Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review
Abstract
:1. Introduction
2. Structure and Synthesis
2.1. Classification of PCs
2.2. Production of PCs
2.3. Storage Sites of PCs
3. Role of PCs in Plant Defense Mechanisms
3.1. Plant-Environment Interactions and Functions of PCs
3.2. Antioxidant Properties of PCs in Plants
3.3. Role of PCs in HR
3.4. Plant-Plant Interactions and the Accumulation of PCs
4. PCs and Plant Tumors
4.1. Anti-Tumor Effect of PCs
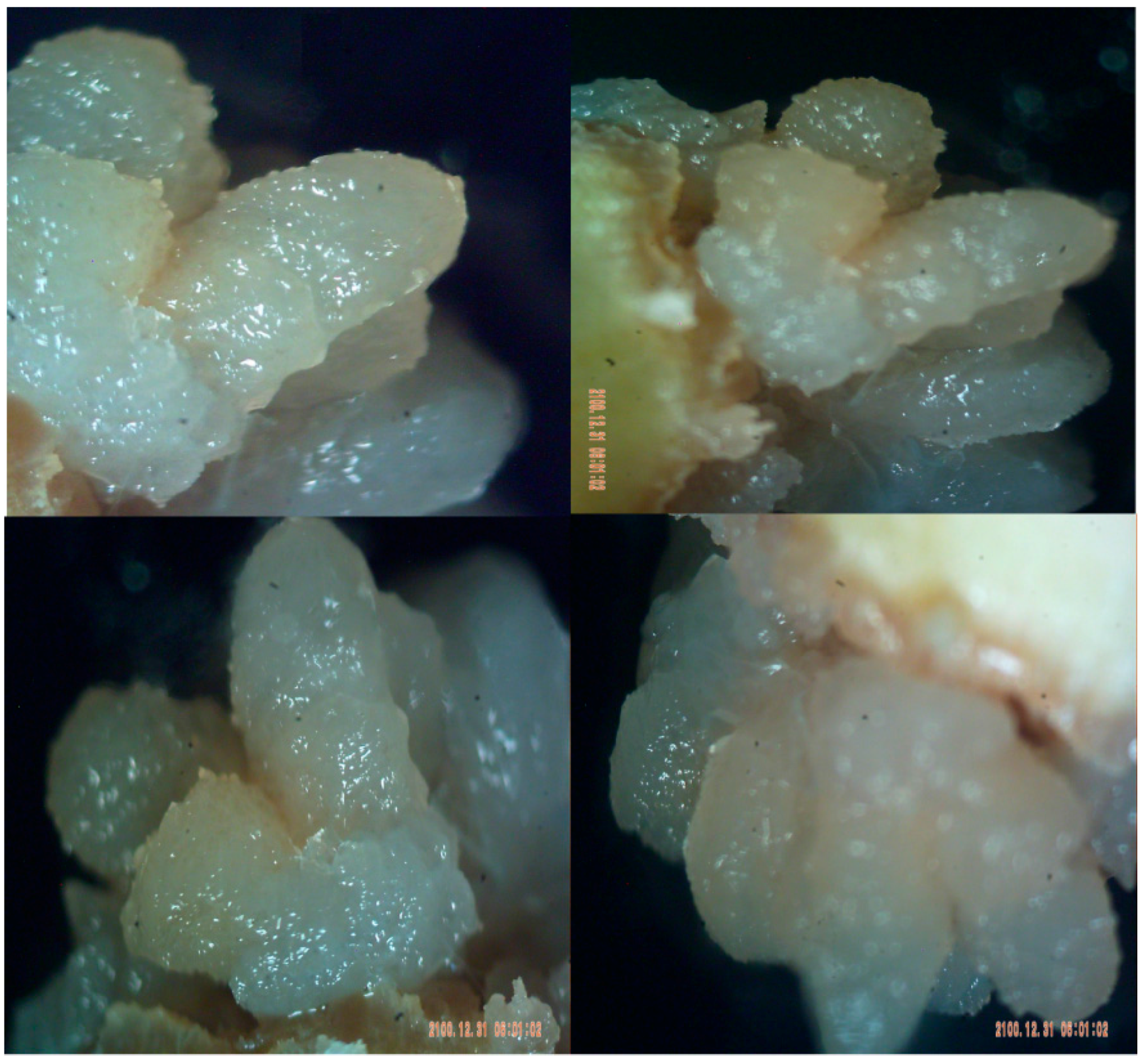
4.2. Tumor in Plant Cells
4.3. Roles of PCs during Plant Growth and Plant Tissue Culture
4.4. PCs as Inhibitor of Seed Germination
4.5. Do PCs Suppress Tumors in Plants?
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PCs | Phenolic compounds |
| EGFR | Epidermal Growth Factor Receptor |
| EGF | Epidermal Growth Factor |
| Her2/neu | Human Epidermal Growth Factor Receptor 2 |
| HR | Hypersensitive response |
| PCD | Programed cell death |
| PAL | Phenylalanine ammonia-lyase |
| PPO | Polyphenol oxidase |
| POH | Polyphenolic antioxidants |
| POX | Peroxidase |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| H2O2 | Hydrogen Peroxide |
| OH | Hydroxyl Radicals |
| SA | Salicylic acid |
| NPA | Nephthylphtalamic acid |
| IAA | Indoleacetic acid |
| ABA | Abscisic acid |
| PVP | Polyvinylpyrrolidone |
| NAA | Naphthaleneacetic acid |
| MAPK | mitogen-activated protein kinase |
| CDKs | Cyclin-dependent kinases |
| 5HTMF | 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone |
| PO• | Phenoxyl radical |
| ABCBs | ATP-binding cassette subfamily B |
References
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Boudet, A. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. The role of phenolic compounds in the fight against cancer—A review. Anticancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Fraga, C.G. (Ed.) Plant Phenolics and Human Health: Biochemistry, Nutrition and Pharmacology, 1st ed.; John Whiley & Sons: Hoboken, NJ, USA, 2009.
