New Deferoxamine Glycoconjugates Produced upon Overexpression of Pathway-Specific Regulatory Gene in the Marine Sponge-Derived Streptomyces albus PVA94-07
Abstract
:1. Introduction
2. Results and Discussion
2.1. Expression of the LysR-Type Cluster-Associated Regulatory Gene in Marine Sponge-Derived S. albus
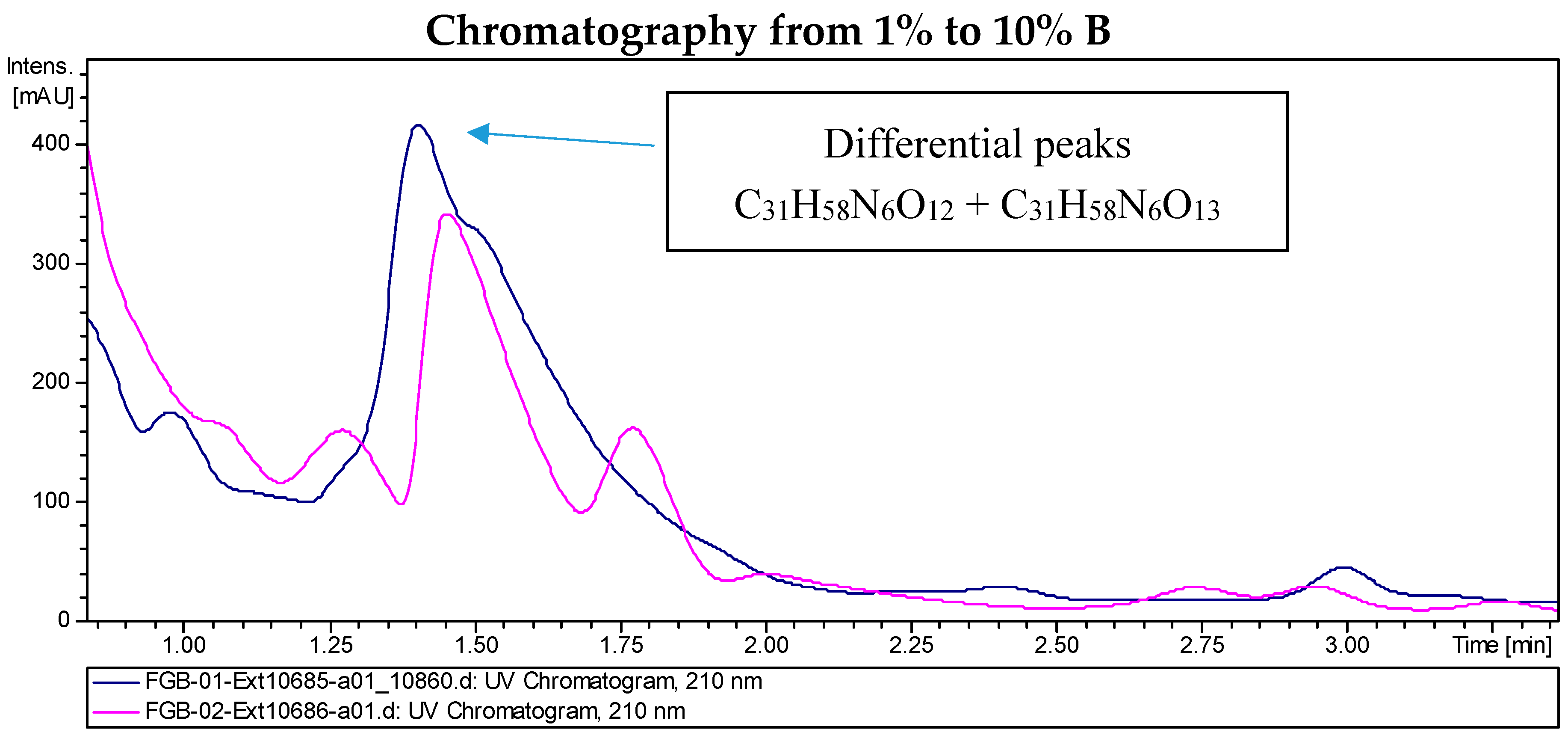
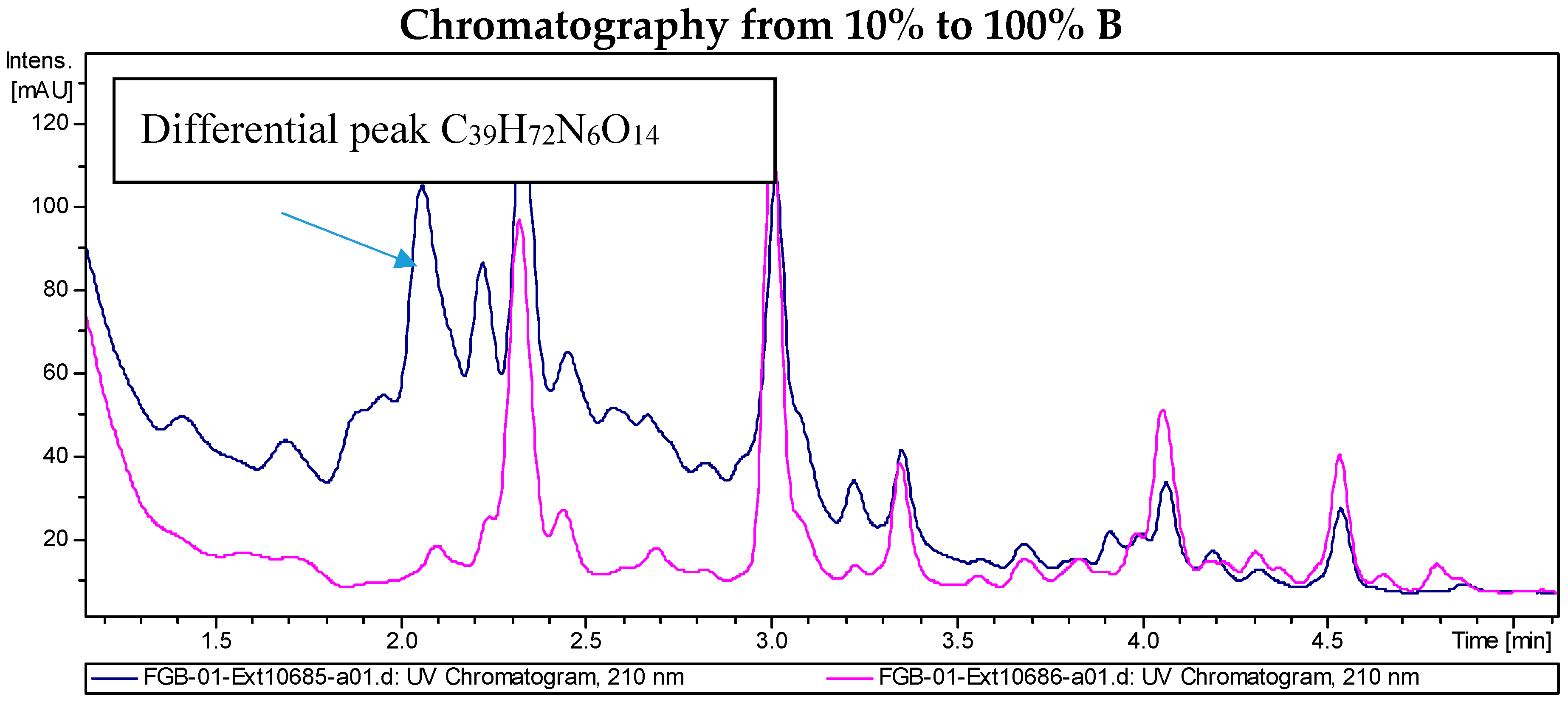
2.2. Recombinant PVA94-07 Overexpressing LysR Regulator Produces Novel Derivatives of Deferoxamine
2.3. Biological Activity of the New Deferoxamine Glycoconjugates
2.4. Investigations into the Biosynthetic Origin of the New Deferoxamine Glycoconjugates
3. Materials and Methods
3.1. DNA Manipulation and Construction of Recombinant Strains
3.2. Cultivation of Streptomyces albus PVA94-07
3.3. Extraction and Qualitative LC-DAD Analysis of Fermentation Broth
3.4. General Analytical Procedures
3.5. Isolation of Deferoxamine Glycoconjugates
3.6. Biological Activity Testing
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Strain improvement in actinomycetes in the postgenomic era. J. Ind. Microbiol. Biotechnol. 2011, 38, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Romero-Rodríguez, A.; Robledo-Casados, I.; Sánchez, S. An overview on transcriptional regulators in Streptomyces. Biochim. Biophys. Acta 2015, 1849, 1017–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, Q.; Bu, Q.; Guo, Y.; Liu, S.; Liu, Y.; Du, Y.; Li, Y. Genome mining-directed activation of a silent angucycline biosynthetic gene cluster in Streptomyces chattanoogensis. Chembiochem 2015, 16, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Aigle, B.; Corre, C. Waking up Streptomyces secondary metabolism by constitutive expression of activators or genetic disruption of repressors. Methods Enzymol. 2012, 517, 343–366. [Google Scholar] [PubMed]
