New Phenylpropanoid and Coumarin Glycosides from the Stems of Hydrangea paniculata Sieb
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification and Characterization
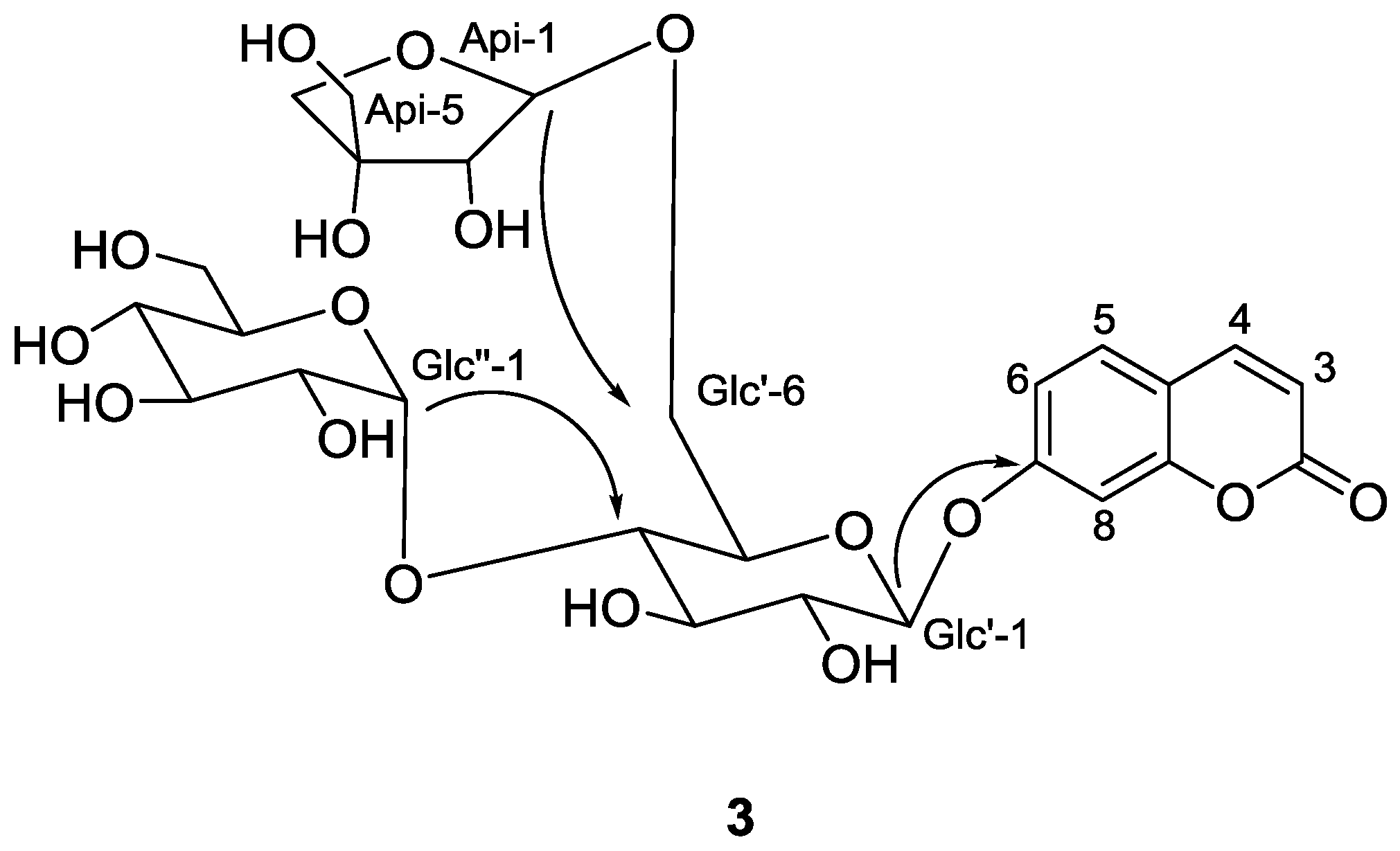
2.2. Structure Identification of the Known Isolates
2.3. Hepatoprotective Effect of Compounds 1–3
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis and Sugar Analysis
3.5. Hepatoprotective Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Herbal Medicine Company. Chinese Traditional Medicine Resource Records; Science Publishing House: Beijing, China, 1994; p. 480. [Google Scholar]
- Zhang, D.M.; Chen, X.G.; Yang, J.Z.; Li, Y.; Zheng, X.G. Effective fraction of hydrangea paniculata and its preparation method, pharmaceutical composition comprising the same, and uses thereof. CN1690069A, 2 November 2005. [Google Scholar]
- Zhang, S.; Xin, H.Q.; Li, Y.; Zhang, D.M.; Shi, J.; Yang, J.Z.; Chen, X.G. Skimmin, a coumarin from Hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evid-based Compl. Alt.: eCAM. 2013, 819296. [Google Scholar] [CrossRef]
- Shi, J.; Li, C.J.; Yang, J.Z.; Ma, J.; Wang, C.; Tang, J.; Li, Y.; Chen, H.; Zhang, D.M. Hepatoprotective coumarins and secoiridoids from Hydrangea paniculata. Fitoterapia 2014, 96, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, C.J.; Yang, J.Z.; Yuan, Y.H.; Chen, N.H.; Zhang, D.M. Coumarin Glycosides and Iridoid Glucosides with Neuroprotective Effects from Hydrangea paniculata. Planta Med. 2012, 78, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, J.Z.; Li, H.Y.; Li, Y.; Liu, Y.; Zhang, D.M.; Zhang, F.R.; Zhou, W.Q.; Chen, X.G. Skimmin, a coumarin, suppresses the streptozotocin-induced diabetic nephropathy in wistar rats. Eur. J. Pharmacol. 2012, 692, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Sakagami, M.; Hashiuchi, F.; Zhou, J.L.; Yoshikawa, M.; Ren, J. Apioglycyrrhizin and araboglycyrrhizin, two new sweet oleanene-type triterpene oligoglycosides from the root of Glycyrrhiza inflate. Chem. Pharm. Bull. 1989, 37, 551–553. [Google Scholar] [CrossRef]
