The in Vitro and in Vivo Antitumor Activities of Tetracyclic Triterpenoids Compounds Actein and 26-Deoxyactein Isolated from Rhizome of Cimicifuga foetida L.
Abstract
:1. Introduction
2. Results
2.1. Both Actein and 26-Deoxyactein Inhibited the Growth of the 12 Human Tumor Cell Lines Tested
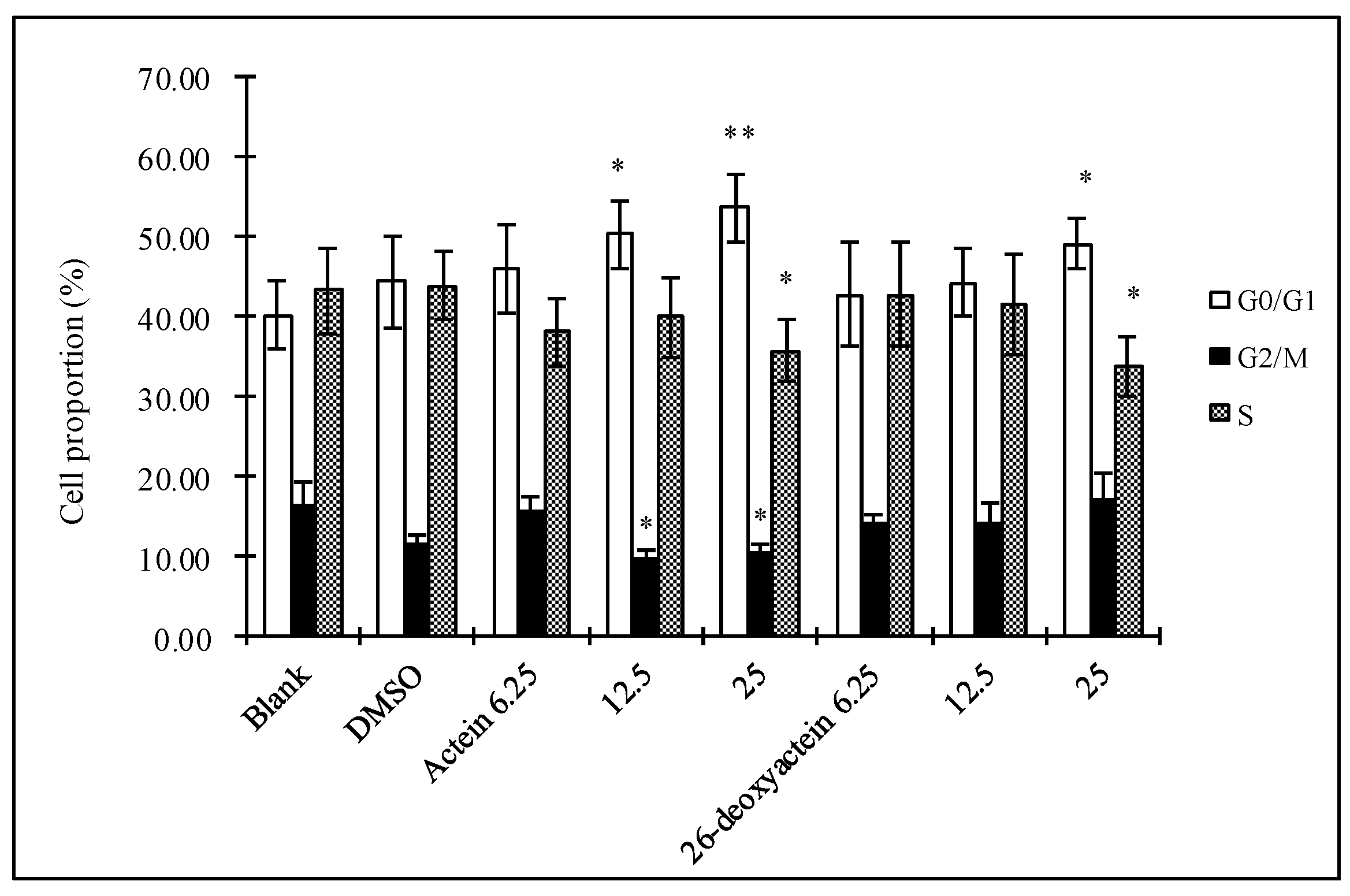
2.2. Actein and 26-Deoxyactein Arrest the HL-60 Cells at G1 Phase
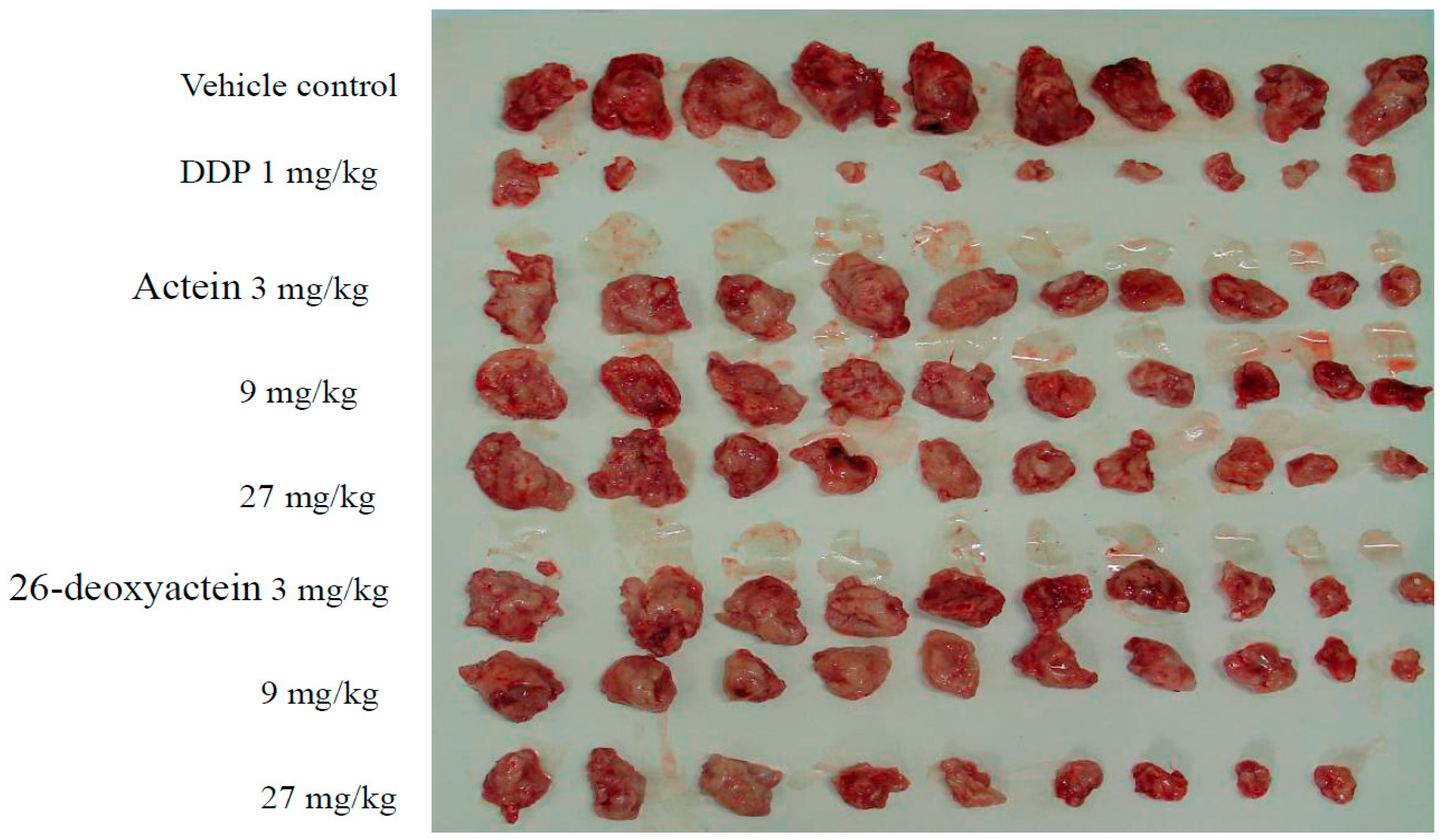
2.3. Both Actein and 26-Deoxyactein Inhibit the Growth of Implanted S180 in the Mice
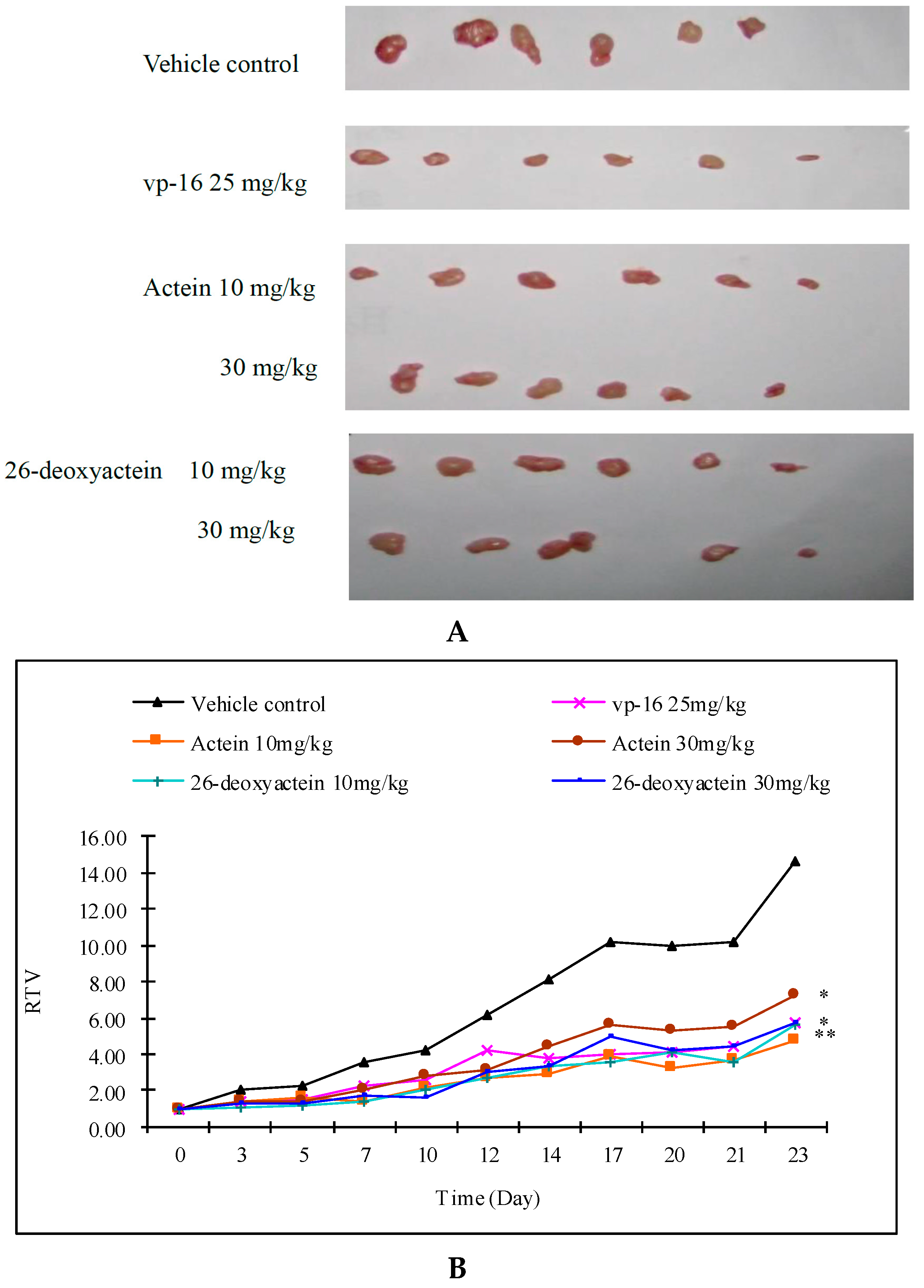
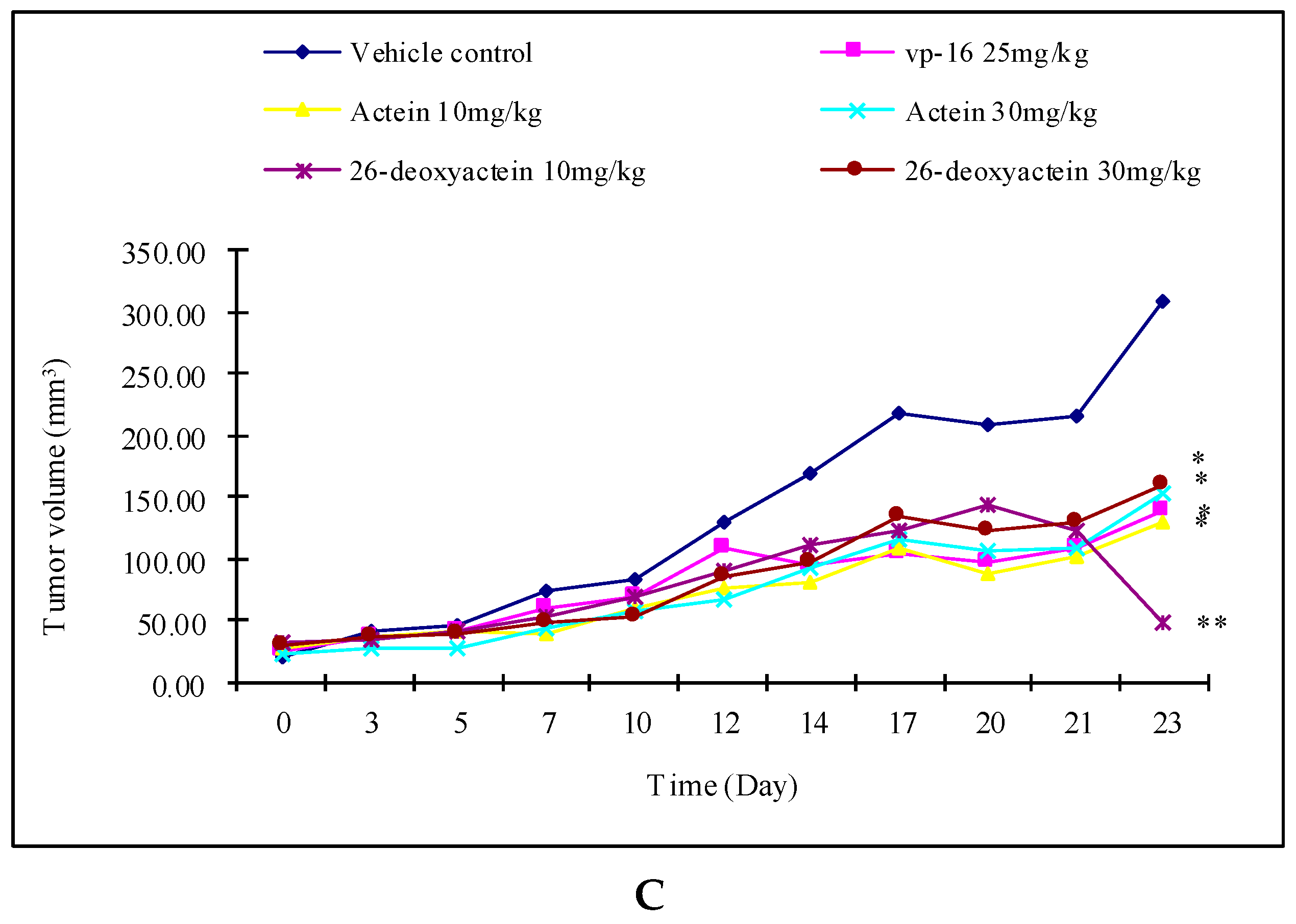
2.4. Actein and 26-Deoxyactein Inhibit the Growth of the Implanted A549 Tumor in the Nude Mice
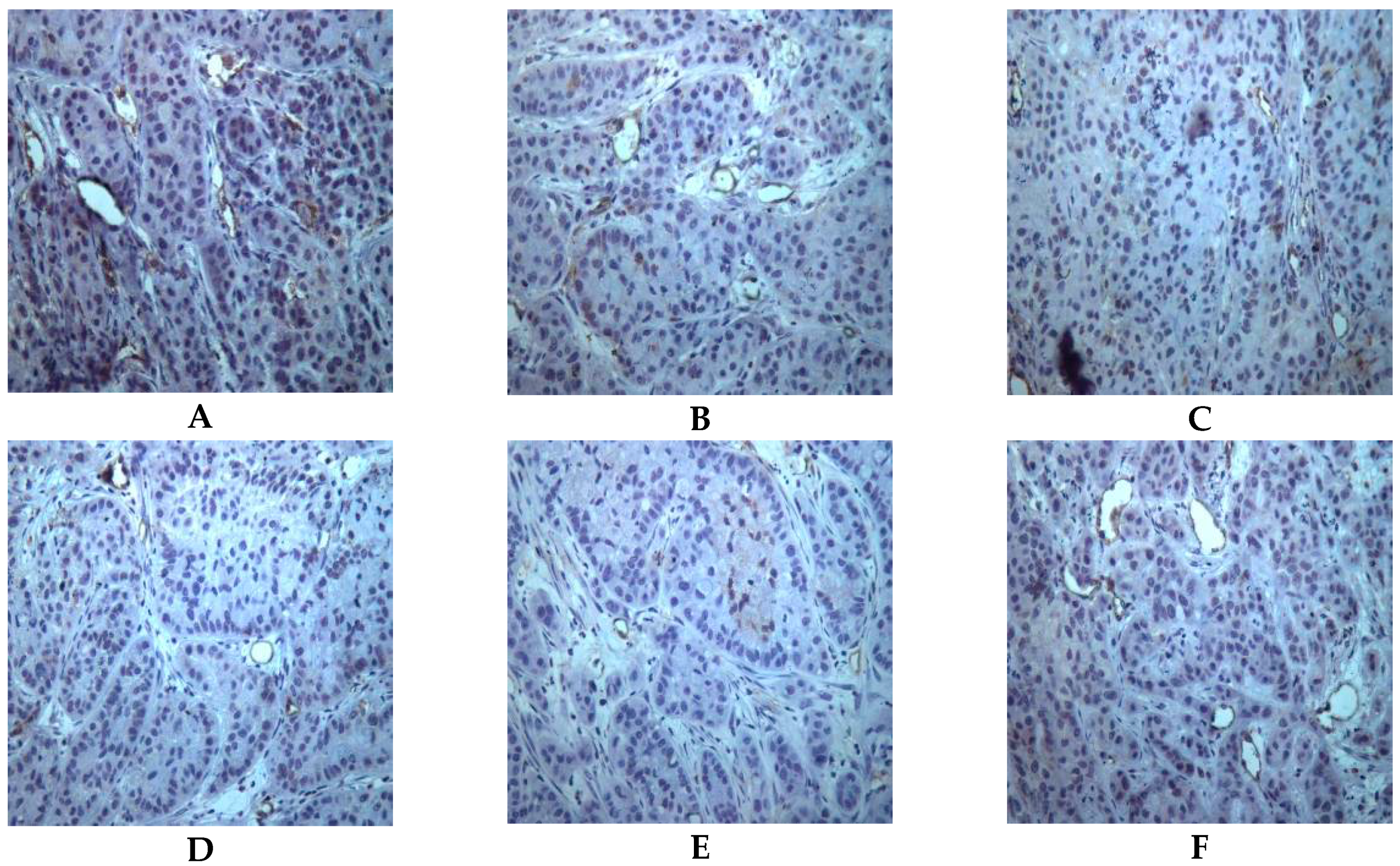
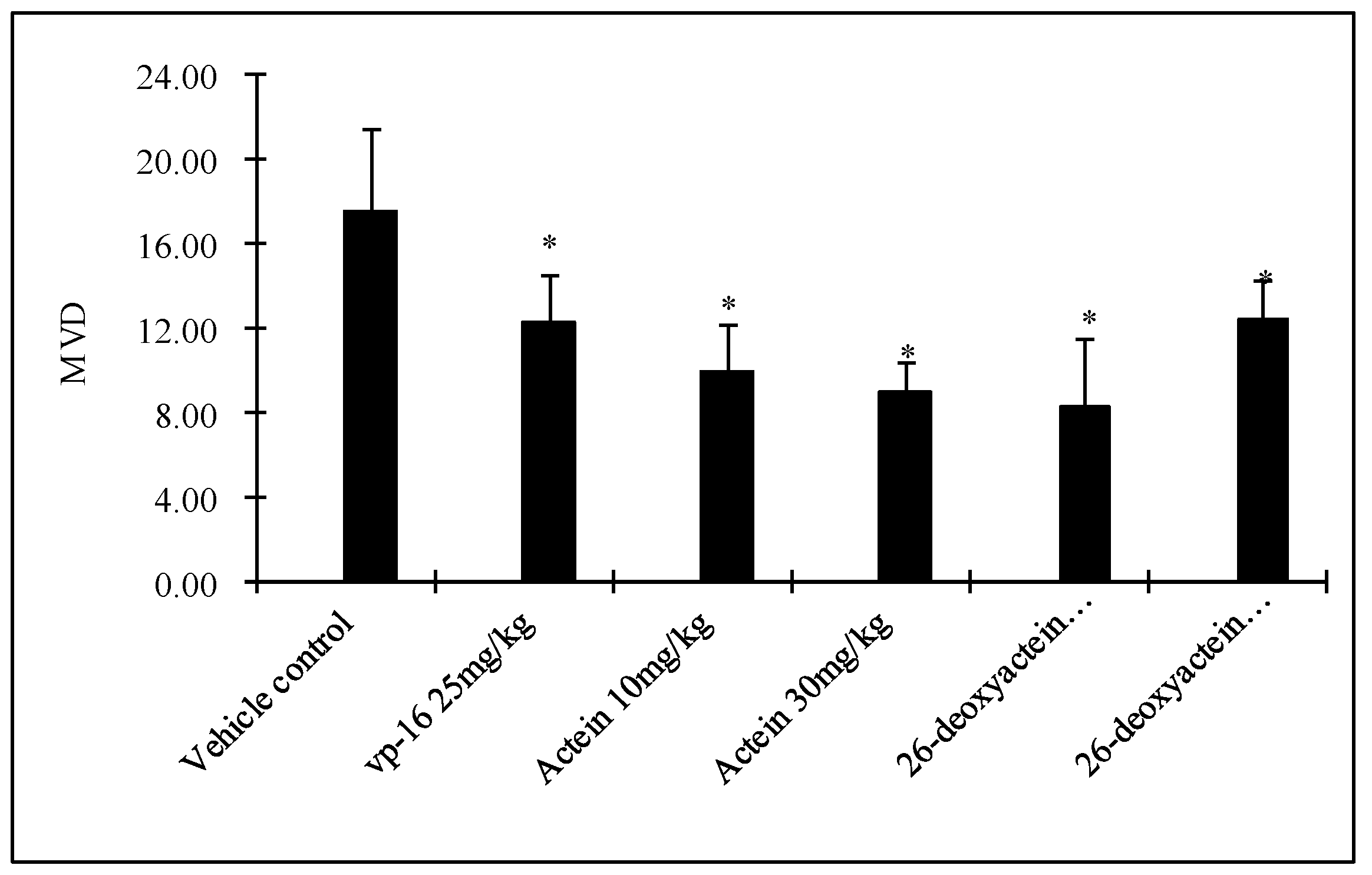
2.5. Both Actein and 26-Deoxyactein Downregulate Cd31-Positive Expression in the Implanted Tumor of the Nude Mice
2.6. Preliminary Safety Evaluation of Actein and 26-Deoxyactein
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Main Reagents