- Huang, J.; Shi, J.; Molle, V.; Sohlberg, B.; Weaver, D.; Bibb, M.J.; Karoonuthaisiri, N.; Lih, C.J.; Kao, C.M.; Buttner, M.J.; Cohen, S.N. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Mol. Microbiol. 2005, 58, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tian, Y.; Yang, H.; Tan, H. A pathway-specific transcriptional regulatory gene for nikkomycin biosynthesis in Streptomyces ansochromogenes that also influences colony development. Mol. Microbiol. 2005, 55, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Ian, E.; Malko, D.B.; Sekurova, O.N.; Bredholt, H.; Rückert, C.; Borisova, M.E.; Albersmeier, A.; Kalinowski, J.; Gelfand, M.S.; Zotchev, S.B. Genomics of sponge-associated Streptomyces spp. closely related to Streptomyces albus J1074: Insights into marine adaptation and secondary metabolite biosynthesis potential. PLoS ONE 2014, 9, e96719. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Oyston, P.C. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 2008, 154, 3609–3623. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Siegl, T.; Luzhetska, M.; Petzke, L.; Jilg, C.; Welle, E.; Erb, A.; Leadlay, P.F.; Bechthold, A.; Luzhetskyy, A. Site-specific recombination strategies for engineering actinomycete genomes. Appl. Environ. Microbiol. 2012, 78, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Angyal, S.K.; Bethell, G.S. Conformational analysis in Carbohydrate chemistry. III. The 13C-NMR spectra of the hexuloses. Aus. J. Chem. 1976, 29, 1249–1265. [Google Scholar] [CrossRef]
