Abstract
An efficient PdII/Pd0-p-benzoquinone/hydroquinone-CuCl2/CuCl catalyst system was developed that uses environmentally friendly molecular oxygen as the terminal oxidant to catalyze the cyclization-carbonylation-cyclization coupling reaction (CCC-coupling reaction) of (o-alkynyl phenyl) (methoxymethyl) sulfides.
1. Introduction
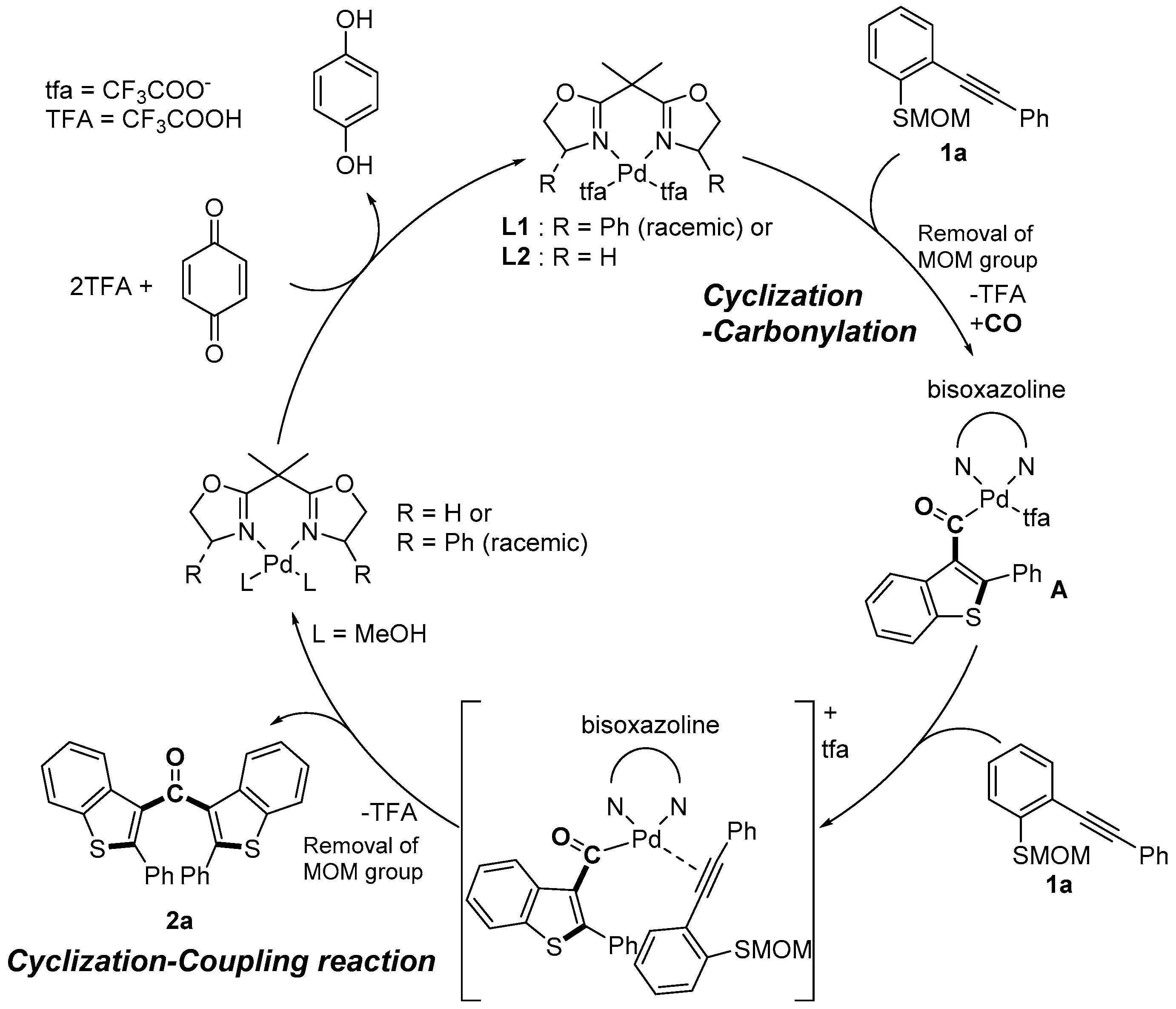
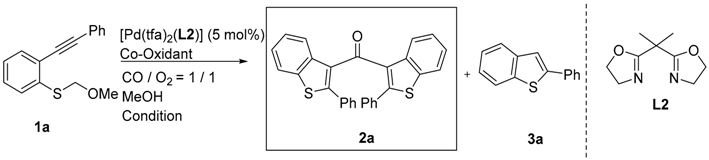
Cascade reactions are important tools for constructing a variety of heterocycles in one step starting from simple compounds [1,2,3,4]. Recently, we reported that the cyclization-carbonylation-cyclization coupling reaction (CCC-coupling reaction) of (o-alkynyl phenyl) (methoxymethyl) sulfides 1 catalyzed by palladium(II)-bisoxazoline (box) complexes afforded bis(benzothiophen-3-yl) methanones 2 in good yield (Scheme 1) [5]. Nucleophilic attack by the sulfur atom at the electrophilically activated triple bond is followed by CO insertion to produce the acyl palladium intermediate A. The methoxymethyl group may be removed by acetal exchange (or hydrolysis) during the formation of intermediate A. Coordination of the triple bond of a second molecule induces the second cyclization, and reductive elimination then leads to the formation of a ketone bearing two benzothiophene groups. The efficient regeneration of the PdII species from Pd0 is the crucial step for obtaining a high yield of the product, and stoichiometric p-benzoquinone was employed as a re-oxidant in this transformation. However, there is a disadvantage to using p-benzoquinone: a stoichiometric amount of hydroquinone is formed as unwanted waste. Molecular oxygen is considered an ideal oxidant because it is naturally abundant, inexpensive (or free if used as present in the atmosphere), and environmentally friendly, and does not generate any waste products, thereby fulfilling the requirements of a “green chemistry” reactant [6]. Bäckvall and coworkers have conducted extensive studies of the palladium(II)-mediated oxidative 1,4-addition of nucleophiles to conjugated dienes [7,8]. p-Benzoquinone is the most common stoichiometric oxidant used in these reactions, but Bäckvall and coworkers also developed a redox-coupled catalytic system to enable the use of molecular oxygen as the terminal oxidant for aerobic palladium-catalyzed oxidations [9,10,11].
