Synthesis and Characterization of a Novel Borazine-Type UV Photo-Induced Polymerization of Ceramic Precursors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of a-CSEB Molecule
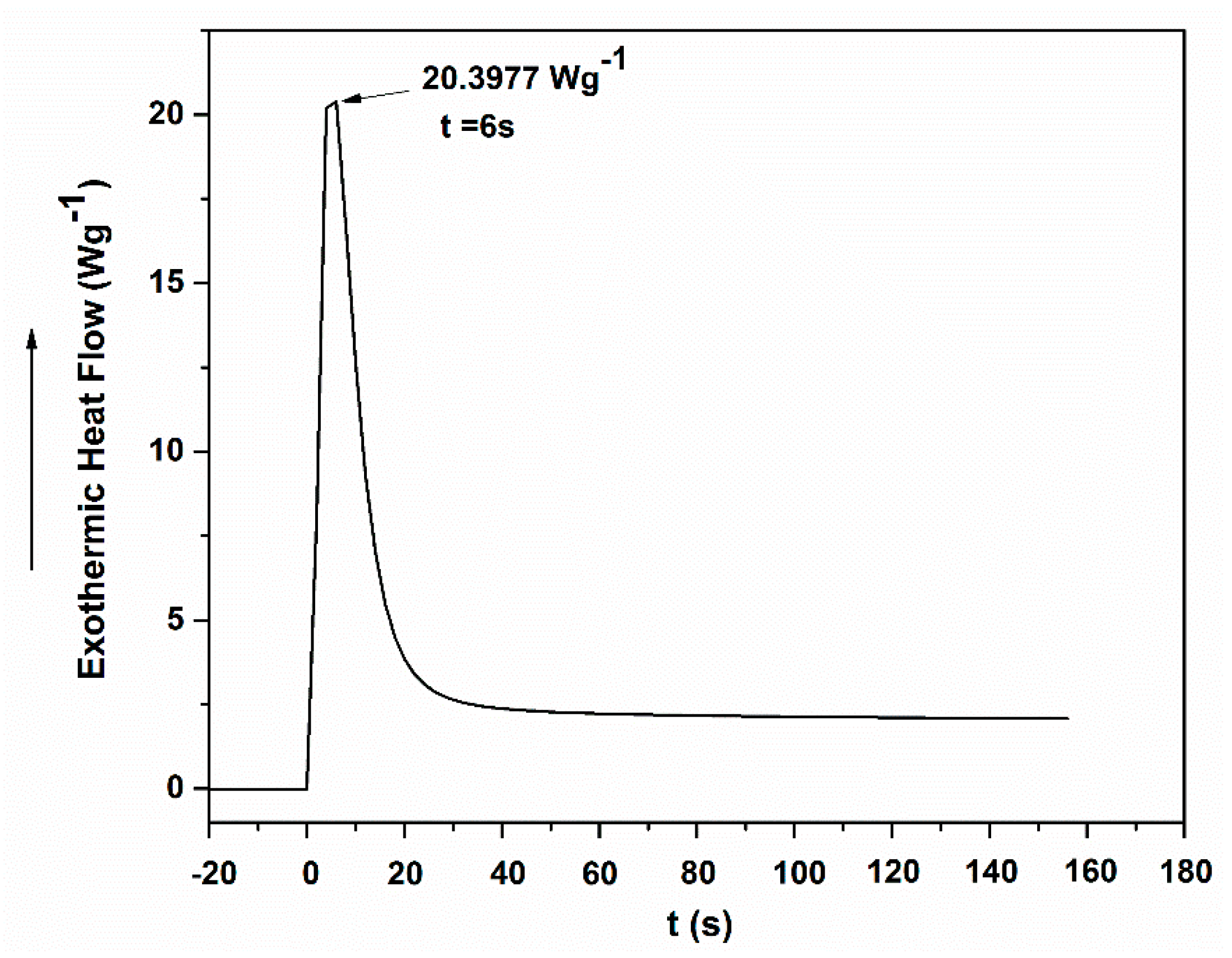
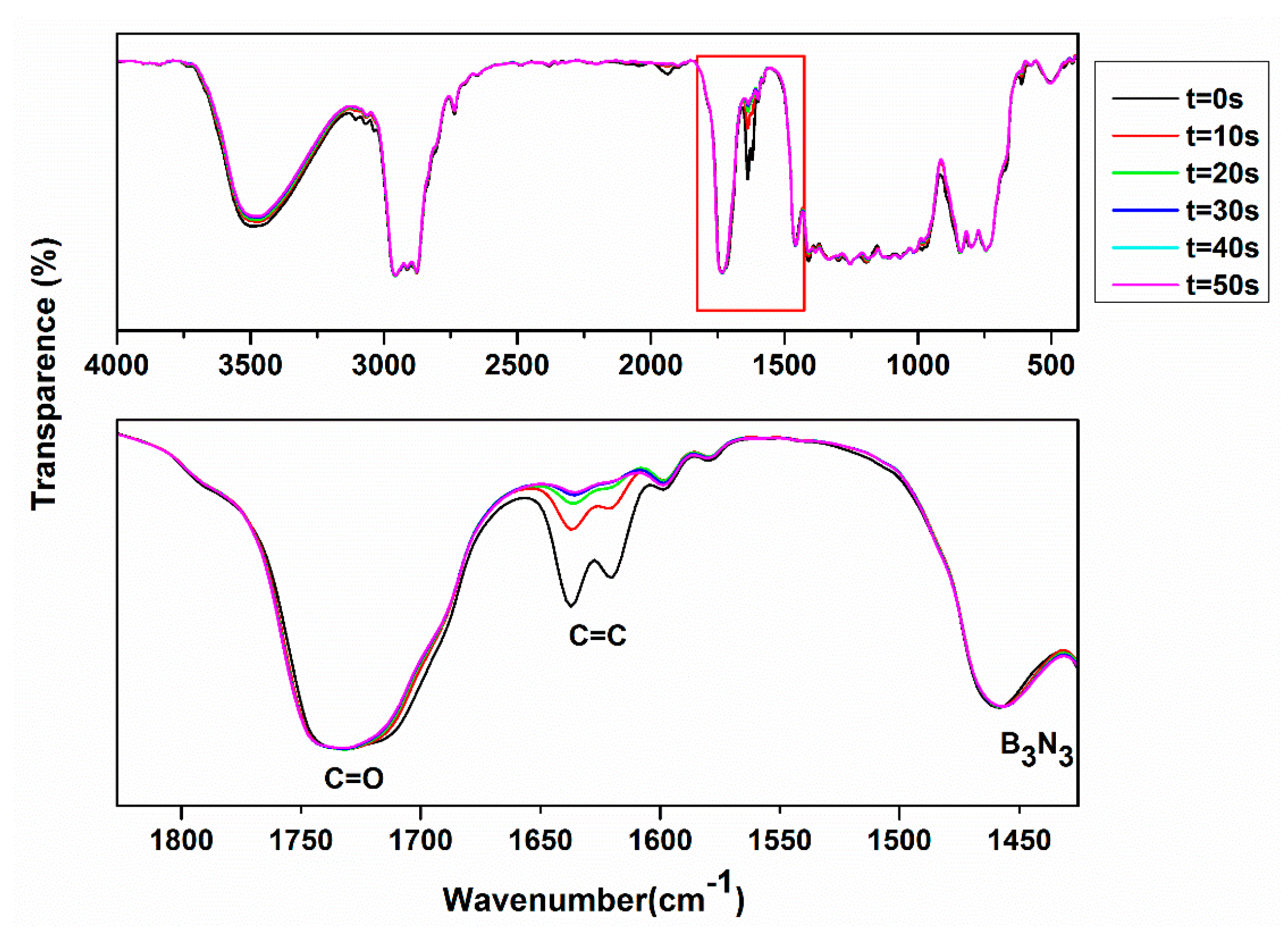
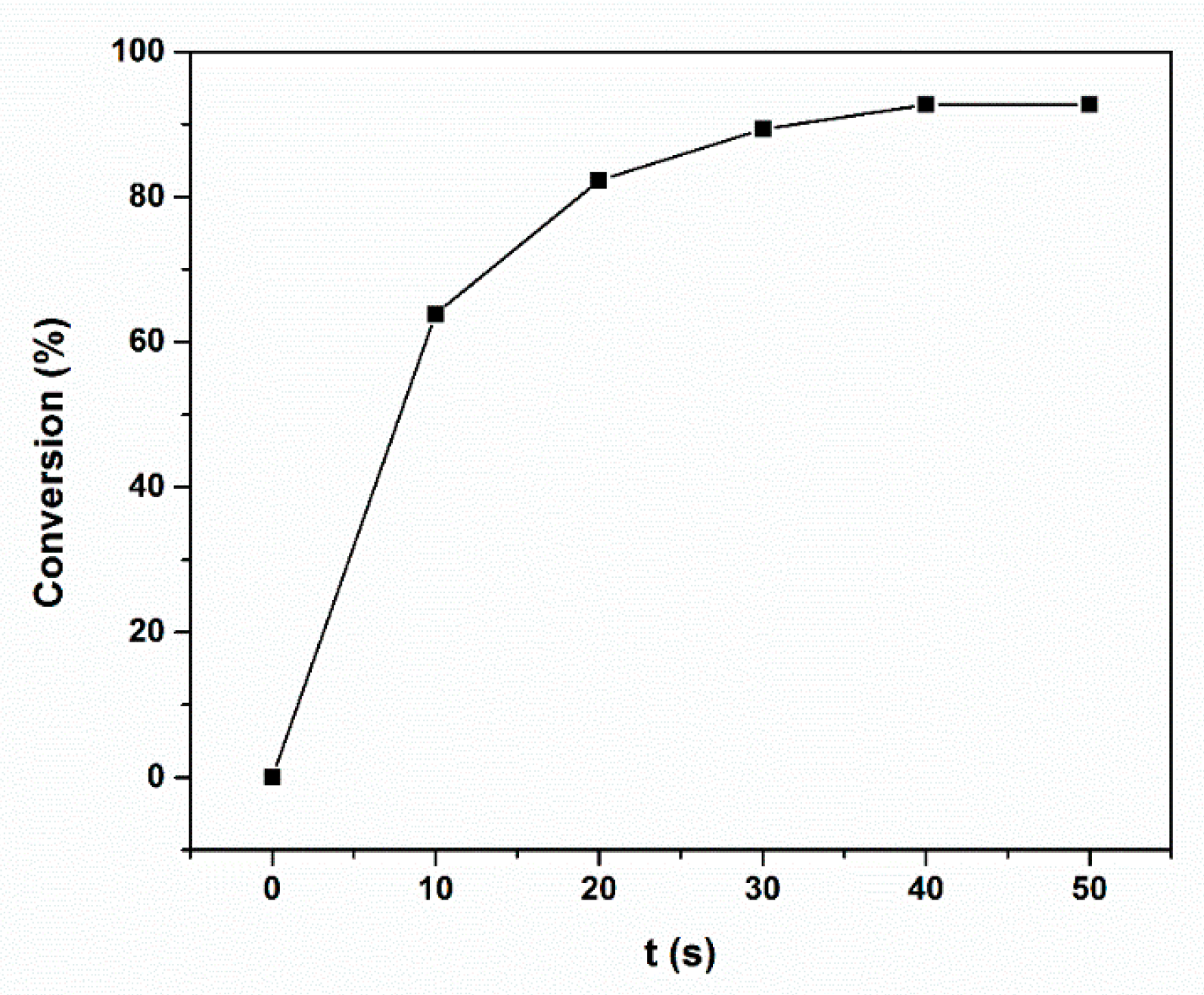
2.2. UV Polymerization Properties
2.3. Characterization of the a-CSEB Polymer
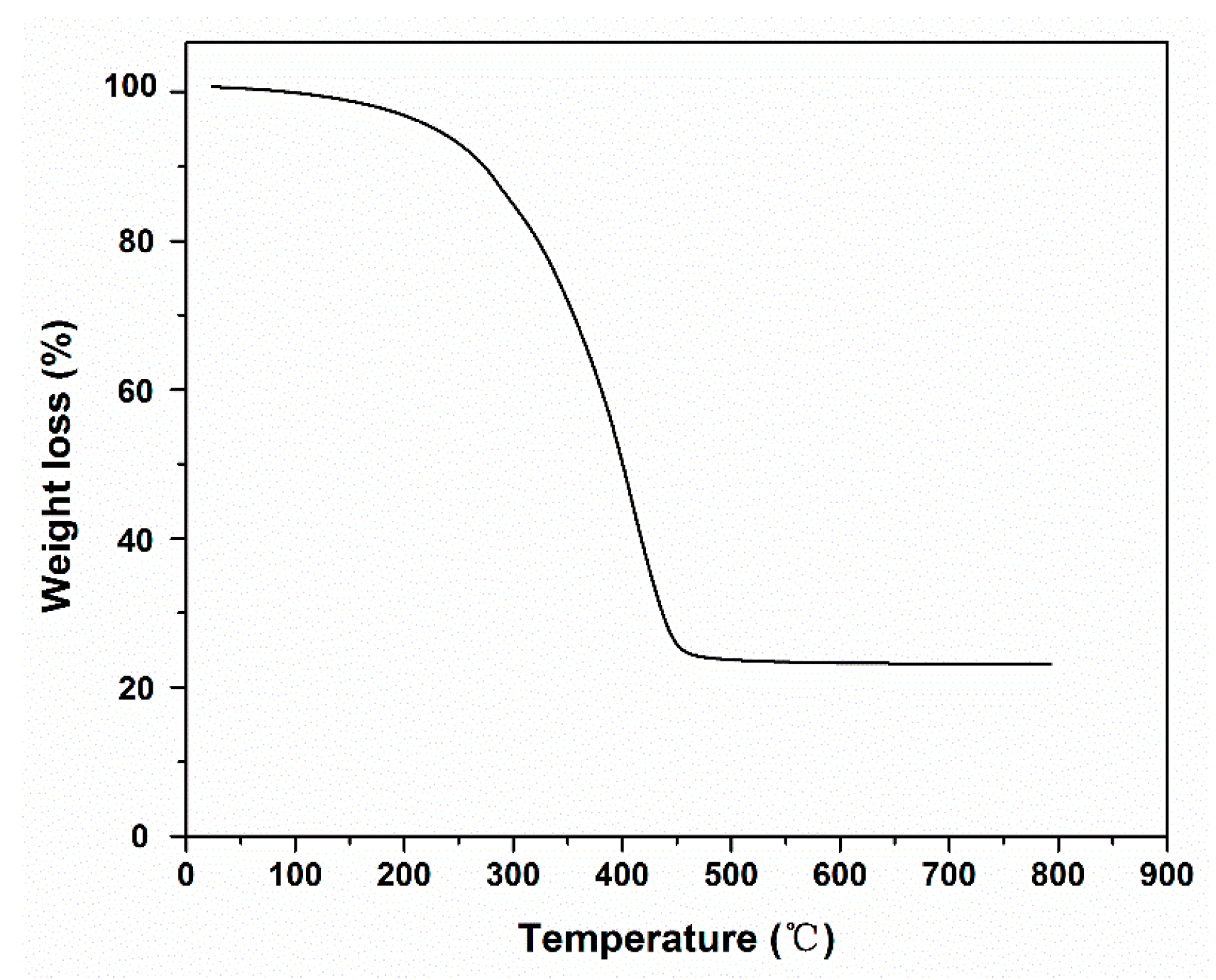
2.4. Pyrolysis of the a-CSEB Polymer
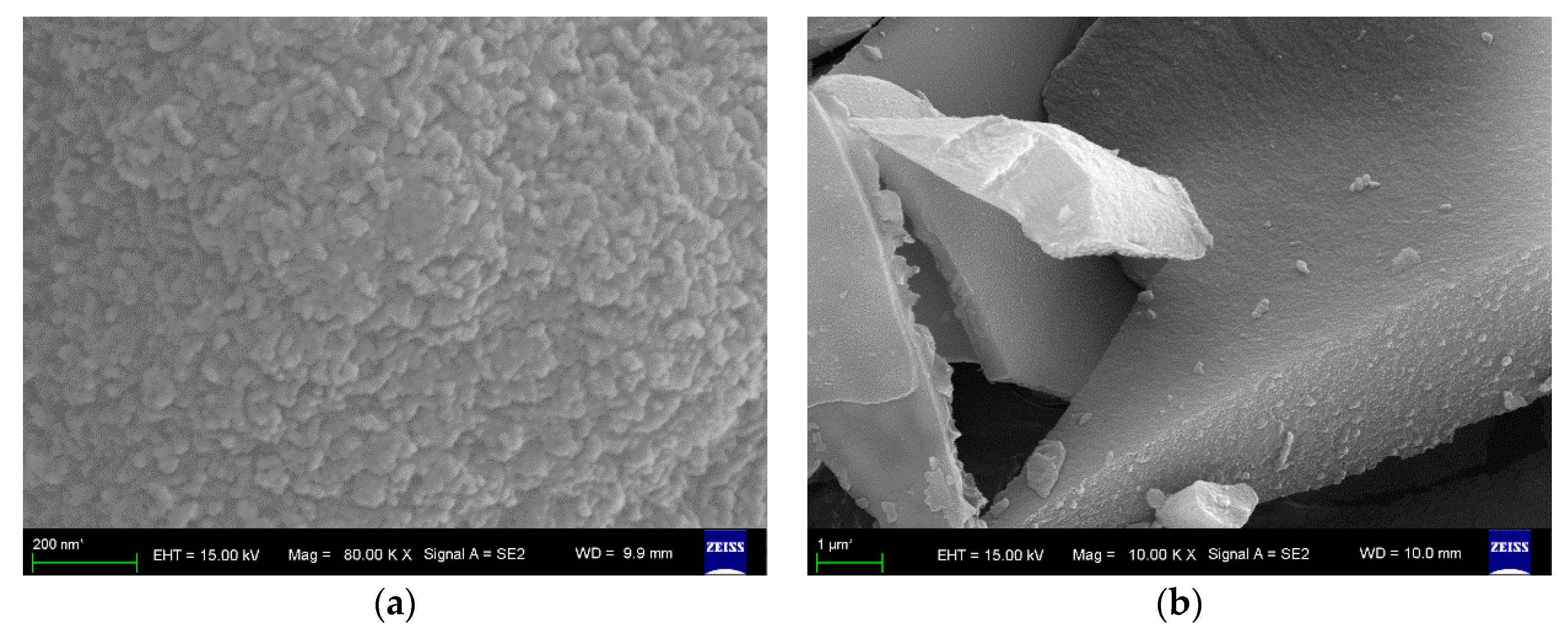
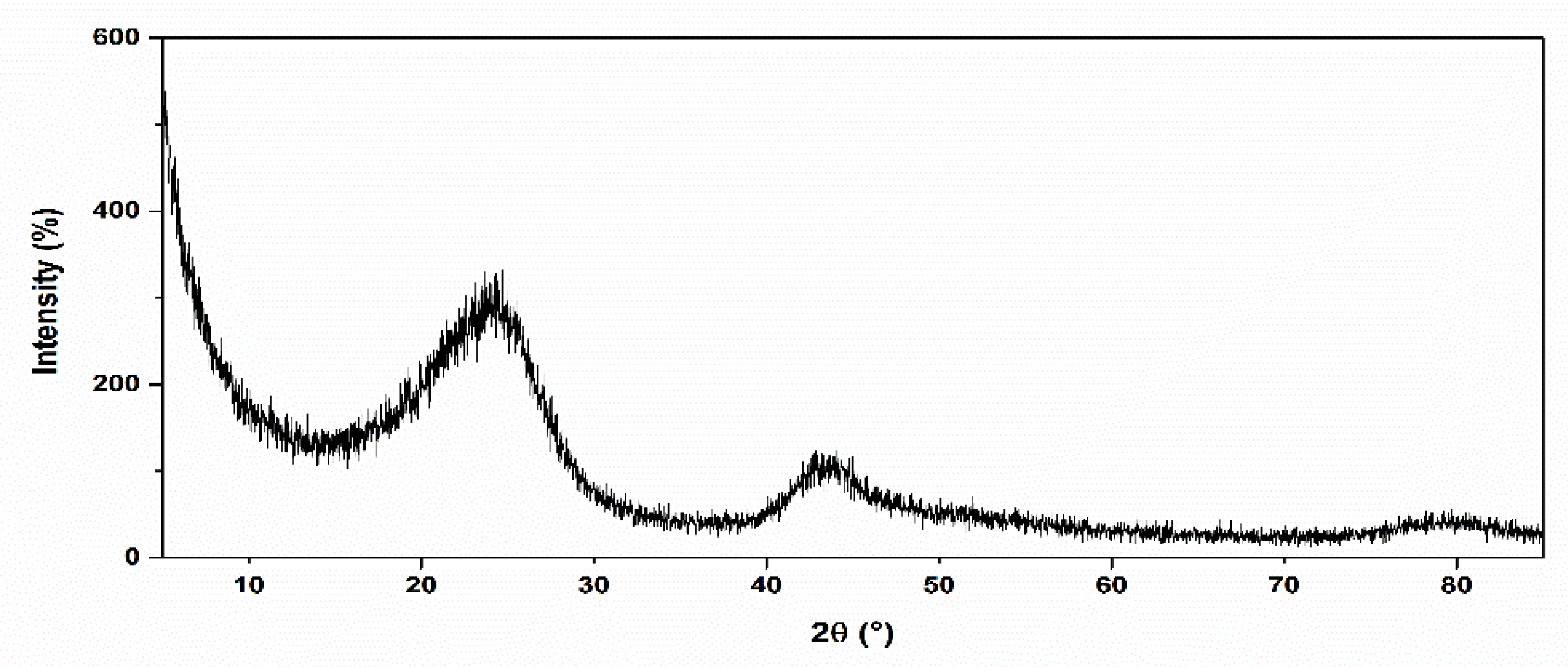
2.5. Characterization of the a-CSEB Ceramic
3. Experimental Section
3.1. Materials
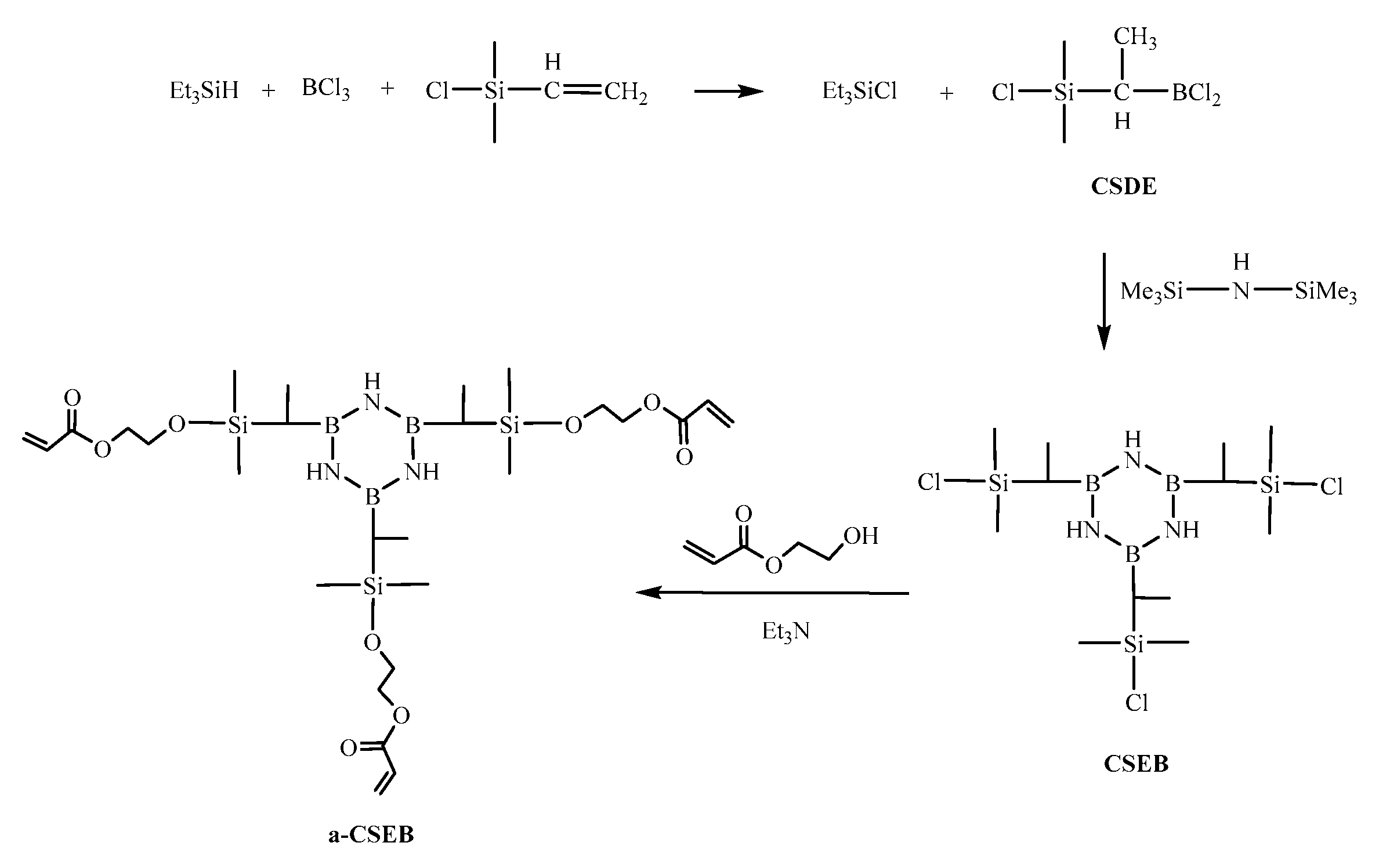
3.2. Synthesis of Single-source Ceramic Precursors
3.2.1. Synthesis of Chloro(dimethyl)silyldichloroborylethane (CSDE)
3.2.2. Synthesis of B,B′,B′′-chloro(dimethyl)silylethylborazine (CSEB)
3.2.3. Synthesis of B,B′,B′′-(dimethyl)ethylacrylatesilyloxyethylborazine (a-CSEB)
3.3. Ultraviolet Light Polymerization
3.4. Pyrolysis
3.5. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramicsl. J. Am. Ceram. Soc. 2010, 97, 1805–1837. [Google Scholar] [CrossRef]
