Synthesis and Single Crystal Structures of Substituted-1,3-Selenazol-2-amines †
Abstract
:1. Introduction
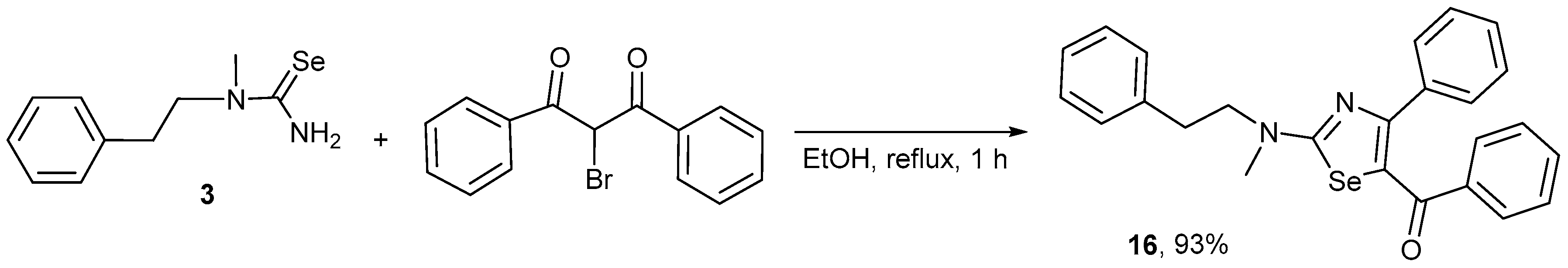
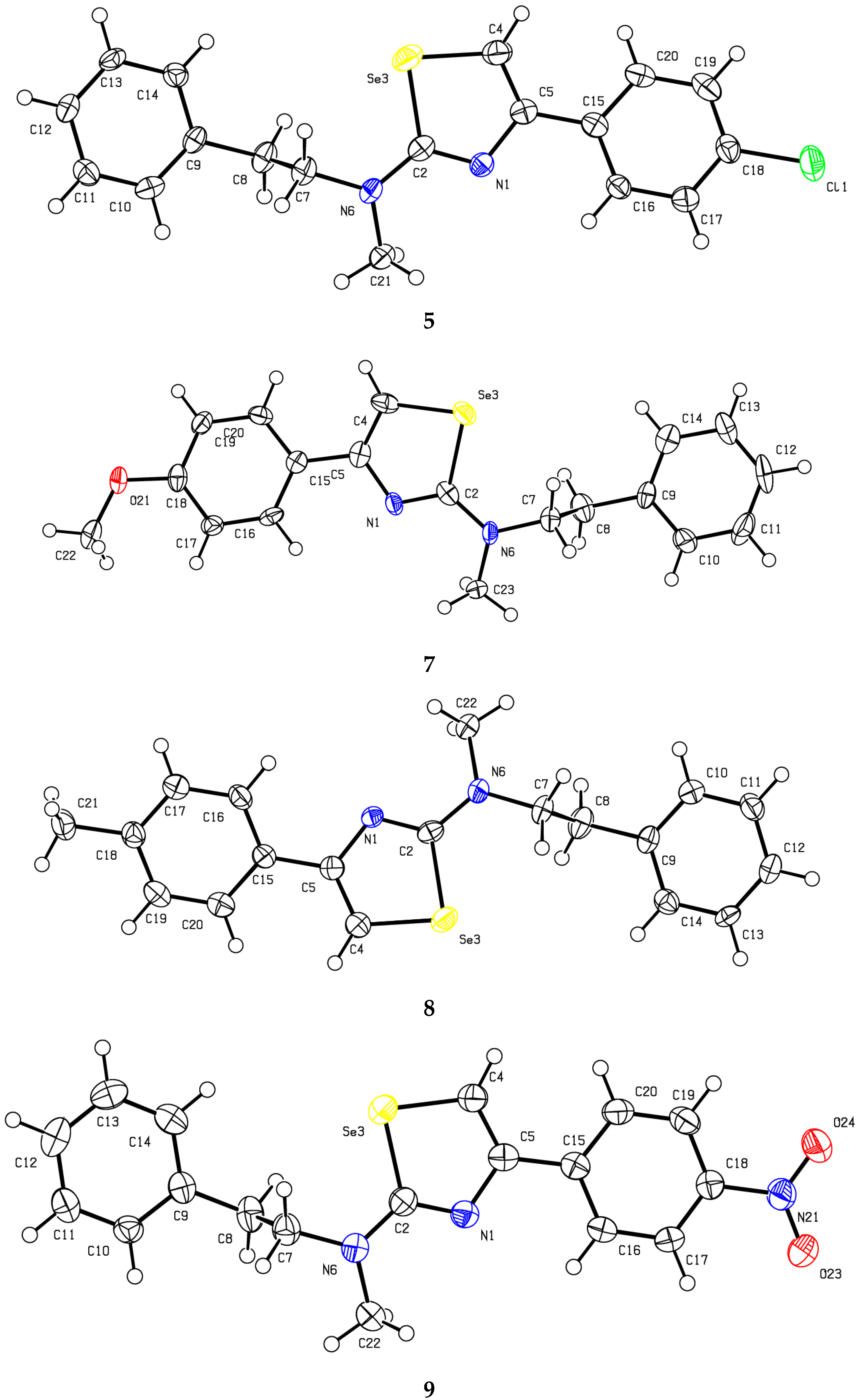
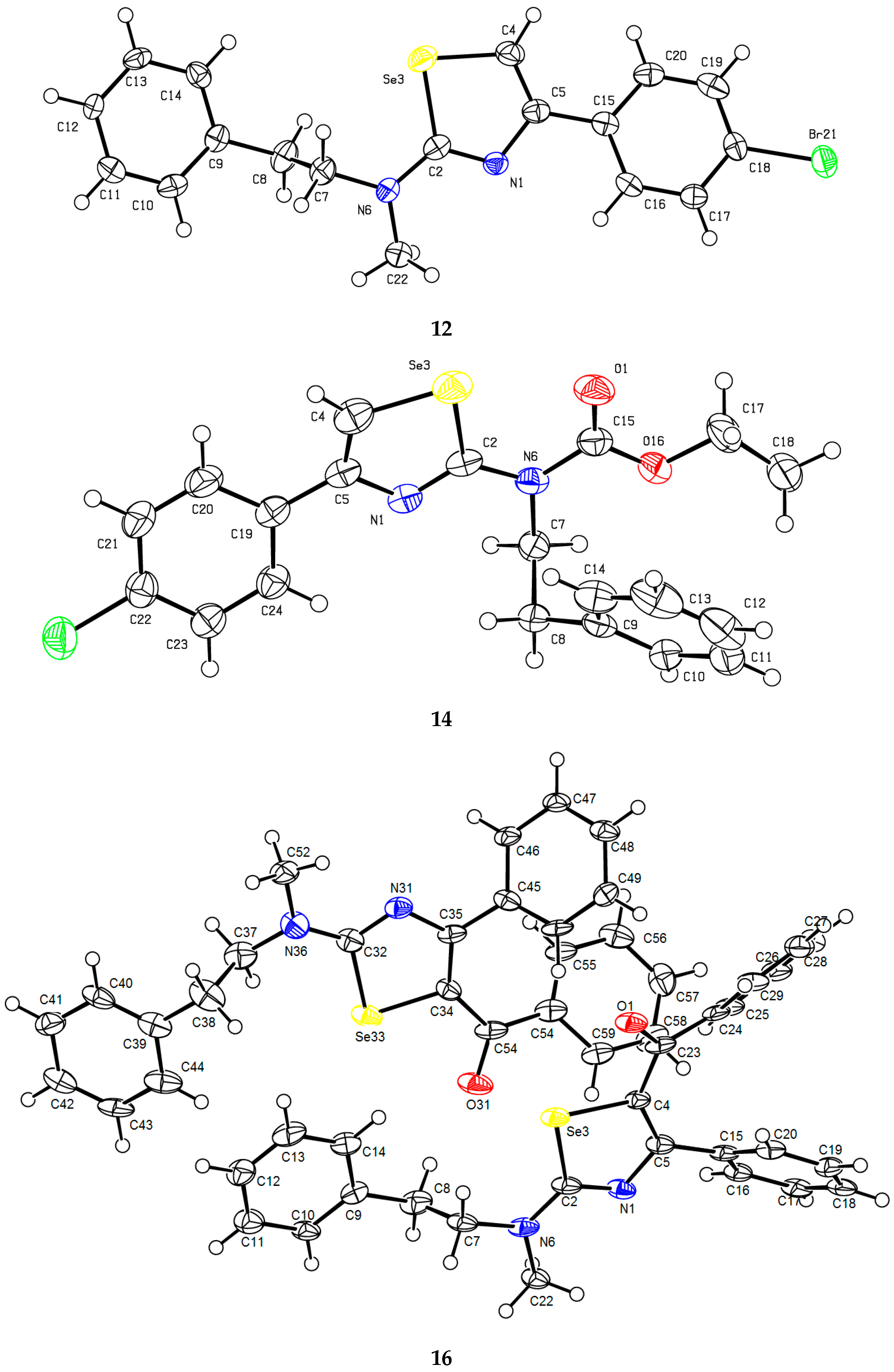
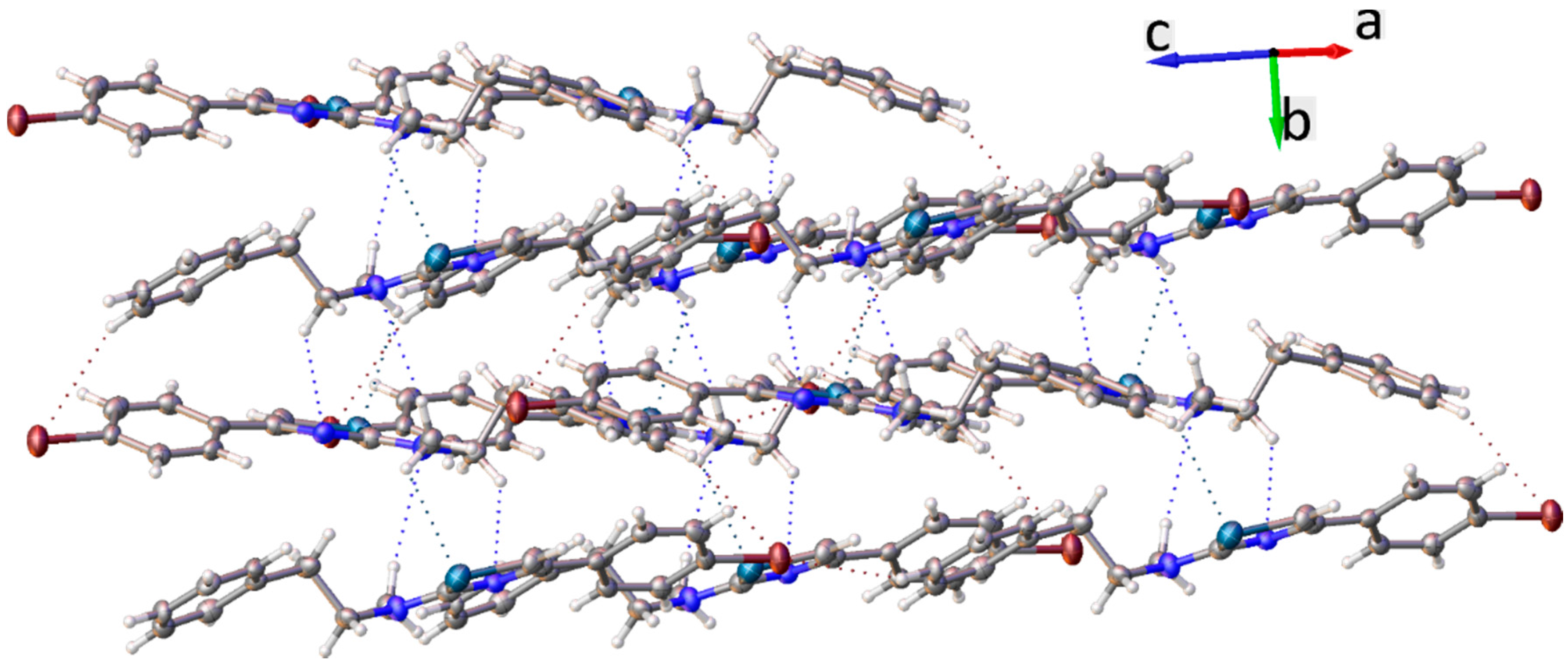
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis
General Procedure for the Synthesis of Compounds 5–16
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casar, Z.; Majcen-Le Marechal, A.; Lorey, D. A novel approach to a substituted 1,3-selenazole core as a precursor of electron rich olefins: Diselenadiazafulvalene and azino-diselenadiazafulvalene. New J. Chem. 2003, 27, 1622–1626. [Google Scholar] [CrossRef]
- Koketsu, M.; Ishihara, H. Synthesis of 1,3-selenazine and 1,3-selenazole and their biological activities. Curr. Org. Chem. 2003, 7, 175–185. [Google Scholar] [CrossRef]
- Duddeck, H.; Bradenahl, R.; Stefaniak, L.; Jazwinski, J.; Kamienski, B. Synthesis and multinuclear magnetic resonance investigation of some 1,3-selenazole and 1,3-selenazoline derivatives. Magn. Reson. Chem. 2001, 39, 709–713. [Google Scholar] [CrossRef]
- Archer, S.; McGarry, R. Diazotization of a 2-amino-1,3-selenazole. J. Heterocycl. Chem. 1982, 19, 1245–1246. [Google Scholar] [CrossRef]
- Koketsu, M.; Choi, S.Y.; Ishihara, H.; Lim, B.O.; Kim, H.; Kim, S.Y. Inhibitory effects of 1,3-selenazol-4-one derivatives on mushroom tyrosinase. Chem. Pharm. Bull. 2002, 50, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.M.; Kennedy, S.D.; Hennen, W.J. Selenium-77 NMR and crystallographic studies of selenazofurin and its 5-amino derivative. J. Am. Chem. Soc. 1990, 112, 8265–8268. [Google Scholar] [CrossRef]
- Shafiee, A.; Shafaati, A.; Khamench, B.H. Selenium heterocycles. XXXIX. Synthesis of thieno[3,4-d]thiazole, thieno[3,4-d]selenazole, selenolo[3,4-d]thiazole and selenolo[3,4-d]selenazole. J. Heterocycl. Chem. 1989, 26, 709–711. [Google Scholar] [CrossRef]
- Sekhiguchi, A.; Nishina, A.; Kimura, H.; Fukumoto, R.H.; Koichi, K.; Ishihara, H.; Koketsu, M. Superoxide anion-scavenging effect of 2-amino-1,3-selenazoles. Chem. Pharm. Bull. 2005, 53, 1439–1442. [Google Scholar] [CrossRef]
