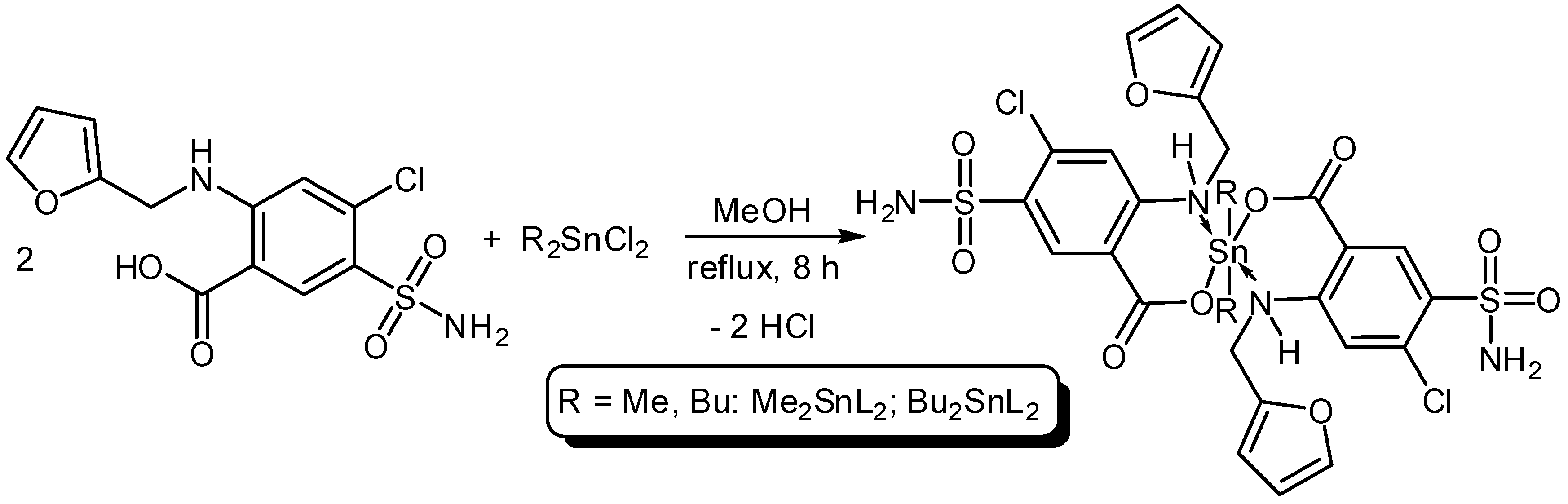Abstract
Three organotin complexes containing furosemide as a ligand (L), Ph3SnL, Me2SnL2 and Bu2SnL2, were synthesized and characterized. Octahedral geometry was proposed for the Me2SnL2 and Bu2SnL2, while the Ph3SnL complex has trigonal bipyramid geometry. The synthesized organotin complexes (0.5% by weight) were used as additives to improve the photostability of poly(vinyl chloride), PVC, (40 μm thickness) upon irradiation. The changes imposed on functional groups, weight loss and viscosity average molecular weight of PVC films were monitored. The experimental results show that the rate of photodegradation was reduced in the presence of the organotin additives. The quantum yield of the chain scission was found to be low (9.8 × 10−7) when Ph3SnL was used as a PVC photostabilizer compared to controlled PVC (5.18 × 10−6). In addition, the atomic force microscope images for the PVC films containing Ph3SnL2 after irradiation shows a smooth surface compared to the controlled films. The rate of PVC photostabilization was found to be highest for Ph3SnL followed by Bu2SnL2 and Me2SnL2. It has been suggested that the organotin complexes could act as hydrogen chloride scavengers, ultraviolet absorbers, peroxide decomposers and/or radical scavengers.
1. Introduction
Poly(vinyl chloride) (PVC) has unique physical and mechanical properties and is widely utilized as a thermoplastic material [1]. PVC polymeric materials come next to polyolefins in terms of global production and consumption [2]. It has various outdoor applications, mainly in construction materials [3,4,5]. PVC however undergoes photochemical degradation when exposed to sunlight or high temperatures for long periods of time [6]. This photodegradation process can lead to changes in the mechanical and physical properties of PVC [7]. Conjugated double bonds could be formed within PVC chains due to dehydrochlorination [8]. This process commonly takes place due to the presence of impurities and/or defects produced within the polymeric chains during synthesis [9,10]. Cross-linking and a reduction in the average molecular weight also occur within PVC chains due to photodegradation processes [6,7,8]. The poor stability of PVC hinders its use in outdoor applications. It is therefore important to photostabilize PVC to enable its uses in harsh conditions such as high temperatures.
Commercial stabilizers such as plasticizers can be used to enhance photostability of PVC [11]. Photostabilizers including Schiff base complexes [12,13,14], aromatics [15,16,17] and heterocycles [18,19,20] have been used in various studies in order to increase PVC photostability. Other additives include inorganic salts and metal complexes are also common [21,22,23,24,25].
Organotin complexes can be used in a variety of different applications. The diorganotin(IV) additives containing benzamidoacetic acid have been used to stabilize the PVC [26]. In this work, we report the use of di- and triorganotin(IV) complexes containing furosemide as photostabilizers for PVC as part of our general interest in the field of polymeric materials [27,28,29,30,31].
2. Results and Discussion
2.1. Synthesis
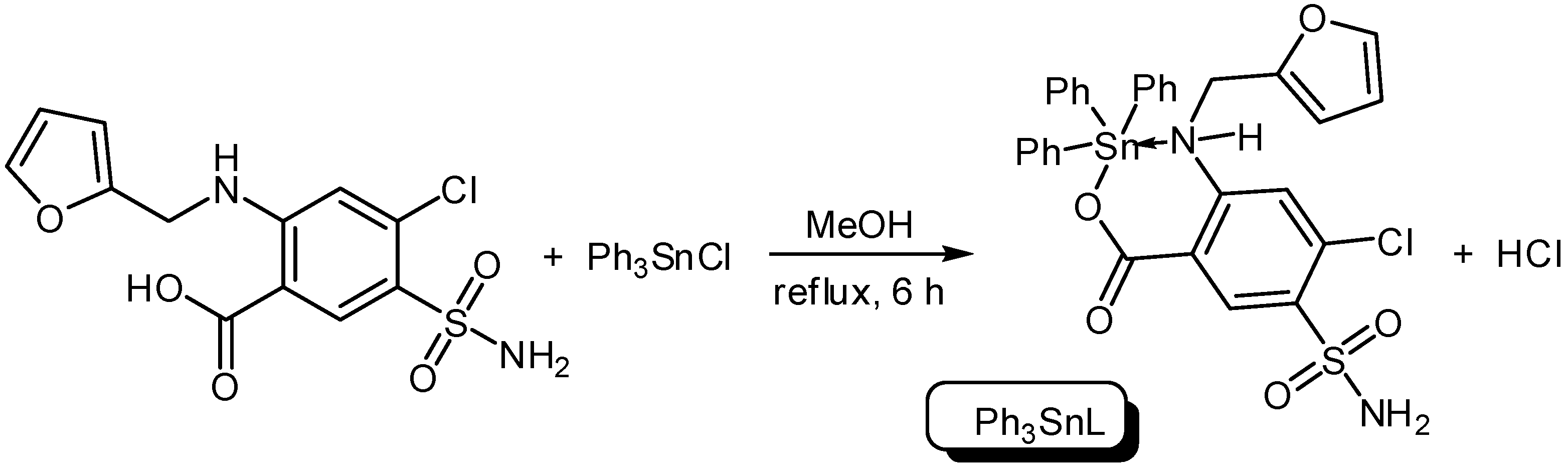
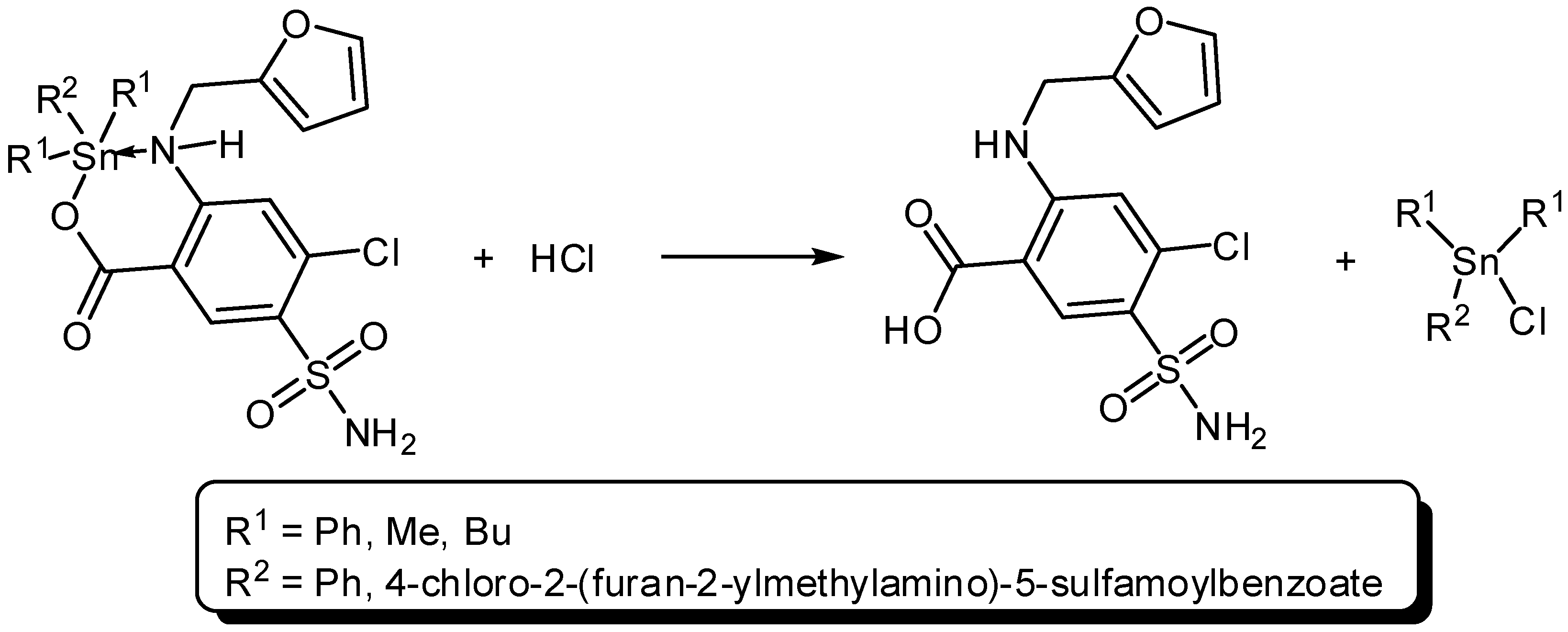
Three organotin complexes, Ph3SnL, Me2SnL2 and Bu2SnL2, were synthesized from reactions between diorganotin dichlorides or triorganotin chloride with furosemide as a ligand (L) in methanol under reflux conditions for 6–8 h (Scheme 1 and Scheme 2). In the case of Ph3SnL, a 1:1 molar ratio of L and Ph3SnCl was used, while, a 2:1 molar ratio of L and R2SnCl2 (R = Me, Bu) was used for the production of Me2SnL2 and Bu2SnL2. The structures of Sn(IV) complexes were characterized by elemental analysis and various spectroscopy methods.
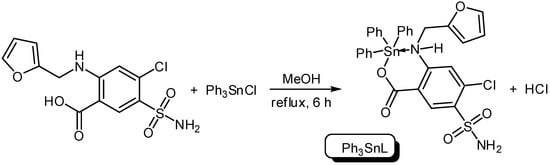
Scheme 1.
Synthesis of triphenyltin (Ph3SnL) complex.
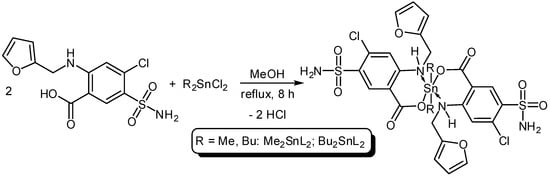
Scheme 2.