- Widgeon, S.; Mera, G.; Gao, Y.; Sen, S.; Navrotsky, A.; Riedel, R. Effect of Precursor on Speciation and Nanostructure of SiBCN Polymer-Derived Ceramics. J. Am. Ceram. Soc. 2013, 5, 1651–1659. [Google Scholar] [CrossRef]
- Toutois, P.; Miele, P.; Jacques, S.; Cornu, D.; Bernard, S. Structural and Mechanical Behavior of Boron Nitride Fibers Derived from Poly[(Methylamino)Borazine] Precursors: Optimization of the Curing and Pyrolysis Procedures. J. Am. Ceram. Soc. 2006, 89, 43–49. [Google Scholar] [CrossRef]
- Jorg Haug, P.L.; Weinmann, M.; Aldinger, F. Diffraction study on the atomic structure and phase separation of amorphous ceramics in the Si–(B)–C–N system. 2. Si–B–C–N ceramicsl. Chem. Mater. 2004, 16, 83–92. [Google Scholar] [CrossRef]
- Sarkar, S.; Gan, Z.; An, L.; Zhai, L. Structural Evolution of Polymer-Derived Amorphous SiBCN Ceramics at High Temperaturel. J. Phys. Chem. C. 2011, 115, 24993–25000. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, J.; Li, X.D.; Li, W.H.; Wang, H.; Wang, X.Z. Thermal stability of polymer derived SiBNC ceramicsl. Ceram. Int. 2009, 35, 2871–2876. [Google Scholar] [CrossRef]
