, G. Preparation of high-stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat. Phys. Chem. 2009, 78, 251–255. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Nanoparticles as antimicrobial agents: their toxicity and mechanisms of action. J. Nanosci. Nanotechnol. 2014, 14, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.T.; Din, M.I.; Bashir, S.; Qadir, M.A.; Rashid, F. Green Synthesis and Characterization of Silver Nanoparticles Using Ferocactus echidne Extract as a Reducing Agent. Anal. Lett. 2015, 48, 1180–1189. [Google Scholar] [CrossRef]
- Velusamy, P.; Das, J.; Pachaiappan, R.; Vaseeharan, B.; Pandian, K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind. Crops Prod. 2015, 66, 103–109. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Chen, X.; Yan, J.-K.; Wu, J.-Y. Characterization and antibacterial activity of silver nanoparticles prepared with a fungal exopolysaccharide in water. Food Hydrocoll. 2016, 53, 69–74. [Google Scholar] [CrossRef]
- Huang, Q.-L.; Siu, K.-C.; Wang, W.-Q.; Cheung, Y.-C.; Wu, J.-Y. Fractionation, characterization and antioxidant activity of exopolysaccharides from fermentation broth of a Cordyceps sinensis fungus. Process Biochem. 2013, 48, 380–386. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Z.Y.; Wang, W.Q.; Siu, K.C.; Wu, J.Y. An antioxidative galactomannan–protein complex isolated from fermentation broth of a medicinal fungus Cs-HK1. Carbohydr. Polym. 2014, 112, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Lehermayr, C.; Mahler, H.C.; Mader, K.; Fischer, S. Assessment of Net Charge and Protein-Protein Interactions of Different Monoclonal Antibodies. J. Pharm. Sci. 2011, 100, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem. 2013, 48, 1099–1106. [Google Scholar] [CrossRef]
- Sondi, I.; Goia, D.V.; Matijevic, E. Preparation of highly concentrated stable dispersions of uniform silver nanoparticles. J. Colloid Interface Sci. 2003, 260, 75–81. [Google Scholar] [CrossRef]
- Panigrahi, S.; Praharaj, S.; Basu, S.; Ghosh, S.K.; Jana, S.; Pande, S.; Vo-Dinh, T.; Jiang, H.; Pal, T. Self-assembly of silver nanoparticles: Synthesis, stabilization, optical properties, and application in surface-enhanced raman scattering. J. Phys. Chem. B 2006, 110, 13436–13444. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.G.; Salvarezza, R.C.; Arvia, A.J. The role of a slow phase formation process in the growth of anodic silver oxide layers in alkaline solutions—I. Electroformation of Ag(I) oxide layer. Electrochim. Acta 1988, 33, 1431–1437. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W. Shape control of silver nanoparticles by stepwise citrate reduction. J. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Rizzello, L.; Cingolani, R.; Pompa, P.P. Nanotechnology tools for antibacterial materials. Nanomedicine 2013, 8, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.E.; Ahmed, H.B. Polysaccharides templates for assembly of nanosilver. Carbohydr. Polym. 2016, 135, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S.; Singh, V.; Shamsi, S.; Fatma, A.; Mehta, B. Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnol. Res. Int. 2010. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Seal, S.; Megan, E.; Ramsdell, J.; Scammon, K.; Lelong, G.; Lachal, L.; Richardson, K.A. Physical and optical properties of sol-gel nano-silver doped silica film on glass substrate as a function of heat-treatment temperature. J. Appl. Phys. 2003, 93, 9553–9561. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Rustamova, N.; Yili, A.; Zhao, H.-Q.; Aisa, H.A. Synthesis of silver nanoparticles on the basis of low and high molar mass exopolysaccharides of Bradyrhizobium japonicum 36 and its antimicrobial activity against some pathogens. Folia Microbiol. 2016, 61, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Deschatre, M.; Ghillebaert, F.; Guezennec, J.; Simon-Colin, C. Study of biosorption of copper and silver by marine bacterial exopolysaccharides. WIT Trans. Ecol. Environ. 2015, 196, 549–559. [Google Scholar]
- Deschatre, M.; Lescop, B.; Colin, C.S.; Ghillebaert, F.; Guezennec, J.; Rioual, S. Characterization of exopolysaccharides after sorption of silver ions in aqueous solution. J. Environ. Chem. Eng. 2015, 3, 210–216. [Google Scholar] [CrossRef]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Impact of exopolysaccharides on the stability of silver nanoparticles in water. Water Res. 2011, 45, 5184–5190. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, M.F. Monosaccharides. In Carbohydrate Analysis: A Practical Approach; Chaplin, M.F., Kennedy, J.F., Eds.; IRL Press Ltd.: Oxford, UK, 1994. [Google Scholar]
- Crisan, E.V.; Sands, A. Nutritional Value. In The Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayes, W.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 137–165. [Google Scholar]
- Siu, K.-C.; Xu, L.; Chen, X.; Wu, J.-Y. Molecular properties and antioxidant activities of polysaccharides isolated from alkaline extract of wild Armillaria ostoyae mushrooms. Carbohydr. Polym. 2016, 137, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Jian, W.; Siu, K.-C.; Wu, J.-Y. Effects of pH and temperature on colloidal properties and molecular characteristics of Konjac glucomannan. Carbohydr. Polym. 2015, 134, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Smith, J.A.; Oyanedel-Craver, V. The effect of natural water conditions on the anti-bacterial performance and stability of silver nanoparticles capped with different polymers. Water Res. 2012, 46, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds, exopolysaccharide (EPS) fractions) are available from the authors. The EPS samples will be provided in the form of dry solid.