Synthesis of dimethyltin (Me2SnL2) and dibutyltin (Bu2SnL2) complexes.
2.2. Elemental Analysis
The purity of the Sn(IV) complexes was checked by elemental analyses. In addition, flame atomic absorption spectroscopy was used to estimate the tin content within the Sn(IV) complexes. The observed values were generally in agreement with the calculated ones, although it should be noted that the observed carbon content in the Ph3SnL complex was significantly less than the calculated one. This could be due to the presence of some impurities and/or solvent. The elemental analyses data (C, H, N, S and Sn %), yields and melting points for Sn(IV) complexes along with those for the ligand are reported in Table 1.

Table 1.
Melting points, yields and elemental analyses for L and its Sn(IV) complexes.
2.3. Infrared Spectroscopy
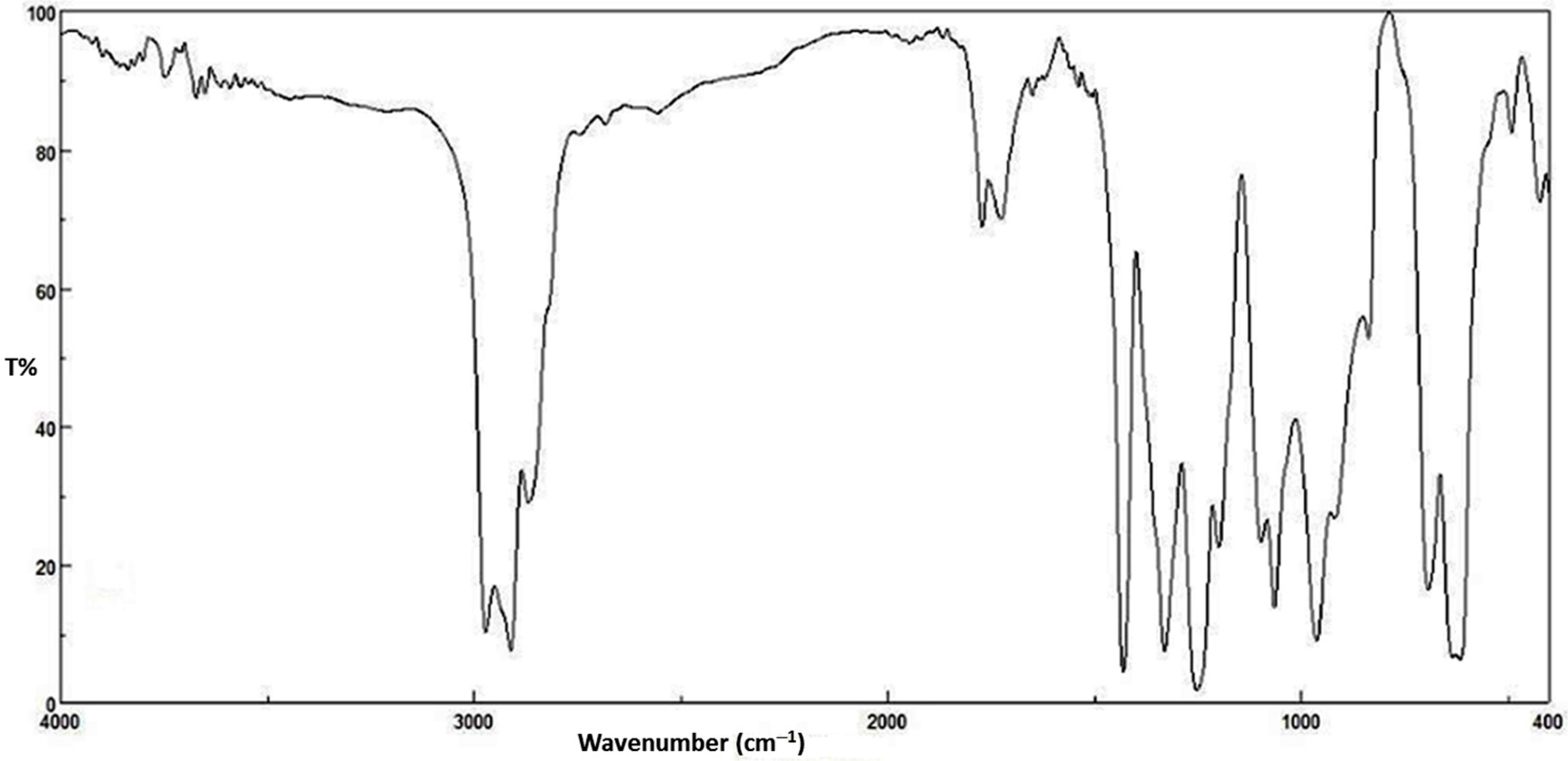
FTIR spectroscopy gives valuable information about the coordination modes within organotin complexes. The FTIR measurements (4000–400 cm−1) were performed using the KBr disc method. The FTIR spectroscopic data for L and synthesized organotin(IV) complexes are reported in Table 2.

Table 2.
FTIR spectroscopic data for L and its organotin(IV) complexes.
The IR spectral data for L were compared with those for di- and triorganotin(IV) complexes in order to confirm the binding modes within the synthesized complexes. The IR spectrum of the ligand showed sharp characteristic absorption bands due to asymmetric NH2 (3398 cm−1) and symmetric NH2 (3282 cm−1) stretching vibrations. The IR spectra of organotin complexes suggest that the –SO2NH2 group was not involved in the coordination process with the Sn atom. The peak appearing at 3352 cm−1 (secondary NH stretching) for the ligand was shifted slightly in the organotin complexes which is an indication that the NH has coordinated to the Sn(IV) atom. The asymmetric vibrations band for carbonyl group (νas COO) in ligand appeared at 1670 cm−1. This peak shifted to higher frequencies for the tin complexes (1672–1679 cm−1). The symmetric stretching vibrations band (1562 cm−1) for the carboxyl group (νs COO) in the ligand has been shifted to higher frequencies (1566 cm−1) in the case of Ph3SnL and Bu2SnL2 and to lower frequency (1558 cm−1) in the case of Me2SnL2. The interaction between the carboxylate oxygen and tin atom can be established from the IR stretching frequencies, Δv (COO) [32]. If Δv [vas (COO) − vs (COO)] is >350 cm−1, the carboxylate group binds in a monodentate mode. The bidentate mode is common when Δv (COO) < 200 cm−1. For Δv (COO) less than 200 and more than 350 cm−1 an intermediate state between bidentate and monodentate takes place. The Δv (COO) values in the bridging mode have been reported to be more than those in the chelating mode [33].
Three new absorption bands appeared in the IR spectra of the organotin complexes. These bands are attributed to v (Sn–N), v (Sn–O) and v (Sn–C) that resonate within the 408–416, 447–426 and 516–540 cm−1 regions, respectively. The appearance of such peaks provides further evidence for the coordination between ligand and Sn(IV). The coordination thus takes place between the tin atom and a secondary amine, carboxylate and alkyl or phenyl groups [34,35].
2.4. Ultraviolet-Visible Spectroscopy and Conductivity
The absorption spectra (200–600 nm) for the ligand and its organotin complexes were recorded in DMF (Table 3). The electronic spectrum of ligand shows two absorption bands. The first resonates at 274 nm due to π–π* electronic transition of the aromatic moieties. This band was slightly shifted to higher wavelength after complexation. The second band resonates at 341 nm due to n–π* electronic transition for the carbonyl oxygen non-bonding electrons and has been shifted slightly to higher wavelength within organotin complexes. The molar conductivity for organotin complexes (10−3 M) were measured in ethanol at room temperature. The conductivity for Sn(IV) complexes was found to be in the range of 2.1–4.4 μS/cm (Table 3). The organotin complexes were found to be non-electrolyte.

Table 3.
Electronic spectral data and conductivity for L and its organotin(IV) complexes.
2.5. 1H-NMR Spectroscopy
The 1H-NMR spectra L and its Sn(IV) complexes were recorded in DMSO-d6 (Table 4) and were consistent with their chemical structures. The changes in the chemical shift values between the ligand and its tin complexes are largely dependent on the environment and can be used as evidence to show that complexation has taken place [36]. The 1H-NMR spectrum of the ligand showed an exchangeable singlet which resonates at 13.35 ppm due to the carboxylate proton. Such a signal was absent in the 1H-NMR spectra of the organotin complexes. The aromatic protons in Sn(IV) complexes was slightly shifted up-field compared to the corresponding ones in the ligand [37]. In contrast, the NH signal was shifted down-field upon chelation of ligand with Sn(IV) atom. This could be a result of a charge transfer towards the carboxylate group that binds to the highly electropositive Sn atom [35]. The 1H-NMR spectra of organotin complexes showed two singlet aromatic protons that resonated within the 7.06–7.10 and 8.38–8.44 ppm regions which were attributed to H-3 and H-6, respectively. They also showed an apparent triplet (J = 3.2 Hz) that resonated at 6.38–6.42 ppm which is characteristic for H-4 of a furan. The H-3 of the furan moiety resonated a doublet (J = 3.2 Hz) within the 6.31–6.37 ppm region. Moreover, the CH2 protons resonated as a doublet (J = ca. 6 Hz) at 4.44–4.58 ppm.

Table 4.
1H-NMR spectral data for ligand and organotin complexes.
2.6. Evaluation of Stabilizing Efficiency of PVC by Weight Loss
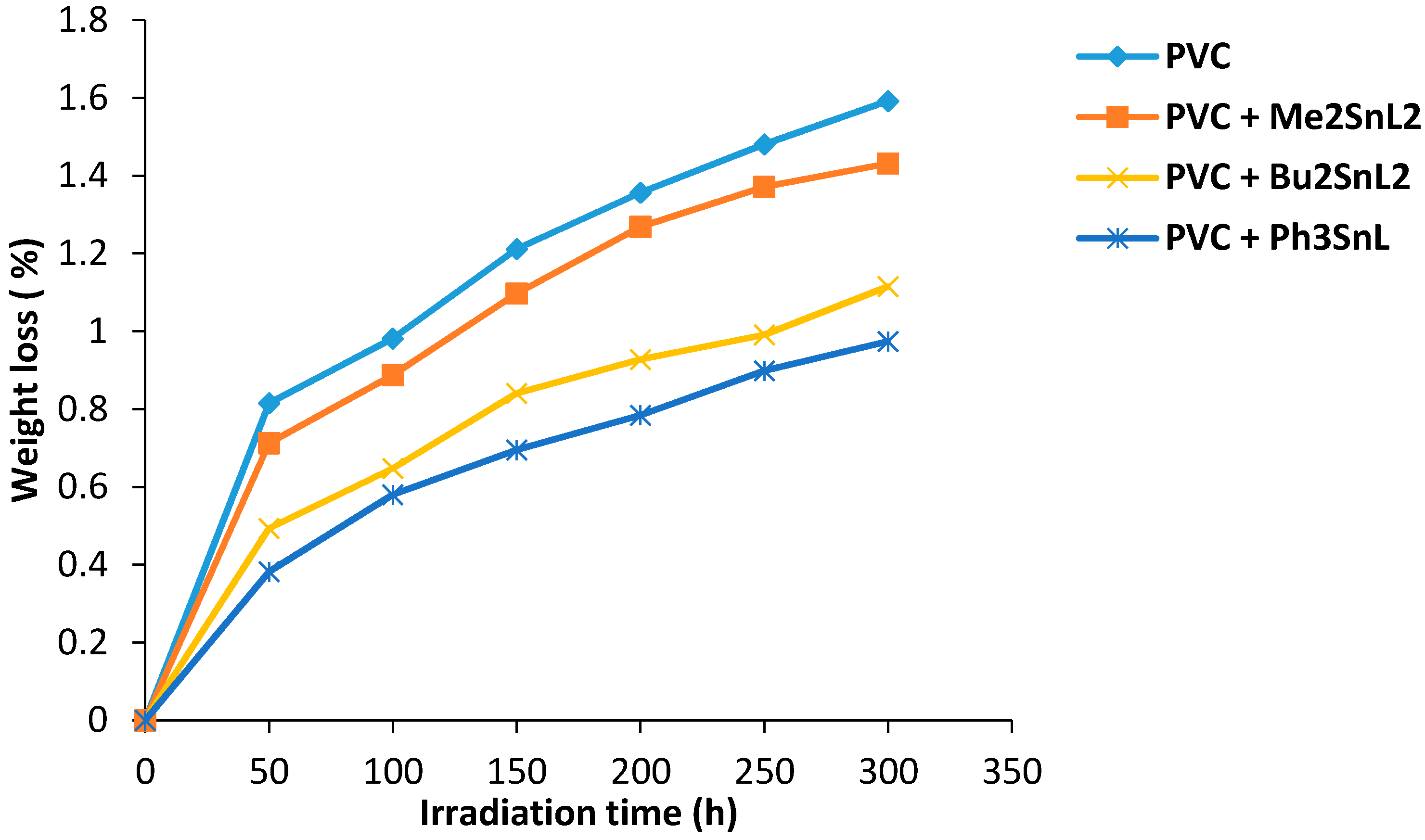
Photodegradation of PVC is associated with the evaluation of HCl gas (dehydrochlorination) and leads to weight loss. Therefore, the weight loss percentage can be used to measure the degree of PVC photodegradation. It has been reported that additives with a concentration of 0.5% by weight showed the most efficient photostabilization effect when added to PVC [20]. Therefore, di- and triorganotin(IV) additives (0.5% by weight) were added to PVC to produce the films. Their photostabilization effect was investigated for PVC films (40 μm thickness) on irradiation for up to 300 h. The effect of irradiation time (300 h) on weight loss of PVC films (40 μm thickness) is shown in Figure 1. Evidently, the PVC weight loss percentage was lower in the presence of organotin complexes when compared to the blank PVC film. The Ph3SnL complex shows the most efficient stabilizing effect compared to the other additives. The Ph3SnL complex is believed to be a better radical scavenger compared to other complexes since the extra phenyl ring increases the photostabilization of PVC films on irradiation via resonance. The PVC photostabilization efficiency of organotin(IV) complexes was found to follow the order Ph3SnL > Bu2SnL2 > Me2SnL2.