- Li, W.H.; Wang, J.; Xie, Z.F.; Wang, H. A novel polyborosilazane for high-temperature amorphous Si–B–N–C ceramic fibresl. Ceram. Int. 2012, 38, 6321–6326. [Google Scholar] [CrossRef]
- Zhang, X.F.; Chen, L.X.; Meng, L.L.; Chen, F.F.; Kong, J. Nickel silicide nanocrystal-containing magnetoceramics from the bulk pyrolysis of polysilazane and nickelocenel. Ceram. Int. 2014, 40, 6937–6947. [Google Scholar] [CrossRef]
- Lei, Y.P.; Wang, Y.D.; Song, Y.C. Boron nitride by pyrolysis of the melt-processable poly[tris(methyl-amino)borane]: Structure, composition and oxidation resistancel. Ceram. Int. 2012, 38, 271–276. [Google Scholar] [CrossRef]
- Gao, Y.; Mera, G.; Nguyen, H.; Morita, K.; Kleebe, H.J.; Riedel, R. Processing route dramatically influencing the nanostructure of carbon-rich SiCN and SiBCN polymer-derived ceramics. Part I: Low temperature thermal transformationl. J. Eur. Ceram. Soc. 2012, 32, 1857–1866. [Google Scholar] [CrossRef]