- Satyanarayana, P.; Subrahmanyam, P.; Kasai, R.; Tanaka, O. An apiose-containing coumarin glycoside from Gmelina arborea root. Phytochemistry 1985, 24, 1862–1863. [Google Scholar] [CrossRef]
- Wu, L.J. Practical Spectral Analysis of Organic Compounds; People’s Medical Publishing House: Beijing, China, 2009; p. 123. [Google Scholar]
- Niwa, M.; Twadare, Y.; Wu, Y.C.; Hirata, Y. Two new phenylpropanoid glycosides from Wikstroemia sikokiana. Chem. Pharm. Bull. 1988, 36, 1158–1161. [Google Scholar] [CrossRef]
- Sugiyama, M.; Nagayama, E.; kikuchi, M. Lignan and phenylpropanoid glycosides from Osmanthus asiaticus. Phytochemistry 1993, 33, 1215–1219. [Google Scholar] [CrossRef]
- Hao, Z.Y.; Liang, D.; Luo, H.; Liu, Y.F.; Ni, G.; Zhang, Q.J.; Li, L.; Si, Y.K.; Sun, H. Bioactive sesquiterpenoids from the rhizomes of Acorus calamus. J. Nat. Prod. 2012, 75, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–5 are available from the authors.

| Position | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | 130.6 (s) | |||||
| 2 | 7.07 (d, 1.5) | 109.9 (d) | 160.3 (s) | 160.6 (s) | ||
| 3 | 149.1 (s) | 6.63 (d, 9.6) | 113.4 (d) | 6.32 (d, 9.6) | 113.7 (d) | |
| 4 | 146.4 (s) | 7.99 (d, 9.6) | 144.3 (d) | 7.98 (d, 9.6) | 144.6 (d) | |
| 5 | 7.01 (d, 8.5) | 115.2 (d) | 7.64 (d, 8.4) | 129.6 (d) | 7.65 (d, 9.0) | 129.9 (d) |
| 6 | 6.91 (dd, 8.5, 1.5) | 119.5 (d) | 7.01 (dd, 8.4, 1.8) | 113.5 (d) | 7.02 (dd, 9.0, 2.4) | 113.8 (d) |
| 7 | 6.58 (d, 16.0) | 131.7 (d) | 160.1 (s) | 160.4 (s) | ||
| 8 | 6.26 (dt, 16.0, 6.0) | 124.4 (d) | 7.03 (d, 1.8) | 103.4 (d) | 7.03 (d, 2.4) | 103.7 (d) |
| 9 | 4.38 (dd, 13.5, 6.0); 4.16 (dd, 13.5, 6.0) | 68.8 (t) | 155.1 (s) | 155.4 (s) | ||
| 10 | 3.77(s, MeO-C(3)) | 55.7 (q) | 113.4 (s) | 113.6 (s) | ||
| Glc′ | ||||||
| 1 | 4.20 (d, 7.5) | 102.0 (d) | 5.10 (d, 7.2) | 99.8 (d) | 5.08 (d, 7.8) | 99.9 (d) |
| 2 | 2.98 c | 73.5 (d) | 3.42 c | 71.7 (d) | 3.48 c | 73.9 (d) c |
| 3 | 3.14 c | 76.7 (d) | 3.48 (m) | 85.7 (d) | 3.56 c | 76.3 (d) |
| 4 | 2.98 c | 70.4 (d) | 3.41 c | 69.4 (d) | 3.38 c | 80.5 (d) |