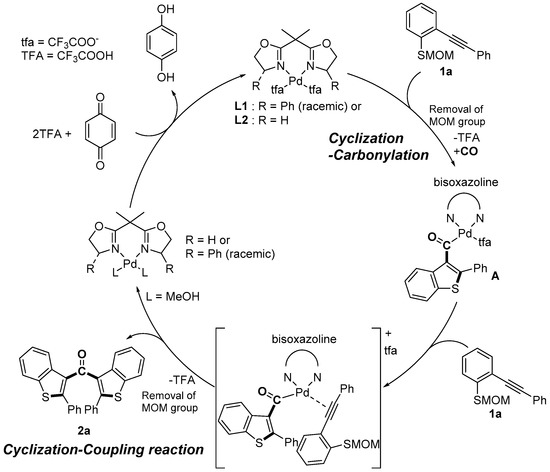
Scheme 1.
Our previous work: catalytic cycle of cyclization-carbonylation-cyclization coupling reaction (CCC-coupling reaction) reaction. Ph, phenyl; MOM, methoxymethyl.
p-Benzoquinone and macrocyclic metal complexes are employed in these oxidations as electron-transfer mediators (ETMs). ETMs usually facilitate the oxidation reaction by transporting electrons from the catalyst to the oxidant along a low-energy pathway, thereby increasing the efficiency of oxidation and thus complementing direct oxidation reactions [12]. Herein, we report a PdII/Pd0-p-benzoquinone/hydroquinone-CuCl2/CuCl system that uses environmentally friendly molecular oxygen as the terminal oxidant to catalyze the CCC-coupling reaction of (o-alkynyl phenyl) (methoxymethyl) sulfides.
2. Results and Discussion
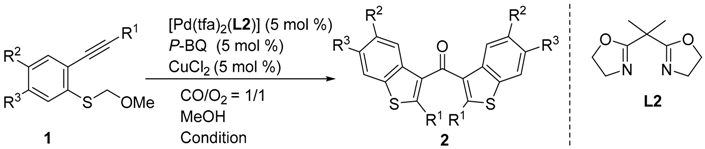
The required starting materials 1 were prepared as described previously [5]. Initially, we selected 1a as a standard substrate to search for potential co-oxidants. The reaction of 1a with [Pd(tfa)2(L2)] (5 mol %) in methanol under a CO/O2 atmosphere (1:1, balloon) generated the dimeric ketone 2a in 12% yield, along with 2-phenylbenzo[b]thiophene 3a (65% yield) (Table 1, Entry 1). The presence of reduced metal (Pd0, black) showed that electron transfer between Pd0 and O2 is too slow compared with decomposition. Next, five co-oxidants (ETMs) were tested in the reaction (p-benzoquinone, CuCl2, FeCl3·6H2O, and VO(acac)2; acac, acetyl acetonate) (Table 1, Entries 2–5), of which p-benzoquinone and CuCl2 gave encouraging but still unacceptable yields (Table 1, Entries 2 and 3). These results suggested that one ETM alone has insufficient oxidation potential to oxidize Pd0 by O2 and therefore the simultaneous use of two co-oxidants was investigated (Table 1, Entries 6–9). Fortunately, the dimeric ketone 2a was obtained in 87% yield by using p-benzoquinone (10 mol %) and CuCl2 (5 mol %) as co-oxidants (Table 1, Entry 6). We next attempted to reduce the amount of p-benzoquinone required by investigating the reaction temperature (Table 1, Entries 7–9). The best result was obtained by using p-benzoquinone (5 mol %) and CuCl2 (5 mol %) as co-oxidants at 0 °C, affording 2a in 88% yield (Table 1, Entry 8). Having optimized the reaction conditions, we examined the reaction of various (o-alkynyl phenyl) (methoxymethyl) sulfide derivatives (Table 2), starting with the reaction of substrates 1b–h bearing aryl substituents at the alkyne terminus (Table 2, Entries 1–8). Neither electron-donating nor electron-withdrawing groups affected the reaction, and 2b–d were obtained in good yield, similar to that of the parent substrate 1a (Table 2, Entries 2–4). Three different halogen substituents (F, Cl, and Br) and a thiophene ring were tolerated under the reaction conditions used (Table 2, Entries 5–8). Substrates 1i–l bearing alkyl substituents at the alkyne terminus were transformed to the corresponding ketones 2j–l in 71%–92% yield (Table 2, Entries 9–12). Free hydroxyl groups were also tolerated. The scope of the substrate for the CCC-coupling reaction was expanded further by investigating the reactions of substrates bearing R2 substituents (Table 2, Entries 13–14). The reactions of 1m–o bearing a Cl substituent, methyl group, and methoxy group in an aromatic moiety proceeded well.