- Jäschke, T.; Jansen, M. Synthesis and characterization of new amorphous Si/B/N/C ceramics with increased carbon content through single-source precursorsl. C.R. Chim. 2004, 7, 471–482. [Google Scholar] [CrossRef]
- Jansen, M.; Jaschke, T. Amorphous Multinary Ceramics in the Si–B–N–C System. Struct. Bond. 2002, 101, 137–191. [Google Scholar]
- Johnson, P.M.; Stansbury, J.W.; Bowman, C.N. FTIR Microscopy for Kinetic Measurements in High-Throughput Photopolymerization: Experimental Design and Applicationl. Macromol. React. Eng. 2009, 3, 522–528. [Google Scholar] [CrossRef]
- Charles Nason, T.R.; Hoyle, C.; Pojman, J.A. UV-Induced Frontal Polymerization of Multifunctional (Meth)acrylatesl. Macromolecules 2005, 38, 5506–5512. [Google Scholar] [CrossRef]
- Chiou, B.S.; Khan, S.A. Real-Time FTIR and in Situ Rheological Studies on the UV Curing Kinetics of Thiol-ene Polymersl. Macromolecules 1997, 30, 7322–7328. [Google Scholar] [CrossRef]
- Kong, J.; Fan, X.D.; Zhang, G.B. Synthesis and UV-curing behaviors of novel rapid UV-curable polyorganosilazanesl. Polymer 2006, 47, 1519–1525. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, G.B.; Fan, X.D. UV-activited hydrosilylation: A facile approach for synthesis of hyperbranched polycarbosilanesl. Appl. Organomet. Chem. 2009, 23, 277–282. [Google Scholar]
- He, W.Q.; Chen, L.X.; Xu, T.T.; Wei, D.; Wang, Y. Synthesis of Silicon Molecular Precursor Chlorosilyl Dichloroboryl Ethane (CSDE) through Experiment Optimization. Chem. Lett. 2014, 44, 70–72. [Google Scholar] [CrossRef]
- Chemtob, A.; Belon, C.; Croutxe´-Barghorn, C.; Rigolet, S. Concomitant Organic-Inorganic UV-Curing Catalyzed by Photoacidsl. Macromolecules 2008, 41, 7390–7398. [Google Scholar] [CrossRef]
- Jäschke, T.; Jansen, M. Synthesis, Crystal Structure, and Spectroscopic Characterization of the Borazine Derivatives [B{CH2(SiCl3)}NH]3 and [B{CH2(SiCl2CH3)}NH]3. ZAAC 2004, 630, 239–243. [Google Scholar] [CrossRef]
- Jäschke, T.; Jansen, M. A new borazine-type single source precursor for Si/B/N/C ceramicsl. J. Mater. Chem. 2006, 16, 2792–2799. [Google Scholar] [CrossRef]