| EPS Fractions | Composition (Man:Glc:Gal) | MW (kDa) 1 | PD 2 | Rm (nm) 3 | Conformation (Plot Slope) | A2 (1 × 10−3 mol·mL/g2) |
|---|---|---|---|---|---|---|
| P0.5 | 1.3:10.8:1 | 1022 ± 41.9 | 5.170 ± 0.38 | 147.3 ± 5.60 | Random coil (0.50 ± 0.01) | −0.51 ± 0.02 |
| P2.0 | 16:1:7.3 | 85.16 ± 4.34 | 10.8 ± 0.43 | 31.0 ± 1.30 | Compact sphere (0.25 ± 0.02) | 4.85 ± 0.04 |
| P5.0 | 15:1:15.5 | 21.67 ± 0.85 | 3.287 ± 0.12 | 18.2 ± 1.80 | Compact sphere (0.21 ± 0.03) | 8.79 ± 0.05 |
| EPS-AgNPs | pH | Atom Contents (weight%) | |||
|---|---|---|---|---|---|
| C | N | O | Ag | ||
| P0.5-AgNPs | 5.5 | 34.6 ± 3.32 | 6.76 ± 2.13 | 2.23 ± 0.96 | 56.4 ± 3.89 |
| 7.0 | 33.9 ± 2.98 | 6.55 ± 2.43 | 2.05 ± 1.03 | 57.5 ± 4.41 | |
| 8.0 | 32.8 ± 3.07 | 7.03 ± 2.18 | 1.94 ± 1.27 | 58.2 ± 3.76 | |
| P2.0-AgNPs | 5.5 | 14.9 ± 1.98 | 10.0 ± 1.87 | 2.67 ± 0.89 | 72.4 ± 3.65 |
| 7.0 | 13.1 ± 2.04 | 11.4 ± 1.74 | 2.98 ± 0.93 | 73.5 ± 2.97 | |
| 8.0 | 11.5 ± 1.97 | 11.7 ± 1.89 | 2.74 ± 1.01 | 74.1 ± 3.06 | |
| 4.0 | 9.47 ± 1.04 | 10.2 ± 1.76 | 1.87 ± 1.05 | 78.5 ± 4.43 | |
| P5.0-AgNPs | 5.5 | 7.34 ± 1.16 | 10.4 ± 2.03 | 1.89 ± 1.21 | 80.3 ± 3.57 |
| 7.0 | 6.28 ± 1.21 | 10.1 ± 2.17 | 2.15 ± 0.99 | 81.5 ± 3.29 | |
| 8.0 | 7.11 ± 1.18 | 10.1 ± 1.87 | 2.03 ± 1.06 | 80.8 ± 3.87 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, W.; Zhang, L.; Siu, K.-C.; Song, A.; Wu, J.-Y. Formation and Physiochemical Properties of Silver Nanoparticles with Various Exopolysaccharides of a Medicinal Fungus in Aqueous Solution. Molecules 2017, 22, 50. https://doi.org/10.3390/molecules22010050
Jian W, Zhang L, Siu K-C, Song A, Wu J-Y. Formation and Physiochemical Properties of Silver Nanoparticles with Various Exopolysaccharides of a Medicinal Fungus in Aqueous Solution. Molecules. 2017; 22(1):50. https://doi.org/10.3390/molecules22010050
Chicago/Turabian StyleJian, Wenjie, Lu Zhang, Ka-Chai Siu, Angxin Song, and Jian-Yong Wu. 2017. "Formation and Physiochemical Properties of Silver Nanoparticles with Various Exopolysaccharides of a Medicinal Fungus in Aqueous Solution" Molecules 22, no. 1: 50. https://doi.org/10.3390/molecules22010050
APA StyleJian, W., Zhang, L., Siu, K.-C., Song, A., & Wu, J.-Y. (2017). Formation and Physiochemical Properties of Silver Nanoparticles with Various Exopolysaccharides of a Medicinal Fungus in Aqueous Solution. Molecules, 22(1), 50. https://doi.org/10.3390/molecules22010050