- Sayed, H.M.; Mohamed, M.H.; Farag, S.F.; Mohamed, G.A.; Omobuwajo, O.R.; Proksch, P. Fructose-amino acid conjugate and other constituents from Cyperus. rotundus L. Nat. Prod. Res. 2008, 22, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Mossine, V.V.; Glinsky, G.V.; Feather, M.S. The preparation and characterization of some Amadori compounds (1-amino-1-deoxy-d-fructose derivatives) derived from a series of ω-amino acids. Carbohydr. Res. 1994, 262, 257–270. [Google Scholar] [CrossRef]
- Flett, F.; Mersinias, V.; Smith, C.P. High efficiency intergeneric conjugal transfer of plasmid DNA from Escherichia coli to methyl DNA-restricting streptomycetes. FEMS Microbiol. Lett. 1997, 155, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zotchev, S.; Haugan, K.; Sekurova, O.; Sletta, H.; Ellingsen, T.E.; Valla, S. Identification of a gene cluster for antibacterial polyketide-derived antibiotic biosynthesis in the nystatin producer Streptomyces noursei ATCC 11455. Microbiology 2000, 146, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Traxler, M.F.; Watrous, J.D.; Alexandrov, T.; Dorrestein, P.C.; Kolter, R. Interspecies interactions stimulate diversification of the Streptomyces coelicolor secreted metabolome. MBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Ihnat, P.M.; Vennerstrom, J.L.; Robinson, D.H. Synthesis and solution properties of deferoxamine amides. J. Pharmacol. Sci. 2000, 89, 1525–1536. [Google Scholar] [CrossRef]
- Barona-Gómez, F.; Wong, U.; Giannakopulos, A.E.; Derrick, P.J.; Challis, G.L. Identification of a cluster of genes that directs desferrioxamine biosynthesis in Streptomyces coelicolor M145. J. Am. Chem. Soc. 2004, 126, 16282–16283. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Durr, C.; Schnell, H.J.; Luzhetskyy, A.; Murillo, R.; Weber, M.; Welzel, K.; Vente, A.; Bechthold, A. Biosynthesis of the terpene phenalinolactone in Streptomyces sp Tu6071: Analysis of the gene cluster and generation of derivatives. Chem. Biol. 2006, 13, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.; Degnes, K.F.; Kemmler, M.; Bredholt, H.; Fjaervik, E.; Klinkenberg, G.; Sletta, H.; Ellingsen, T.E.; Zotchev, S.B. Production of a New Thiopeptide Antibiotic, TP-1161, by a Marine Nocardiopsis Species. Appl. Environ. Microb. 2010, 76, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Crespo, G.; González-Menéndez, V.; Pérez-Moreno, G.; Sánchez-Carrasco, P.; Pérez-Victoria, I.; Ruiz-Pérez, L.M.; González-Pacanowska, D.; Vicente, F.; Genilloud, O.; et al. MDN-0104, an Antiplasmodial Betaine Lipid from Heterospora chenopodii. J. Nat. Prod. 2014, 77, 2118–2123. [Google Scholar]
- Pérez-Victoria, I.; Martín, J.; Reyes, F. Combined LC/UV/MS and NMR Strategies for the Dereplication of Marine Natural Products. Planta Med. 2016, 82, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Audoin, C.; Bonhomme, D.; Ivanisevic, J.; de la Cruz, M.; Cautain, B.; Monteiro, M.C.; Reyes, F.; Rios, L.; Perez, T.; Thomas, O.P. Balibalosides, an original family of glucosylated sesterterpenes produced by the mediterranean sponge Oscarella balibaloi. Mar. Drugs 2013, 11, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Corine, V.; Coiffard, C.; Coifard, L.J.M.; Rivalland, P.; de Roeck-Holtzhauer, Y. In vitro correlation between two colorimetric assays and the pyruvic acid consumption by fibroblasts cultured to determine the sodium laurylsulfate cytotoxicity. J. Pharmacol. Toxicol. 1998, 39, 143–146. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors, but the producer strains can be provided upon request.