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, T.; Akpinar-Bayizit, A.; Ylimaz-Ersan, L.; Delikanli, B. Phenolics in human health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Siah, M.; Farzaei, M.H.; Ashrafi-Kooshk, M.R.; Adibi, H.; Arab, S.S.; Rashidi, M.R.; Khodarahmi, R. Inhibition of guinea pig aldehyde oxidase activity by different flavonoid compounds: An in vitro study. Bioorg. Chem. 2016, 64, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Masanori, H.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Giacoppo, S.; Soundara Rajan, T.; Bramanti, P.; Mazzon, E. Natural phytochemicals in the treatment and prevention of dementia: An overview. Molecules 2016, 21, 518. [Google Scholar] [CrossRef] [PubMed]
- Serna, D.M.O.; Martínez, J.H.I. Phenolics and polyphenolics from melastomataceae species. Molecules 2015, 20, 17818–17847. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as adjunctive with conventional anticancer therapies. Curr. Pharm. Des. 2016, 22, 1–18. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Mahmoudi, M.; Rezaei, N. Dietary anthocyanins as a complementary medicinal approach for management of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 807–820. [Google Scholar] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Kamiloglu, S.; Ozdal, T.; Boyacioglu, D.; Capanoglu, E. Potential use of turkish medicinal plants in the treatment of various diseases. Molecules 2016, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; du Plessis, L.H.; Gerber, M.; Hamman, J.H.; du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed]
- Wahle, W.J.K.; Rotondo, D.; Brown, I.; Heys, D.S. Plant phenolics in the prevention and treatment of cancer. In Bio-Farms for Nutraceuticals; Springer US: New York, NY, USA, 2010; pp. 36–51. [Google Scholar]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical and hypochlorous acid. J. Agric. Food Chem. 2002, 50, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.I.; Said, R.B.; Kontek, B.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Stochmal, A.; Olas, B. LC-ESI-MS/MS profile of phenolic and glucosinolate compounds in samh flour (Mesembryanthemum forsskalei Hochst. ex Boiss) and the inhibition of oxidative stress by these compounds in human plasma. Food Res. Int. 2016, 85, 282–290. [Google Scholar] [CrossRef]
- Thipyapong, P.; Stout, M.J.; Attajarusit, J. Functional analysis of polyphenol oxidases by antisense/sense technology. Molecules 2007, 12, 1569–1595. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Simões, M.; Borges, F. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilm. Molecules 2016, 21, 877. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.E. Primary, secondary metabolites, H2O2, malondialdehyde and photosynthetic responses of Orthosiphon stimaneus benth. to different irradiance levels. Molecules 2012, 17, 1159–1176. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.G. From lignins to tannins: Forty years of enzyme studies on the biosynthesis of phenolic compounds. Phytochemistry 2008, 69, 3018–3031. [Google Scholar] [CrossRef] [PubMed]
- Kefeli, V.I.; Kalevitch, M.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]
- Jørgensen, K.; Rasmussen, A.V.; Morant, M.; Nielsen, A.H.; Bjarnholt, N.; Zagrobelny, M.; Bak, S.; Møller, B.L. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr. Opin. Plant Biol. 2005, 8, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefit. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenbo, G.; Schnitzler, J.P. Tissue localization of phenolic compounds in plants by confocal laser scanningmicroscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Li, Z.; Tang, T.; Liang, S.; Ning, X.; Bai, M.; Wu, H. The synthesis and storage sites of phenolic compounds in the root and rhizome of echinacea purpurea. Am. J. Plant Sci. 2012, 3, 551–558. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during agrobacterium and rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Zapprometv, M. The formation of phenolic compounds in plant cell and tissue cultures and possibility of regulation. Adv. Cell Cult. 1989, 7, 240–245. [Google Scholar]
- Iakimova, E.T.; Lech, M.; Woltering, J.E. Hypersensitive cell death in plants—Its mechanism and role in plant defence against pathogens. J. Fruit Ornam. Plant Res. 2005, 13, 135–158. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.I.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.L. Controlled cell death, plant survival and development. Nat. Rev. Mol. Cell Biol. 2004, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reape, T.J.; McCabe, P.F. Apoptotic-like programmed cell death in plants. New Phytol. 2008, 180, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Danon, A.; Delorme, D.; Mailhac, N.; Gallois, P.G. Plant programmed cell death: A common way to die. Plant Physiol. Biochem. 2000, 38, 647–655. [Google Scholar] [CrossRef]
- Lois, R.; Buchanan, B.B. Severe sensitivity to ultraviolet radiation in an arabidopsis mutant deficient in flavonoid accumulation. Planta 1994, 194, 504–509. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.H.; Dennis, E.S.; Peacock, J.W. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999, 119, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G.; Woltering, J.E. Many ways to exit? Cell death categories in plants. Trends Plant Sci. 2005, 10, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Kato, N.; Lawton, M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature 2001, 411, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Buckner, B.; Janick-Buckner, D.; Gray, J.; Johal, G.S. Cell-death mechanisms in maize. Trends Plant Sci. 1998, 3, 218–223. [Google Scholar] [CrossRef]
