Rate of Homologous Desensitization and Internalization of the GLP-1 Receptor
Abstract
:1. Introduction
2. Results
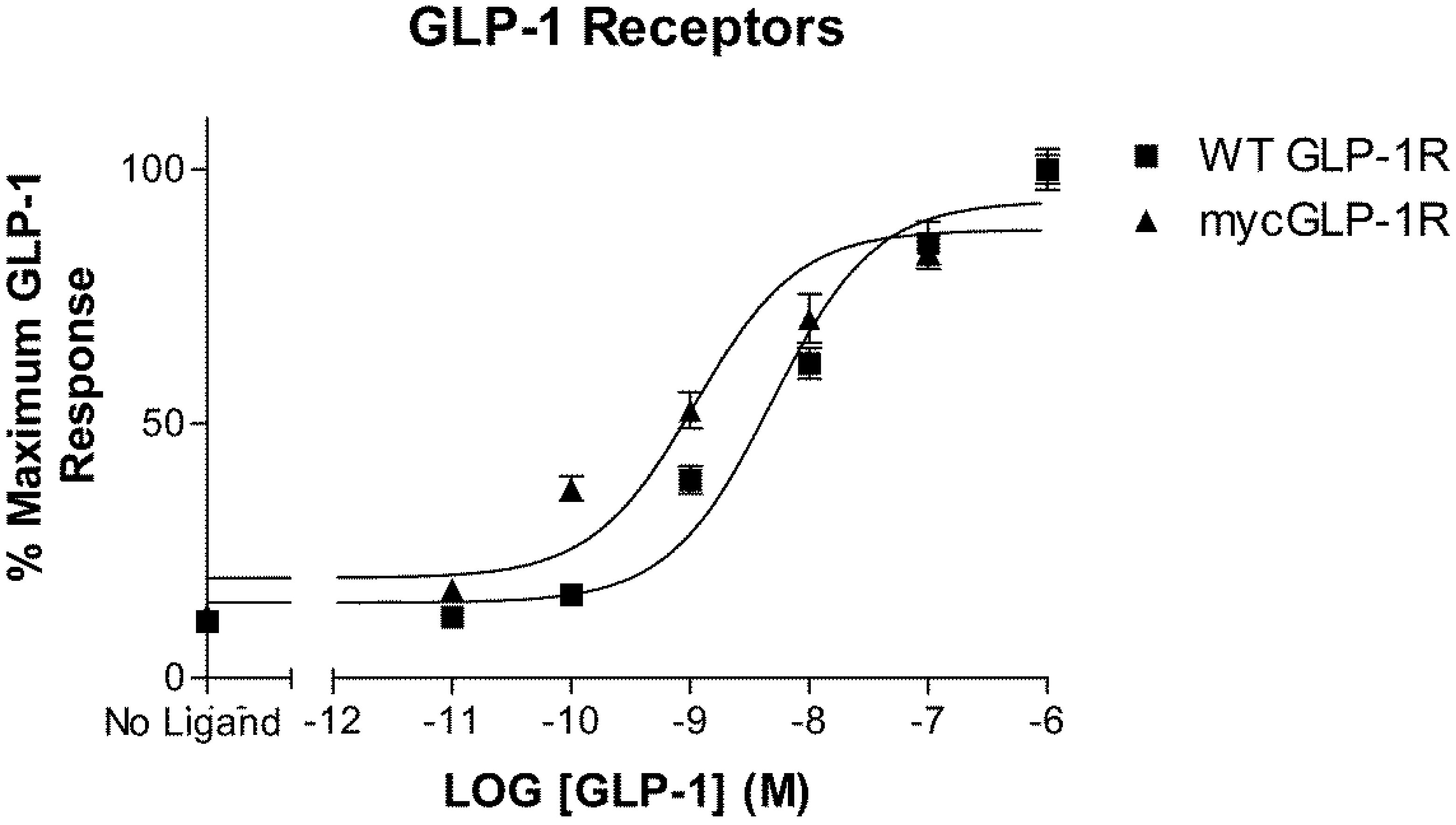
2.1. Validation of N-Terminally Labelled GLP-1R
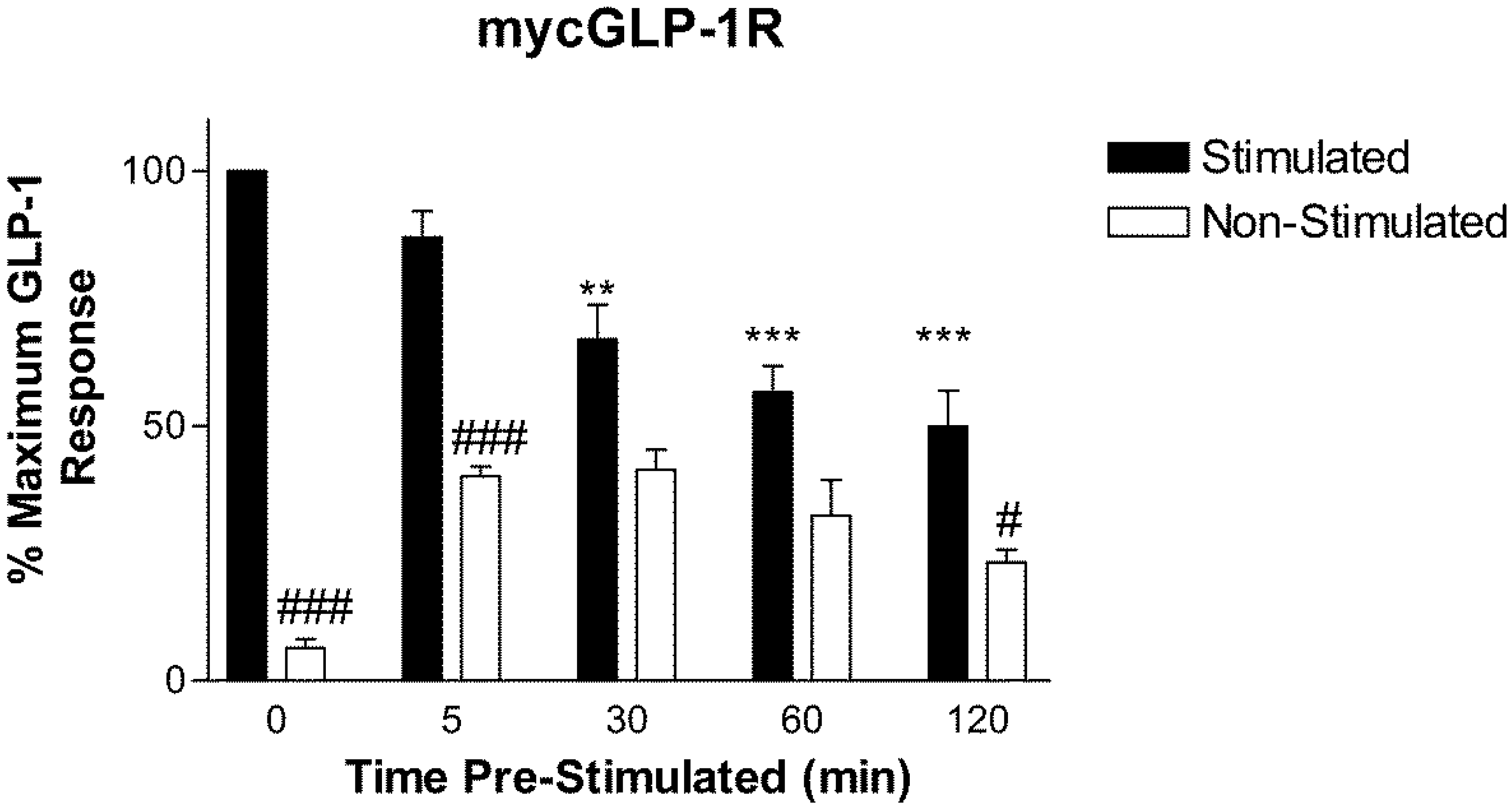
2.2. Homologous Desensitization of GLP-1R
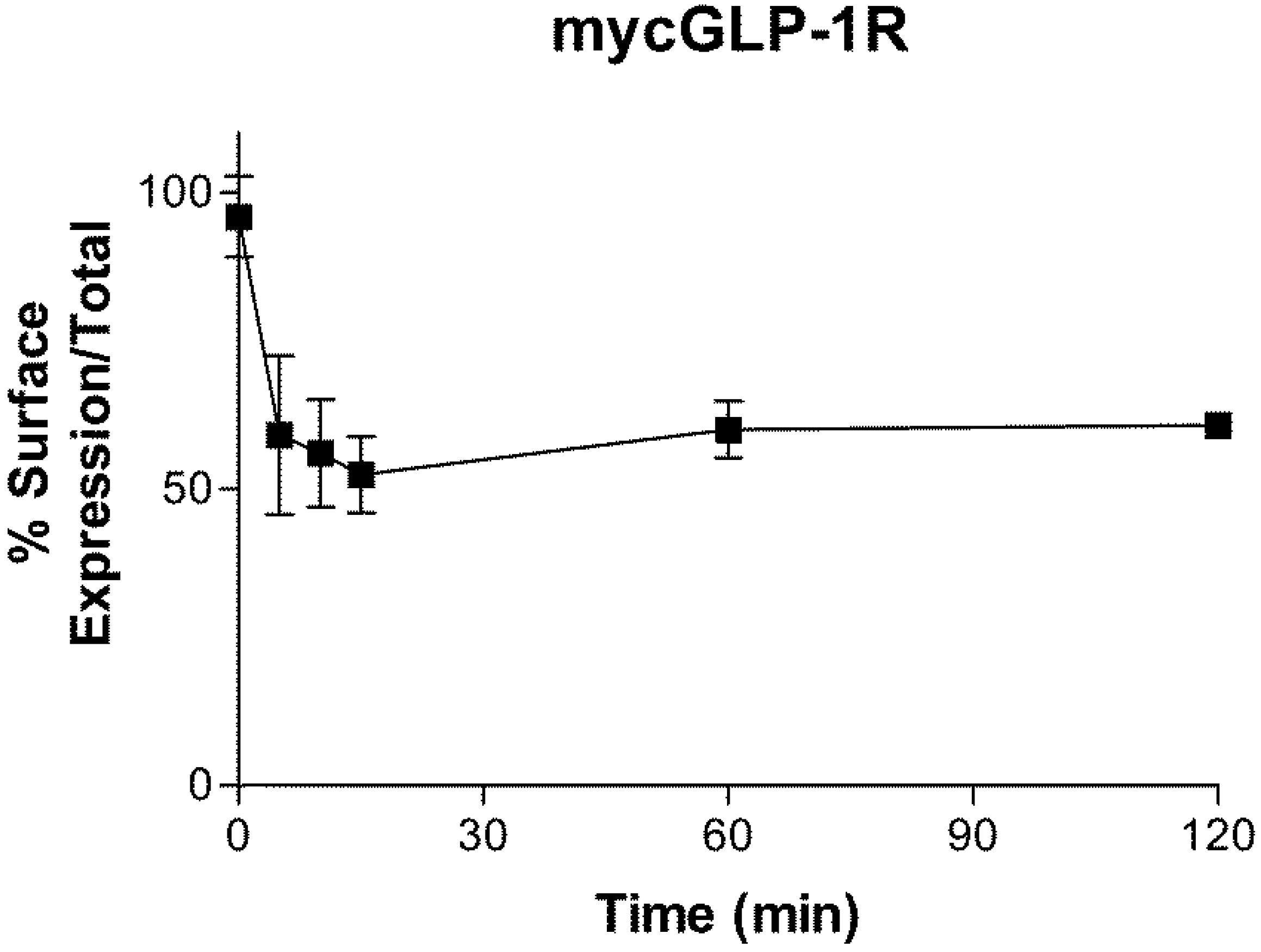
2.3. Internalization of GLP-1R
2.4. Visualization of Internalized GLP-1R
3. Discussion
4. Materials and Methods
4.1. Construction of cDNA
4.2. Cell Culture and Transfection of Cells
4.3. Luciferase Assay
4.4. Desensitization Assay
4.5. Internalization Assay
4.6. Confocal Microscopy
4.7. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, C.H.; Widenmaier, S.; Kim, S.J. Pleiotropic actions of the incretin hormones. Vitam. Horm. 2010, 84, 21–79. [Google Scholar] [PubMed]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Knop, F.K.; Vilsboll, T.; Krarup, T.; Madsbad, S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011, 34, S251–S257. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. Pharmacology of GLP-1-based therapies. Br. J. Diabetes Vasc. Dis. 2008, 8 (Suppl. 2), S10–S18. [Google Scholar] [CrossRef]
- Ahren, B. The future of incretin-based therapy: Novel avenues—novel targets. Diabetes Obes. Metab. 2011, 13, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br. J. Pharmacol. 2012, 166, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Montrose-Rafizadeh, C.; Avdonin, P.; Garant, M.J.; Rodgers, B.D.; Kole, S.; Yang, H.; Levine, M.A.; Schwindinger, W.; Bernier, M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 1999, 140, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.J.; Benovic, J.L.; Codina, J.; Caron, M.G.; Lefkowitz, R.J. Beta-Arrestin: A protein that regulates beta-adrenergic receptor function. Science 1990, 248, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.S. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev. 2001, 53, 1–24. [Google Scholar] [PubMed]