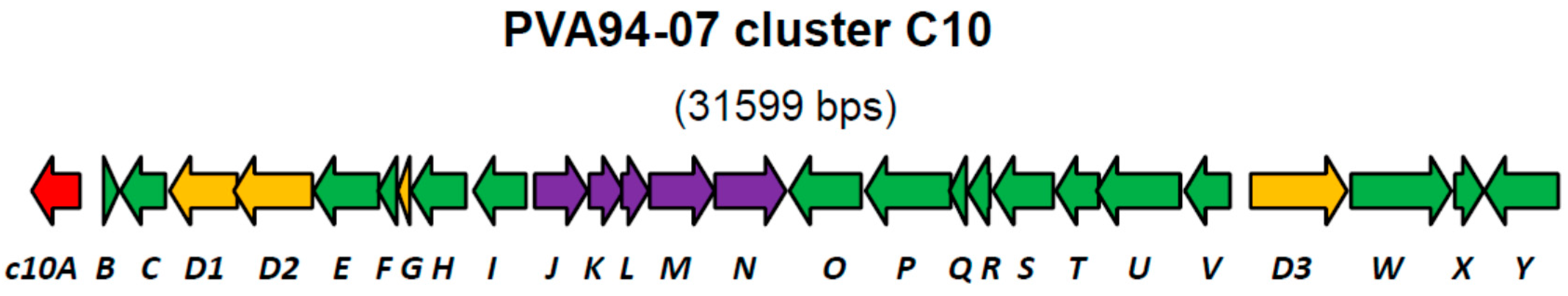

| Gene | Protein, aa | Putative Product | Closest Similarity in the Databases |
|---|---|---|---|
| orf1 | 257 | Allantoicase | Streptomyces sp. MBRL 601, 99% |
| c10A | 303 | LysR family of transcriptional regulators | Streptomyces sp. CNQ431, 99% |
| c10B | 97 | l-lysine tRNA ligase | Streptomyces sp. CNQ431, 98% |
| c10C | 281 | NADH pyrophosphatase | Streptomyces griseus subsp. griseus NBRC 13350, 75% |
| c10D1 | 423 | Non-ribosomal peptide synthetase, C-domain only | Streptomyces sp. CNQ431, 99% |
| c10D2 | 491 | Non-ribosomal peptide synthetase, A-domain (Phe) | Streptomyces sp. CNQ431, 99% |
| c10E | 408 | Major facilitator superfamily protein | Streptomyces sp. CNQ431, 99% |
| c10F | 116 | Keto-acyl-synthetase 2 motif | Streptomyces sp. CNQ431, 99% |
| c10G | 76 | Peptidyl carrier protein | Streptomyces sp. CNQ431, 99% |
| c10H | 346 | Acetyltransferase (GNAT) | Streptomyces sp. CNQ431, 98% |
| c10I | 330 | Monooxygenase, luciferase-like | Streptomyces griseus subsp. griseus NBRC 13350, 85% |
| c10J | 330 | Galactose mutarotase | Streptomyces sp. CNQ431, 99% |
| c10K | 216 | GAT1 peptidase E | Streptomyces sp. CNQ431, 99% |
| c10L | 168 | Acetyltransferase (GNAT) family | Streptomyces griseus subsp. griseus NBRC 13350, 85% |
| c10M | 406 | Radical SAM superfamily protein | Streptomyces sp. CNQ431, 99% |
| c10N | 445 | Glycosyltransferase, MGT family | Streptomyces sp. CNQ431, 99% |
| c10O | 448 | Aldehyde dehydrogenase | Streptomyces sp. CNQ431, 99% |
| c10P | 533 | Acyl CoA-ligase | Streptomyces sp. CNQ431, 99% |
| c10Q | 106 | Acyl carrier protein | Streptomyces griseus subsp. griseus NBRC 13350, 73% |
| c10R | 140 | Cupin 2 superfamily protein | Streptomyces griseus subsp. griseus NBRC 13350, 94% |
| c10S | 378 | Sugar phosphate isomerase | Streptomyces griseus subsp. griseus NBRC 13350, 83% |
| c10T | 271 | TIM-barrel enzyme | Streptomyces sp. CNQ431, 99% |
| c10U | 523 | Aspartate aminotransferase | Streptomyces griseus subsp. griseus NBRC 13350, 88% |
| c10V | 282 | Clavaminic acid synthetase (CAS)-like | Streptomyces griseus subsp. griseus NBRC 13350, 82% |
| c10D3 | 603 | Non-ribosomal peptide synthetase, A- and T-domains | Streptomyces sp. CNQ431, 99% |
| c10W | 627 | Hypothetical protein | Streptomyces sp. CNQ431, 99% |
| c10X | 180 | Mrr superfamily DNA binding protein | Streptomyces sp. CNQ431, 99% |
| c10Y | 460 | Glutamyl-tRNA synthetase | Streptomyces sp. CNQ431, 99% |
| orf2 | 76 | Hypothetical protein | Streptomyces albus J1074, 100% |
| Bacterial Strains | Genotype/Phenotype | Source/Reference |
| Escherichia coli DH5α | General cloning host: (luxS supE44 ΔlacU169, (ϕ80 lacZΔM15) hsdR17, recA1, endA1, gyrA96, thi-1, relA1) | BRL |
| Escherichia coli ET125671 (pUZ8002) | Mediates conjugative DNA transfer from RP4 oriT with helper plasmid pUZ8002 (KanR, CmR); methylation deficient (dam−, dcm−, hsdM−) | [14] |