4.2. Experimental Animals
4.3. Cell Proliferation Assay
4.4. Cell Cycle Assay
4.5. Establishment of the S180 Cell-Implanted Tumor Mouse Model and Treatments
4.6. Establishment of the A549 Xenogrft Tumor Model and Treatments
4.7. Immunohistochemistry Assay of CD31-Positive Expression in the Xenograft Tumor Tissue in the Nude Mice
4.8. Preliminary Safety Valuation of Actein and 26-Deoxyactein
4.9. Data Presentation and Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Zeng, H.; Zhang, S.; He, J. Annual report on status of cancer in China, 2011. Chin J. Cancer. Res. 2011, 27, 2–12. [Google Scholar]
- Yoshimitsu, H.; Nishida, M.; Nohara, T. Three new 15,16-seco-cycloartane glycosides from Cimicifuga rhizome. Chem. Pharm. Bull. (Tokyo) 2007, 55, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Nagai, M. Chemical constituents of original plants of Cimicifugae rhizoma in Chinese medicine. Yakugaku Zasshi 1996, 116, 850–865. [Google Scholar] [PubMed]
- Yoshimitsu, H.; Nishida, M.; Sakaguchi, M.; Nohara, T. Two new 15-deoxycimigenol-type and three new 24-epi-cimigenol-type glycosides from Cimicifuga rhizome. Chem. Pharm. Bull. (Tokyo) 2006, 54, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Yoshimitsu, H. Six new cycloartane glycosides from Cimicifuga rhizome. Chem. Pharm. Bull. (Tokyo) 2011, 59, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Yoshimitsu, H.; Nohara, T. Three cycloartane glycosides from Cimicifuga rhizome and their immunosuppressive activities in mouse allogeneic mixed lymphocyte reaction. Chem. Pharm. Bull. (Tokyo) 2003, 51, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Si, J.Y.; Chen, S.B.; Yang, M.S.; Xiao, P.G.; Wu, E.X. Cytotoxicity and mechanism of 23-O-acetylcimigenol-3-O-β-d-xylopyranoside on HepG2 cells. Zhongguo Zhong Yao Za Zhi 2006, 31, 1818–1821. [Google Scholar] [PubMed]