- Krasel, C.; Bunemann, M.; Lorenz, K.; Lohse, M.J. Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J. Biol. Chem. 2005, 280, 9528–9535. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.S.; Zhang, J.; Barak, L.S.; Caron, M.G. G-protein-coupled receptor kinases and arrestins: Regulators of G-protein-coupled receptor sequestration. Biochem. Soc. Trans. 1996, 24, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabah, S.; Al-Fulaij, M.; Shaaban, G.; Ahmed, H.A.; Mann, R.J.; Donnelly, D.; Bunemann, M.; Krasel, C. The GIP receptor displays higher basal activity than the GLP-1 receptor but does not recruit GRK2 or arrestin3 effectively. PLoS ONE 2014, 9, e106890. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, R.; Martini, L.; Schwartz, T.W.; Elling, C.E. Characterization of glucagon-like peptide-1 receptor beta-arrestin2 interaction: A high-affinity receptor phenotype. Mol. Endocrinol. 2005, 19, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Kanamarlapudi, V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Galphaq pathway. Biochem. Pharmacol. 2015, 93, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wilkinson, G.F.; Willars, G.B. Role of the signal peptide in the synthesis and processing of the glucagon-like peptide-1 receptor. Br. J. Pharmacol. 2010, 159, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Widmann, C.; Dolci, W.; Thorens, B. Desensitization and phosphorylation of the glucagon-like peptide-1 (GLP-1) receptor by GLP-1 and 4-phorbol 12-myristate 13-acetate. Mol. Endocrinol. 1996, 10, 62–75. [Google Scholar] [PubMed]