Table 1.
Optimization of the CCC-coupling reaction of 1a.

Table 2.
Scope of suitable substrates for the CCC-coupling reaction.
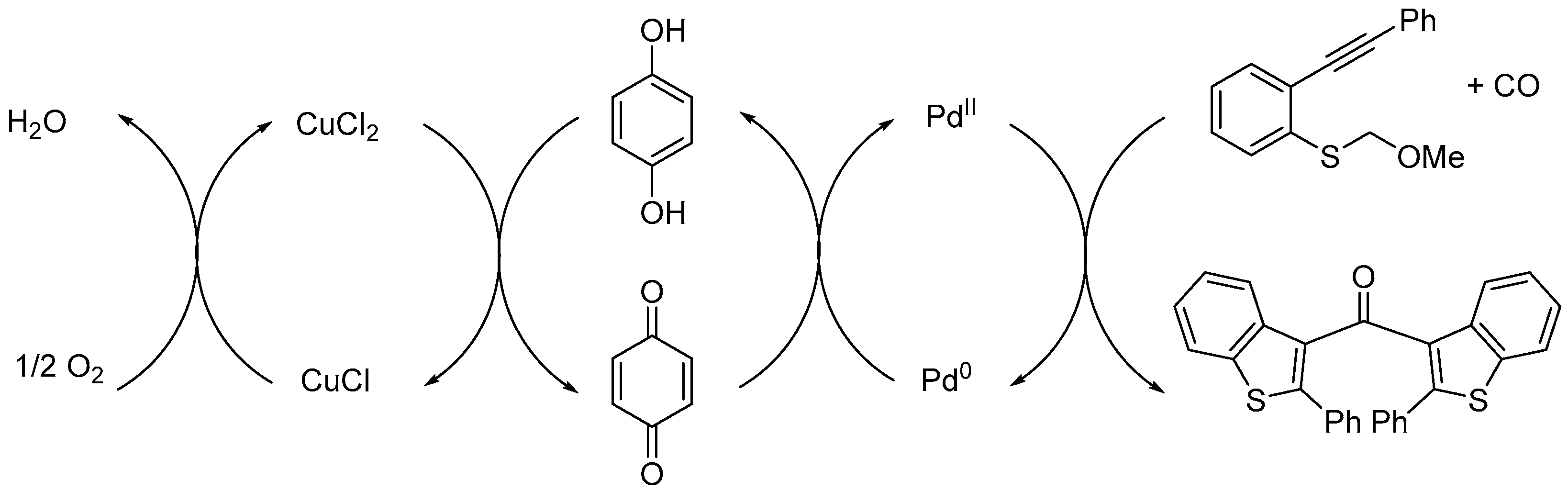
This redox-coupled PdII/Pd0-p-benzoquinone/hydroquinone-CuCl2/CuCl triple catalytic system can be described according to Scheme 2. The initial steps of the CCC-coupling reaction are mediated by PdII [5] and p-benzoquinone acts as a co-oxidant (ETM) to transfer protons and electrons from palladium to CuCl2. Finally, CuCl is re-oxidized by molecular oxygen, the terminal oxidant [13,14].
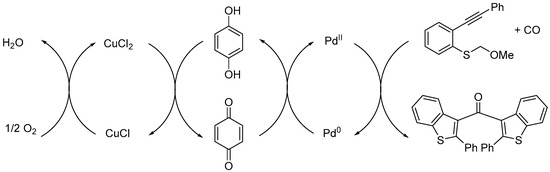
Scheme 2.
Proposed redox cycles.