- Tian, Z.; Si, J.; Chang, Q.; Zhou, L.; Chen, S.; Xiao, P.; Wu, E. Antitumor activity and mechanisms of action of total glycosides from aerial part of Cimicifuga dahurica targeted against hepatoma. BMC Cancer 2007, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Golias, C.H.; Charalabopoulos, A.; Charalabopoulos, K. Cell proliferation and cell cycle control: A mini review. Int. J. Clin. Pract. 2004, 58, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Campioni, M.; D'Avino, A.; Caruso, G.; Citro, G.; Baldi, A. Cell-cycle molecules in mesothelioma: An overview. J. Exp. Clin. Cancer Res. 2007, 26, 443–449. [Google Scholar] [PubMed]
- Santamaria, D.; Ortega, S. Cyclins and CDKS in development and cancer: Lessons from genetically modified mice. Front. Biosci. 2006, 11, 1164–1188. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclins and related kinases in cancer cells. J. BUON 2007, 12, S45–S52. [Google Scholar] [PubMed]
- Santo, L.; Siu, K.T.; Raje, N. Targeting Cyclin-Dependent Kinases and Cell Cycle Progression in Human Cancers. Semin. Oncol. 2015, 42, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Wesierska-Gadek, J.; Schmid, G. Dual action of the inhibitors of cyclin-dependent kinases: targeting of the cell-cycle progression and activation of wild-type p53 protein. Expert. Opin. Investig. Drugs 2006, 15, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Bakkar, N. Modulating cell cycle: Current applications and prospects for future drug development. Curr. Cancer Drug Targets 2002, 2, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Pour, L.; Hájek, R.; Buchler, T.; Maisnar, V.; Smolej, L. Angiogenesis and antiangiogenic cancer therapy. Vnitr. Lek. 2004, 50, 930–938. [Google Scholar] [PubMed]
- DeLisser, H.M.; Newman, P.J.; Albelda, S.M. Platelet endothelial cell adhesion molecule (CD31). In Adhesion in Leukocyte Homing and Differentiation; (Current Topics in Microbiology and Immunology); Springer: Berlin, Germany, 1993; Volume 184, pp. 37–45. [Google Scholar]
- Muller, W.A. The role of PECAM-1 (CD31) in leukocyte emigration: Studies in vitro and in vivo. J. Leukoc. Biol. 1995, 57, 523–528. [Google Scholar] [PubMed]