- Widmann, C.; Dolci, W.; Thorens, B. Internalization and homologous desensitization of the GLP-1 receptor depend on phosphorylation of the receptor carboxyl tail at the same three sites. Mol. Endocrinol. 1997, 11, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Kim, J.G.; Drucker, D.J. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes 2004, 53, S205–S214. [Google Scholar] [CrossRef] [PubMed]
- Widmann, C.; Dolci, W.; Thorens, B. Agonist-induced internalization and recycling of the glucagon-like peptide-1 receptor in transfected fibroblasts and in insulinomas. Biochem. J. 1995, 310, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Roed, S.N.; Wismann, P.; Underwood, C.R.; Kulahin, N.; Iversen, H.; Cappelen, K.A.; Schaffer, L.; Lehtonen, J.; Hecksher-Soerensen, J.; Secher, A.; et al. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol. Cell. Endocrinol. 2014, 382, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, M.; Hausdorff, W.P.; De Blasi, A.; O’Dowd, B.F.; Kobilka, B.K.; Caron, M.G.; Lefkowitz, R.J. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature 1988, 333, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Barak, L.S.; Tiberi, M.; Freedman, N.J.; Kwatra, M.M.; Lefkowitz, R.J.; Caron, M.G. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J. Biol. Chem. 1994, 269, 2790–2795. [Google Scholar] [PubMed]
- Shenoy, S.K.; Lefkowitz, R.J. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J. Biol. Chem. 2005, 280, 15315–15324. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, N.; Imamura, T.; Yoshizaki, T.; Babendure, J.L.; Lu, J.C.; Olefsky, J.M. Beta-Arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6614–6619. [Google Scholar] [CrossRef] [PubMed]