- Zhu, H.H.; Yao, Q. Localized and systemic increase of phenols in tomato roots induced by glomus versiforme inhibits ralstonia solanacearum. J. Phytopathol. 2004, 152, 537–542. [Google Scholar] [CrossRef]
- Mandal, S.; Mitra, A. Reinforcement of cell wall in roots of lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol. Mol. Plant Pathol. 2007, 71, 201–209. [Google Scholar] [CrossRef]
- Panina, Y.; Fravel, D.R.; Baker, C.J.; Shcherbakova, L.A. Biocontrol and plant pathogenic fusarium oxysporum-induced changes in phenolic compounds in tomato leaves and roots. J. Phytopathol. 2007, 155, 475–481. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Monteiro, S.; Freitas, R.; Santos, C.N.; Chen, Z.; Batista, L.M.; Duarte, J.; Borges, A.; Teixeira, A.R. The role of plant defence proteins in fungal pathogenesis. Mol. Plant Pathol. 2007, 8, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 2008, 20, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Managing phenol contents in crop plants by phytochemical farming and breeding—Visions and constraints. Int. J. Mol. Sci. 2010, 11, 807–857. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Zhu, L.; Huang, Y.; Lu, J. Influence of growing season on phenolic compounds and antioxidant properties of grape berries from vines grown in subtropical climate. J. Agric. Food Chem. 2011, 59, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Bittel, P.; Robatzek, S. Microbe-associated molecular patterns (MAMPs) probe plant immunity. Curr. Opin. Plant Biol. 2007, 10, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Del Rı́o, J.A.; Báidez, A.G.; Botı́a, J.M.; Ortuño, A. Enhancement of phenolic compounds in olive plants (Olea europaea L.) and their influence on resistance against phytophthora sp. Food Chem. 2003, 83, 75–78. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–67. [Google Scholar]
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Fiorenza, C. Phenolic compounds and saponins in plants grown under different irrigation regimes. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Faria, N.C.G.; Kim, J.H.; Gonçalves, L.A.P.; de L. Martins, M.; Chan, K.L.; Campbell, B.C. Enhanced activity of antifungal drugs using natural phenolics against yeast strains of candida and cryptococcus. Lett. Appl. Microbiol. 2011, 52, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, B.; Mahoney, N.; Chan, K.; Molyneux, R.; May, G. Chemosensitization prevents tolerance of Aspergillus fumigatus to antimycotic drugs. Biochem. Biophys. Res. Commun. 2008, 372, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.F.; Johnston, A.; Balcerzak, M.; Rocheleau, H.; Harris, L.J.; Long, X.Y.; Wei, Y.M.; Zheng, Y.L.; Ouellet, T. Effect of salicylic acid on Fusarium graminearum, the major causal agent of fusarium head blight in wheat. Fungal Biol. 2012, 116, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha Neto, A.C.; Maraschin, M.; di Piero, R.M. Antifungal activity of salicylic acid against Penicillium expansum and its possiblemechanisms of action. Int. J. Food Microbiol. 2015, 215, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Báidez, A.G.; del Río, J.A.; Gómez, P.; Ortuño, A. Antifungal capacity of major phenolic compounds of Olea europaea L. against Phytophthora megasperma drechsler and Cylindrocarpon destructans (Zinssm.) scholten. Physiol. Mol. Plant Pathol. 2006, 69, 224–229. [Google Scholar] [CrossRef]
- Lattanzio, V.; Venere, D.D.; Linsalata, V.; Lima, G.; Ippolito, A.; Salerno, M. Antifungal activity of 2,5-dimethoxybenzoic acid on postharvest pathogens of strawberry fruits. Postharvest Biol. Technol. 1996, 9, 325–334. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dong, H.H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.Y.; Jin, Y.S. The synthesis and synergistic antifungal effects of chalcones against drug resistant candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C 2006, 61, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Notices 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Syafni, N.; Putra, D.P.; Arbain, A. 3,4-Dihydroxybenzoic acid and 3,4-dihydroxybenzaldehyde from the fern Trichomanes hinense L.; Isolation, antimicrobial and antioxidant properties. Indones. J. Chem. 2012, 12, 273–278. [Google Scholar]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Hématy, K.; Cherk, C.; Somerville, S. Host-pathogen warfare at the plant cell wall. Curr. Opin. Plant Biol. 2009, 12, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.L. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Fritig, B.; Kauffmann, S.; Dumas, B.; Geoffroy, P.; Kopp, M.; Legrand, M. Mechanism of the hypersensitivity reaction of plants. Novartis Found. Symp. 1987, 133, 92–108. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Tamang, B.G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant. Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, N. Role of flavonoids in oxidative stress. Curr. Trends Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Phys. India 2004, 52, 794–804. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Donahue, J.L.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant 1997, 100, 224–233. [Google Scholar] [CrossRef]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997, 113, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Dicko, M.H.; Gruppen, H.; Barro, C.; Traore, A.S.; van Berkel, W.J.H.; Voragen, A.G.J. Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 2005, 31, 2671. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Chávez, R.A.; Martínez-Peniche, R.Á.; Hernández-Iturriaga, M.; Teixidó-Espasa, N.; UsallRodié, J.; Viñas-Almenar, I.; Torres-Sanchis, R. Mechanisms of resistance in postharvest fruit-pathogen interaction. Rev. Chapingo Ser. Hortic. 2015, 21, 185–198. [Google Scholar] [CrossRef]