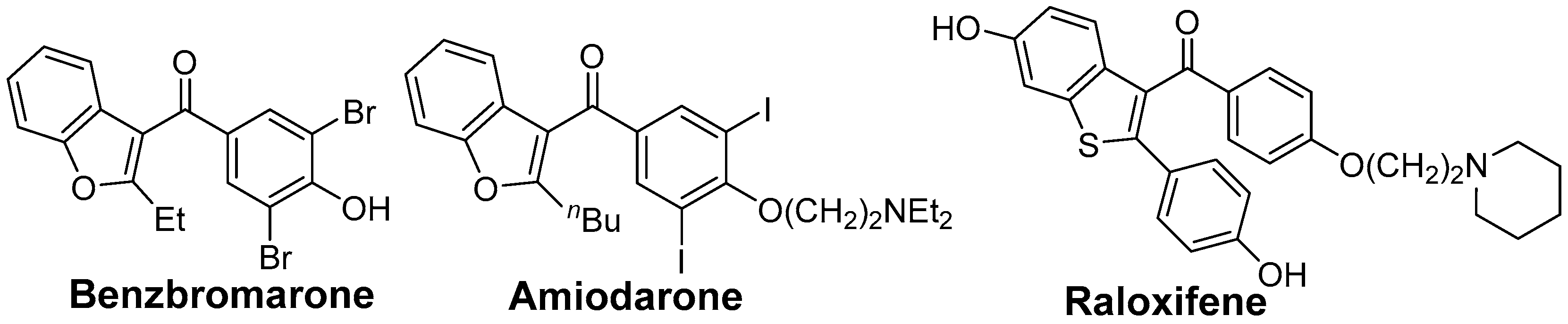
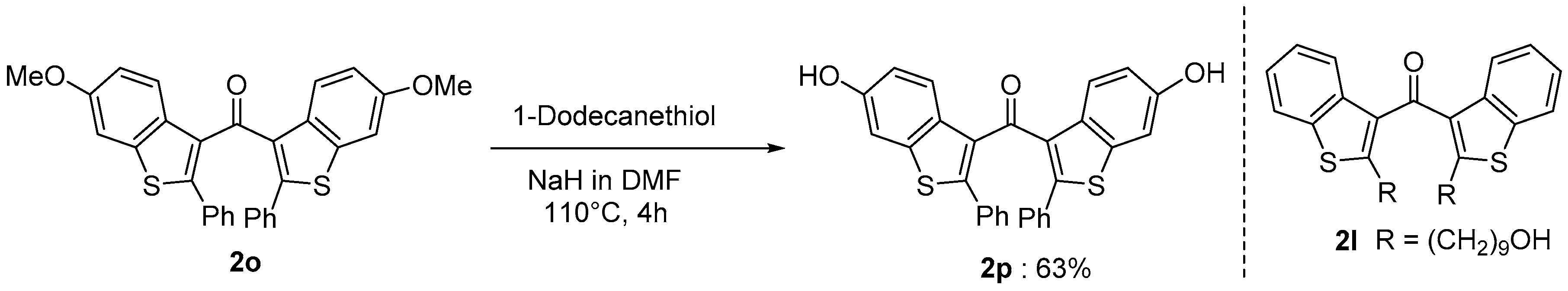
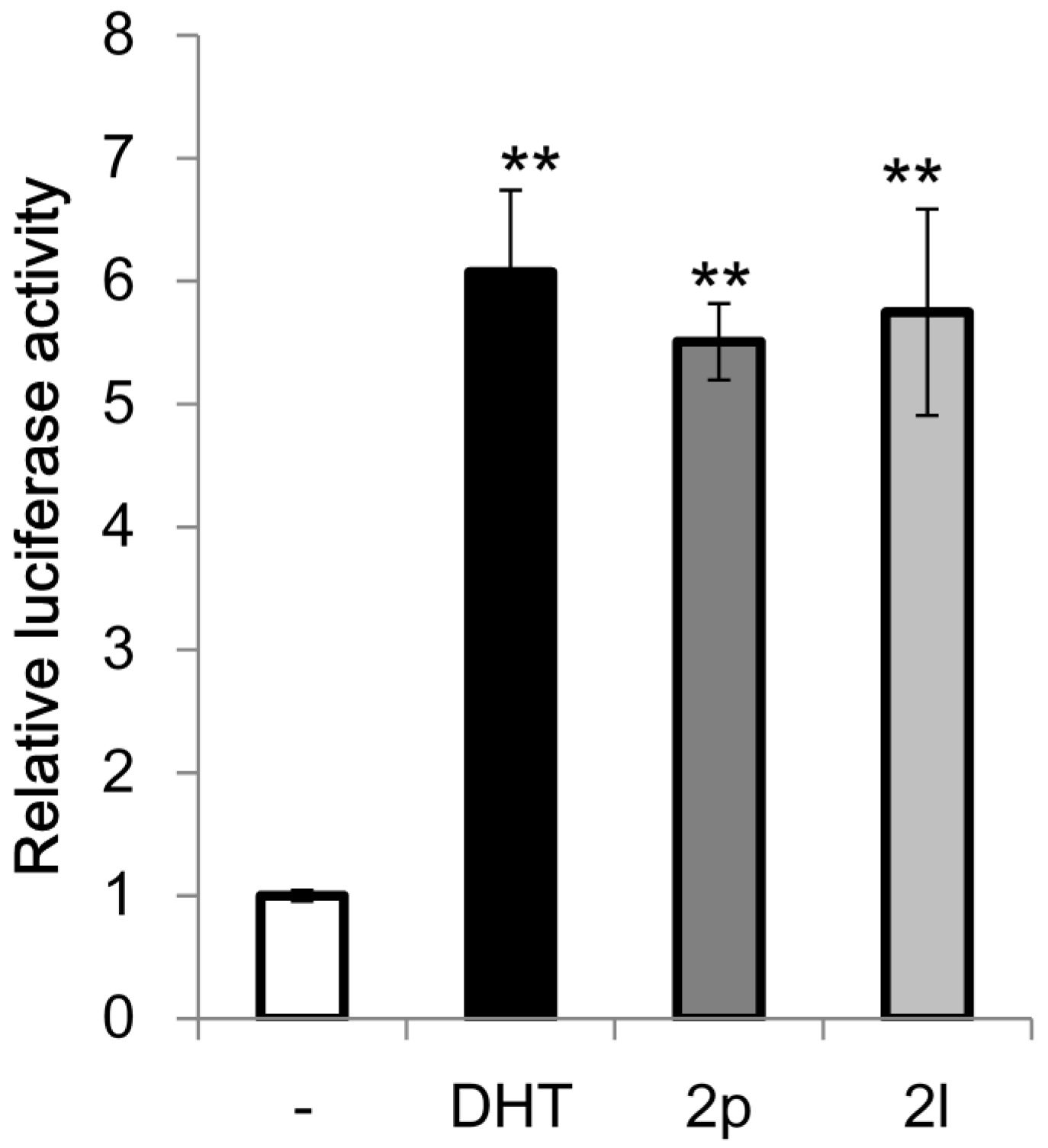
Benzo[b]thiophene skeletons are an important class of S-heterocycles [15,16,17,18] and are found in a variety of drugs, pesticides, and biologically active compounds that exhibit various interesting biological properties [19,20,21,22,23,24]. Diaryl ketone scaffolds are also important motifs in natural products and pharmaceuticals [25,26,27,28,29,30] (Figure 1). Androgens are known to have beneficial anabolic actions on various tissues such as bone and muscle. However, the clinical use of androgens has been limited because of their undesirable sexually actions. Recently, non-steroidal androgens have been investigated in many laboratories. As a preliminary study, we tested the androgen receptor (AR) agonistic activity of 2p and 2l. Demethylation of 2o afforded 2p in 63% yield (Scheme 3). We performed androgen response element (ARE)-driven luciferase reporter assay (ARE-luc.) in human kidney derived HEK293 cells. A portion of 10 μM of 2p and 2l were examined for their ability to activate the transcription of the ARE-luc. reporter gene (Figure 2). Both 2p and 2l elicited ARE-luciferase reporter activity similarly to dihydrotestosterone (DHT, 10 nM). This observation suggested that dibenzo[b]thiophenyl ketone scaffolds (such as 2p and 2l) may expect to be pharmacophores for non-steroidal AR-agonist. We are currently investigating further biological studies of the synthesized compounds 2.
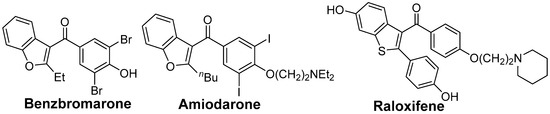