- Kazzouli, S.E.; Raboin, S.B.; Mouadbib, A.; Guillaumet, G. Solid support synthesis of 2,4-disubstituted thiazoles and aminothiazoles. Tetrahedron Lett. 2002, 43, 3193–3196. [Google Scholar] [CrossRef]
- Bailey, N.; Dean, A.W.; Judd, D.B.; Middlemiss, D.; Storer, R.; Stephen, P.W. A convenient procedure for solution phase preparation of 2-aminothiazole combinatorial libraries. Bioorg. Med. Chem. Lett. 1996, 6, 1409–1414. [Google Scholar] [CrossRef]
- Kearney, P.C.; Fernandez, M.; Flygare, J.A. Solid-phase synthesis of 2-aminothiazoles. J. Org. Chem. 1998, 63, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.; Fernandez, J. The preparation of 2,4-disubstituted thiazoles on solid support. Tetrahedron Lett. 1999, 40, 423–426. [Google Scholar] [CrossRef]
- Narender, M.; Somi Reddy, M.; Kumar, V.P.; Reddy, V.P.; Nageswar, Y.V.D.; Rao, K.R. Supramolecular synthesis of selenazoles using selenourea in water in the presence of β-cyclodextrin under atmospheric pressure. J. Org. Chem. 2007, 72, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Narender, M.; Somi Reddy, M.; Sridhar, R.; Nageswar, Y.V.D.; Rao, K.R. Aqueous phase synthesis of thiazoles and aminothiazoles in the presence of β-cyclodextrin. Tetrahedron Lett. 2005, 46, 5953–5955. [Google Scholar] [CrossRef]
- Dalip, K.; Kumar, N.M.; Patel, G.; Gupta, S.; Varma, R.S. A facile and eco-friendly synthesis of diarylthiazoles and diarylimidazoles is described utilizing a facile reaction of α-tosyloxyketones in water. Tetrahedron Lett. 2011, 52, 1983–1986. [Google Scholar]
- Madhav, B.; Narayana Murthy, S.; Anil Kumar, B.S.P.; Ramesh, K.; Nageswar, Y.V.D. A tandem one-pot aqueous phase synthesis of thiazoles/selenazoles. Tetrahedron Lett. 2012, 53, 3835–3838. [Google Scholar] [CrossRef]
- Klayman, D.L.; Griffins, T.S. Reaction of selenium with sodium borohydride in protic solvents. A facile method for the introduction of selenium into organic molecules. J. Am. Chem. Soc. 1973, 95, 197–199. [Google Scholar] [CrossRef]