- Kavitha, R.; Umesha, S. Regulation of defense-related enzymes associated with bacterial spot resistance in tomato. Phytoparasitica 2008, 36, 144–159. [Google Scholar] [CrossRef]
- Poiatti, V.A.D.; Dalmas, F.R.; Astarita, L.V. Defense mechanisms of Solanum tuberosum L. in response to attack by plant-pathogenic bacteria. Biol. Res. 2009, 42, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.I.; Cho, H.J.; Kim, S.R.; Park, O.K. The Rab GTPase RabG3b positively regulates autophagy and immunity-associated hypersensitive cell death in arabidopsis. Plant Physiol. 2013, 161, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Houot, V.; Etienne, P.; Petitot, A.S.; Barbier, S.; Blein, J.P.; Lydie, S. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J. Exp. Bot. 2001, 52, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Hofius, D.; Munch, D.; Bressendorff, S.; Mundy, J.; Petersen, M. Role of autophagy in disease resistance and hypersensitive response-associated cell death. Cell Death Differ. 2011, 18, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Hatsugai, N.; Kuroyanagi, M.; Nishimura, M.; Nishimura, H. A cellular suicide strategy of plants: Vacuole-mediated cell death. Apoptosis 2006, 11, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, A.; Fragasso, M.; Platani, C.; Narducci, A.; Miullo, V.; Papa, R. Dynamics of release of allelochemical compounds from roots of wild oat (Avena fatua L.). Agrochimica 2012, 56, 1–8. [Google Scholar]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.A. The representation of allelopathy in ecosystem-level forest models. Ecol. Model. 2007, 209, 65–77. [Google Scholar] [CrossRef]
- Fabbro, C.D.; Prati, D. The relative importance of immediate allelopathy and allelopathic legacy in invasive plant species. Basic Appl. Ecol. 2015, 16, 28–35. [Google Scholar] [CrossRef]
- Iftikhar Hussain, M.; Gonzalez-Rodriguez, L.; Reigosa Roger, M. Germination and growth response of four plant species to different allelochemicals and herbicides. Allelopath. J. 2009, 22, 101–110. [Google Scholar]
- Reigosa, M.J.; Sánchez-Moreiras, A.M. Role of phenolics in allelopathy in the soil. In Soil Phenols; Muscolo, A., Sidari, M., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 87–115. [Google Scholar]
- Weir, L.T.; Park, W.S.; Vivanco, M.J. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Strandberg, M.; Strandberg, B. Ecological Effects of Allelopathic Plants—A Review; National Environmental Research Institute: Silkeborg, Denmark, 2000. [Google Scholar]
- Zeinali, A.; Esmaeili, M.; Heidarzade, A. Salicylic acid and abiotic stress influence allelochemicals and inhibitory potential of root exudates of two rice(Oryza sativa) cultivars against barnyardgrass (Echinochloa crus-galli L.). Int. J. Farm. Allied Sci. 2013, 2, 779–784. [Google Scholar]
- Esmaeili, M.; Heidarzadeh, A.; Pirdashti, H.; Esmaeili, F. Inhibitory activity of pure allelochemicals on barnyardgrass (Echinochloa crus-galli L.) seed and seedling parameters. Int. J. Farm. Allied Sci. 2012, 4, 274–279. [Google Scholar]
- Nardi, S.; Pizzeghello, D.; Bragazza, L.; Gerdol, R. Low-molecular-weight organic acids and hormone-like activity of dissolved organic matter in two forest soils in N italy. J. Chem. Ecol. 2003, 29, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Mallik, A.U. Effect of phenolic compounds on selected soil properties. For. Ecol. Manag. 1997, 92, 11–18. [Google Scholar] [CrossRef]
- Ting, Y.; Chiou, Y.; Pan, M.H.; Ho, C.T.; Huang, Q. In vitro and in vivo anti-cancer activity of tangeretin against colorectal cancer was enhanced by emulsion-based delivery system. J. Funct. Foods 2015, 15, 264–273. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Giuliano, A.E.; van Herle, A.J. Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. Thyroid 1999, 9, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Rakariyatham, K.; Zheng, J.; Guo, S.; Tang, Z.; Zhou, S.; Xiao, H. Anti-inflammatory effects of 4′-demethylnobiletin, a major metabolite of nobiletin. J. Funct. Foods 2015, 19, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Bhatia, J.; Suchal, K.; Gamad, N.; Dinda, A.K. Nobiletin ameliorates cisplatin-induced acute kidney injury due to its anti-oxidant, anti-inflammatory and anti-apoptotic effects. Exp. Toxicol. Pathol. 2015, 67, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Takara, K.; Toyozato, T.; Wada, K. A novel bioactive chalcone of morus australis inhibits tyrosinase activity and melanin biosynthesis in b16 melanoma cells. J. Oleo Sci. 2012, 61, 582–592. [Google Scholar] [CrossRef]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahkar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef]
- Nath, R.; Roy, S.; De, B.; Choudhury, M.D. Anticancer and antioxidant activity of croton: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 63–70. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Adjakly, M.; Ngollo, M.; Boiteux, J.P.; Bignon, Y.J.; Guy, L.; Bernard-Gallon, D. Genistein and daidzein: Different molecular effects on prostate cancer. Anticancer Res. 2013, 33, 39–44. [Google Scholar] [PubMed]