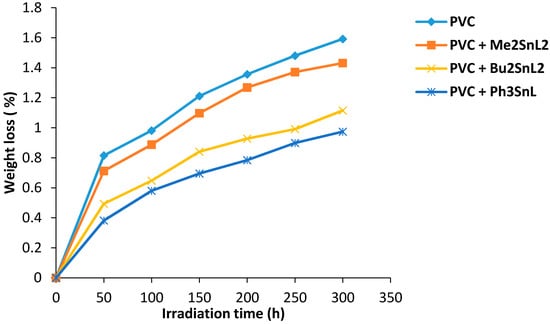
Figure 1.
Changes in weight loss of PVC films versus irradiation time.
2.7. Evaluation of Stabilizing Efficiency of PVC by FTIR Spectroscopy
The photochemical activity of organotin complexes as additives for PVC films photostabilization was investigated using FTIR spectroscopy. The PVC films were irradiated with UV light (λmax = 365 nm) for 300 h. The irradiation of PVC leads to the formation of various functional groups. The IR spectrum of irradiated PVC shows three absorption bands due to formation of carbonyl, polyene and hydroxyl groups. These bands resonate at 1772 (C=O), 1604 (C=C) and 3500 cm−1 (OH), respectively [38]. The growth rate of such peaks related to a reference peak (1328 cm−1) could be considered as a measure for the photodegrading of PVC. The FTIR spectra of PVC films containing Bu2SnL2 complex before and after irradiation are represented in Figure 2 and Figure 3, respectively.
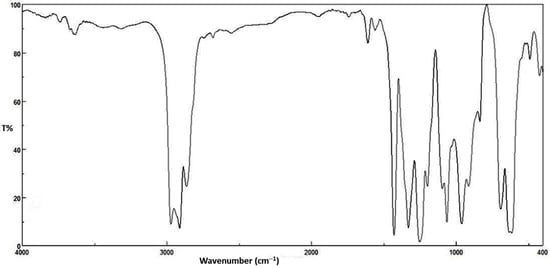
Figure 2.
FTIR spectrum of PVC film containing Bu2SnL2 complex before irradiation.
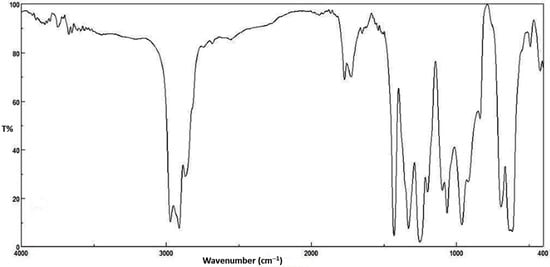
Figure 3.
FTIR spectrum of PVC film containing Bu2SnL2 complex after irradiation.
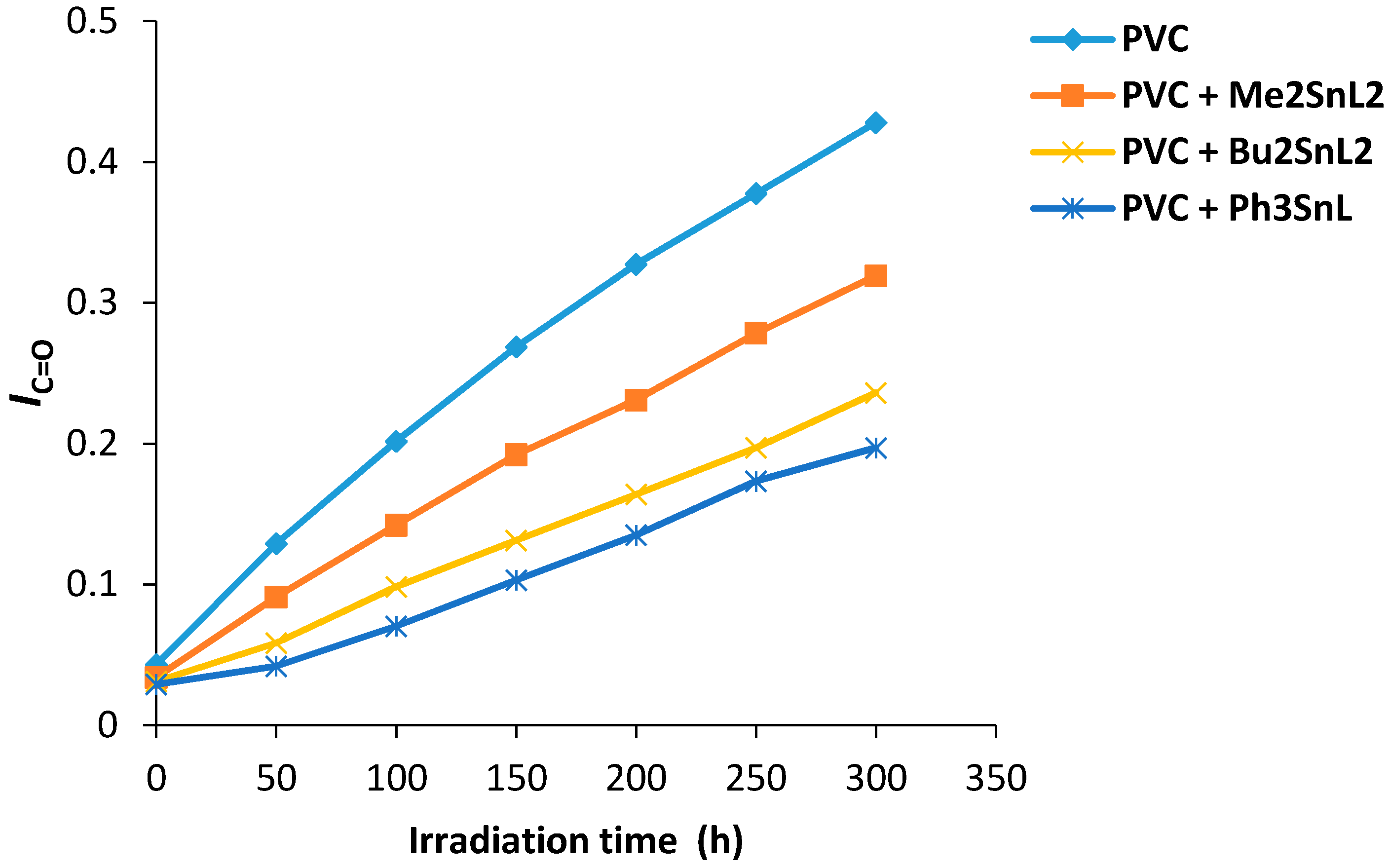
The changes in carbonyl group intensity (1722 cm−1) for PVC films containing organotin(IV) was monitored on irradiation. The carbonyl index (IC+O) was calculated and plotted against irradiation time. The growth rate of carbonyl group was lower when Ph3SnL, Me2SnL2 and Bu2SnL2 (0.5 wt %) were used as additives compared to PVC without additives (Figure 4). The Ph3SnL complex was the most efficient additive amongst the ones used in photostabilization of PVC followed by Bu2SnL2 and Me2SnL2.
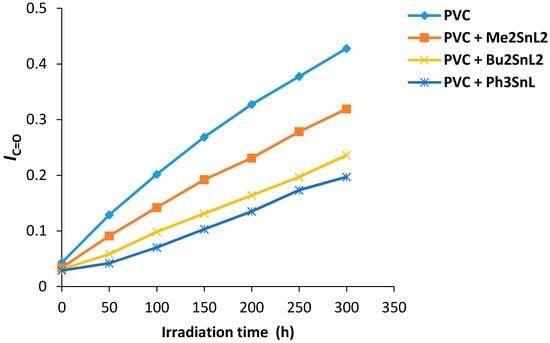
Figure 4.
Changes in the IC=O index for PVC films versus irradiation time.