- Lai, L.L.; Reid, D.H. Synthesis of primary selenocarboxamides and conversion of alkyl selenocarboxamides into selenazoles. Synthesis 1993, 1993, 870–872. [Google Scholar] [CrossRef]
- Koketsu, M.; Fukuta, Y.; Nada, F. Reaction of lithium aluminum hydride with elemental selenium: Its application as a selenating reagent into organic molecules. J. Am. Chem. Soc. 2001, 123, 8408–8409. [Google Scholar]
- Koketsu, M.; Fukuta, Y.; Ishihara, H. Preparation of N,N-unsubstituted selenoureas and thioureas from cyanamides. Tetrahedron Lett. 2001, 42, 6333–6335. [Google Scholar] [CrossRef]
- Ogawa, A.; Miyaka, J.; Karasaki, Y.; Murai, S.; Sonoda, N. Synthesis utilizing reducing ability of carbon selenocarboxamides: Reaction of nitriles with selenium, carbon monoxide, and water. J. Org. Chem. 1985, 50, 384–386. [Google Scholar] [CrossRef]
- Geisler, K.; Jacobs, A.; Kunzler, A.; Mathes, M.; Girrleit, H.; Zimmermann, B.; Bulka, E.; Pferffer, W.D.; Langer, P. Efficient synthesis of primary selenocarboxylic amides by reaction of nitriles with phosphorous(V) selenide. Synlett 2002, 2002, 1983–1986. [Google Scholar] [CrossRef]
- Kamminski, R.; Glass, R.S.; Skowronska, A. A convenient synthesis of selenocarboxamides from nitriles. Synthesis 2001, 2001, 1308–1310. [Google Scholar] [CrossRef]
- Cohen, V.J. Synthesis of unsubstituted aromatic and heterocyclic selenocarboxamides. Synthesis 1978, 1978, 668–669. [Google Scholar] [CrossRef]
- Shimada, K.; Hikage, S.; Takeishi, Y.; Takigawa, Y. A Novel synthesis of primary selenoamides from nitriles by the treatment of bis(trimethylsilyl) selenide and BF3·OEt2. Chem. Lett. 1990, 19, 1403–1406. [Google Scholar] [CrossRef]
- Ishihara, H.; Yosimuura, K.; Kouketsu, M. A facile preparation of aliphatic and aromatic primary selenoamides using 4-methylselenobenzoate as a new selenating reagent. Chem. Lett. 1998, 27, 1287–1288. [Google Scholar] [CrossRef]