| Streptomyces albus PVA94-07 | Wild type, isolated from marine sponge Phakellia ventilabrum, Trondheim fjord, Norway | [8] |
| S. albus PVA94-07 C10A1C10A1 | Wild type strain harboring C10A1pC10A1 for c10A expression | This work |
| S. albus PVA94-07 C10ND | Wild type strain harboring pC10gtKO for c10N disruption | This work |
| S. albus PVA94-07 C10Nex | Wild type strain harboring pC10Nex for c10N expression | This work |
| S. albus PVA94-07 C10JNex | Wild type strain harboring pC10Jnex for c10JKLMN gene operon expression | This work |
| S. albus PVA94-07 (C10ND) pC10Nex | S. albus PVA94-07 C10ND strain harboring pC10Nex | This work |
| S. albus PVA94-07 (C10ND) pC10Jnex | S. albus PVA94-07 C10ND strain harboring pC10Jnex | This work |
| Plasmids | Genotype | Source/Reference |
| pUWLoriT | pIJ101 minimal replicon, ThioR, AmpR, RP4 oriT, ColEI replication origin, ermE*p | [10] |
| pSOK201 | pSG5 minimal replicon, AmR, RP4 oriT, ColEI replication origin | [15] |
| pC10A1 | pIJ101 minimal replicon, ThioR, AmpR, RP4 oriT, ColEI replication origin, ermE*p, c10R | This work |
| pC10gtKO | pSG5 minimal replicon, AmR, RP4 oriT, ColEI replication origin, an internal part of the c10N gene from cluster C10 | This work |
| pC10Nex | pIJ101 minimal replicon, ThioR, AmpR, RP4 oriT, ColEI replication origin, ermE*p, c10N | This work |
| pC10JNex | pIJ101 minimal replicon, ThioR, AmpR, RP4 oriT, ColEI replication origin, ermE*p, c10JKLMN gene operon | This work |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekurova, O.N.; Pérez-Victoria, I.; Martín, J.; Degnes, K.F.; Sletta, H.; Reyes, F.; Zotchev, S.B. New Deferoxamine Glycoconjugates Produced upon Overexpression of Pathway-Specific Regulatory Gene in the Marine Sponge-Derived Streptomyces albus PVA94-07. Molecules 2016, 21, 1131. https://doi.org/10.3390/molecules21091131
Sekurova ON, Pérez-Victoria I, Martín J, Degnes KF, Sletta H, Reyes F, Zotchev SB. New Deferoxamine Glycoconjugates Produced upon Overexpression of Pathway-Specific Regulatory Gene in the Marine Sponge-Derived Streptomyces albus PVA94-07. Molecules. 2016; 21(9):1131. https://doi.org/10.3390/molecules21091131
Chicago/Turabian StyleSekurova, Olga N., Ignacio Pérez-Victoria, Jesús Martín, Kristin F. Degnes, Håvard Sletta, Fernando Reyes, and Sergey B. Zotchev. 2016. "New Deferoxamine Glycoconjugates Produced upon Overexpression of Pathway-Specific Regulatory Gene in the Marine Sponge-Derived Streptomyces albus PVA94-07" Molecules 21, no. 9: 1131. https://doi.org/10.3390/molecules21091131
APA StyleSekurova, O. N., Pérez-Victoria, I., Martín, J., Degnes, K. F., Sletta, H., Reyes, F., & Zotchev, S. B. (2016). New Deferoxamine Glycoconjugates Produced upon Overexpression of Pathway-Specific Regulatory Gene in the Marine Sponge-Derived Streptomyces albus PVA94-07. Molecules, 21(9), 1131. https://doi.org/10.3390/molecules21091131