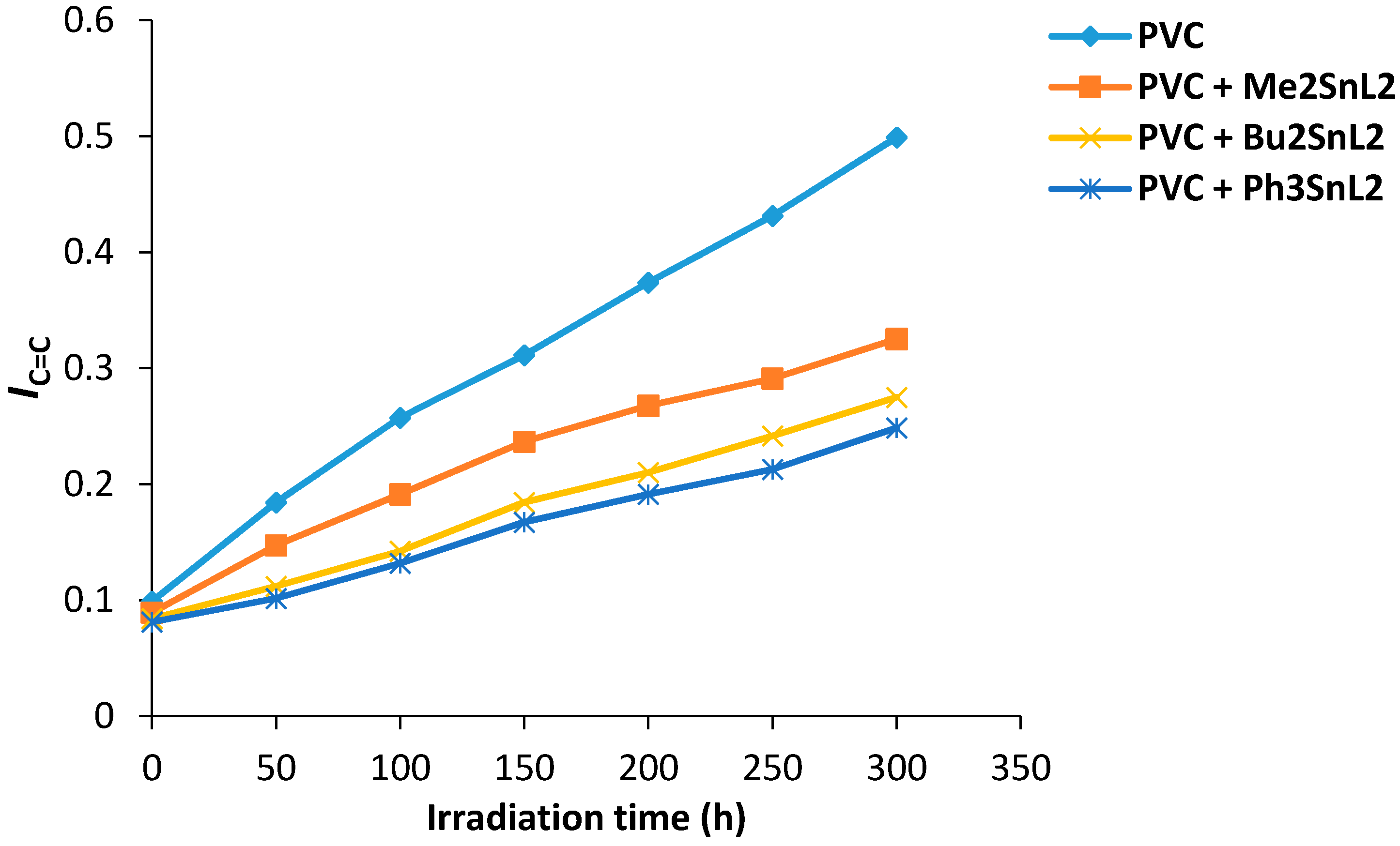
Similarly, the changes in the C=C group intensity was monitored and the polyene index (IC=C) was calculated. Figure 5 shows the changes in IC=C of PVC films upon irradiation. All the additives used show stabilization for PVC on irradiation in which triphenyltin(IV) complex was the most effective additive against photodegradation of PVC.
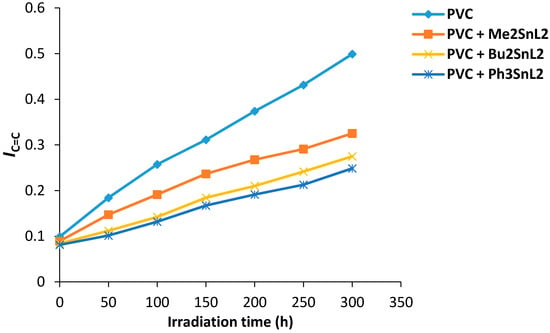
Figure 5.
Changes in the IC=C index for PVC films versus irradiation time.
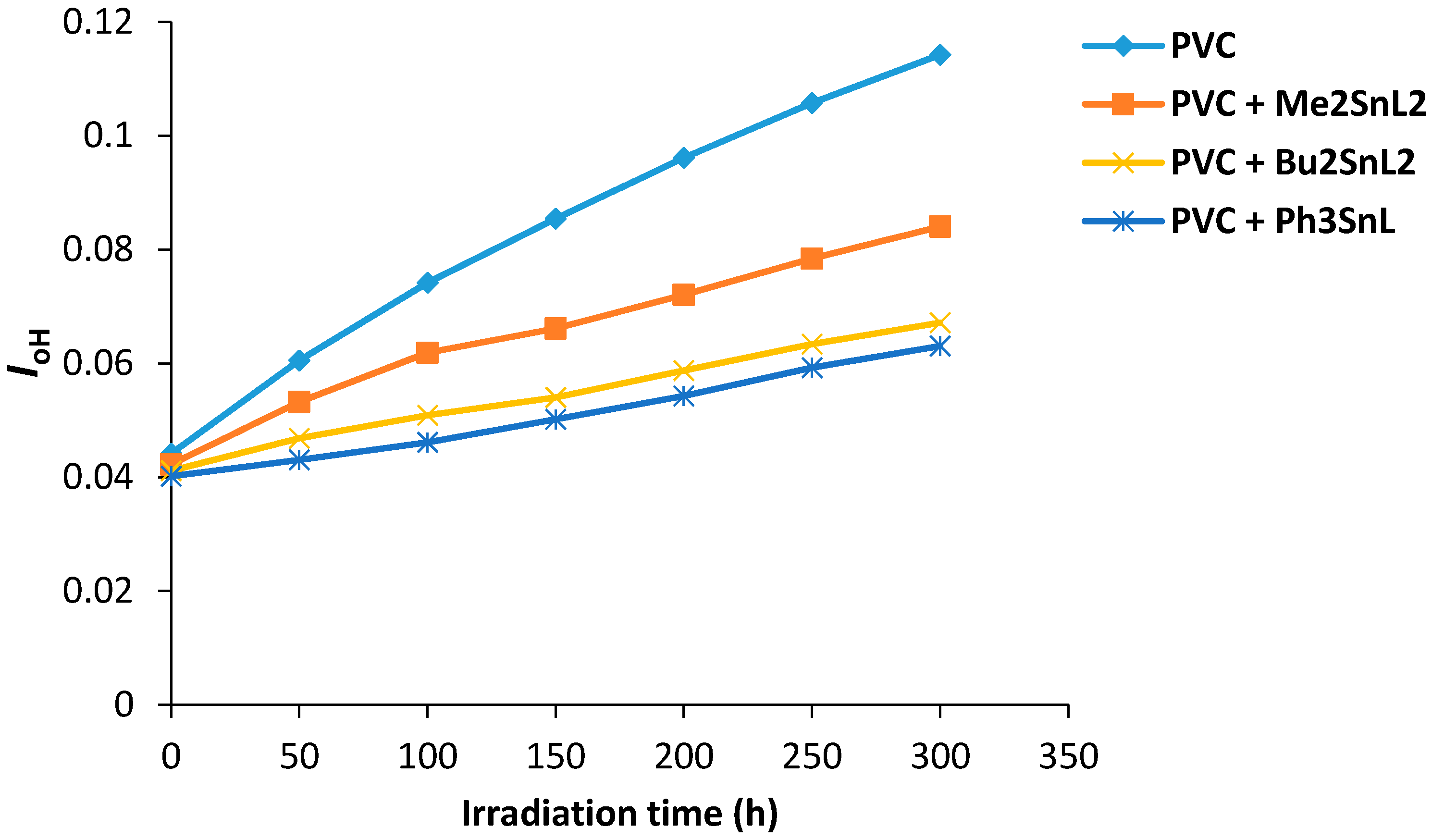
Figure 6 shows the changes in hydroxyl index (IOH) of PVC films containing organotin(IV) complexes during irradiation. Again the additives used, Ph3SnL, Me2SnL2 and Bu2SnL2, showed lower IOH growth compared to PVC (control).
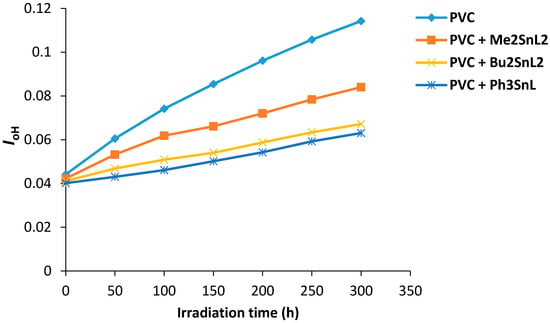
Figure 6.
Changes in the IOH index for PVC films versus irradiation time.
2.8. Evaluation of Stabilizing Efficiency of PVC by Variation in Molecular Weight
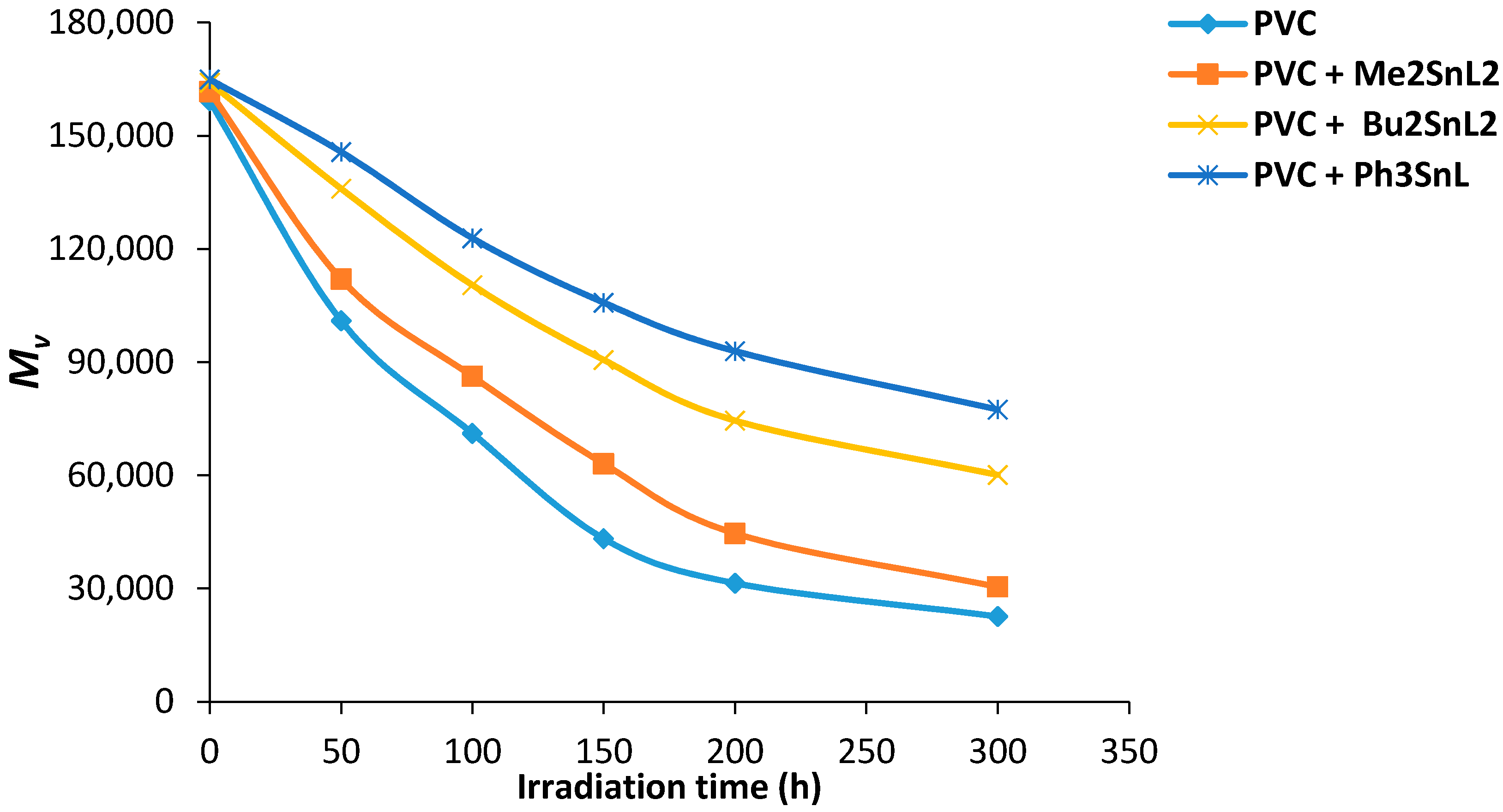
Viscosity average molecular weight () can be measured from intrinsic viscosity using Equation (5). The changes in for PVC films (40 μm thickness) containing organotin complexes (0.5 wt %) was plotted against irradiation time (Figure 7) with a light intensity of 1.052 × 10−8 ein·dm−2·s−1 (THF, 25 °C).