Figure 1.
Some drugs having diarylketone scaffolds.
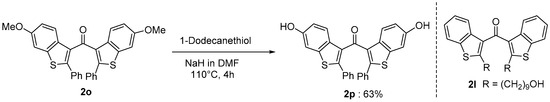
Scheme 3.
Preparation of 2p and the structure of 2l.
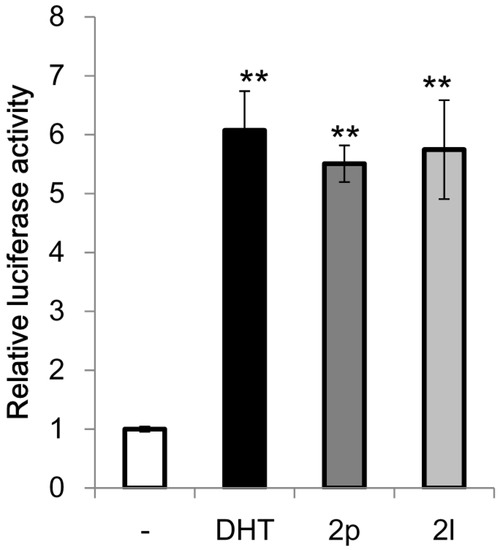
Figure 2.
Effect of 2p and 2l on ARE-luciferase reporter activity in HEK293 cells. The results are shown as the mean ± S.D. (n = 4). ** p < 0.01 compared with the solvent control.
The HEK293 cells were transfected with the ARE-luciferase reporter, AR expression, and pGL4.74 plasmids. The next day, cells were treated with dihydrotestosterone (DHT) (10 nM), 2p (10 μM), or 2l (10 μM) for 24 h. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System. The results are shown as the mean ± S.D. (n = 4). Statistically significant differences were determined using one-way analysis of variance followed by Dunnett’s multiple comparison test as the post-hoc test.
3. Experimental
3.1. General Information
1H and 13C-NMR spectra was recorded on JEOL ECS 400 (JEOL, Tokyo, Japan) and JEOL ECA 500 spectrometers (JEOL, Tokyo, Japan) in CDCl3 with Me4Si as an internal reference. When the solvent was DMSO-d6, solvent peak was used as a reference (2.50 ppm for 1H, and 39.5 ppm for 13C). 13C-NMR spectra were recorded at 100 MHz. All reagents were purchased from commercial sources and used without purification. All evaporations were performed under reduced pressure. Silica gel (Kieselgel 60, Merck, Kenilworth, NJ, USA) was used for column chromatography. CO and O2 were measured and injected into a balloon using a jumbo syringe (SGE Analytical Science, Milton Keynes, UK).