- Syme, C.A.; Zhang, L.; Bisello, A. Caveolin-1 regulates cellular trafficking and function of the glucagon-like Peptide 1 receptor. Mol. Endocrinol. 2006, 20, 3400–3411. [Google Scholar] [CrossRef] [PubMed]
- Kuna, R.S.; Girada, S.B.; Asalla, S.; Vallentyne, J.; Maddika, S.; Patterson, J.T.; Smiley, D.L.; DiMarchi, R.D.; Mitra, P. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E161–E170. [Google Scholar] [CrossRef] [PubMed]
- Merlen, C.; Fabrega, S.; Desbuquois, B.; Unson, C.G.; Authier, F. Glucagon-mediated internalization of serine-phosphorylated glucagon receptor and Gsalpha in rat liver. FEBS Lett. 2006, 580, 5697–5704. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, S.; Feinstein, T.N.; Castro, M.; Wang, B.; Bouley, R.; Potts, J.T.; Gardella, T.J.; Vilardaga, J.P. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 2009, 5, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Gherardi, M.J.; Froese, A.; Zanoun, M.; Gigoux, V.; Clerc, P.; Gaits-Iacovoni, F.; Steyaert, J.; Nikolaev, V.O.; Fourmy, D. Internalized Receptor for Glucose-dependent Insulinotropic Peptide stimulates adenylyl cyclase on early endosomes. Biochem. Pharmacol. 2016, 120, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, T.N.; Wehbi, V.L.; Ardura, J.A.; Wheeler, D.S.; Ferrandon, S.; Gardella, T.J.; Vilardaga, J.P. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol. 2011, 7, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Dubois-Vedrenne, I.; Laval, M.; Tikhonova, I.G.; D’Angelo, R.; Sanchez, C.; Clerc, P.; Gherardi, M.J.; Gigoux, V.; Magnan, R.; et al. Internalization and desensitization of the human glucose-dependent-insulinotropic receptor is affected by N-terminal acetylation of the agonist. Mol. Cell. Endocrinol. 