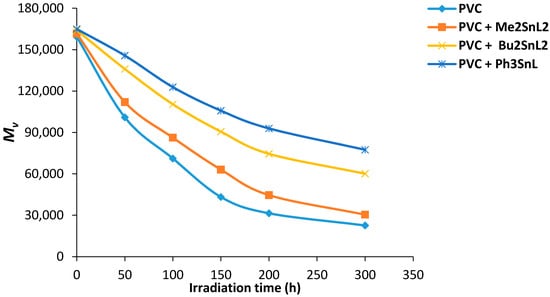
Figure 7.
Changes in for PVC films versus irradiation time.
It was noted that some insoluble PVC traces in THF was produced during photolysis process. It was suggested that these residues could possibly be an indication for PVC crosslinking or branching that occurs on irradiation [39]. To clarify this possibility, the number of average chain scission (S) [40] was calculated using Equation (1). The S value is highly dependent on the viscosity average molecular weight at start () and at t irradiation time ().
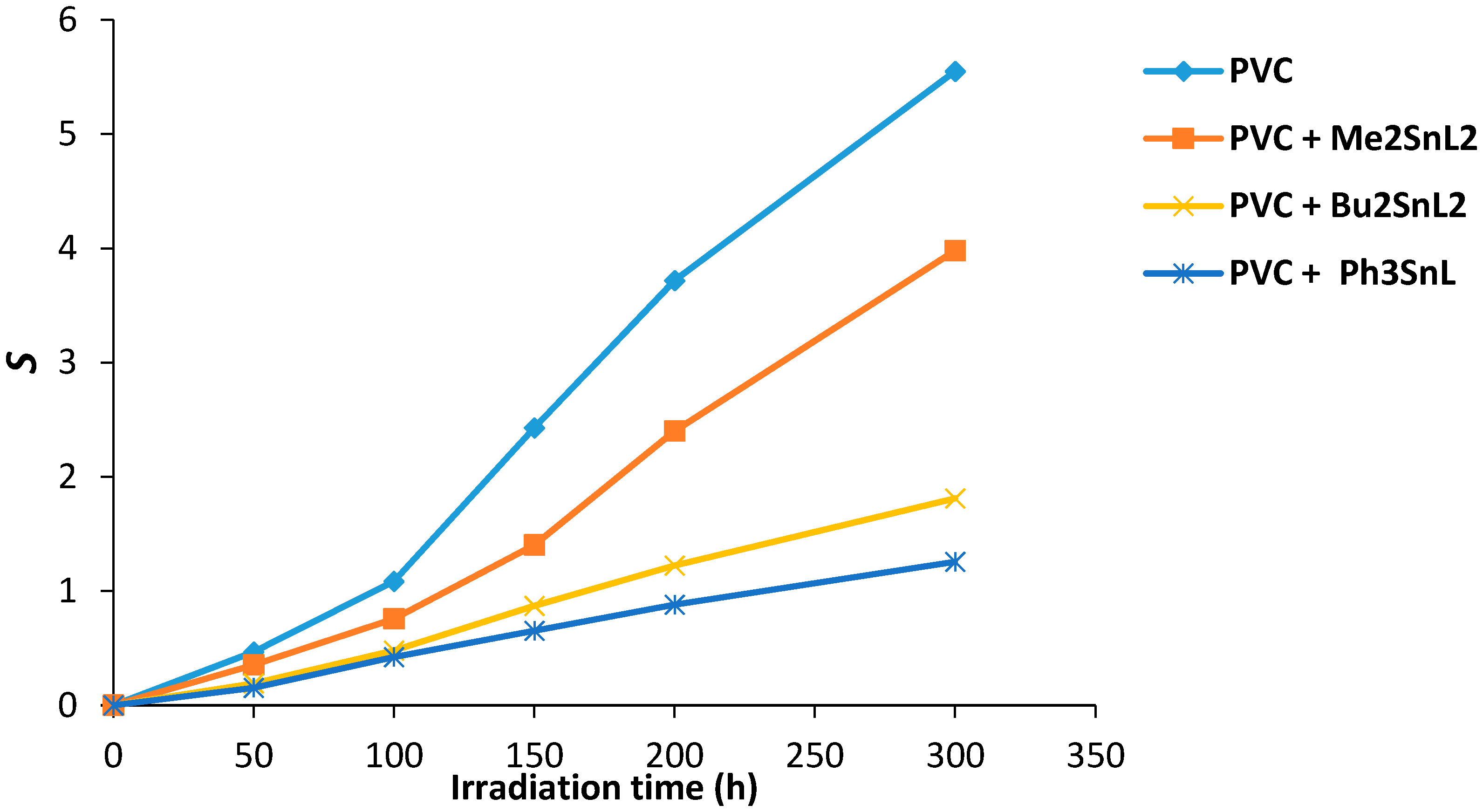
Figure 8 shows the effect of irradiation time on the value of S for PVC films containing organotin(IV) complexes. The PVC (control) on irradiation shows a higher degree of branching and/or cross-linking compared to the ones containing additives. The increases in S value was sharp for control PVC between 100 and 300 h. Less insoluble residual polymers were observed in PVC containing complexes and in particular those that contain Ph3SnL.
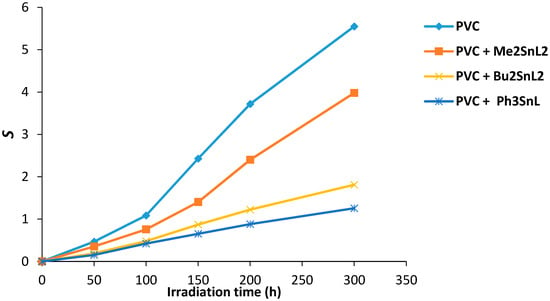
Figure 8.
Changes in the main chain scission (S) for PVC films versus irradiation time.
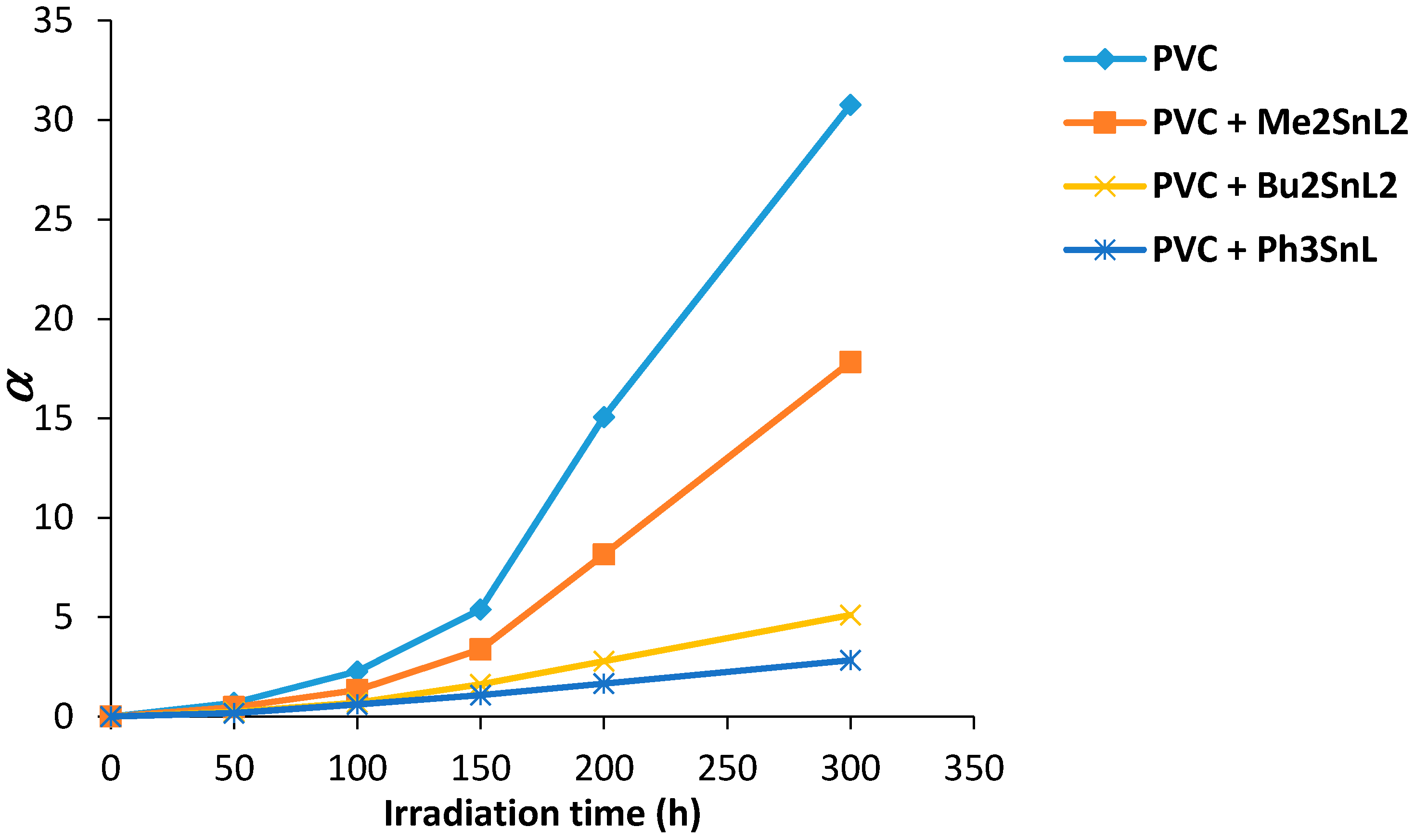
The degree of deterioration (α) for PVC films can be calculated using Equation (2). It is dependent on the initial molecular weight (m), S and . The value of α is proportional to the break of randomly distributed weak bond which is fast in the initial stage of irradiation.
The effect of irradiation on PVC films is shown in Figure 9. The change in α value was increased with irradiation time. The increase in α value for PVC (control) was steep at 150 °C and reached a maximum after 300 h. It seems likely that random bond breakage is occurring within PVC chain. The α value was lower for PVC containing Sn(IV) complexes and was minimum in the case of triphenyltin(IV) complex. It is clear that the use of such additives has reduced the photodegradation of PVC films upon irradiation.
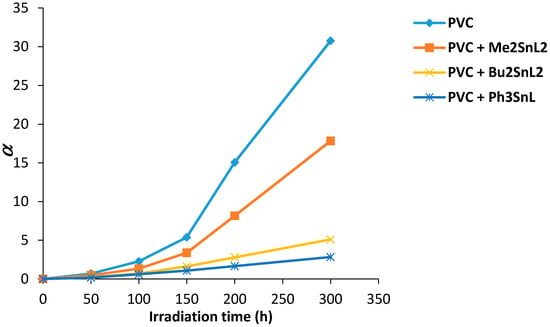
Figure 9.
Changes in the degree of deterioration (α) for PVC films versus irradiation time.
The quantum yield of chain scission (Φcs) for PVC films was measured using Equation (6) and represented in Table 5. The Φcs for PVC (control) was found to be higher (5.18 × 10−6) compared to the ones containing Sn(IV) complexes (1.58 × 10−6–9.8 × 10−7). The higher the Φcs value the greater the photodegrading of PVC films [13]. The PVC film which contains Ph3SnL complex shows the lowest Φcs value. It can therefore be suggested that this additive inhibits the photodegrading of PVC upon irradiation. The quantum yield (Φcs) increases with increasing temperature [13]. It reaches peak around the melting temperature and glass transition temperature (Tg; 80 °C) of the crystalline and amorphous polymers, respectively. The PVC photolysis was carried out at 35–45 °C which is lower than the Tg of PVC. Therefore, the dependence of Φcs on temperature is not significant [41].

Table 5.
Quantum yield (Φcs) for the chain scission for PVC film (40 µm) containing metal complexes (0.5 wt %) after irradiation (300 h).