- Wang, L.; Wang, J.; Fang, L.; Zheng, Z.; Zhi, D.; Wang, S.; Li, S.; Ho, C.T.; Zhao, H. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. BioMed Res. Int. 2014, 2014, 453972. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Lang, Q.; Zhou, M.; Zhang, H.; Zhang, Y.; Wan, B.; Huang, Q.; Yu, L. The dietary flavonoid luteolin inhibits aurora b kinase activity and blocks proliferation of cancer cells. Eur. J. Pharm. Sci. 2012, 45, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Zhang, L.; Chen, L.; Du, Y.; Ye, T.; Shi, X. Naringenin exerts anti-angiogenic effects in human endothelial cells: Involvement of ERRα/VEGF/KDR signaling pathway. Fitoterapia 2016, 11, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kim, T.W.; Shin, S.Y.; Park, M.J.; Yong, Y.; Kim, D.W.; Islam, T.; Lee, Y.H.; Jung, K.Y.; Lim, Y. Design, synthesis and inhibitory activities of naringenin derivatives on human colon cancer cells. Bioorg. Med. Chem. Lett. 2013, 23, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Marković, S.; Tošović, J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chem. 2016, 210, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P.M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Yajko, D.M.; Hegeman, G.D. Tumor induction by Agrobacterium tumefaciens: Specific transfer of bacterial deoxyribonucleic acid to plant tissue. J. Bacteriol. 1971, 108, 973–979. [Google Scholar] [PubMed]
- Lee, C.W.; Efetova, M.; Engelmann, J.C.; Kramell, R.; Wasternack, C.; Ludwig-Muller, J.; Hedrich, R.; Deeken, R. Agrobacterium tumefaciens promotes tumor induction by modulating pathogen defense in arabidopsis thaliana. Plant Cell 2009, 21, 2948–2962. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, T.V.; Lutova, L.A.; Nester, Y. Tumor formation in plants. Russ. J. Genet. 2001, 37, 993–1001. [Google Scholar] [CrossRef]
- Bruce, A.S.; Saville, J.B.; N, E. Ustilago maydis produces cytokinins and abscisic acid for potential regulation of tumor formation in maize. J. Plant Growth Regul. 2011, 30, 51–63. [Google Scholar] [CrossRef]
- Macoy, M.D.; Kim, W.Y.; Lee, Y.S.; Kim, G.M. Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant Biotechnol. Rep. 2015, 9, 269–278. [Google Scholar] [CrossRef]
- Kefeli, V.I.; Kadyrov, C.S. Natural growth inhibitors, their chemical and physiological properties. Annu. Rev. Psychol. 1971, 22, 185–196. [Google Scholar] [CrossRef]
- Demos, E.K.; Woolwine, M.; Wilson, R.H.; McMillan, C. The effects of ten phenolic compounds on hypocotyl growth and mitochondrial metabolism of mung bean. Am. J. Bot. 1975, 62, 97–102. [Google Scholar] [CrossRef]
- Da Silva, T.J.A.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. In Vitro Cell. Dev. Biol. Plant 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Sarker, D.; Naik, P.S. Phloroglucinol enhances growth and rate of axillary shoot proliferation in potato shoot tip cultures in vitro. Plant Cell Tissue Organ Cult. 2000, 60, 139–149. [Google Scholar] [CrossRef]
- George, E.F. Plant Propagation by Tissue Culture: In Practice Pt.2; Exegetics Ltd.: Edington, UK, 1996. [Google Scholar]
- Ndakidemi, C.F.; Mneney, E.; Ndakidemi, P.A. Effects of ascorbic acid in controlling lethal browning in in vitro culture of brahylaena huillensis using nodal segments. Am. J. Plant Sci. 2014, 5, 187–191. [Google Scholar] [CrossRef]
- Lichtenthale, H.K.; Schweiger, J. Cell wal bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J. Plant Phys. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Ozeker, E. Phenolic compounds and their importance. Anadolu. J. AARI 1999, 9, 114–124. [Google Scholar]
- Arora, A.; Byrem, T.M.; Nari, M.G.; Strasburg, G.M. Modulation of liposomal membranes fluidity by fla vonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Lavola, A.; Julkunen-Tiitto, R.; de la Rosa, T.M.; Aphalo, P.J. Allocation of carbon to growth and secondary metabolites in birch seedlings under UV-B radiation and CO2 exposure. Physiol. Plant 2000, 109, 260–267. [Google Scholar] [CrossRef]
- Ozyigit, I.I. Phenolic changes during in vitro organogenesis of cotton (Gossypium hirsutum L.) shoot tips. Afr. J. Biotechnol. 2008, 7, 1145–1150. [Google Scholar]
- Ibañez, M.; Amo-Marco, J.B. Promotion by phloroglucinol of micropropagation of minuartia valentina, an endangered and endemic spanish plant. Plant Growth Regul. 1998, 26, 49–56. [Google Scholar] [CrossRef]
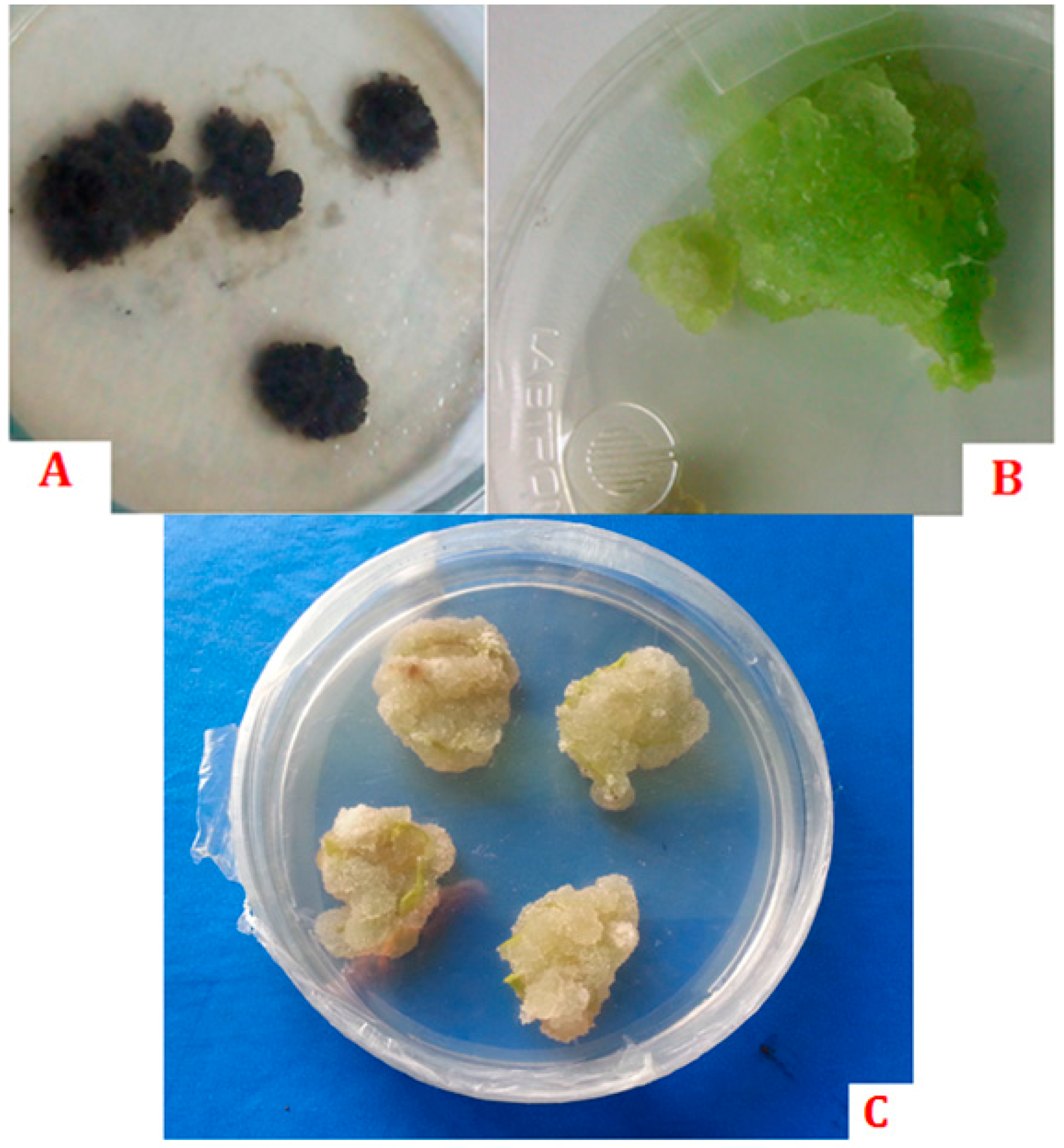
- Coppede, J.S.; Pina, E.S.; Paz, T.A.; Fachin, A.L.; Marins, M.A.; Bertoni, B.W.; França, S.C.; Pereira, A.M.S. Cell cultures of Maytenus ilicifolia Mart. are richer sources of quinone-methide triterpenoids than plant roots in natura. Plant Cell Tissue Organ Cult. 2014, 118, 33–43. [Google Scholar]
- Rasouli, H.; Fazeli-Nasab, B. Production of callus structure from different cultivars of wheat and medical plants. Ilam University: Ilam, Pazhohesh Blvd, Iran, Unpublished Project. 2012. [Google Scholar]
- Smith, D.L.; Miransari, M. Plant hormones and seed germination. Env. Exp. Botany 2014, 99, 110–121. [Google Scholar]
- Mohamadi, N.; Rajaie, P. Effects of aqueous eucalyptus (E. camadulensis Labill) extracts on seed germination, seedling growth and physiological responses of phaseolus vulgaris and sorghum bicolor. Res. J. Biol. Sci. 2009, 4, 1292–1296. [Google Scholar] [CrossRef]
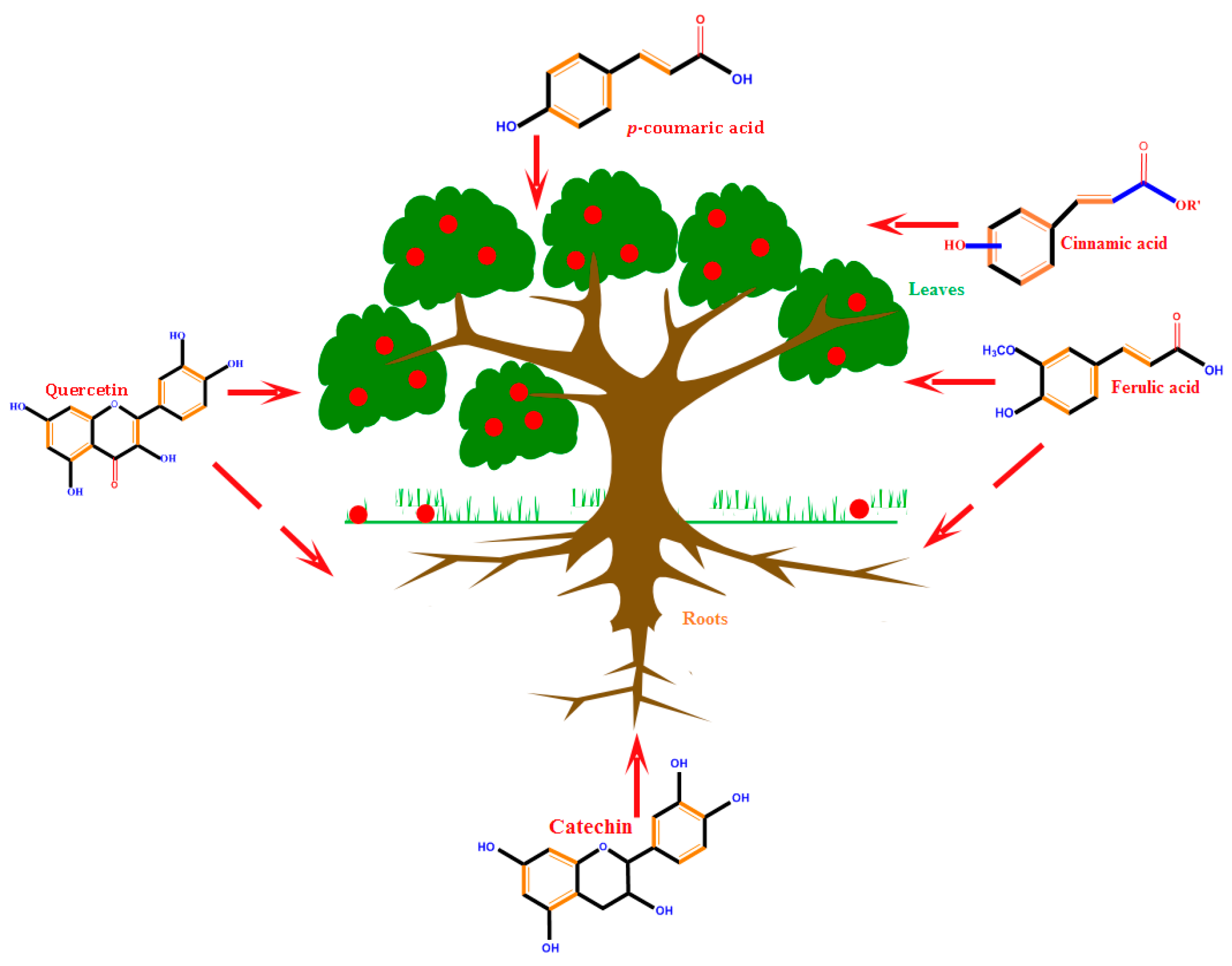