| 5 | 3.25 c | 75.7 (d) | 3.69 (m) | 74.9 (d) | 3.78 (m) | 74.2 (d) |
| 6 | 3.86 (dd, 11.5, 4.5); 3.41 c | 67.8 (t) | 3.47 (m);3.85 (dd,11.5,4.2) | 67.2 (t) | 3.88 c; 3.59 (m) | 67.6 (t) |
| Glc′′ | ||||||
| 1 | 4.89 (d, 6.5) | 100.0 (d) | 5.01(d, 3.0) | 100.3 (d) | 4.99 (d, 2.8) | 101.7 (d) |
| 2 | 3.24 c | 73.3 (d) | 3.24 c | 72.9 (d) | 3.24 (m) | 72.9 (d) c |
| 3 | 3.25 c | 77.0 (d) | 3.45 c | 73.5 (d) | 3.36 c | 73.7 (d) |
| 4 | 3.14 c | 69.7 (d) | 3.11 (m) | 70.1 (d) | 3.12 (m) | 70.0 (d) |
| 5 | 3.25 c | 77.1 (d) | 3.73 (m) | 72.6 (d) | 3.47 (m) | 72.9 (d) |
| 6 | 3.65 (dd, 12.0, 5.0); 3.42 c | 60.7 (t) | 3.61 (dd,12.0, 4.8); 3.45 c | 60.7 (t) | 3.64 c; 3.50 c | 60.9 (t) |
| Api | ||||||
| 1 | 4.88 (d, 3.0) | 109.4 (d) | 4.80 (d, 3.0) | 109.4 (d) | 4.84 (d, 2.8) | 109.7 (d) |
| 2 | 3.76 (d, 3.0) | 76.0 (d) | 3.75 (d, 3.0) | 76.0 (d) | 3.73 (d, 2.8) | 76.4 (d) |
| 3 | 78.9 (s) | 78.8 (s) | 79.1 (s) | |||
| 4 | 3.86 (d, 9.5); 3.58 (d, 9.5) | 73.3 (t) | 3.88 (d, 9.6); 3.58 (d, 9.6) | 73.5 (t) | 3.87 (d, 9.6) c; 3.57 (d, 9.6) c | 73.8 (t) |
| 5 | 3.32 (m) | 63.1 (t) | 3.34 (m) | 63.2 (t) | 3.34 (m) | 63.6 (t) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Li, C.-J.; Yang, J.-Z.; Sun, H.; Zhang, D.-M. New Phenylpropanoid and Coumarin Glycosides from the Stems of Hydrangea paniculata Sieb. Molecules 2017, 22, 133. https://doi.org/10.3390/molecules22010133
Ma J, Li C-J, Yang J-Z, Sun H, Zhang D-M. New Phenylpropanoid and Coumarin Glycosides from the Stems of Hydrangea paniculata Sieb. Molecules. 2017; 22(1):133. https://doi.org/10.3390/molecules22010133
Chicago/Turabian StyleMa, Jie, Chuang-Jun Li, Jing-Zhi Yang, Hua Sun, and Dong-Ming Zhang. 2017. "New Phenylpropanoid and Coumarin Glycosides from the Stems of Hydrangea paniculata Sieb" Molecules 22, no. 1: 133. https://doi.org/10.3390/molecules22010133
APA StyleMa, J., Li, C.-J., Yang, J.-Z., Sun, H., & Zhang, D.-M. (2017). New Phenylpropanoid and Coumarin Glycosides from the Stems of Hydrangea paniculata Sieb. Molecules, 22(1), 133. https://doi.org/10.3390/molecules22010133