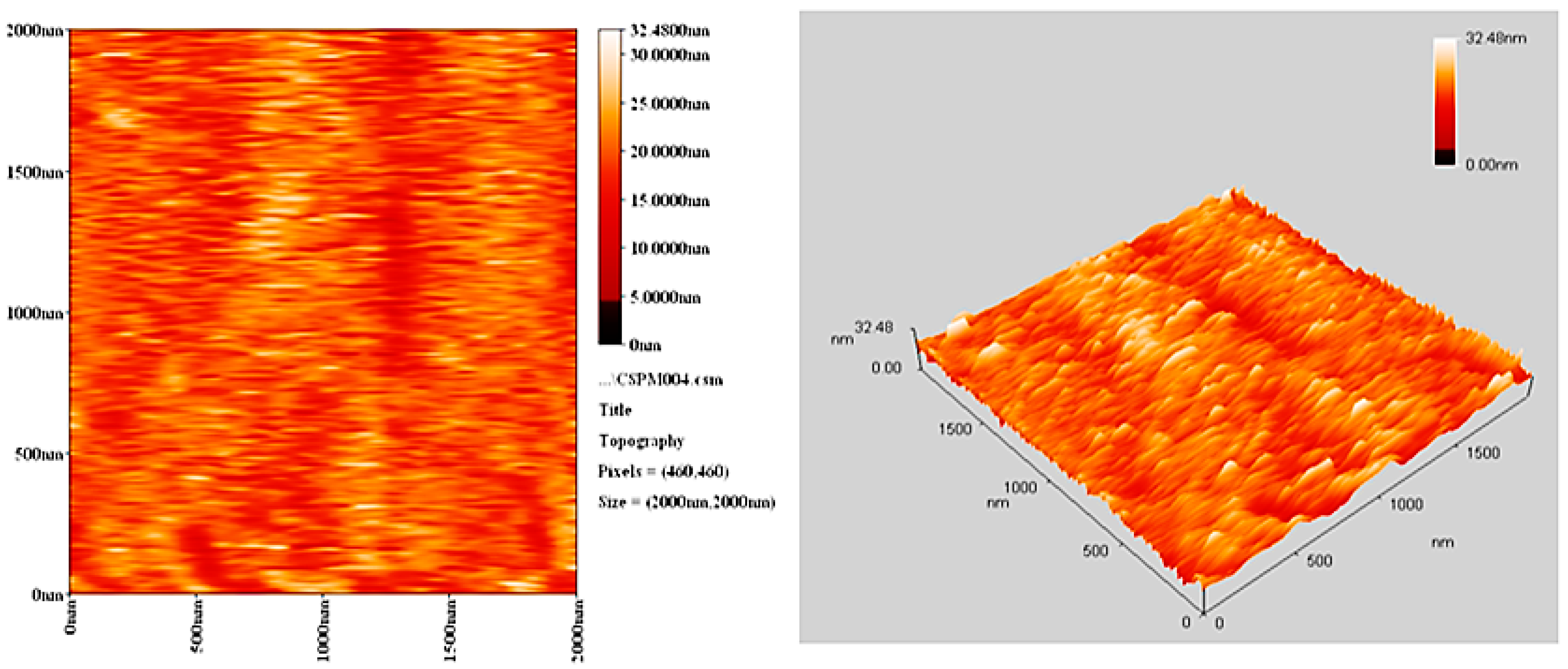
2.9. Surface Analysis
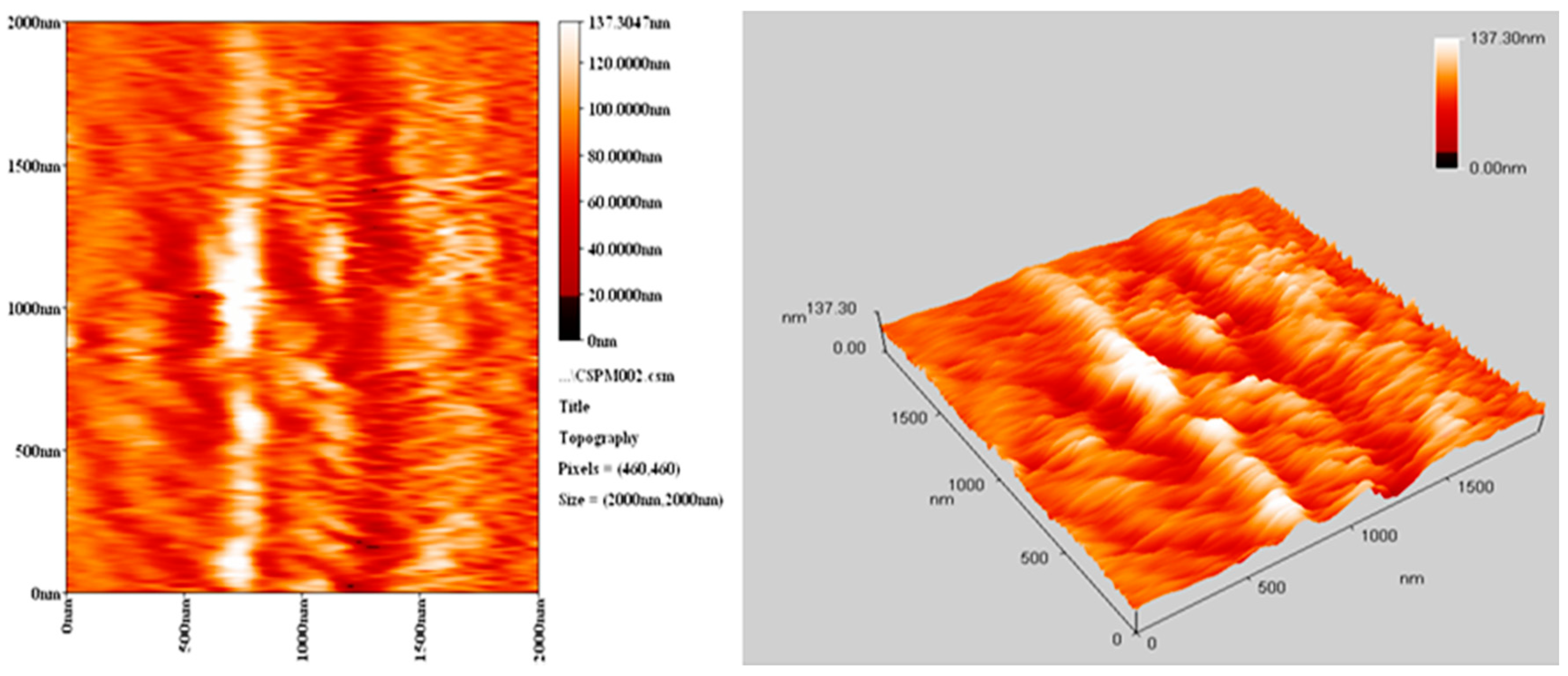
The morphology of PVC surface (area = 5.0 × 5.0 μm2) was inspected using an atomic force microscope (AFM). It provides two- and three-dimensional topographic images of the PVC surface on irradiation (300 h). AFM is helpful to measure the pores size and roughness factor (Rq) of the PVC film [42]. The AFM images for PVC films (control; 40 μm thickness) and that contains Ph3SnL (0.5 wt %) as a photostabilizer after irradiation (300 h) are given in Figure 10 and Figure 11, respectively.
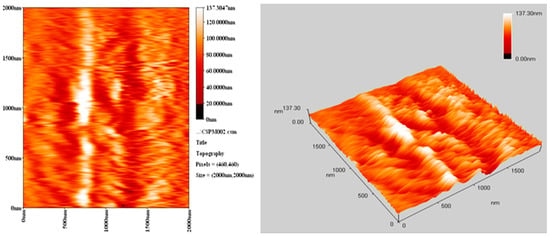
Figure 10.
AFM images for PVC film (control) after irradiation (300 h).
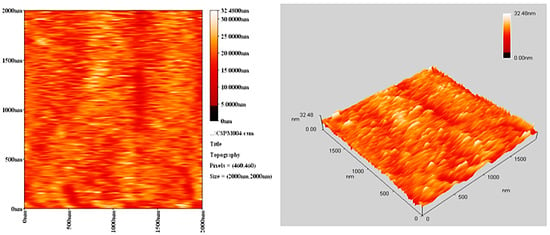
Figure 11.
AFM images for PVC film containing Ph3SnL complex after irradiation (300 h).
The surface of PVC film containing Ph3SnL after irradiation was very smooth (Rq = 2.61). In contrast, the blank PVC film after irradiation has a rough surface (Rq = 17.3). UV irradiation may have led to bond breakage within polymeric chains and hence the removal of leachable constituents from the PVC surface which can result in a roughened surface [43]. Irradiation of PVC in the absence of photostabilizers could lead to dehydrochlorination which is common at 150 °C and above [44]. It seems likely that the Sn(IV) complexes inhibits the dehydrochlorination process.
2.10. Suggested Mechanisms of Photostabilization of PVC
The di- and triorganotin(IV) complexes act as photostabilizers for PVC films and their efficiencies follow the order Ph3SnL > Bu2SnL2 > Me2SnL2. The three Sn(IV) utilized all reduced PVC photodegradation. Such additives can stabilize the polymeric films based on various mechanisms. Tin is a strong Lewis acid which is also known as a secondary stabilizer and therefore acts as HCl scavenger (Scheme 3). The photostabilization effect could be due to displacement of chlorine atom within PVC chains by carboxylate oxygen. Various organic compounds are known as long-term PVC stabilizers [21].
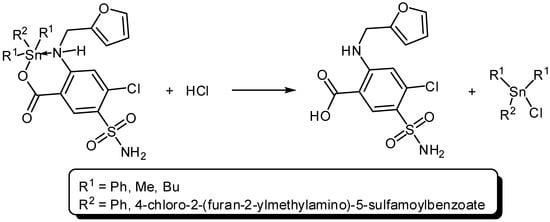
Scheme 3.
Organotin complexes act as HCl scavengers.
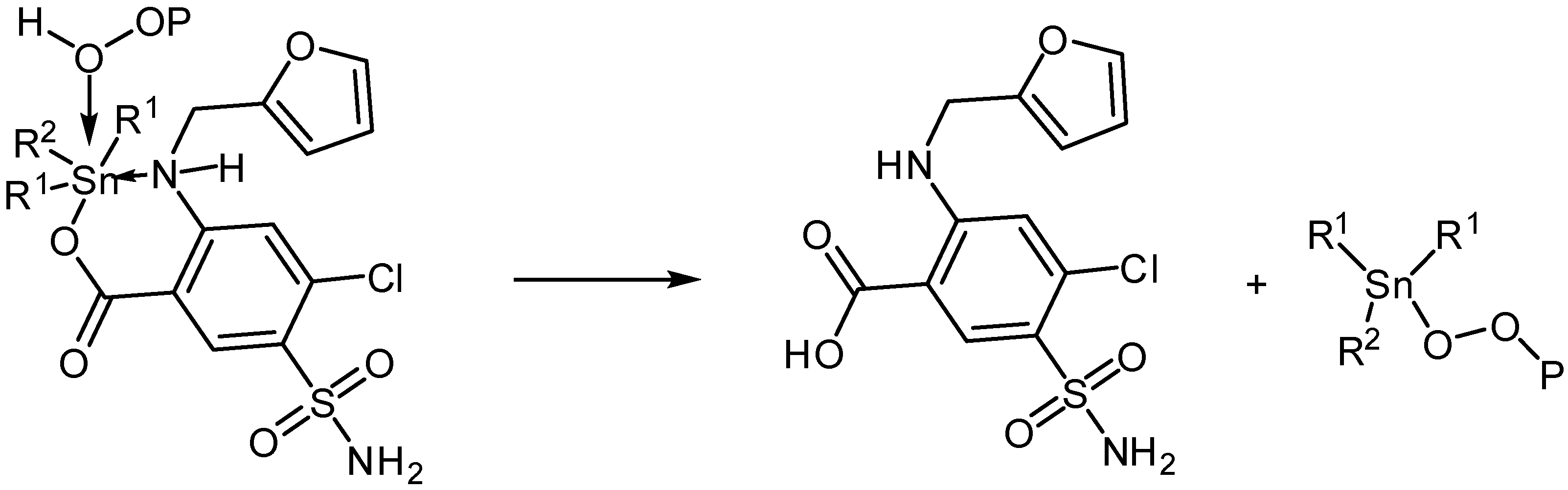
Photooxidation of PVC can take place in the presence of hydroperoxides. Various additives are known for their reactions with hydroperoxides and therefore inhibit photodegradation of polymers [45]. The organotin(IV) complexes are expected to decompose peroxides (Scheme 4) and therefore, stabilize PVC polymeric chains.