3.2. Preparation of Substrates
The (o-alkynyl phenyl) (methoxymethyl) sulfides 1a–n were prepared from known o-iodoanilines using a published procedure, and the spectral data were identical to those described in the literature [5].
3.3. General Procedure for the Reaction of (o-Alkynyl Phenyl) (Methoxymethyl) Sulfides 1
A 30 mL two-necked round-bottom flask containing a magnetic stir bar, (o-alkynyl phenyl) (methoxymethyl) sulfide 1 (0.4 mmol), p-benzoquinone (2.2 mg, 0.02 mmol), CuCl2 (3.4 mg, 0.02 mmol), and MeOH (3 mL) was fitted with a rubber septum and a three-way stopcock connected to a balloon filled with CO and O2 (500 mL:500 mL). The apparatus was purged with the gas from the balloon by pump-filling via the three-way stopcock. A MeOH (1 mL) suspension of [Pd(tfa)2(L2)] (10.3 mg, 0.02 mmol) was added to the stirred solution at an appropriate temperature via a syringe. The residual catalyst was washed with MeOH (1 mL) twice, and the reaction mixture was stirred for 24–72 h. In most cases, the dimeric ketones 2 precipitated from the reaction mixture. The resulting precipitate was collected by filtration and washed with cold MeOH (1.5 mL × 2) to yield dimeric ketones 2. The small amount of 2 remaining in the filtrate was recovered by diluting the filtrate with CH2Cl2 (50 mL) and washing with 5% NaOH (40 mL). The aqueous layer was extracted with CH2Cl2 (25 mL) and the combined organic layers were dried over MgSO4 and concentrated in vacuo. The crude product was purified by column chromatography on silica gel. The fraction eluted with hexane/EtOAc (100:1) afforded small amounts of dimeric ketones 2. The spectral data of products 2a–o were identical to those described in the literature [5].
Preparation of bis(6-hydroxy-2-phenylbenzo[b]thiophen-3-yl)methanone, 2p
To a suspension of sodium hydride (58 mg, 1.2 mmol, 50% in mineral oil) and 1-dodecanethiol (243 mg, 1.2 mmol) in anhydrous DMF (5 mL) under Ar was added 2o (101.2 mg, 0.2 mmol), and the mixture was heated at 110 °C for 4 h. The mixture was allowed to cool, and was then diluted with ice-water. The mixture was extracted with CH2Cl2 (30 mL) twice. The combined organic layers were dried over MgSO4 and concentrated in vacuo. The crude product was purified by column chromatography on silica gel. The fraction eluted with EtOAc afforded 2p (60 mg, 63% yield) as white solid.mp: 259–260 °C; 1H-NMR (DMSO-d6): δ 6.89–6.97 (8H, m), 7.03 (2H, dd, J = 2.4, 8.0 Hz), 7.08–7.14 (4H, m), 7.98 (2H, d, J = 8.8 Hz), 9.79 (2H, s); 13C-NMR (DMSO-d6): δ 106.7 (2C), 115.7 (2C), 124.3 (2C), 127.6 (4C), 128.4 (2C),128.5 (4C), 132.1 (2C), 132.3 (2C), 132.5 (2C), 139.2 (2C), 146.4 (2C), 155.5 (2C), 188.5; IR (KBr): 3651, 2925, 1734, 1617, 1560, 1541, 1523, 1508 cm−1; HRMS-EI: m/z: [M+] calcd for C29H18O3S2 478.0697 found 478.0696 (See Supplementary Materials for more details).
3.4. Agonistic Activity of 2p and 2l
The cells were seeded in 48-well plates and transfected with appropriate expression plasmids, the ARE-luciferase reporter plasmid, AR expression plasmid, and a Renilla pGL4.74 [hRluc/TK] (Promega, Madison, WI, USA) as an internal standard by the reverse-transfection method using the PEI Max reagent (Polysciences Inc., Warrington, PA, USA). After overnight incubation in phenol red-free DMEM containing 5% charcoal-stripped FBS (Promega, Madison, WI, USA), the cells were treated with various compounds for 24 h before luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). Firefly luciferase activities were normalized against Renilla luciferase activities.
4. Conclusions
In summary, we developed a multistep electron-transfer process involving a “triple-catalysis” system: a PdII/Pd0-p-benzoquinone/hydroquinone-CuCl2/CuCl catalytic system that uses environmentally friendly molecular oxygen as the terminal oxidant to effectively catalyze the CCC-coupling reaction of (o-alkynyl phenyl) (methoxymethyl) sulfides 2. Synthesized compounds 2p and 2l showed the androgen receptor (AR) agonistic activity. Dibenzo[b]thiophenyl ketone scaffold may expect to pharmacophore for non-steroidal AR-agonist. We are currently investigating further biological studies of the synthesized compounds 2.