2015, 414, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabah, S.; Al-Fulaij, M.; Ahmed, H.A. Selectivity of peptide ligands for the human incretin receptors expressed in HEK-293 cells. Eur. J. Pharmacol. 2014, 741, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the receptor constructs are available from the authors.


| Control (Media) ↓ | Control (Media) ↓ | Pre-Stimulate with 1 µM GLP-1 (5–120) min ↓ | Pre-Stimulate with 1 µM GLP-1 (5–120) min ↓ |
| Wash ↓ | Wash ↓ | Wash ↓ | Wash ↓ |
| Stimulate 1 µM GLP-1 15 min (Stimulated) ↓ | Media (Non-Stimulated) ↓ | Stimulate 1 µM GLP-1 15 min (Stimulated) ↓ | Media (Non-Stimulated) ↓ |
| Assay | Assay | Assay | Assay |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaaban, G.; Oriowo, M.; Al-Sabah, S. Rate of Homologous Desensitization and Internalization of the GLP-1 Receptor. Molecules 2017, 22, 22. https://doi.org/10.3390/molecules22010022
Shaaban G, Oriowo M, Al-Sabah S. Rate of Homologous Desensitization and Internalization of the GLP-1 Receptor. Molecules. 2017; 22(1):22. https://doi.org/10.3390/molecules22010022
Chicago/Turabian StyleShaaban, Ghina, Mabayoje Oriowo, and Suleiman Al-Sabah. 2017. "Rate of Homologous Desensitization and Internalization of the GLP-1 Receptor" Molecules 22, no. 1: 22. https://doi.org/10.3390/molecules22010022
APA StyleShaaban, G., Oriowo, M., & Al-Sabah, S. (2017). Rate of Homologous Desensitization and Internalization of the GLP-1 Receptor. Molecules, 22(1), 22. https://doi.org/10.3390/molecules22010022