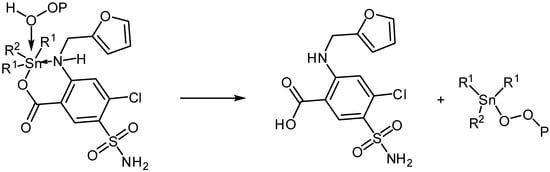
Scheme 4.
Organotin complexes as peroxide decomposers.
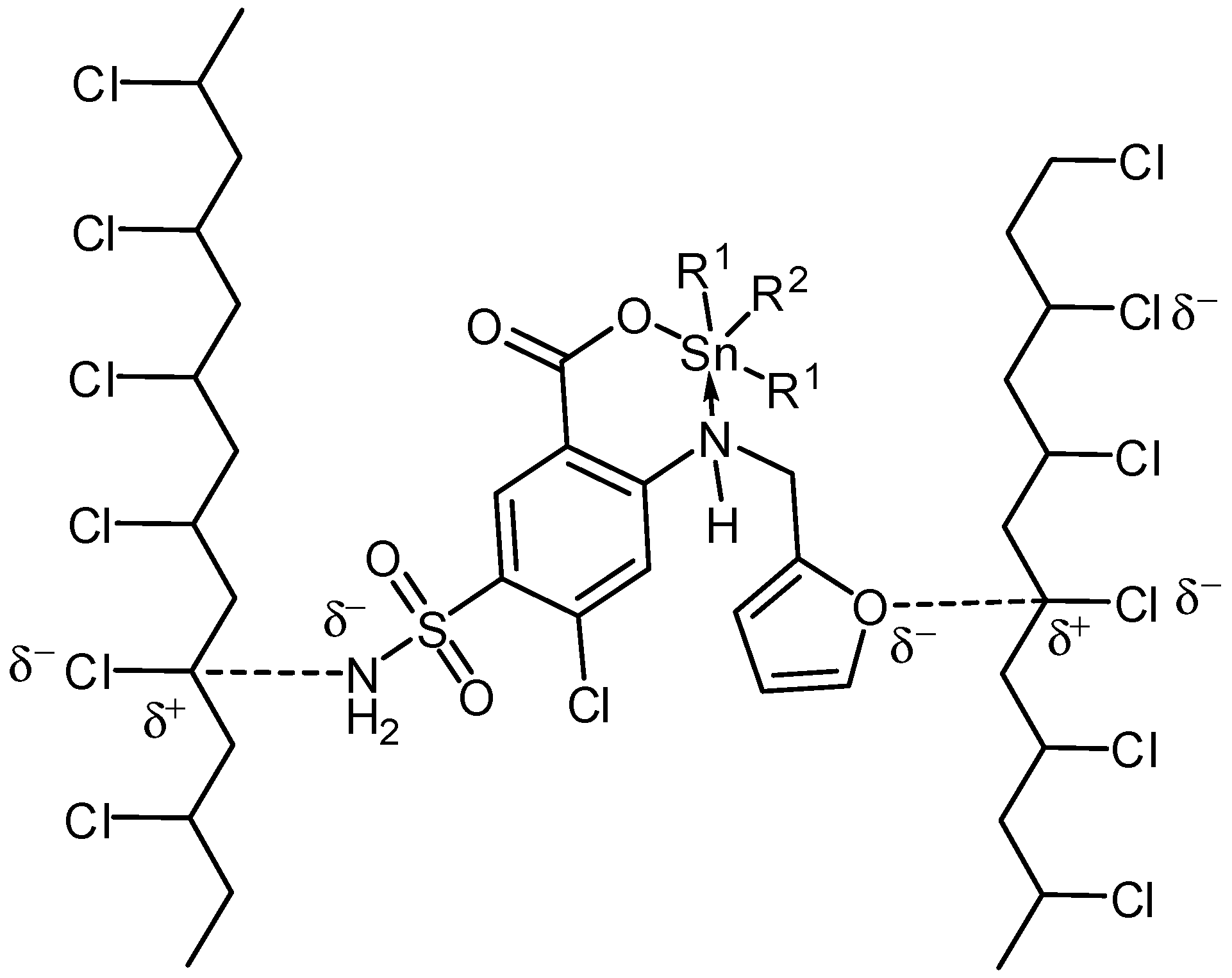
Attraction between polarized C–O bonds in furan moiety and S–NH2 group within Sn(IV) complexes and polarized C–Cl within PVC chains could stabilize the polymer (Scheme 5). This attraction can aid the conversion of the excited state PVC energy to a level of energy that does not harm the polymeric chain [20].
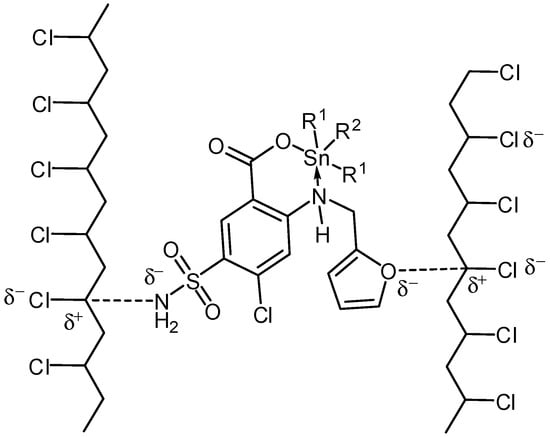
Scheme 5.
Organotin complexes as primary stabilizers.
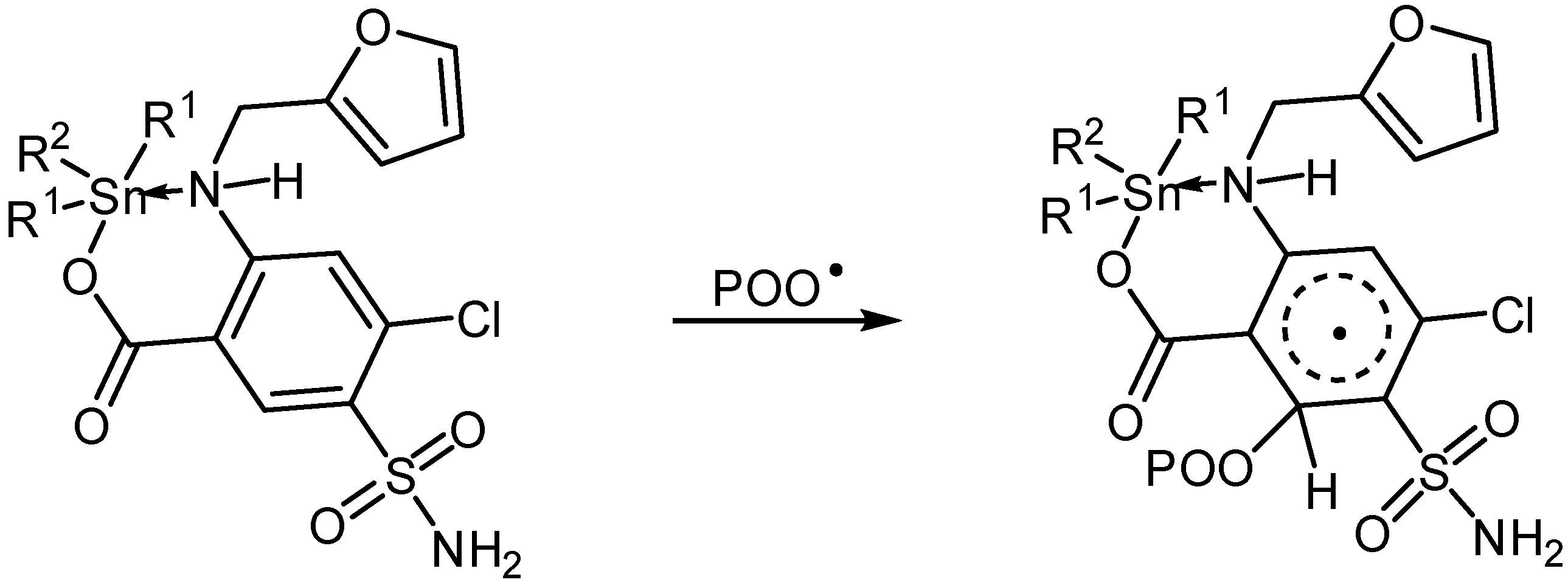
Sn(IV) complexes could also act as radical scavengers (Scheme 6). The additives could form a complex with a chromophore (POO•) in the excited state [46]. This complex could be stabilized via resonance of aryl and furan moieties (Scheme 6).
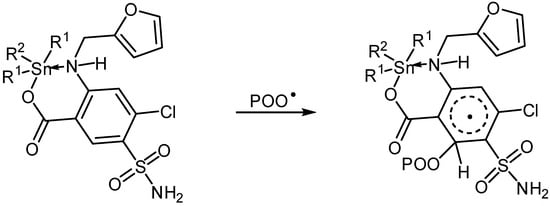
Scheme 6.
Organotin complexes as radical scavengers.
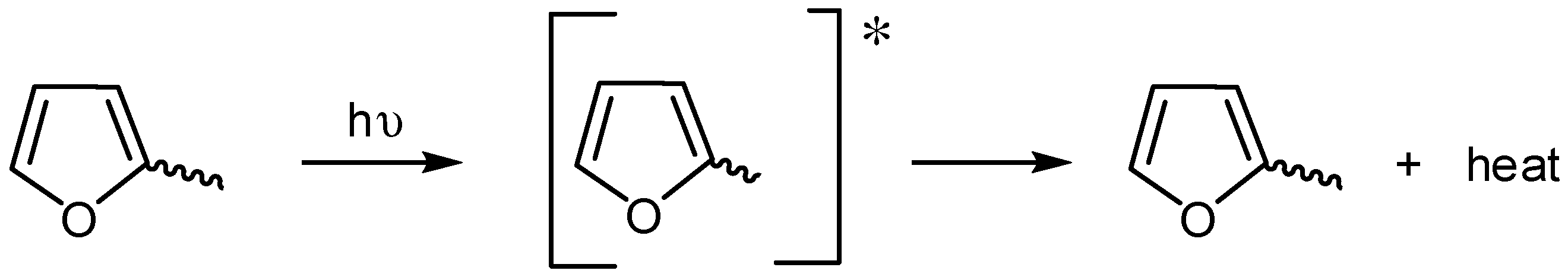
Electron rich aromatic moieties within the PVC backbone can absorb UV radiation [20]. These additives can convert the absorbed UV radiation energy into heat energy that is not harmful to PVC. Therefore, it is believed that both furan and aryl ring within Sn(VI) complexes act as UV absorbers (Scheme 7).
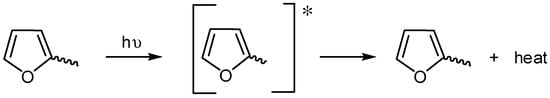
Scheme 7.
Furan moiety acts as a UV absorber.
3. Experimental
3.1. Materials
Furosemide, reagents and solvents were purchased from Sigma-Aldrich Chemical Company (Gillingham, UK) and used as received. PVC (K value = 67, degree of polymerization = 800) was supplied from Petkim Petrokimya (Istanbul, Turkey).
3.2. Synthesis of Triphenyltin(IV) Complex
To a stirred solution of furosemide (0.33 g, 1.0 mmol) in methanol (15 mL) a solution of chlorotriphenylstannane (0.39 g, 1.0 mmol) in methanol (10 mL) was added in a dropwise manner. The mixture was stirred at room temperature for 10 min and then heated under reflux for 6 h. The mixture was filtered and the solvent was removed under reduced pressure. The solid obtained was purified by crystallization using methanol to give white powder of triphenyltin(IV) complex.