- Gray, I.P.; Bhattacharyya, P.; Slawin, A.M.Z.; Woollins, J.D. A new synthesis of (PhPSe2)2 (Woollis reagent) and its use in the synthesis of novel P-Se heterocycles. Chem. Eur. J. 2005, 11, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Woollins, J.D. Formation and reactivity of phosphorus-selenium rings. Angew. Chem. Int. Ed. 2009, 48, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.J.A.; Romano, R.M.; Beckers, H.; Willner, H.; Della, V.C.O. Trifluoroselenoacetic acid, CF3C(O)SeH: Preparation and properties. Inorg. Chem. 2010, 49, 9972–9977. [Google Scholar] [CrossRef] [PubMed]
- Abdo, M.; Zhang, Y.; Schramm, V.L. Electrophilic aromatic selenylation: New OPRT inhibitors. Org. Lett. 2010, 12, 2982–2985. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.C.S.; Ooi, M.L. A new approach to coordination chemistry involving phosphorus-selenium based ligands. Ring opening, deselenation and phosphorus–phosphorus coupling of Woollins’ reagen. Inorg. Chim. Acta 2011, 366, 350–356. [Google Scholar] [CrossRef]
- Hua, G.; Griffin, J.M.; Ashbrook, S.E.; Slawin, A.M.Z.; Woollins, J.D. Octaselenocyclododecane. Angew. Chem. Int. Ed. 2011, 50, 4123–4126. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Du, J.; Slawin, A.M.Z.; Woollins, J.D. Fluorinated phosphorus-selenium heteroatom compounds: Phenylphosphonofluorodiselenoic salts, adducts, and esters. Inorg. Chem. 2013, 52, 8214–8217. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Randall, R.A.M.; Slawin, A.M.Z.; Cordes, D.B.; Crawford, L.; Bühl, M.; Woollins, J.D. An efficient route for the synthesis of phosphorus-selenium macroheterocycles. Chem. Commun. 2013, 49, 2619–2621. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Du, J.; Slawin, A.M.Z.; Woollins, J.D. One-pot approach to organo-phosphorus-chalcogen macrocycles incorporating double OP(S)SCn or OP(Se)SeCn scoffolds: A synthetic and structural study. Chem. Eur. J. 2016, 22, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Li, Y.; Slawin, A.M.Z.; Woollins, J.D. Synthesis of primary arylselenoamides by reaction of aryl nitriles with Woollins’ reagent. Org. Lett. 2006, 8, 5251–5254. [Google Scholar] [CrossRef] [PubMed]
