MASM, a Matrine Derivative, Offers Radioprotection by Modulating Lethal Total-Body Irradiation-Induced Multiple Signaling Pathways in Wistar Rats
Abstract
:1. Introduction
2. Results and Discussion
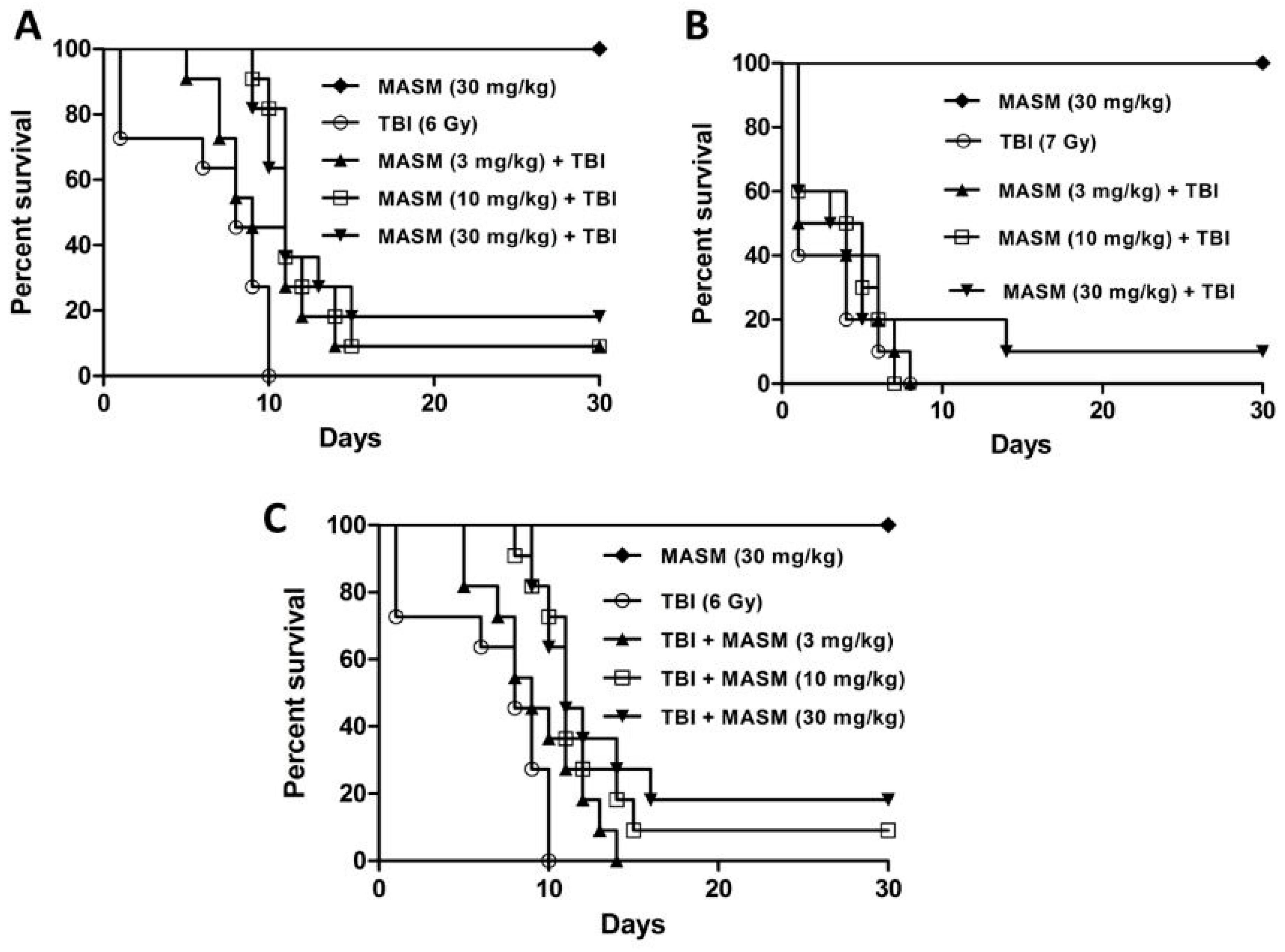
2.1. Effect of MASM on 30 Day Survival
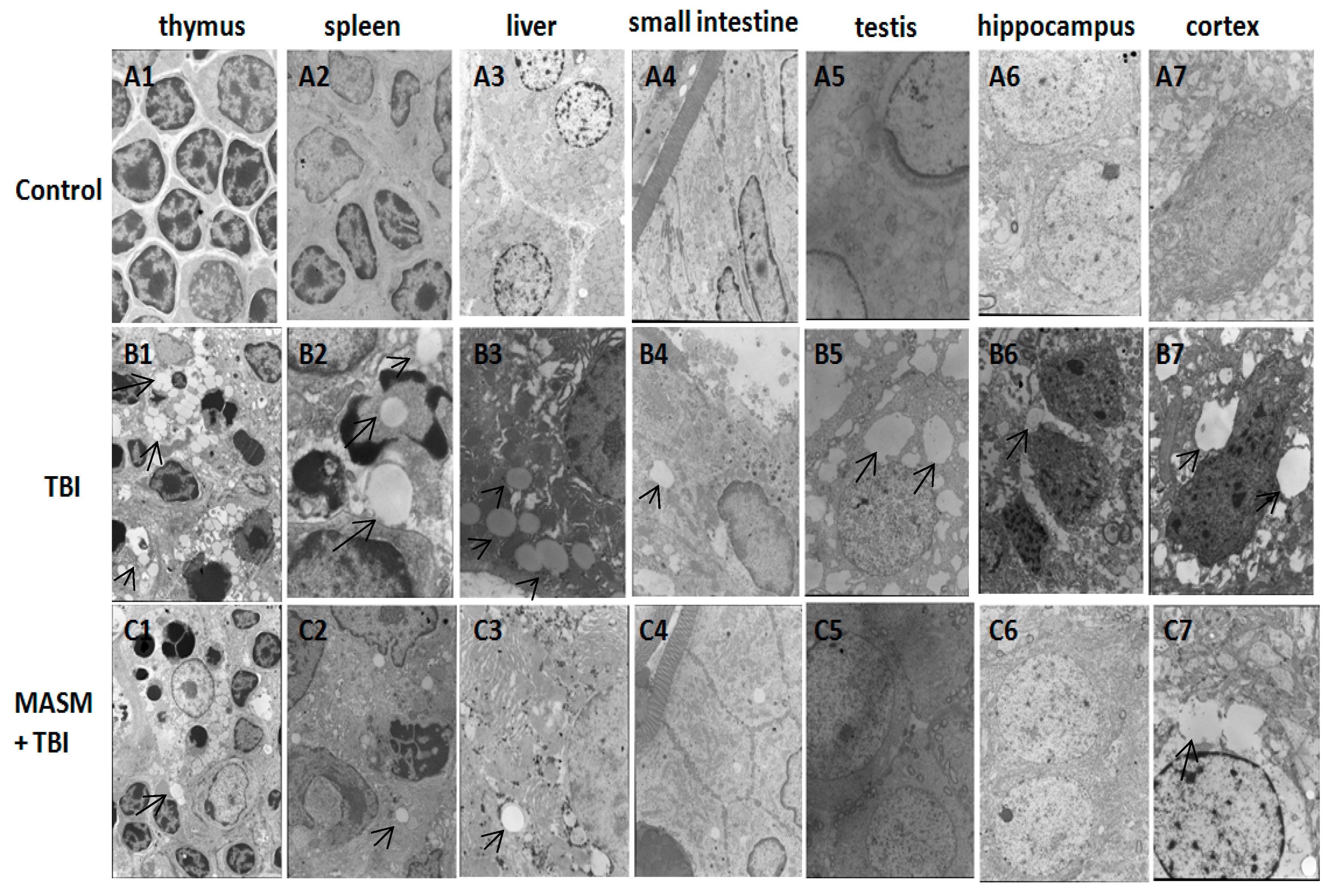
2.2. Effect of MASM on Radiation-Induced Tissues Injury Using Transmission Electron Microscopy
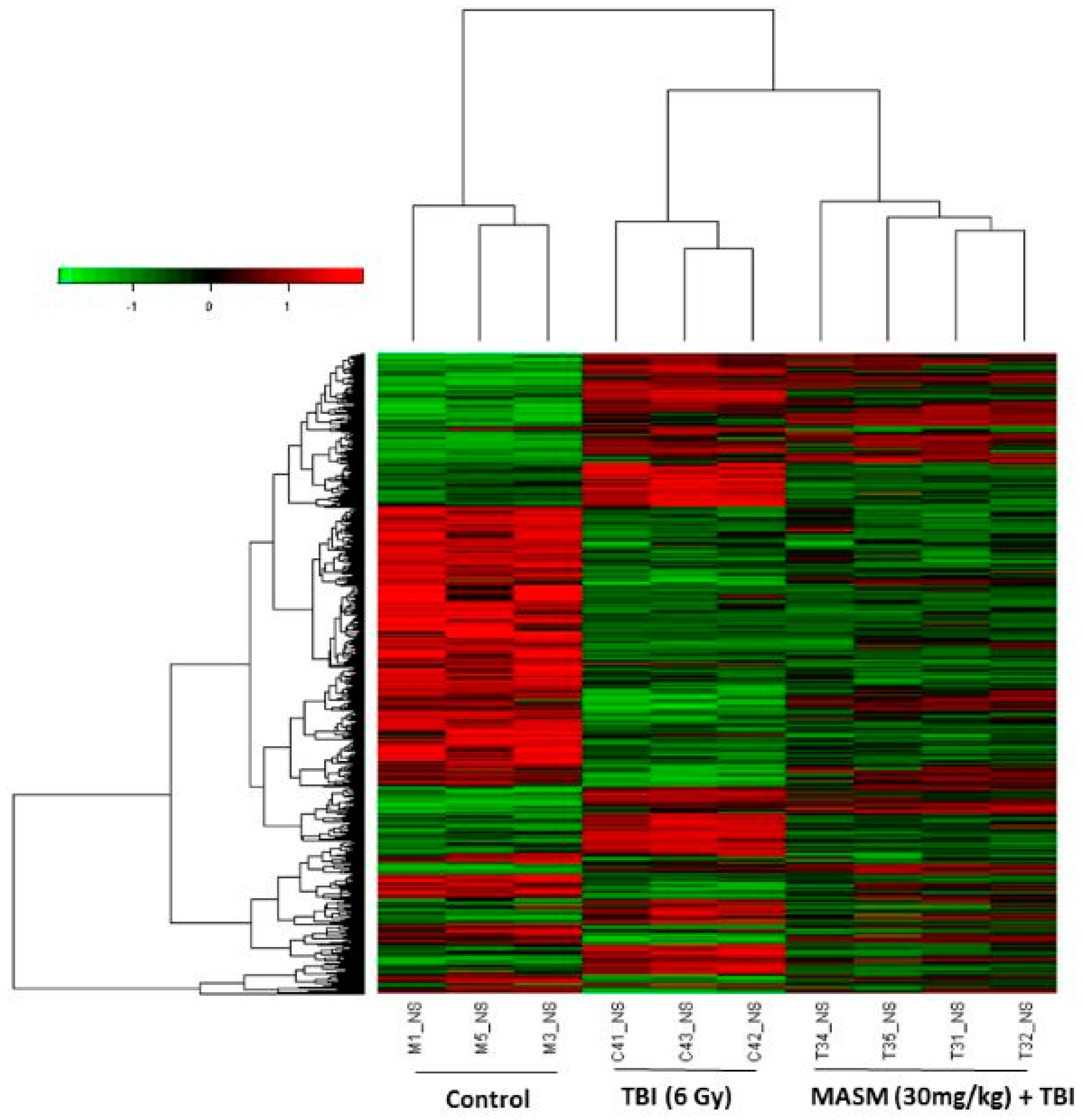
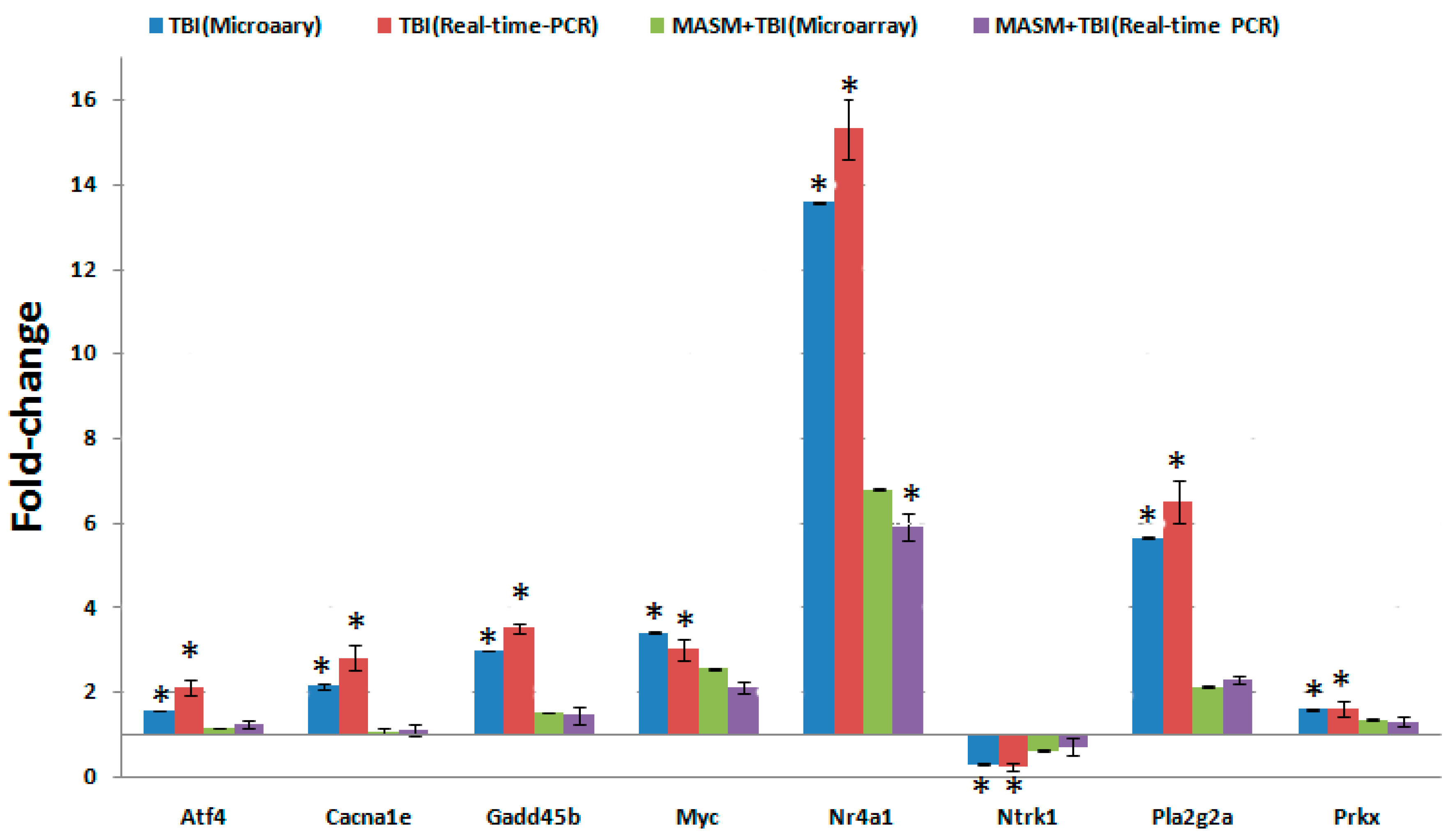