3.3. Synthesis of Diorganotin(IV) Complexes
To a stirred solution of furosemide (0.66 g, 2.0 mmol) in methanol (20 mL) a solution of dichlorodimethylstannane or dibutyldichlorostannane (1.0 mmol) in methanol (10 mL) was added in a dropwise manner. The mixture was heated under reflux for 8 h. The mixture was filtered and the solvent was removed under reduced pressure. The solid obtained was purified by crystallization using methanol to give the organotin(IV) complexes as off white powders.
3.4. Films Preparation
A solution of PVC in tetrahydrofuran (5 g/100 mL) was re-precipitated using ethanol. The PVC was dried under reduced pressure at room temperature for 24 h. PVC films (40 µm thickness) was prepared. The thickness was measured using a Digital Caliper Vernier (Kevelaer, Germany). The PVC films containing organotin complexes (0.5% by weight) were prepared by the evaporation technique at room temperature for 24 h. The residual tetrahydrofuran was removed and the PVC films containing additives were fixed using aluminum plate stands supplied by Q-Panel Company (Homestead, FL, USA) [12].
3.5. Accelerated Testing Technique
Accelerated weather-meter QUV tester (Philips, Saarbrücken, Germany) was used to irradiate PVC films. The weather-meter is equipped with two UV-B 313 lamps (290–360 nm) with λmax of 313 nm [13].
3.6. Measuring the PVC Photodegradation Rate by FTIR Spectrophotometry
The progress of PVC photodegradation due to irradiation was investigated using a FTIR 8300 Spectrophotometer (Shimadzu, Tokyo, Japan, 4000–400 cm−1). The changes in carbonyl (1722 cm−1), polyene (1602 cm−1) and hydroxyl (3500 cm−1) absorption peaks intensity were recorded [47]. The carbonyl (ICO), polyene (IPO) and hydroxyl (IOH) indices were calculated by comparison of the FTIR absorption peaks with the reference peak that resonates at 1328 cm−1. The functional group index (Is) is the functional group peak absorbance (As) divided by the reference peak absorbance (Ar). Is can be calculated using the baseline method [47] as shown in Equation (3):
3.7. Measuring the Photodegradation by Weight Loss
The PVC film weight loss percentage was calculated using Equation (4). It can be calculated from PVC weight before irradiation (W1) and that after irradiation (W2) [48].
3.8. Measuring the Photodegradation by Morphology Study
The morphology of PVC surface was inspected before and after irradiation using atomic force microscopy (AFM; Veeco, (Plainview, NY, USA)) and a microscope (Meiji Techno, Tokyo, Japan).
3.9. Determination of the Average Molecular Weight () by the Viscometry Method
The intrinsic viscosity, [], was calculated using Equation (5).
The quantum yield of main chain scission (Φcs) [49] is dependent on intrinsic viscosity of PVC before irradiation (), irradiation time (t), incident intensity (Io), , concentration (C) and Avogadro’s number (A). The Φcs was calculated using Equation (6):
4. Conclusions
Three organotin complexes (Ph3SnL, Me2SnL2 and Bu2SnL2) were synthesized and characterized. The complexes have been used as additives to stabilize PVC films upon irradiation. The PVC photodegradation rate has been reduced in the presence of organotin complexes (0.5% by weight) upon UV irradiation for 300 h. In addition, the AFM images show a smooth surface for the PVC films containing Ph3SnL complex. The most photostable PVC film was the one that contains Ph3SnL followed by Bu2SnL2 and Me2SnL2. A number of mechanisms was proposed to explain the PVC photostabilization in the presence of Sn(IV) complexes. The additives could act as HCl scavengers, UV absorbers, peroxide decomposers and/or radical scavengers.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for its funding for this research through the research group project RGP-239 and to the Department of Chemistry, College of Science, Al-Nahrain University for continued support.
Author Contributions
Emad Yousif conceived and designed the experiments. Mustafa M. Ali performed the experiments and analyzed the data. Gamal A. El-Hiti, Emad Yousif and Mustafa M. Ali wrote the paper. Gamal A. El-Hiti provided the funds and revised the paper. All the authors discussed the results and improved the final text of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, Z.; Ding, A.; Guo, H.; Lu, G.; Huang, X. Construction of nontoxic polymeric UV-absorber with great resistance to UV-photoaging. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Emura, T. Technical progresses for PVC production. Prog. Polym. Sci. 2002, 27, 2055–2131. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, T.; Pi, H.; Guo, S. Mechanochemical preparation of a novel polymeric photostabilizer for poly(vinyl chloride). J. Appl. Polym. Sci. 2010, 116, 3079–3086. [Google Scholar] [CrossRef]
- Real, L.E.P.; Ferraria, A.M.; Botelho de Rego, A.M. Comparison of different photo-oxidation conditions of poly(vinyl chloride) for outdoor applications. Polym. Test. 2008, 27, 743–751. [Google Scholar] [CrossRef]
- Nicholson, J.W. The Chemistry of Polymers, 3rd ed.; RSC Pub.: Cambridge, UK, 2012. [Google Scholar]
- Starnes, W.H. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Braun, D. Thermal Degradation of Poly (vinyl chloride). In Developments in Polymer Degradation; Grassie, N., Ed.; Applied Science Publishers: London, UK, 1981. [Google Scholar]
- Fahmy, M.M.; Mohamed, R.R.; Mohamed, N.A. Novel antimicrobial organic thermal stabilizer and co-stabilizer for rigid PVC. Molecules 2012, 17, 7927–7940. [Google Scholar] [CrossRef] [PubMed]
- Iván, B.; Kennedy, J.P.; Kélen, T.; Tüdòs, F.; Nagy, T.T.; Turcsanyi, B. Degradation of PVCs Obtained by Controlled Chemical Dehydrochlorination. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 2177–2188. [Google Scholar] [CrossRef]
- Caraculacu, A.; Bezdadea, E.C.; Istrate, G. Structure of Branching in PVC. J. Polym. Sci. 1970, 8, 1239–1246. [Google Scholar] [CrossRef]
- Titow, W.V. PVC Technology, 4th ed.; Springer: Essex, UK, 1984. [Google Scholar]
- Yousif, E.; Hameed, A.; Rasheed, R.; Mansoor, H.; Farina, Y.; Graisa, A.; Salih, N.; Salimon, J. Synthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ring. Int. J. Chem. 2010, 2, 65–80. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Tomohito, K.; Masahiko, O.; Guido, G.; Tadaaki, M.; Toshiaki, Y. Antibacterial effect of thiocyanate substituted poly (vinyl chloride). J. Polym. Res. 2011, 18, 945–947. [Google Scholar]
- Sabaa, M.W.; Mikhael, M.G.; Mohamed, N.A.; Yassin, A.A. N-substituted maleimides as thermal stabilizers for rigid poly (vinyl chloride). Angew. Makromol. Chem. 1989, 168, 23–25. [Google Scholar] [CrossRef]
- Yousif, E.A.; Aliwi, S.M.; Ameer, A.A.; Ukal, J.R. Improved photostability of PVC films in the presence of 2-thioacetic acid-5-phenyl-1,3,4-oxadiazole complexes. Turk. J. Chem. 2009, 33, 399–410. [Google Scholar]
- Yousif, E.; Salih, N.; Salimon, J. Improvement of the photostabilization of PVC films in the presence of 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole. J. Appl. Polym. Sci. 2011, 120, 2207–2214. [Google Scholar] [CrossRef]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar]
- Deanin, R.D.; Reynolds, H.H.; Ozcayir, Y. Thermal stabilization of polyvinyl chloride by group II metal laurates. J. Appl. Polym. Sci. 1969, 13, 1247–1252. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Zhang, L.; Xu, S.; Zhou, Q.; Zhu, Y.; Qu, X. Main factors in preparation of antibacterial particles /PVC composite. China Particuol. 2004, 2, 226–229. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Birmingham, J.N. The effect of surface oxidation and titanium dioxide on exterior PVC color retention. J. Vinyl Addit. Technol. 1995, 1, 84–87. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N. New photostabilizers for PVC based on some diorganotin(IV) complexes. J. Saudi Chem. Soc. 2015, 19, 133–141. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl)propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Poly(propylene sulfide)-borane: Convenient and versatile reagent for organic synthesis. Tetrahedron 2012, 68, 7834–7839. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; Pardasani, R.T.; El-Hiti, G.A. New polymeric sulfide-borane complexes: Convenient hydroborating and reducing reagents. J. Sulfur Chem. 2011, 32, 287–295. [Google Scholar] [CrossRef]
- Singh, H.L.; Singh, J. Synthesis, spectroscopic, molecular structure, and antibacterial studies of dibutyltin(IV) Schiff base complexes derived from phenylalanine, isoleucine, and glycine. Bioinorg. Chem. Appl. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Mohamad, T.; Saad, E.E.; Farina, Y.; Graisa, A.; Yousif, E. Synthesis and characterization and fungicidal activity of triorganotin (IV) with benzamidomethionine. Eur. J. Sci. Res. 2009, 34, 212–217. [Google Scholar]
- Nath, M.; Singh, H.; Kumar, P.; Song, X.; Eng, G. Organotin(IV) tryptophanylglycinates: Potential non-steroidal antiinflammatory agents; crystal structure of dibutyltin(IV) tryptophanylglycinate. Appl. Organomet. Chem. 2009, 23, 347–358. [Google Scholar] [CrossRef]
- Masood, H.; Ali, S.; Mazhar, M. 1H, 13C, 119Sn NMR, mass, Mössbauer and biological studies of tri-, di- and chlorodiorganotin(IV) carboxylates. Turk. J. Chem. 2004, 28, 75–85. [Google Scholar]
- Coşkun, A. The Synthesis of 4-phenoxyphenylglyoxime and 4,4-oxybis(phenylglyoxime) and their complexes with Cu(II), Ni(II) and Co(II). Turk. J. Chem. 2006, 30, 461–469. [Google Scholar]
- Shahid, K.; Ali, S.; Bhatti, M.H.; Mazhar, M.; Mahmood, S.; Rehman, S. Synthesis, characterization and thermal analysis of organotin(IV) derivatives of 4-(N-maleoyl) butanoate. Turk. J. Chem. 2002, 26, 589–597. [Google Scholar]
- Andrady, A.L.; Searle, N.D. Photodegradation of rigid PVC formulations. II. Spectral sensitivity to light-induced yellowing by polychromatic light. J. Appl. Polym. Sci. 1989, 37, 2789–2802. [Google Scholar] [CrossRef]
- Braun, D.; Rabie, S.T.; Khaireldin, N.Y.; Abd El-Ghaffar, M.A. Preparation and evaluation of some benzophenone terpolymers as photostabilizers for rigid PVC. J. Vinyl Addit. Technol. 2011, 17, 147–155. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells—An overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Júnior, G.C.; Silva, A.P.S.; Guinesi, L.S. Synthesis, characterization and electropolymerization of a new polypyrrole Iron(II) Schiff-base complex. Polyhedron 2004, 23, 1953–1960. [Google Scholar] [CrossRef]
- Kara, F.; Aksoy, E.A.; Yuksekdagd, Z.; Hasirci, N.; Aksoy, S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydr. Polym. 2014, 112, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, pp. 48–49. [Google Scholar]
- Shyichuk, A.V.; White, J.R. Analysis of chain-scission and crosslinking rates on the photooxidation of polystyrene. J. Appl. Polym. Sci. 2000, 77, 3015–3023. [Google Scholar] [CrossRef]
- Rabek, J.; Ranby, B. Photodegradation, Photooxidation and Photostabilization of Polymers; John Wiley: New York, NY, USA, 1975. [Google Scholar]
- Sabaa, M.W.; Oraby, E.H.; Abdul Naby, A.S.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]
- Torikai, A.; Ohno, M.; Fueki, K. Photodegradation of poly(methyl methacrylate) by monochromatic light: Quantum yield, effect of wavelengths, and light intensity. J. Appl. Polym. Sci. 1990, 41, 1023–1032. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the organotin complexes are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).