Supplementary Materials
The following are available online at http://www.mdpi.com/1420-3049/21/9/1177/s1. 1H and 13C-NMR spectra of the synthesized compounds.
Acknowledgments
This research was supported by Grant-in-Aid for Scientific Research (C) (15K07871).
Author Contributions
K.K. conceived and designed the experiments; T.Y., Y.K. and K.N. performed the biological experiments; T.K. analyzed the data; K.T. contributed reagents and materials; R.S. performed the chemical experiments, and wrote the paper. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vlaar, T.; Ruijter, E.; Orru, R.V.A. Recent Advances in Palladium-Catalyzed Cascade Cyclizations. Adv. Synth. Catal. 2011, 353, 809–841. [Google Scholar] [CrossRef]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Alkenes and Alkynes. ChemCatChem 2009, 1, 28–41. [Google Scholar] [CrossRef]
- Wu, X.-F. Acylation of (Hetero) Arenes throuth C-H Activation with Aroyl Surrogates. Chem. Eur. J. 2015, 21, 12252–12265. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-Catalyzed Oxidative Carbonylation Reactions. ChemSusChem 2013, 6, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kusakabe, T.; Takahashi, K.; Kato, K. A cyclization-carbonylation-cyclization coupling reaction of (ortho-alkynyl phenyl) (methoxymethyl) sulfides with the palladium(II)-bisoxazoline catalyst. Org. Biomol. Chem. 2014, 12, 3380–3385. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.W.C.E.; ten Brink, G.-J.; Dijksman, A. Green, Catalytic Oxidations of Alcohols. Acc. Chem. Res. 2002, 35, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Bäckvall, J.-E.; Hopkins, R.B.; Grennberg, H.; Mader, M.; Awasthi, A.K. Multistep electron transfer in palladium-catalyzed aerobic oxidations via a metal macrocycle quinone system. J. Am. Chem. Soc. 1990, 112, 5160–5166. [Google Scholar] [CrossRef]
- Wöltinger, J.; Bäckvall, J.-E.; Zsigmond, Á. Zeolite-Encapsulated Cobalt Salophen Complexes as Efficient Oxygen-Activating Catalysts in Palladium-Catalyzed Aerobic 1,4-Oxidation of 1,3-Dienes. Chem. Eur. J. 1999, 5, 1460–1467. [Google Scholar] [CrossRef]
- Bäckval, J.-E.; Awasthi, A.K.; Renko, Z.D. Biomimetic aerobic 1,4-oxidation of 1,3-dienes catalyzed by cobalt tetraphenylporphyrin-hydroquinone-palladium(II). An example of triple catalysis. J. Am. Chem. Soc. 1987, 109, 4750–4752. [Google Scholar] [CrossRef]
- Grennberg, H.; Faizon, S.; Bäckvall, J.-E. Cobalt Tetra(hydroquinone)porphyrin: An Efficient Electron Transfer Reagent in Aerobic Pd-Catalyzed 1,4-Diacetoxylation of 1,3-Cyclohexadiene. Angew. Chem. Int. Ed. 1993, 32, 263–264. [Google Scholar] [CrossRef]
- Verboom, R.C.; Slagt, V.F.; Bäckvall, J.-E. Fast and mild palladium(II)-catalyzed 1,4-oxidation of 1,3-dienes via activation of molecular oxygen with a designed cobalt(II) porphyrin. Chem. Commun. 2005, 10, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Piera, J.; Bäckvall, J.-E. Catalytic Oxidation of Organic Substrates by Molecular Oxygen and Hydrogen Peroxide by Multistep Electron Transfer—A Biomimetic Approach. Angew. Chem. Int. Ed. 2008, 47, 3506–3523. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, A.E.; Stahl, S.S. Quinone-Catalyzed Selective Oxidation of Organic Molecules. Angew. Chem. Int. Ed. 2015, 54, 14638–14658. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-Z.; Andreasson, U.; Bäckvall, J.-E. Aerobic oxidation of secondary alcohols via ruthenium-catalysed hydrogen transfer involving a new triple catalytic system. J. Chem. Soc. Chem. Commun. 1994, 9, 1037–1038. [Google Scholar] [CrossRef]
- Cho, C.-H.; Neuenswander, B.; Lushington, G.H.; Larock, R.C. Solution-Phase Parallel Synthesis of a Multi-substituted Benzo[b]thiophene Library. J. Comb. Chem. 2009, 11, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H.; Neuenswander, B.; Larock, R.C. Diverse Methyl Sulfone-Containing Benzo[b]thiophene Library via Iodocyclization and Palladium-Catalyzed Coupling. J. Comb. Chem. 2010, 12, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Berrade, L.; Aisa, B.; Ramirez, M.J.; Galiano, S.; Guccione, S.; Moltzau, L.R.; Levy, F.O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; et al. Novel Benzo[b]thiophene Derivatives as New Potential Antidepressants with Rapid Onset of Action. J. Med. Chem. 2011, 54, 3086–3090. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Shao, H.Y.; Yang, Z.Y.; Xue, S.T.; Li, X.; Liu, Z.Y.; He, X.B.; Jiang, J.D.; Zhang, Y.Q.; Si, S.Y.; et al. Substituted Benzothiophene or Benzofuran Derivatives as a Novel Class of Bone Morphogenetic Protein-2 Up-Regulators: Synthesis, Structure-Activity Relationships, and Preventive Bone Loss Efficacies in Senescence Accelerated Mice (SAMP6) and Ovariectomized Rats. J. Med. Chem. 2010, 53, 1819–1829. [Google Scholar] [PubMed]