- Sulusoglu, M. Phenolic compounds and uses in fruit growing. Turk. J. Agric. Nat. Sci. 2014, 1, 947–956. [Google Scholar]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development and Germination, 3rd ed.; Springer-Verlag: New York, NY, USA, 2013. [Google Scholar]
- Giannakoula, A.E.; Ilias, I.F.; Maksimović, J.J.D.; Maksimović, V.M.; Živanović, B.D. The effects of plant growth regulators on growth, yield, and phenolic profile of lentil plants. J. Food Compost. Anal. 2012, 28, 46–53. [Google Scholar] [CrossRef]
- Arteca, R.N. Plant growth substances, principles and application; Springer US: New York, NY, USA, 1996. [Google Scholar]
- Moore, T.C. Biochemistry and Physiology of Plant Hormones, 2nd ed.; Springer-Verlag: New York, NY, USA, 1991. [Google Scholar]
- McCann, M.C.; Roberts, K.; Carpita, N.C. Plant cell growth and elongation. Encycl. Life Sci. 2001, 1, 1–8. [Google Scholar]
- Morris, J.W.; Doumas, P.; Marris, O.R.; Zaerr, J.P. Cytokinins in vegetative and reproductive buds of Pseudotsuga menziesii. Plant Physiol. 1990, 93, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar]
- Petrášek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Häsler, J.; Wüest, J.; Gaspar, T.; Crèvecoeur, M. Long term in vitro-cultured plant cells show typical neoplastic features at the cytological level. Biol. Cell 2003, 95, 357–364. [Google Scholar] [CrossRef]
- Moreno, P.R.H.; van der heijden, R.; Verpoorte, R. Cell and tissue cultures of catharanthus roseus: A literature survey. Plant Cell Tissue Organ Cult. 1995, 42, 1–25. [Google Scholar] [CrossRef]
- Gaspar, T. Plants can get cancer. Plant Physiol. Biochem. 1998, 36, 203–204. [Google Scholar] [CrossRef]
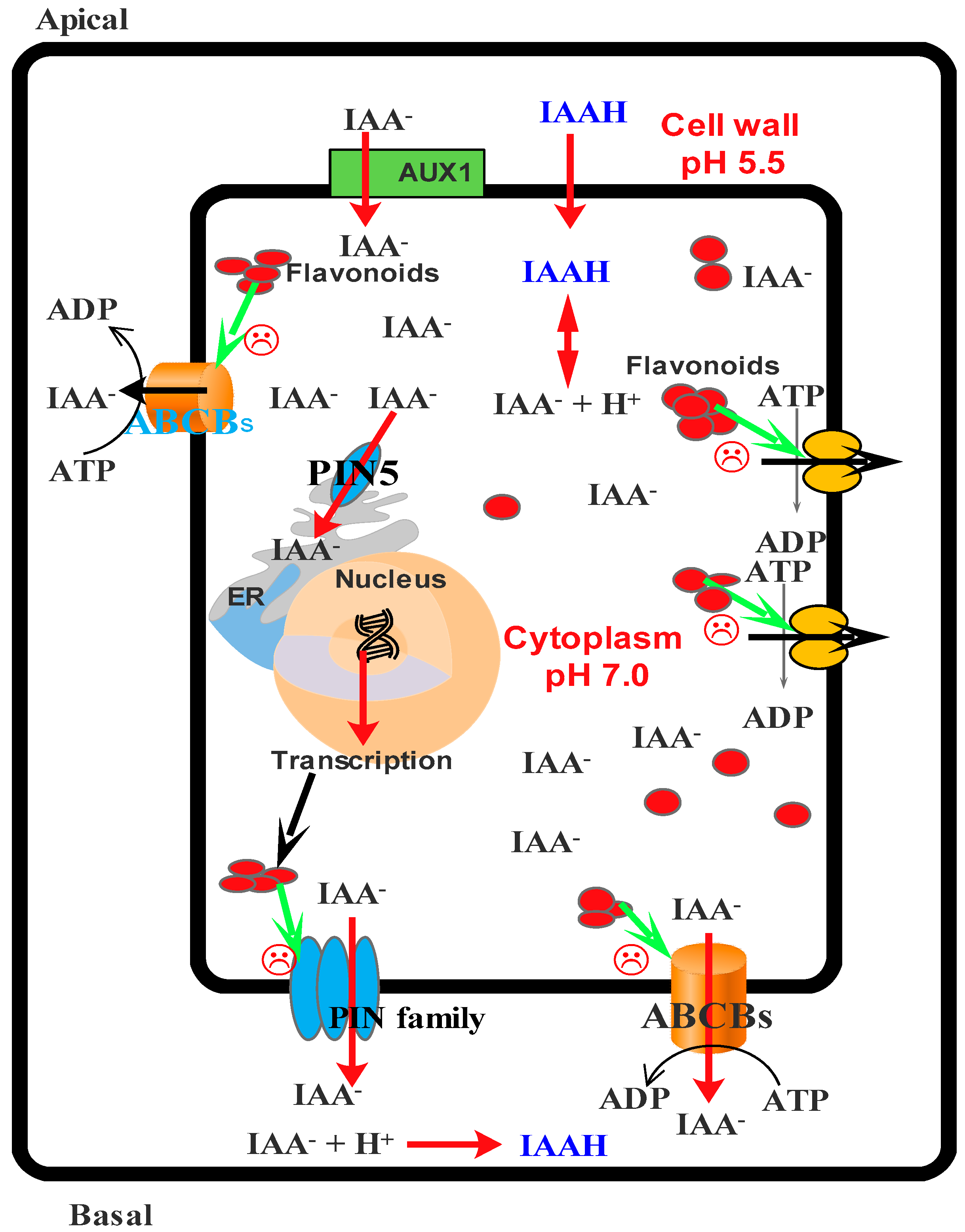
- Vieten, A.; Sauer, M.; Brewer, P.B.; Friml, J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 2007, 12, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Santelia, D.; Henrichs, S.; Vincenzetti, V.; Sauer, M.; Bigler, L.; Kelin, M.; Bailly, A.; Lee, Y.; Friml, J.; Geisler, M.; et al. Flavonoids redirect pin-mediated polar auxin fluxes during root gravitropic response. J. Biol. Chem. 2008, 283, 31218–31226. [Google Scholar] [CrossRef] [PubMed]
- Blakeslee, J.J.; Bandyopadhyay, A.; Lee, O.R.; Mravec, J.; Titapiwatanakun, B.; Sauer, M.; Makam, S.N.; Cheng, Y.; Bouchard, R.; Adamec, J.; et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 2007, 19, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Peer, W.A.; Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 2000, 211, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.E.; Rashotte, M.A.; Murphy, S.A.; Normanly, J.; Tague, W.B.; Peer, A.W.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; du Toit, E.S.; Reinhardt, C.F.; Rimando, A.M.; van der Kooy, F.; Meyer, J.J.M. The phenolic, 3,4-dihydroxybenzoic acid, is an endogenous regulator of rooting in protea cynaroides. Plant Growth Regul. 2007, 52, 207–215. [Google Scholar] [CrossRef]
- Younis, M.E.B.; Hasaneen, M.N.A.G.; Abdel-Aziz, H.M.M. An enhancing effect of visible light and UV radiation on phenolic compounds and various antioxidants in broad bean seedlings. Plant Signal. Behav. 2010, 5, 1197–1203. [Google Scholar] [CrossRef]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal-plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N.; Chaimovitch, D.; Dudai, N. Effect of irrigation with secondary treated effluent on essential oil, antioxidant activity, and phenolic compounds in oregano and rosemary. Agron. J. 2009, 101, 1–10. [Google Scholar] [CrossRef]
- Min, K.; Freeman, C.; Kang, H.; Choi, S.U. The regulation by phenolic compounds of soil organic matter dynamics under a changing environment. BioMed Res. Int. 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
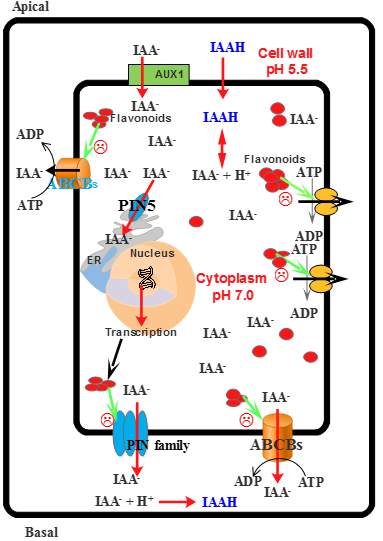
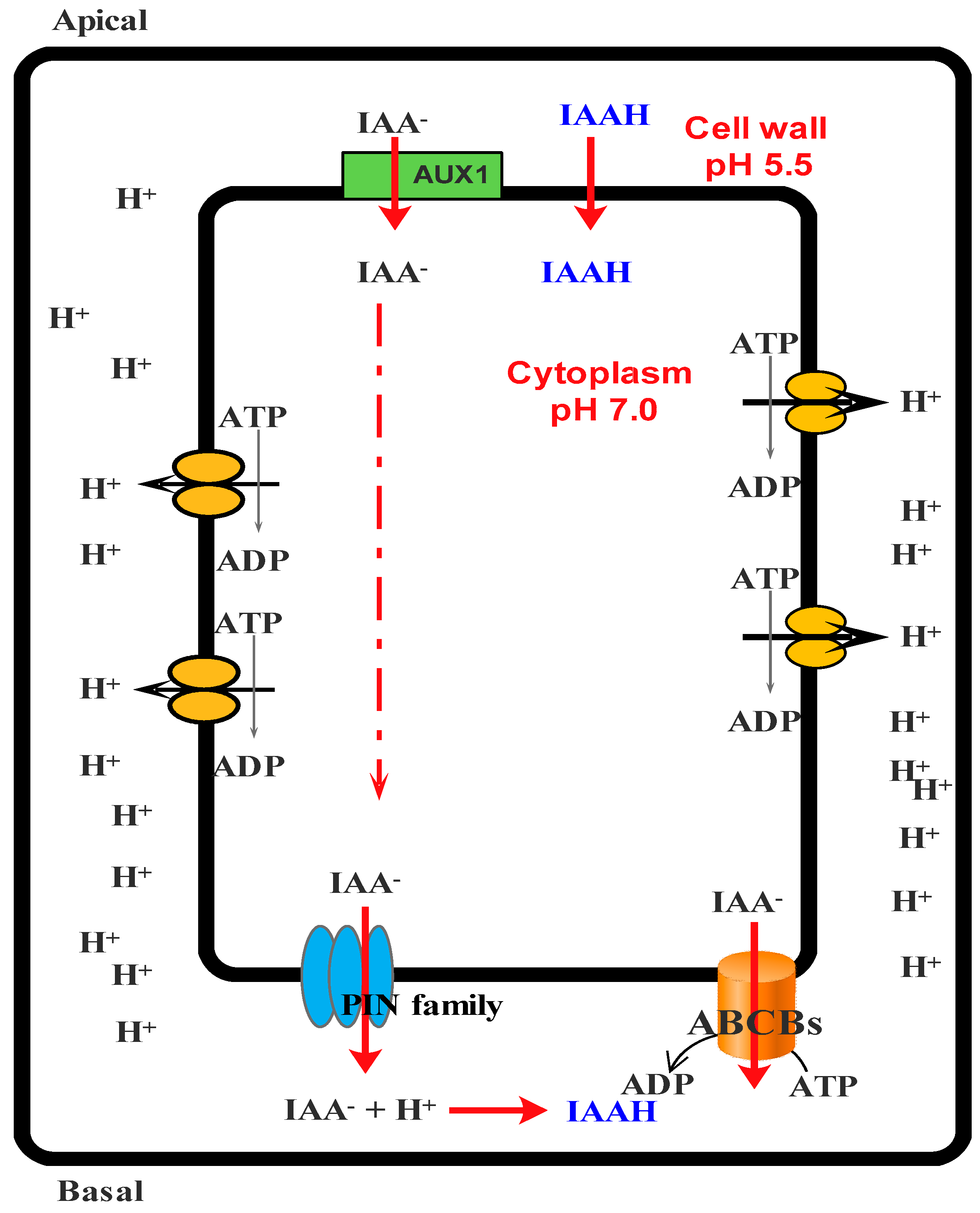
- Jacobs, M.; Rubery, P.H. Naturally occurring auxin transport regulators. Science 1988, 241, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Brown, D.E.; Tague, B.W.; Muday, G.K.; Taiz, L.; Murphy, A.S. Flavonoid accumulation patterns of transparent testa mutants of arabidopsis. Plant Physiol. 2001, 126, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, B.S.; Ribeiro, W.C.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
| Polyphenols | Basic structure | Examples | ||
|---|---|---|---|---|
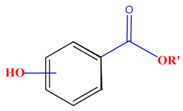
| Phenolic acids | Hydroxybenzoic acids | Vanillic acid | Gallic acid | Syringic acid |
 | 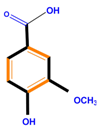 | 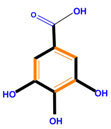 | 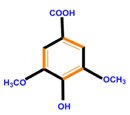 | |
| Hydroxycinnamic acids | Caffeic acid | Ferulic acid | p-Coumaric acid | |
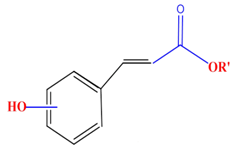 | 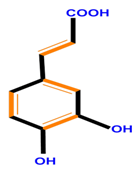 | 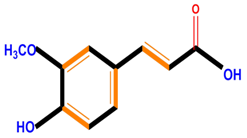 | 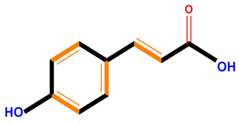 | |
| Flavonoids | Flavones | Chrysin | Luteolin | Apigenin |
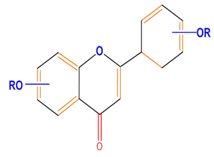 | 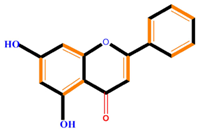 | 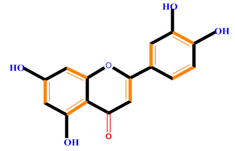 | 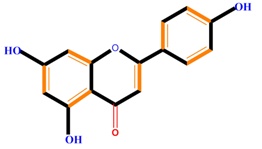 | |
| Flavonoids | Flavonols | Galangin | Kaempferol | Quercetin |
 |  | 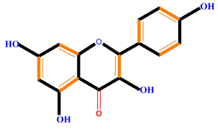 | 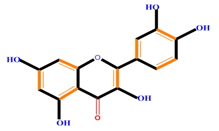 | |
| Flavanones | Naringenin | Hesperetin | Eriodictyol | |
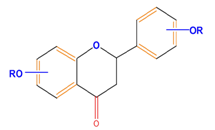 | 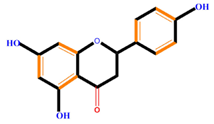 | 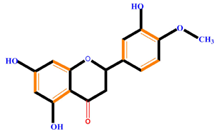 | 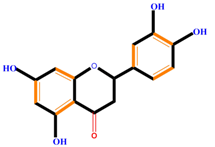 | |
| Flavan-3-ols | Catechin | Epicatechin | Epigallocatechin (EGC) | |
