Biphenyl Phytoalexin in Sorbus pohuashanensis Suspension Cell Induced by Yeast Extract
Abstract
:1. Introduction
2. Results
2.1. Establishment of Cell Suspension Cultures
2.2. Study of Growth Properties of Suspension Cells
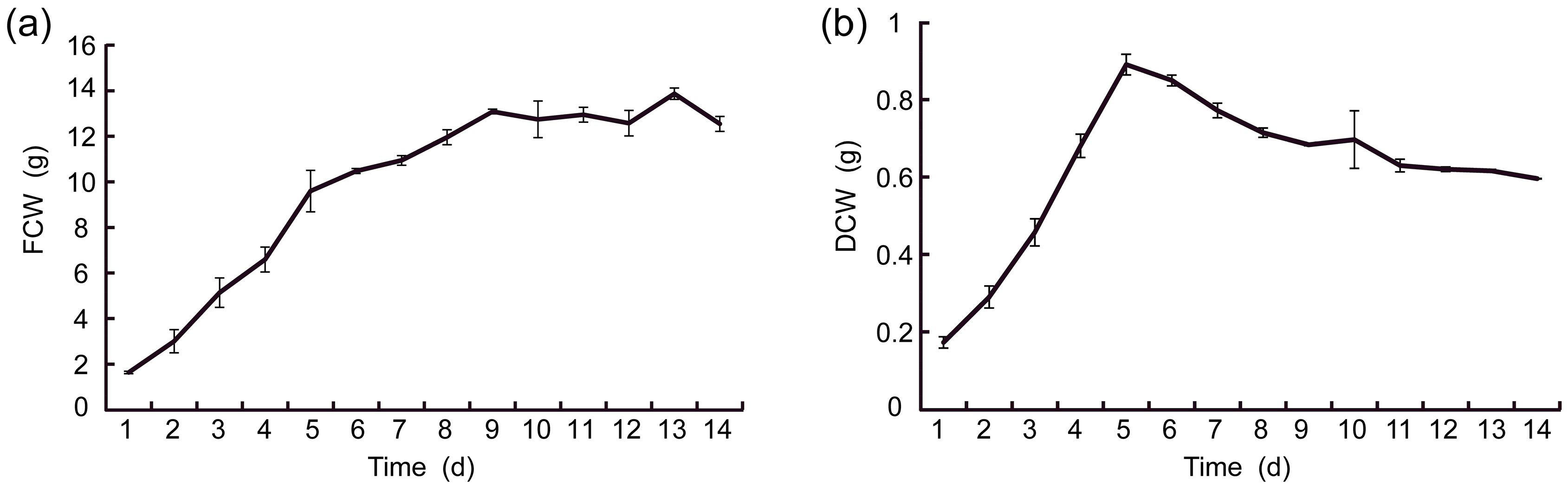
2.2.1. Growth Curves of Suspension Cells
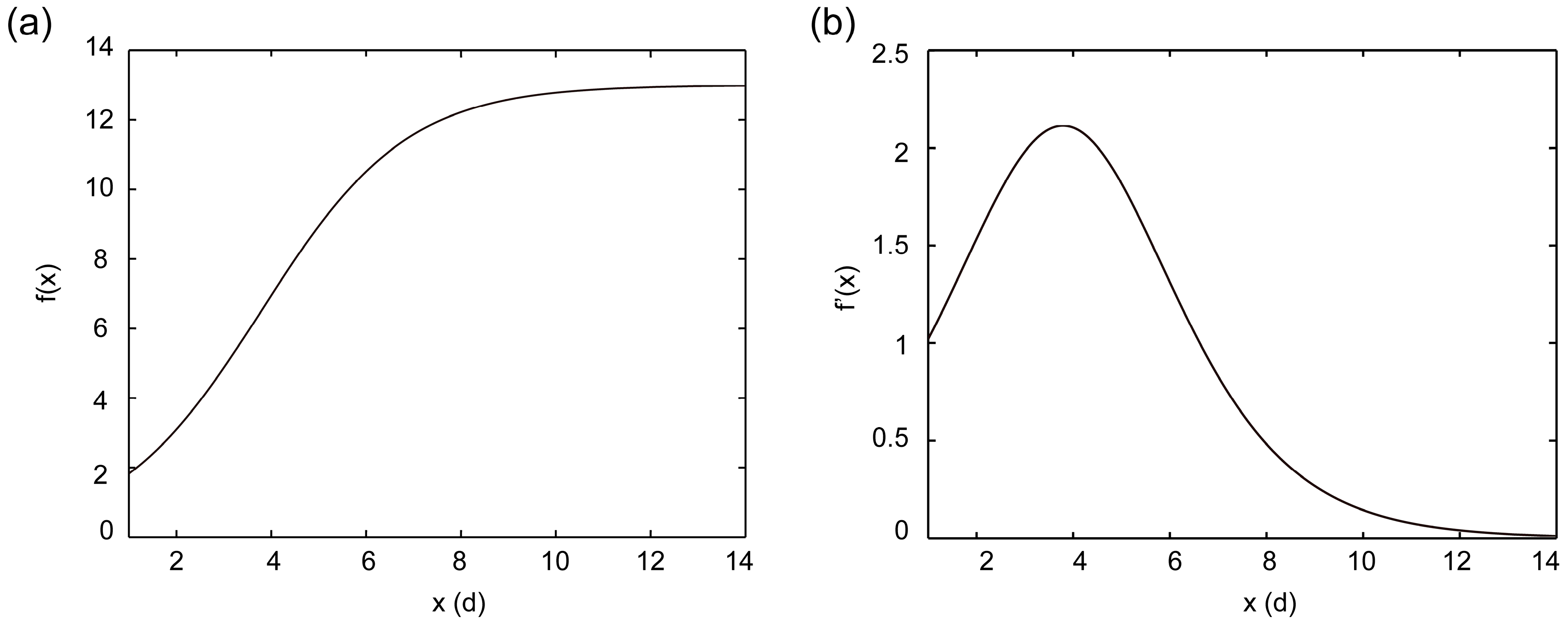
2.2.2. Fitting of Growth Curve for Suspension Cell
2.2.3. Solving Key Point of the Logistic Growth Model
2.3. Analyses of Metabolites in YE-Treated S. pohuashanensis Suspension Cells
2.3.1. Analyses of Time Course of Metabolites Differentially Accumulated in YE-Treated S. pohuashanensis Suspension Cells
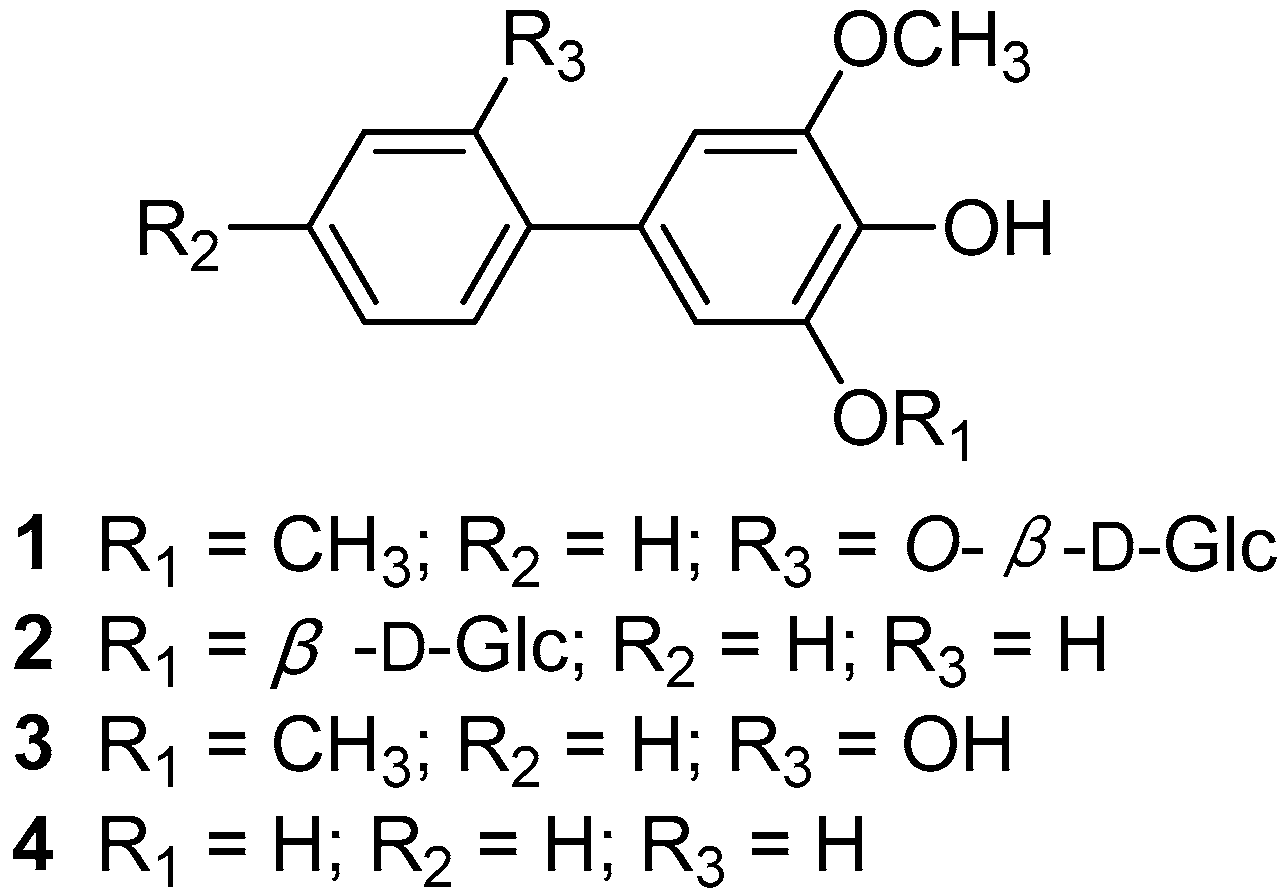
2.3.2. Characterization of Biphenyls Compounds
2.3.3. Calibration Curves, Limit of Detection (LOD), and Limit of Quantitation (LOQ)
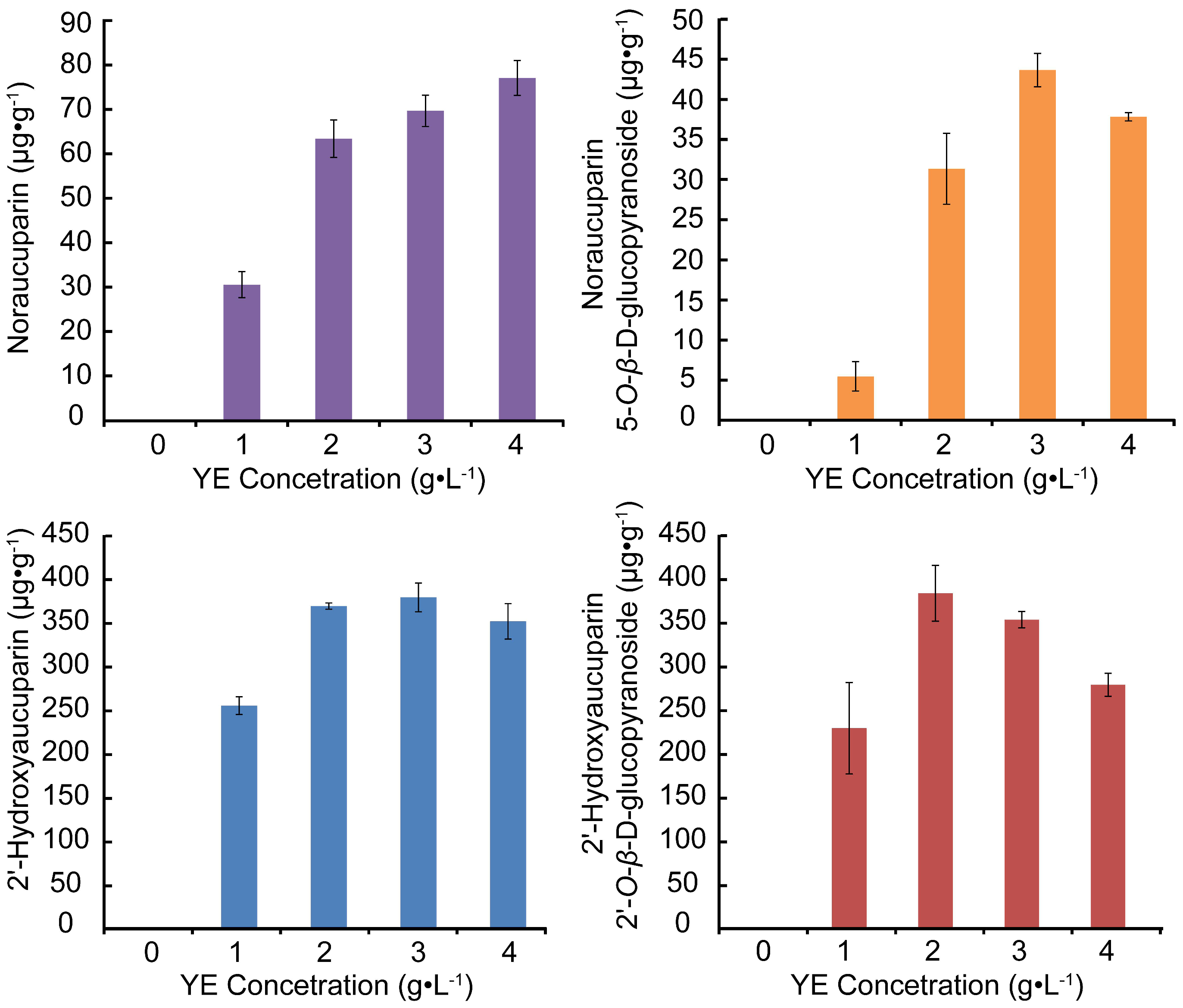
2.3.4. Analyses of Biphenyls Accumulated in S. pohuashanensis Suspension Cells Treated with Different Concentrations of YE
2.3.5. Determination of Time Course of Biphenyls Accumulation in YE-Treated S. pohuashanensis Suspension Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagent
4.3. Construction of Cell Suspension Cultures
4.4. Growth Property Study of the Suspension Cells
4.5. Effects of Metabolites in Cell Cultures of S. pohuashanensis Treated at Different Times and with Different Concentrations of YE
4.6. Sample Preparation
4.7. UPLC-Q-TOF-MS Analysis
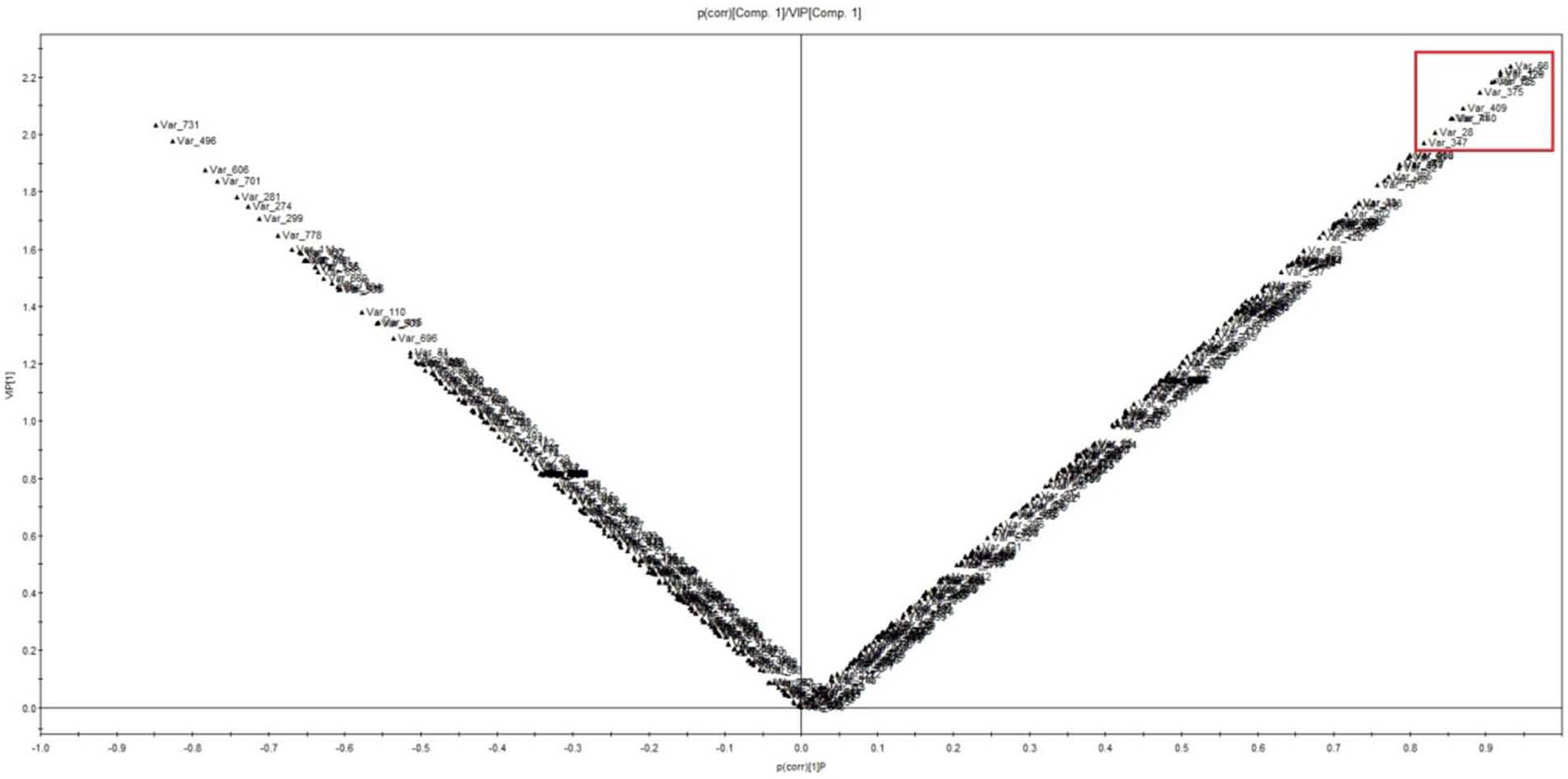
4.8. Multivariate Data Analysis
4.9. Extraction and Isolation
4.10. LC-MS/MS Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Bonn, W.G.; van der Zwet, T. Distribution and economic importance of fire blight. In Fire Blight: The Disease Causative Agent, Erwinia Amylovora; Vanneste, J.L., Ed.; CAB International: Wallingford, UK, 2000; pp. 37–53. [Google Scholar]
- Hrazdina, G.; Borejsza-Wysocki, W.; Lester, C. Phytoalexin production in an apple cultivar resistant to Venturia inaequalis. Phytopathology 1997, 87, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, T.; Harborne, J.; Eagles, J.; Waterman, P. Antifungal biphenyl compounds are the phytoalexins of the sapwood of Sorbus aucuparia. Phytochemistry 1995, 40, 57–59. [Google Scholar] [CrossRef]