- Jarak, I.; Kralj, M.; Šuman, L.; Pavlović, G.; Dogan, J.; Piantanida, I.; Žinić, M.; Pavelić, K.; Karminski-Zamola, G. Novel Cyano- and N-Isopropylamidino-Substituted Derivatives of Benzo[b]thiophene-2-carboxanilides and Benzo[b]thieno[2,3-c]quinolones: Synthesis, Photochemical Synthesis, Crystal Structure Determination, and Antitumor Evaluation. 2. J. Med. Chem. 2005, 48, 2346–2360. [Google Scholar] [CrossRef] [PubMed]
- Hrib, N.J.; Jurcak, J.G.; Bregna, D.E.; Dunn, R.W.; Geyer, H.M.; Hartman, H.B.; Roehr, J.E.; Rogers, K.L.; Rush, D.K. 3[4-[1-(6-Fluorobenzo[b]thiophen-3-yl)-4-piperazinyl]butyl]-2,5,5-trimethyl-4-thiazolidinone: A new atypical antipsychotic agent for the treatment of schizophrenia. J. Med. Chem. 1992, 35, 2712–2715. [Google Scholar] [CrossRef] [PubMed]
- Boschelli, D.H.; Kramer, J.B.; Khatana, S.S.; Sorenson, R.J.; Connor, D.T.; Ferin, M.A.; Wright, C.D.; Lesch, M.E.; Imre, K. Inhibition of E-Selectin-, ICAM-1-, and VCAM-1-Mediated Cell Adhesion by Benzo[b]thiophene-, Benzofuran-, Indole-, and Naphthalene-2-carboxamides: Identification of PD 144795 as an Antiinflammatory Agent. J. Med. Chem. 1995, 38, 4597–4614. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.T.; Cetenko, W.A.; Mullican, M.D.; Sorenson, R.J.; Unangst, P.C.; Weikert, R.J.; Adolphson, R.L.; Kennedy, J.A.; Thueson, D.O. Novel benzothiophene-, benzofuran-, and naphthalenecarboxamidotetrazoles as potential antiallergy agents. J. Med. Chem. 1992, 35, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.-J.R.P.; Ferreira, I.C.F.R.; Gaetano, Y.D.; Kirsch, G.; Calhelha, R.C.; Estevinho, L.M. Synthesis and antimicrobial activity studies of ortho-chlorodiarylamines and heteroaromatic tetracyclic systems in the benzo[b]thiophene series. Bioorg. Med. Chem. 2006, 14, 6827–6831. [Google Scholar] [CrossRef] [PubMed]
- Pinney, K.G.; Bounds, A.D.; Dingeman, K.M.; Mocharla, V.P.; Pettit, G.R.; Bai, R.; Hamel, E. A new anti-tubulin agent containing the benzo[b]thiophene ring system. Bioorg. Med. Chem. Lett. 1999, 9, 1081–1086. [Google Scholar] [CrossRef]
- Ehrlich, M.; Carell, T. Total Syntheses and Biological Evaluation of 3-O-Methylfunicone and Its Derivatives Prepared by TMPZnCl·LiCl-Mediated Halogenation and Carbonylative Stille Cross-Coupling. Eur. J. Org. Chem. 2013, 2013, 77–83. [Google Scholar] [CrossRef]
- Grossmann, K.; Ehrhardt, T. On the mechanism of action and selectivity of the corn herbicide topramezone: A new inhibitor of 4-hydroxyphenylpyruvate dioxygenase. Pest Manag. Sci. 2007, 63, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Brennführer, A.; Beller, M. A General Synthesis of Diarylketones by Means of a Three-Component Cross-Coupling of Aryl and Heteroaryl Bromides, Carbon Monoxide, and Boronic acids. Chem. Eur. J. 2008, 14, 3645–3652. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Rashidi-Ranjbar, P.; Kashani, A.O. Easy-to-Execute Carbonylative Arylation of Aryl Halides using Molybdenum Hexacarbonyl: Efficient Synthesis of Unsymmetrical Diaryl Ketones. Eur. J. Org. Chem. 2011, 2011, 2128–2132. [Google Scholar] [CrossRef]
- Lo Fiego, M.J.; Silbestri, G.F.; Chopa, A.B.; Lockhart, M.T. Selective Synthetic Routes to Sterically Hindered Unsymmetrical Diaryl Ketones via Arylstannanes. J. Org. Chem. 2011, 76, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nishimura, Y.; Gao, F.; Gotoh, K.; Nishihara, Y.; Takagi, K. Rh-Catalyzed Carbonylation of Arylzinc Compounds Yielding Symmetrical Diaryl Ketones by the Assistance of Oxidizing Agents. J. Org. Chem. 2011, 76, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).