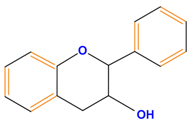 | 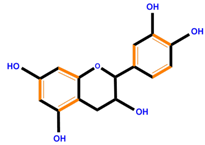 | 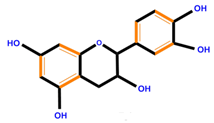 | 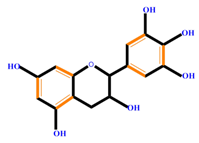 | |
| Isoflavones | Genistein | Daidzein | Neobavaisoflavone | |
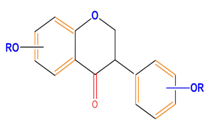 | 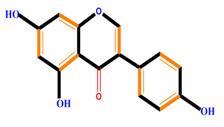 | 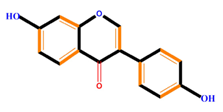 | 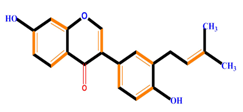 | |
| Flavonoids | Anthocyanidins | Cyanidin | Delphinidin | Pelargonidin |
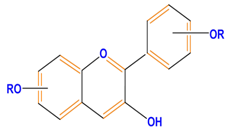 | 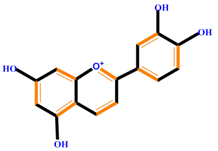 |  |  | |
| Lignans | 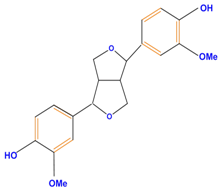 | Pinoresinol | ||
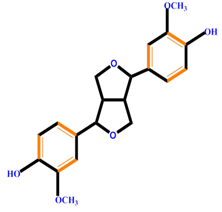 | ||||
| Stilbenes |  | Resveratrol | Polydatin | |
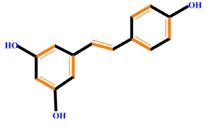 |  | |||
| Substance | Pathogen | Ref. |
|---|---|---|
| Oleuropein | Phytophthora spp. | [58] |
| Nobilietin | Phoma tracheiphila | [59] |
| Genistein | Monilinia fructicola | [60] |
| Biochanin | Monilinia fructicola | [60] |
| 5,8-Dihydroxy-6,7-dimethoxyflavan | Fusarium oxysporum | [60] |
| Thymol | Cryptococcus neoformans, Candida albicans, Rhyzopus sp., Aspergillus sp. | [61,62] |
| Hispidulin | Cladosporium sphaerospermum | [59] |
| Flavone | Aspergillus sp. | [59] |
| Flavanone | Aspergillus sp. | [59] |
| Phloretin | Venturiaina equalis | [59] |
| Kaempferol | Pyriclaria oryzae | [59] |
| 3-and 7-Hydroxyflavone | Penicillium glabrum | [59] |
| p-Coumaric acid | Gelosporium perennas | [59] |
| Rutin | Fusarium oxysporum | [59] |
| Vanillic acid | Phytophthora infestans | [59] |
| Salicylic Acid (SA) | Eutypa lata, Penicillium expansum, Fusarium graminearum | [63,64,65] |
| 2,5-Dimethoxybenzoic acid | Botrytis cinerea | [59,66] |
| Catechol | Colletotrichum circinans, Candida albicans | [67,68] |
| Protocatehuic acid | Colletotrichum circinans | [69] |
| 3,4-Dihydroxybenzaldehyde | Gloesporium musarum | [70] |
| Allelochemical | Distribution | Mechanism | Ref. |
|---|---|---|---|
| p-Hydroxybenzoic acid | Leaves | Inhibiting enzymatic activity | [101] |
| p-Coumaric acid | Leaves | Growth inhibitor | [101] |
| Quercetin | Leaves, Root, Bark | Anti-insect (Aphis craccivora Koch) | [59] |
| 2,4-Dihydroxy-1,4(2H) benzoxazin-3-one | Leaves, Root, Bark | Various actions | [59] |
| (−)-Catechin | Root | Inducing stress responses | [101] |
| Sorgoleone | Root | Photosystem II inhibitor, hydroxyphenyl pyruvate dioxygenase inhibitor | [101] |
| Phenolic acid | Root | Inhibiting seedling growth | [102] |
| Flavonoids | Root | Inhibiting seedling growth | [102] |
| SA | Root | Release of other allelochemicals | [103] |
| Cinnamic acid | Leaves | Inhibiting chlorophyll biosynthesis | [104] |
| Ferulic acid | Leaves, Root | Inhibiting of seed germination | [104] |
| Compound | Mechanism | Type of Cancer | Ref. |
|---|---|---|---|
| Sophoranone | Inhibits cell growth, induces apoptosis | Human stomach cancer MKN7 cells, human leukemia U937 cells | [16,22,78] |
| Kaempferol 3-O-rutinoside | Anti-inflammatory | Gastric cancer | [22] |
| Kaempferol | Anti-inflammatory, induces apoptosis | Gastric cancer, prostate cancer, thyroid cancer (ARO, NPA, WRO cells) | [109] |
| Isoflavonoids (general) | Induces apoptosis | Breast cancer lines, lung cancer lines, | [22] |
| Nobiletin | Cell cycle arrest (G1 phase), inhibits angiogenic differentiation by Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), down-regulation of ERK1/2 and c-Jun N-terminal kinases (c-JNK), induces caspase pathway | Breast cancer cell lines | [110,111] |
| Quercetin | Inhibits cancer metastasis, inhibits MAPK phosphorylation, induces differentiation of HL-60 cells into granulocytes and monocytes | Gastric cancer, lung cancer (SK-LU1, SW900, H441, H661, haGo-K-1, A549 cells) | [112] |
| Chalcones | Inhibits cell growth | B16 mouse melanoma | [22,113] |
| Apigenin | Inhibits cancer metastasis, inhibits MAPK phosphorylation, induces apoptosis, induces differentiation of HL-60 cells into granulocytes and monocytes | Leukemia (HL-60, K562, Jurkat cells) | [114] |
| Flavone | Inhibits proliferation, induces apoptosis | Colon cancer (Caco-2, HT-29, IEC-6, HCT-15 cells) | [22,115] |
| Genistein | Inhibits proliferation, induces apoptosis | Prostate cancer (LNCaP, PC3, DU145 cells) | [116] |
| Daidzein | Inhibits proliferation, induces apoptosis | Breast cancer (MCF-7 cells) | [117] |
| Courcumin | Inhibits proliferation, induces apoptosis | Oral cancer (HSC-2, HSG, SCC-25 cells) | [112] |
| Catechin | Inhibits tumor-invasive activity, inhibits cell shedding, hepatocyte growth factor signaling, cell arrest in S phase, modulates NO signaling, induces killer caspases, inhibits NF-κB signaling | Same effect as genistein | [112] |
| Flavopiridol | Inhibits CDKs, induces cell cycle arrest during G1 or G2/M, induces apoptosis | Prostate, colon, and gastric cancers | [118] |
| Luteolin | Induces differentiation of HL-60 cells into granulocytes and monocytes | Colon cancer cells | [118,119] |
| Hesperetin | Represses CDK2, CDK4, and cyclin D, Induces p21 and p27 expression, blocks cell cycle in G1 phase , promotes apoptosis, suppresses proliferation, increases expression of caspase-3, caspase-8, caspase-9, p53, Bax, Fas | Liver cancer (HepG2 cells), cervical cancer (SiHa cells), leukemia (NALM-6 cells), breast cancer (MCF-7 cells) | [118] |
| 5HTMF | Induces G0/G1 arrest, changes p21and p53 status | Colon cancer cells | [118] |