- Kokubun, T.; Harborne, J.B.; Eagles, J.; Waterman, P.G. Dibenzofuran phytoalexins from the sapwood of Cotoneaster acutifolius and five related species. Phytochemistry 1995, 38, 57–60. [Google Scholar] [CrossRef]
- Watanabe, K.; Ishiguri, Y.; Nonaka, F.; Morita, A. Isolation and identification of aucuparin as a phytoalexin from Eriobotrya japonica L. Agric. Biol. Chem. 1982, 46, 567–568. [Google Scholar] [CrossRef]
- Chizzali, C.; Khalil, M.N.; Beuerle, T.; Schuehly, W.; Richter, K.; Flachowsky, H.; Peil, A.; Hanke, M.-V.; Liu, B.; Beerhues, L. Formation of biphenyl and dibenzofuran phytoalexins in the transition zones of fire blight-infected stems of Malus domestica cv.‘Holsteiner cox’ and Pyrus communis cv.‘Conference’. Phytochemistry 2012, 77, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, T.; Harborne, J.B. Phytoalexin induction in the sapwood of plants of the maloideae (rosaceae): Biphenyls or dibenzofurans. Phytochemistry 1995, 40, 1649–1654. [Google Scholar] [CrossRef]
- Liu, B.; Beuerle, T.; Klundt, T.; Beerhues, L. Biphenyl synthase from yeast-extract-treated cell cultures of Sorbus aucuparia. Planta 2004, 218, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Hüttner, C.; Beuerle, T.; Scharnhop, H.; Ernst, L.; Beerhues, L. Differential effect of elicitors on biphenyl and dibenzofuran formation in Sorbus aucuparia cell cultures. J. Agric. Food Chem. 2010, 58, 11977–11984. [Google Scholar] [CrossRef] [PubMed]
- Chizzali, C.; Beerhues, L. Phytoalexins of the pyrinae: Biphenyls and dibenzofurans. Beilstein J. Org. Chem. 2012, 8, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, H.; Kai, A.; Morozumi, S. A new logistic model for escherichia coli growth at constant and dynamic temperatures. Food Microbiol. 2004, 21, 501–509. [Google Scholar] [CrossRef]
- Tsoularis, A.; Wallace, J. Analysis of logistic growth models. Math. Biosci. 2002, 179, 21–55. [Google Scholar] [CrossRef]
- Goodacre, R.; Shann, B.; Gilbert, R.J.; Timmins, E.M.; McGovern, A.C.; Alsberg, B.K.; Kell, D.B.; Logan, N.A. Detection of the dipicolinic acid biomarker in bacillus spores using curie-point pyrolysis mass spectrometry and fourier transform infrared spectroscopy. Anal. Chem. 2000, 72, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Borejsza-Wysocki, W.; Lester, C.; Attygalle, A.; Hrazdina, G. Elicited cell suspension cultures of apple (Malus domestica) cv. Liberty produce biphenyl phytoalexins. Phytochemistry 1999, 50, 231–235. [Google Scholar] [CrossRef]
- Schmidt, B.; Riemer, M. Suzuki-miyaura coupling of halophenols and phenol boronic acids: Systematic investigation of positional isomer effects and conclusions for the synthesis of phytoalexins from pyrinae. J. Org. Chem. 2014, 79, 4104–4118. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, S.U.; Ha, S.K.; Kim, S.Y.; Lee, K.R. Biphenyls from berberis koreana. J. Nat. Prod. 2009, 72, 2061–2064. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.N.; Beuerle, T.; Müller, A.; Ernst, L.; Bhavanam, V.B.; Liu, B.; Beerhues, L. Biosynthesis of the biphenyl phytoalexin aucuparin in Sorbus aucuparia cell cultures treated with Venturia inaequalis. Phytochemistry 2013, 96, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Raeth, T.; Beuerle, T.; Beerhues, L. Biphenyl synthase, a novel type iii polyketide synthase. Planta 2007, 225, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Degousee, N.; Triantaphylidès, C.; Montillet, J.-L. Involvement of oxidative processes in the signaling mechanisms leading to the activation of glyceollin synthesis in soybean (Glycine max). Plant Physiol. 1994, 104, 945–952. [Google Scholar] [PubMed]
- Guo, Z.J.; Lamb, C.; Dixon, R.A. Potentiation of the oxidative burst and isoflavonoid phytoalexin accumulation by serine protease inhibitors. Plant Physiol. 1998, 118, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Jabs, T.; Tschöpe, M.; Colling, C.; Hahlbrock, K.; Scheel, D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl. Acad. Sci. USA 1997, 94, 4800–4805. [Google Scholar] [CrossRef] [PubMed]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defence—A broad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Qiu, X.; Lei, C.; Huang, L.; Li, X.; Hao, H.; Du, Z.; Wang, H.; Ye, H.; Beerhues, L.; Liu, B. Endogenous hydrogen peroxide is a key factor in the yeast extract-induced activation of biphenyl biosynthesis in cell cultures of Sorbus aucuparia. Planta 2012, 235, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.