- Axelle, R.C.; Sylvie, D.; Celine, P.; David, L.G.; Jean-Luc, B.; Roger, A.; Marie-Agnes, S.; Dennis, S.; Daniel, M. N-Aryl N′-hydroxyguanidines, a new class of NO-donors after selective oxidation by nitric synthases: Structure-activity relationship. J. Med. Chem. 2002, 45, 944–954. [Google Scholar]
- Hiroyo, K.; Masako, I.; Masahiro, S.; Keiro, H.; Keiko, Y.; Hiroko, S.; Tatsuhiro, T.; Tsutomu, I. Chemistry of N-hydroxyguanidines: Photo-sensitized oxygenation and reaction with nitric oxide. Helv. Chim. Acta 2002, 85, 2636–2643. [Google Scholar]
- Garmaise, D.L.; Uchiyama, A. Some stable dimers of substituted benzylcyanamides. Can. J. Chem. 1961, 39, 1054–1058. [Google Scholar] [CrossRef]
- Bi, X.; Lopez, C.; Bacchi, C.J.; Rattendi, D.; Woster, P.M. Novel alkylpolyaminoguanidines and alkylpolyaminobiguanides with potent antitrypanosomal activity. Bioorg. Med. Chem. Lett. 2006, 16, 3229–3232. [Google Scholar] [CrossRef] [PubMed]
- Bakunov, S.A.; Rukavishnikov, A.V.; Kachev, A.V. Modification of the Tieman rearrangement: One-pot synthesis of N,N-disubstituted cyanamides from amidoximes. Synthesis 2000, 2000, 1148–1153. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, Q.; Li, Y.; Slawin, A.M.Z.; Woollins, J.D. Novel heterocyclic selenazadiphospholaminediselenides, zwitterionic carbamidoyl(phenyl)-phosphinodiselenoic acids and selenoureas derived from cyanamides. Tetrahedron 2009, 65, 6074–6082. [Google Scholar] [CrossRef]
- Kurita, E.; Matsuura, H.; Ohno, K. Relationship between force constants and bond lengths for CX (X = C, Si, Ge, N, P, As, O, S, Se, F, Cl and Br) single and multiple bonds: Formulation of Badger’s rule for universal use. Spectrochim. Acta A 2004, 60, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, M.; Kanoh, K.; Ando, H.; Ishihara, H. A facile synthesis of 2-amino-1,3-selenazole by reaction of N,N-unsubstituted selenourea with ketone. Heteroat. Chem. 2006, 17, 88–92. [Google Scholar] [CrossRef]