| Tangeretin | Induces caspase-3 activity, Cell cycle arrest (inhibit G1 phase), suppresses proliferation, inhibits cancer metastasis, Scavenging of ROS | Colon cancer cells, liver cancer (HepG2 cell), cervical cancer (SiHa cell) | [118] |
| Naringenin | Blocks cells in the G0/G1 and G2/M phases, induces metastasis, decreased ROS generation, induces TNF-α | Liver cancer (HepG2 cell), cervical cancer (SiHa cell) | [107,120,121] |
| Sinensetin | Antiangiogenesis, blocks G0/G1 phase, regulates expression of angiogenesis genes flt1, kdrl, and hras | General anticancer substances | [107] |
| Anthocyanins | Reduces inflammatory and tumor initiation, suppresses angiogenesis, minimizes cancer-induced DNA damage (in animal disease model) | General anticancer substances | [122] |
| Flavonols | Direct cellular proliferation inhibitor | Leukemia and pancreatic, breast, cervical, prostate, uterine, and urinary tract cancers. | [22] |
| Caffeoylquinic acids | Antioxidant activity | Limit LDL oxidation, general effect on cancer cell lines | [22] |
| Isoflavonoids | General protective activity | breast and prostate cancers | [123] |
| Resveratrol | Skin cancer, tumors of the gastrointestinal tract | [124] |
| PCs | Roles during Plant Growth | Ref. |
|---|---|---|
| p-Coumaric acid | Cell wall development, seed germination, and dormancy | [129] |
| SA | Effect on accumulation of ABA and IAA, regulation of growth, ion uptake, photosynthetic performance, membrane permeability, response to drought, salt stress, heavy metals, and multiple-stress tolerance. | [132] |
| Ferulic acid | Cell wall development, Allelopathy (germination inhibitors), effect on accumulation of ABA, IAA, response to abiotic stress | [129] |
| Caffeic acid | Antioxidant, light absorption | [136] |
| Cinnamic acid | Effect on accumulation of ABA, IAA, response to abiotic stress | [136] |
| Tyramine | Reduce cell count and dwarfing | [129] |
| Hydroxycinnamic acids | Decrease of lignification during abiotic stress, response to water tension, seed germination, and dormancy | [129] |
| Hydroxycinnamoylquinic acids | Response to water stress | [129] |
| Hydroxycinnamic acid glucosides | Response to water stress | [129] |
| SA glucoside | Response to water stress | [137] |
| Conjugated flavonols (with disaccharides) | Response to water stress | [129] |
| Caffeoylputrescine | Response to water stress | [129] |
| Isoflavonoids | Phytoalexins | [129] |
| Tannins | Defensive properties by binding to proteins, Tolerant to heavy metal | [138] |
| Flavons and Flavonols | Plant growth development by absorb light, protect cells from excessive UV radiation, legume nodulations and nitrogen-fixing, membrane stabilizer during stresses | [138] |
| Anthocyanin | Attracting pollinators | [139] |
| Flavonoids | Flower pigmentation, UV-protection, plant defense, legume nodulations, membranes stabilizer during stress, scavenging of reactive species (ROS, H2O2, etc.) | [140] |
| Lignin | Xylogenesis, defensive response to pathogen, cell wall formation | [139] |
| Apigenin | Compete with IAA and inhibit polar auxin transport | [59] |
| Gallic acid 4-O-(β-d-glucopyranosyl-6′-sulfate) | Control of nyctinastic movement in leaves | [59] |
| Gentisic acid 5-O-β-d-glucopyranoside | Control of nyctinastic movement in leaves | [59] |
| Kaempferol | Compete with IAA and inhibit polar auxin transport | [59] |
| Ascorbic acid | Antioxidant activity and protection of cells | [138] |
| Isoflavone | Response to environmental tensions | [141] |
| o-Dihydroxy phenolics | Anti-herbivore activity | [138] |
| Simple Phenolics | Plant–environment interactions and allelopathy | [139] |
| Phenylpropanoid | Lignin biosynthesis | [139] |
| Monohydroxy B-ring Flavonoids | Decompose IAA hormone, prevent of IAA transport by binding to NPA | [59] |
| Dihydroxy B-ring Flavonoids | Preventers of the IAA action, preventers of IAA transport by binding to NPA | [59] |
| Compound | Activity | Ref. |
|---|---|---|
| Phloroglucinol (1,3,5-trihydroxybenzene) | Increase growth and axillary shoot generation, prevention of vitrification, increase somatic embryogenesis, control of hyperhydricity in lignification | [132] |
| Phloroglucinol + NAA | Higher levels of somatic embryogenesis | [135] |
| Phloroglucinol + BA | Improve number of shoots | [134] |
| Phloroglucinol + any cytokinins | 100% regeneration | [135] |
| Phloretic acid | Increase shoot and root | [135] |
| Phloroglucinol + IAA | Increase rooting | [142] |
| Chlorogenic acid | Stimulate callus growth | [143] |
| Glycoside phloridzin | Same effect as phloroglucinol | [132] |
| Quinone | Negative effect on cell growth (by death/necrosis) | [144] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasouli, H.; Farzaei, M.H.; Mansouri, K.; Mohammadzadeh, S.; Khodarahmi, R. Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review. Molecules 2016, 21, 1104. https://doi.org/10.3390/molecules21091104
Rasouli H, Farzaei MH, Mansouri K, Mohammadzadeh S, Khodarahmi R. Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review. Molecules. 2016; 21(9):1104. https://doi.org/10.3390/molecules21091104
Chicago/Turabian StyleRasouli, Hassan, Mohammad Hosein Farzaei, Kamran Mansouri, Sara Mohammadzadeh, and Reza Khodarahmi. 2016. "Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review" Molecules 21, no. 9: 1104. https://doi.org/10.3390/molecules21091104
APA StyleRasouli, H., Farzaei, M. H., Mansouri, K., Mohammadzadeh, S., & Khodarahmi, R. (2016). Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review. Molecules, 21(9), 1104. https://doi.org/10.3390/molecules21091104