| Parameters | ||||
|---|---|---|---|---|
| a | b | k | R-Square | |
| FCW | 11.75 | 13.01 | 0.6503 | 0.9881 |
| DCW | 20.84 | 0.7068 | 1.367 | 0.7839 |
| Analytes | Calibration Curves | r | Linear Range (μg/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| Noraucuparin | y = 5854.12x − 2346.20 | 0.9993 | 0.510–25.500 | 1.01 | 2.89 |
| Noraucuparin 5-O-β-d-glucopyranoside | y = 6654.89x − 1009.62 | 0.9991 | 0.220–11.000 | 0.74 | 1.91 |
| 2′-Hydroxyaucuparin | y = 5382.90x − 2.39 × 104 | 0.9995 | 5.200–104.000 | 2.47 | 7.65 |
| 2′-Hydroxyaucuparin 2′-O-β-d-glucopyranoside | y = 4365.74x − 3.72 × 104 | 0.9993 | 10.500–210.000 | 1.38 | 3.67 |
| Analytes | MRM Transitions (m/z) | Declustering Potential (DP)/V | Collision Energy (CE)/V | Collision Cell Exit Potential (CXP)/V |
|---|---|---|---|---|
| Noraucuparin | 214.9→199.9 | −91 | −24 | −23 |
| Noraucuparin 5-O-β-d-glucopyranoside | 376.9→214.9 | −95 | −22 | −12 |
| 2′-Hydroxyaucuparin | 245.1→214.9 | −75 | −27 | −15 |
| 2′-Hydroxyaucuparin 2′-O-β-d-glucopyranoside | 406.9→244.9 | −94 | −21 | −24 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Yang, J.; Yang, G.; Kang, C.; Xiao, W.; Lv, C.; Wang, S.; Tang, J.; Guo, L. Biphenyl Phytoalexin in Sorbus pohuashanensis Suspension Cell Induced by Yeast Extract. Molecules 2016, 21, 1180. https://doi.org/10.3390/molecules21091180
Zhou L, Yang J, Yang G, Kang C, Xiao W, Lv C, Wang S, Tang J, Guo L. Biphenyl Phytoalexin in Sorbus pohuashanensis Suspension Cell Induced by Yeast Extract. Molecules. 2016; 21(9):1180. https://doi.org/10.3390/molecules21091180
Chicago/Turabian StyleZhou, Liangyun, Jian Yang, Guang Yang, Chuanzhi Kang, Wenjuan Xiao, Chaogeng Lv, Sheng Wang, Jinfu Tang, and Lanping Guo. 2016. "Biphenyl Phytoalexin in Sorbus pohuashanensis Suspension Cell Induced by Yeast Extract" Molecules 21, no. 9: 1180. https://doi.org/10.3390/molecules21091180
APA StyleZhou, L., Yang, J., Yang, G., Kang, C., Xiao, W., Lv, C., Wang, S., Tang, J., & Guo, L. (2016). Biphenyl Phytoalexin in Sorbus pohuashanensis Suspension Cell Induced by Yeast Extract. Molecules, 21(9), 1180. https://doi.org/10.3390/molecules21091180