- Bernard, S.; Weinmann, M.; Gerstel, P.; Miele, P.; Aldinger, F. Boron-modified Polysilazane as a Novel Single-source Precursor for SiBCN Ceramic Fibers: Synthesis, Melt-spinning, Curing and Ceramic Conversion. J. Mater. Chem. 2005, 15, 289–299. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
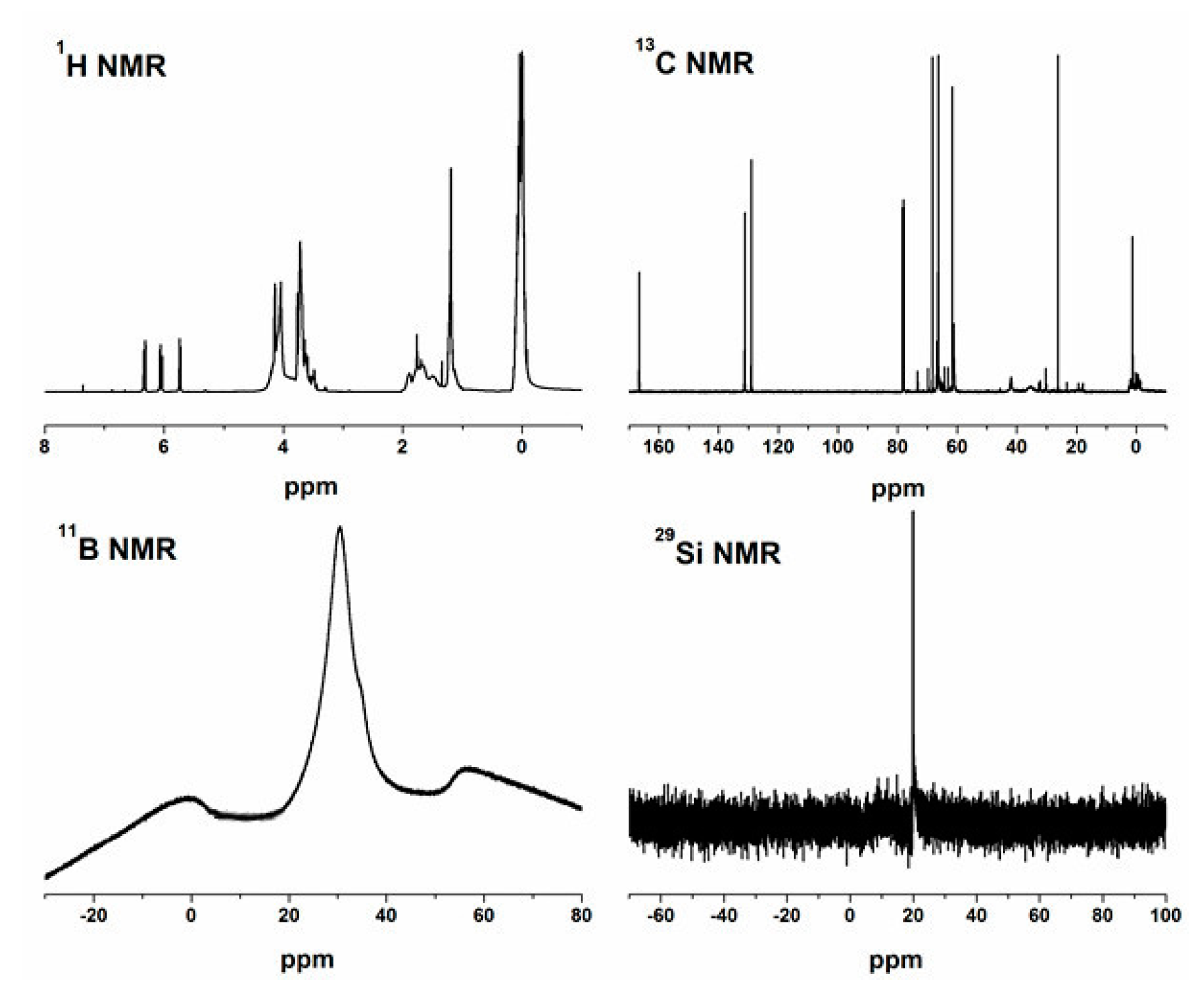

| Nucleus | Observed Multiplicity | δ/ppm | Assignment |
|---|---|---|---|
| 1H | Doublet | 5.72/6.33 | –CH=CH2 |
| 1H | Triplet | 6.02 | –CH=CH2 |
| 1H | Quartet | 1.75 | –CH–CH3 |
| 1H | Doublet | 1.18 | –CH–CH3 |
| 1H | Singlet | 4.0 | NH |
| 1H | Triplet | 4.13 | Si–O–CH2–CH2–O |
| 1H | Triplet | 3.71 | Si–O–CH2–CH2–O |
| 13C | Singlet | 129.03 | –CH=CH2 |
| 13C | Singlet | 131.16 | –CH=CH2 |
| 13C | Singlet | 166.53 | C=O |
| 13C | Singlet | 26.19 | –CH–CH3 |
| 13C | Singlet | 68.36 | –CH–CH3 |
| 13C | Singlet | 61.54 | –CH2–CH2–O– |
| 13C | Singlet | 66.35 | –O–CH2–CH2– |
| 11B | Singlet | 30.00 | |
| 29Si | Singlet | 19.93 |
| Vibrational Mode | a-CSEB Molecule Wave Number/cm−1 | a-CSEB Polymer Wave Number/cm−1 | a-CSEB Ceramic Wave Number/cm−1 |