- Hua, G.; Du, J.; Slawin, A.M.Z.; Woollins, J.D. 2,4-Diaryl-1,3-chalcogen azoles bearing pentafluorosulfanyl SF5 groups: A synthetic and structural study. J. Org. Chem. 2014, 79, 3876–3886. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Du, J.; Slawin, A.M.Z.; Woollins, J.D. A synthetic and structural study of arylselenoamides and 2,4-diaryl-1,3-selenazoles. Synlett 2014, 25, 2189–2195. [Google Scholar]
- Geisler, K.; Pfeiffer, W.D.; Künzler, A.; Below, H.; Bulka, E.; Langer, P. Synthesis of 1,3-selenazoles and bis(selenazoles) from primary selenocarboxylic amides and selenourea. Synthesis 2004, 875–884. [Google Scholar] [CrossRef]
- Murai, T.; Yamaguchi, K.; Hori, F.; Maruyama, T. Reaction of selenoamide dianions with thio- and selenoformamides leading to the formation of 5-aminoselenazoles: Photophysical and electrochemical properties. J. Org. Chem. 2014, 79, 4930–4939. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T. Organoselenium Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Koketsu, M.; Mio, T.; Ishihara, H. Facile preparation of 1,3-selenazole-5-carboxylic acids and the carboxylates by reaction of selenazadienes with chloroacetyl chloride. Synthesis 2004, 2004, 233–236. [Google Scholar] [CrossRef]
- Li, G.M.; Zingaro, R.A.; Segi, M.; Reibenspies, J.H.; Nakajima, T. Synthesis and structure of telluroamides and selenoamides. The first crystallographic study of Telluroamides. Organometallics 1997, 16, 756–762. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
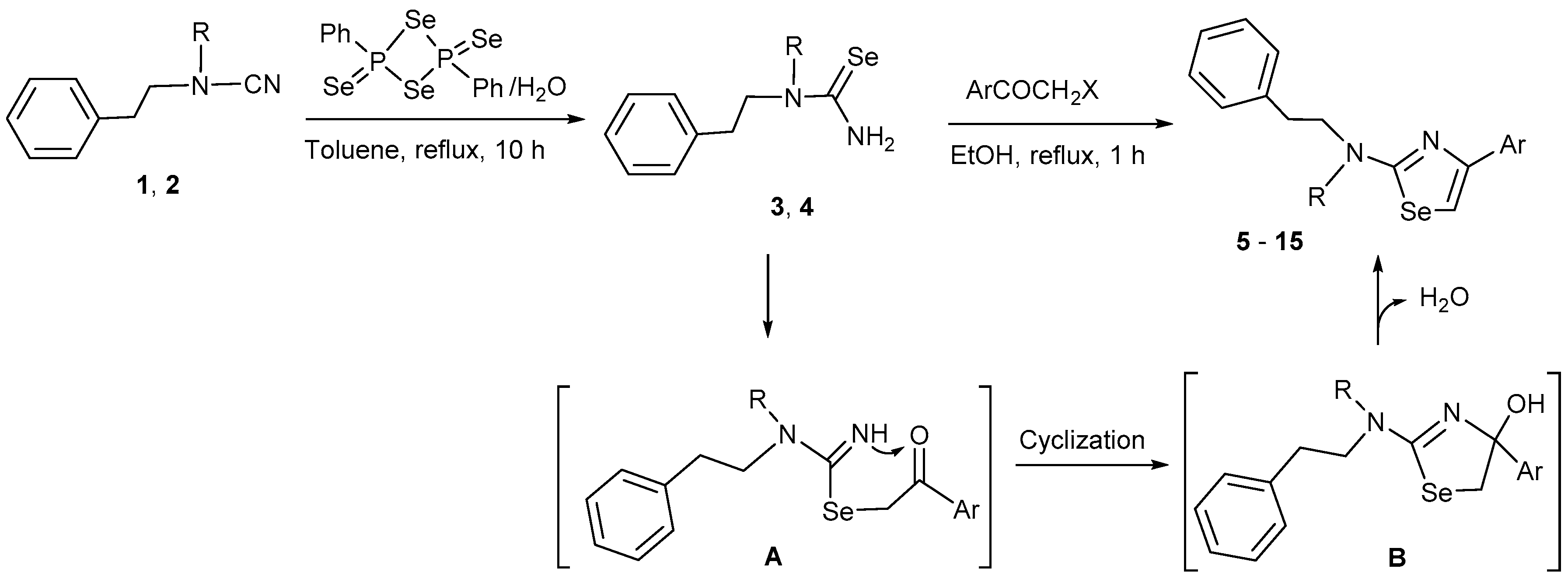

| Compound | X | R | Ar | Yield (%) | 77Se-NMR (δ, ppm) |
|---|---|---|---|---|---|
| 1 | - | CH3 | - | 99 | - |
| 2 | - | C2H5O(O)C | - | 99 | - |
| 3 | - | CH3 | - | 87 | 607.7 |
| 4 | - | C2H5O(O)C | - | 90 | 382.1 |
| 5 | Cl | CH3 | C6H5 | 92 | 575.3 |
| 6 | Br | CH3 | 4-ClC6H4 | 96 | 571.1 |
| 7 | Br | CH3 | 4-MeOC6H4 | 97 | 567.1 |
| 8 | Br | CH3 | 4-MeC6H4 | 98 | 568.1 |
| 9 | Br | CH3 | 4-NO2C6H4 | 93 | 590.1 |
| 10 | Br | CH3 | 2,5-di-MeOC6H3 | 90 | 572.7 |
| 11 | Cl | CH3 | 2,4-di-ClC6H3 | 91 | 578.9 |
| 12 | Br | CH3 | 4-BrC6H4 | 95 | 577.8 |
| 13 | Cl | C2H5O(O)C | C6H5 | 96 | 679.9 |
| 14 | Br | C2H5O(O)C | 4-ClC6H4 | 96 | 684.2 |
| 15 | Br | C2H5O(O)C | 4-MeOC6H4 | 95 | 675.7 |
| Compound | ||||
|---|---|---|---|---|