- Bogen, S.; Pak, J.; Garifallou, M.; Deng, X.; Muller, W.A. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J. Exp. Med. 1994, 179, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
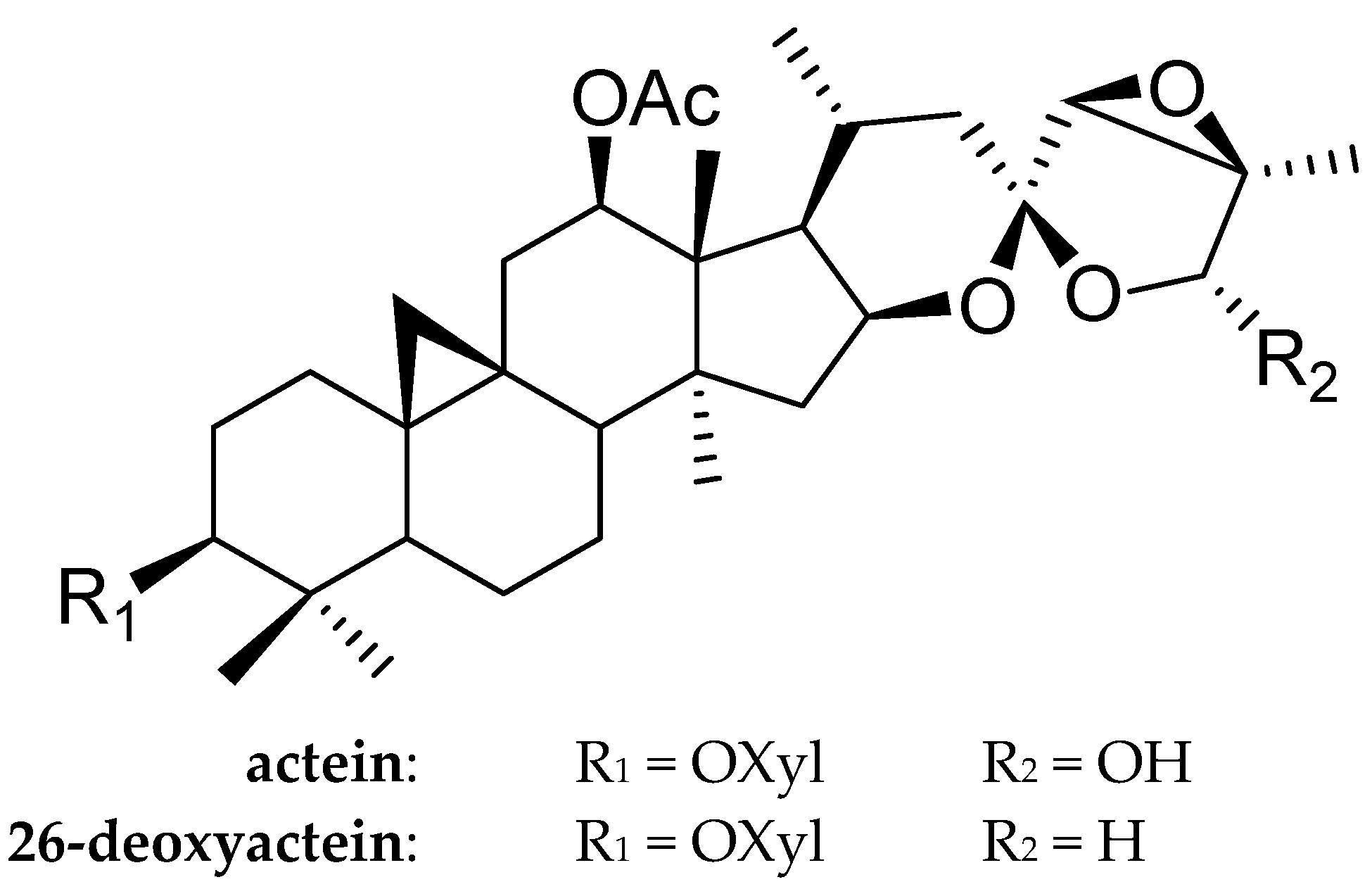
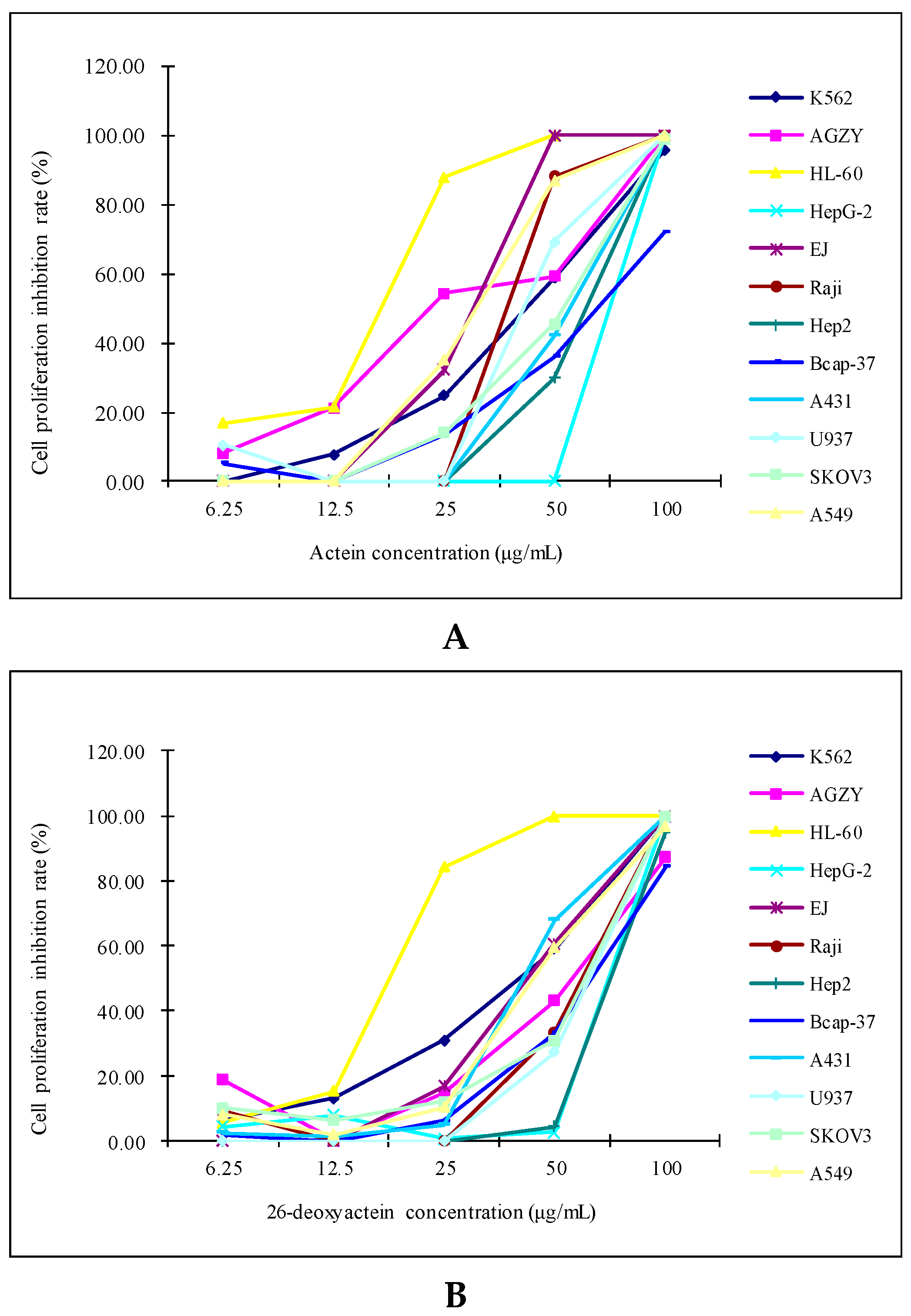
| Cell Line | Actein (μg/mL) | 26-Deoxyactein (μg/mL) |
|---|---|---|
| K562 | 39.83 | 22.15 |
| AGZY | 19.35 | 54.49 |
| HL-60 | 12.29 | 14.54 |
| HepG-2 | 50–100 | 42.43 |
| EJ | 29.14 | 38.04 |
| Raji | 45.4 | 52.72 |
| Hep2 | 52.22 | 88.39 |
| Bcap-37 | 79.17 | 71.04 |
| A431 | 60.28 | 29.86 |
| U937 | 47.08 | 52.61 |
| SKOV3 | 44.71 | 26.36 |
| A549 | 34.68 | 37.72 |
| Group | Dosage (mg/kg) | Tumor Weight (g) | Growth Inhibition (%) |
|---|---|---|---|
| Vehicle control | − | 2.83 ± 0.91 | − |
| DDP | 1 | 0.31 ± 0.22 ** | 88.87 |
| Actein | 3 | 1.69 ± 0.83 * | 40.24 |
| 9 | 1.58 ± 0.79 * | 44.27 | |
| 27 | 1.34 ± 0.74 * | 52.80 | |