|---|---|---|---|
| ν (NH) | 3494 | 3494 | |
| ν (C–C) | 3145 | ||
| ν (CH3) | 2961 | 2961 | |
| ν (CH) | 2875 | 2875 | |
| ν (C=O) | 1735 | 1735 | |
| ν (C=C) | 1640/1410 | ||
| νas (B3N3) | 1462 | 1462 | 1402 |
| δas (CH3) | 1340 | 1340 | |
| δs (CH3) | 1250 | 1250 | |
| ν (BC) | 1133 | 1133 | |
| δ (NH) | 1069 | 1069 | |
| ν (SiO) | 1002 | 1002 | 1105 |
| δ (C=C) | 979 | ||
| νas (B3N3) | 840 | 840 | 810 |
| ν (SiC) | 746 | 746 | |
| γ(NH) | 610 | 610 |
| Nucleus | Observed Multiplicity | δ/ppm | Assignment |
|---|---|---|---|
| 1H | Quartet | 1.75 | –CH–CH3 |
| 1H | Doublet | 1.18 | –CH–CH3 |
| 1H | Singlet | 3.79 | NH |
| 1H | Triplet | 4.11 | Si–O–CH2–CH2–O |
| 1H | Triplet | 3.58 | Si–O–CH2–CH2–O |
| 1H | Triplet | 2.40 | –CH2–CH2–CH2–CH2– |
| 1H | Triplet | 1.70 | –CH2–CH2–CH2–CH2– |
| 13C | Singlet | 175.02 | C=O |
| 13C | Singlet | 26.19 | –CH–CH3 |
| 13C | Singlet | 66.49 | –CH–CH3 |
| 13C | Singlet | 60.51 | –CH2–CH2–O– |
| 13C | Singlet | 65.75 | –O–CH2–CH2– |
| 13C | Singlet | 31.92 | –CH2–CH2–CH2–CH2– |