| 5 | 7 | 8 | 9 | |
| Formula | C18H17ClN2Se | C19H20N2OSe | C19H20N2Se | C18H17N3O2Se |
| M | 375.76 | 371.34 | 355.34 | 386.31 |
| Crystal system | monoclinic | orthorhombic | monoclinic | monoclinic |
| Space group | P21 | P212121 | P21 | P21/n |
| a/Å | 10.657(7) | 6.6544(8) | 10.719(4) | 10.5159(7) |
| b/Å | 7.525(5) | 7.8134(9) | 7.495(3) | 7.5401(5) |
| c/Å | 11.320(8) | 33.372(4) | 11.294(4) | 20.9330(15) |
| α | 90 | 90 | 90 | 90 |
| β | 115.852(8) | 90 | 115.650(6) | 91.879(2) |
| γ | 90 | 90 | 90 | 90 |
| U/A3 | 817.0(10) | 1735.1(4) | 818.0(5) | 1658.9(2) |
| Z | 2 | 4 | 2 | 4 |
| µ/cm−1 | 24.591 | 21.703 | 22.939 | 22.793 |
| Reflections collected | 7051 | 11,930 | 6175 | 12,160 |
| Independent reflections | 2587 | 3043 | 1540 | 2895 |
| Rint | 0.0291 | 0.1535 | 0.0336 | 0.0679 |
| R1 | 0.0249 | 0.0695 | 0.0532 | 0.0394 |
| wR2 [I > 2σ(I)] | 0.0544 | 0.1012 | 0.1398 | 0.0903 |
| Compound | |||
|---|---|---|---|
| 12 | 14 | 16 | |
| Formula | C18H17BrN2Se | C20H19ClN2O2Se | C25H22N2OSe |
| M | 420.21 | 433.80 | 445.42 |
| Crystal system | monoclinic | orthorhombic | monoclinic |
| Space group | P21 | Pbca | P21/c |
| a/Å | 10.6448(10) | 27.974(16) | 10.1342(7) |
| b/Å | 7.4660(7) | 17.910(11) | 14.6025(10) |
| c/Å | 11.5064(11) | 7.817(5) | 27.942(2) |
| α | 90 | 90 | 90 |
| β | 116.039(3) | 90 | 90.421(4) |
| γ | 90 | 90 | 90 |
| U/A3 | 821.64(14) | 3916(4) | 4134.9(5) |
| Z | 2 | 8 | 8 |
| µ/cm−1 | 47.219 | 20.701 | 18.350 |
| Reflections collected | 6350 | 26,522 | 31,646 |
| Independent reflections | 2839 | 3437 | 7262 |
| Rint | 0.0536 | 0.0694 | 0.2391 |
| R1 | 0.0309 | 0.0422 | 0.0701 |
| wR2 [I > 2σ(I)] | 0.0639 | 0.0975 | 0.1456 |
| 5 | 7 | 8 | 9 | 12 | 14 | 16 | |
|---|---|---|---|---|---|---|---|
| N1-C2 | 1.302(5) | 1.295(11) | 1.300(13) | 1.291(4) | 1.300(9) | 1.301(4) | 1.329(12)[1.286(12)] |
| C2-N6 | 1.358(5) | 1.350(11) | 1.348 (12) | 1.350(5) | 1.368(9) | 1.402(4) | 1.348(12)[1.369(12)] |
| C2-Se3 | 1.906(3) | 1.924(8) | 1.914(7) | 1.912(3) | 1.896(5) | 1.899(3) | 1.863(8)[1.886(7)] |