| 26-Deoxyactein | 3 | 1.68 ± 0.84 * | 40.80 |
| 9 | 1.34 ± 0.70 * | 52.59 | |
| 27 | 0.89 ± 0.49 * | 68.66 |
| Group | Dosage (g/kg) | Body Weight (g) | |||
|---|---|---|---|---|---|
| Time after Administration (Day) | |||||
| 0 | 3 | 7 | 14 | ||
| Vehicle | − | 18.9 ± 0.8 | 22.1 ± 1.3 | 25.9 ± 1.5 | 28.9 ± 1.9 |
| Actein | 5.0 | 18.4 ± 0.7 | 19.8 ± 1.4 ** | 24.6 ± 1.7 * | 27.7 ± 2.9 |
| 26-Deoxyactein | 5.0 | 18.8 ± 0.8 | 19.6 ± 1.2 ** | 24.4 ± 2.0 * | 27.6 ± 3.2 |
| Group | Dosage (g/kg) | Liver Toxicity | Kidney Toxicity | Lung Toxicity | |||
|---|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | Present | Absent | ||
| Vehicle | − | 0 | 20 | 0 | 20 | 0 | 20 |
| Actein | 5.0 | 2 | 18 | 1 | 19 | 2 | 18 |
| 26-Deoxyactein | 5.0 | 2 | 18 | 2 | 18 | 1 | 19 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Yao, Q.; Chen, Y.; Hu, X.; Qing, C.; Qiu, M. The in Vitro and in Vivo Antitumor Activities of Tetracyclic Triterpenoids Compounds Actein and 26-Deoxyactein Isolated from Rhizome of Cimicifuga foetida L. Molecules 2016, 21, 1001. https://doi.org/10.3390/molecules21081001
Wu D, Yao Q, Chen Y, Hu X, Qing C, Qiu M. The in Vitro and in Vivo Antitumor Activities of Tetracyclic Triterpenoids Compounds Actein and 26-Deoxyactein Isolated from Rhizome of Cimicifuga foetida L. Molecules. 2016; 21(8):1001. https://doi.org/10.3390/molecules21081001
Chicago/Turabian StyleWu, Desong, Qi Yao, Yajuan Chen, Xiaodong Hu, Chen Qing, and Minghua Qiu. 2016. "The in Vitro and in Vivo Antitumor Activities of Tetracyclic Triterpenoids Compounds Actein and 26-Deoxyactein Isolated from Rhizome of Cimicifuga foetida L." Molecules 21, no. 8: 1001. https://doi.org/10.3390/molecules21081001
APA StyleWu, D., Yao, Q., Chen, Y., Hu, X., Qing, C., & Qiu, M. (2016). The in Vitro and in Vivo Antitumor Activities of Tetracyclic Triterpenoids Compounds Actein and 26-Deoxyactein Isolated from Rhizome of Cimicifuga foetida L. Molecules, 21(8), 1001. https://doi.org/10.3390/molecules21081001