| 13C | Singlet | 22.68 | –CH2–CH2–CH2–CH2– |
| 11B | Singlet | 33.36 | |
| 29Si | Singlet | 21.09 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Chen, L.; Xu, T.; He, W.; Wang, Y. Synthesis and Characterization of a Novel Borazine-Type UV Photo-Induced Polymerization of Ceramic Precursors. Molecules 2016, 21, 801. https://doi.org/10.3390/molecules21060801
Wei D, Chen L, Xu T, He W, Wang Y. Synthesis and Characterization of a Novel Borazine-Type UV Photo-Induced Polymerization of Ceramic Precursors. Molecules. 2016; 21(6):801. https://doi.org/10.3390/molecules21060801
Chicago/Turabian StyleWei, Dan, Lixin Chen, Tingting Xu, Weiqi He, and Yi Wang. 2016. "Synthesis and Characterization of a Novel Borazine-Type UV Photo-Induced Polymerization of Ceramic Precursors" Molecules 21, no. 6: 801. https://doi.org/10.3390/molecules21060801
APA StyleWei, D., Chen, L., Xu, T., He, W., & Wang, Y. (2016). Synthesis and Characterization of a Novel Borazine-Type UV Photo-Induced Polymerization of Ceramic Precursors. Molecules, 21(6), 801. https://doi.org/10.3390/molecules21060801