| Se3-C4 | 1.860(5) | 1.886(9) | 1.858(12) | 1.854(4) | 1.862(8) | 1.872(4) | 1.866(8)[1.898(9)] |
| C4-C5 | 1.349(5) | 1.353(12) | 1.355(12) | 1.359(5) | 1.358(8) | 1.356(5) | 1.366(11)[1.349(11)] |
| C5-N1 | 1.394(4) | 1.395(11) | 1.387(10) | 1.389(4) | 1.387(7) | 1.401(4) | 1.374(11)[1.378(11)] |
| N1-C2-N6 | 123.8(3) | 125.1(8) | 124.5(7) | 124.8(3) | 123.2(5) | 120.3(3) | 120.5(7)[121.5(7)] |
| N1-C2-Se3 | 115.1(3) | 114.5(6) | 114.4(6) | 114.9(3) | 115.7(4) | 116.1(2) | 115.6(6)[117.1(6)] |
| Se3-C2-N6 | 121.1(3) | 120.3(6) | 121.1(7) | 120.3(2) | 121.1(5) | 123.7(2) | 123.8(7)[121.4(6)] |
| C2-Se3-C4 | 83.59(18) | 83.9(4) | 83.6(4) | 83.370(15) | 83.1(3) | 83.10(15) | 84.3(4)[82.5(4)] |
| Se3-C4-C5 | 111.5(3) | 110.1(6) | 111.6(7) | 111.3(3) | 111.8(5) | 111.9(3) | 110.6(6)[110.2(6)] |
| C5-N1-C2 | 112.0(3) | 112.7(7) | 112.9(6) | 112.6(3) | 111.6(4) | 112.0(3) | 111.7(7)[111.6(7)] |
| N1-C5-C15 | 117.1(3) | 117.2(7) | 118.2(7) | 117.5(3) | 117.3(4) | 116.3(3) * | 112.9(7)[113.7(7)] |
| N1-C5-C4 | 117.8(4) | 118.8(8) | 117.4(9) | 117.5(3) | 117.7(6) | 116.9(3) | 117.8(8)[118.4(8)] |
| C4-C5-C15 | 124.9(3) | 123.9(8) | 124.2(8) | 124.9(3) | 124.9(6) | 126.8(3) * | 129.2(8)[127.6(8)] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, G.; Du, J.; Slawin, A.M.Z.; Woollins, J.D. Synthesis and Single Crystal Structures of Substituted-1,3-Selenazol-2-amines. Molecules 2017, 22, 46. https://doi.org/10.3390/molecules22010046
Hua G, Du J, Slawin AMZ, Woollins JD. Synthesis and Single Crystal Structures of Substituted-1,3-Selenazol-2-amines. Molecules. 2017; 22(1):46. https://doi.org/10.3390/molecules22010046
Chicago/Turabian StyleHua, Guoxiong, Junyi Du, Alexandra M. Z. Slawin, and J. Derek Woollins. 2017. "Synthesis and Single Crystal Structures of Substituted-1,3-Selenazol-2-amines" Molecules 22, no. 1: 46. https://doi.org/10.3390/molecules22010046
APA StyleHua, G., Du, J., Slawin, A. M. Z., & Woollins, J. D. (2017). Synthesis and Single Crystal Structures of Substituted-1,3-Selenazol-2-amines. Molecules, 22(1), 46. https://doi.org/10.3390/molecules22010046