2.3. MASM Prevents TBI-Induced Differential Expression of Many Genes
3. Materials and Methods
3.1. Chemicals
3.2. Animals
3.3. Radiation and Administration
3.4. Survival Assays
3.5. Sample Collection
3.6. Transmission Electron Microscopy
3.7. Gene Expression Microarray and Data Analysis
3.8. Quantitative Real Time (qRT)-PCR Array Validation
3.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moores, B.M.; Regulla, D. A review of the scientific basis for radiation protection of the patient. Radiat. Prot. Dosim. 2011, 147, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N. Recent insights into the biological action of heavy-ion radiation. J. Radiat. Res. 2009, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bortfeld, T.; Jeraj, R. The physical basis and future of radiation therapy. Br. J. Radiol. 2011, 84, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.M.; Nair, C.K. Radiation protection by diethyldithiocarbamate: Protection of membrane and DNA in vitro and in vivo against gamma-radiation. J. Radiat. Res. 2004, 45, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Nair, C.K.; Parida, D.K.; Nomura, T. Radioprotectors in radiotherapy. J. Radiat. Res. 2001, 42, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J. Trends in the development of radioprotective agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Wang, Y.; Xing, R.; Yi, C.; Zhu, H.; Chen, X.; Guo, J.; Guo, W.; Li, W.; et al. Matrine, a novel autophagy inhibitor, blocks trafficking and the proteolytic activation of lysosomal proteases. Carcinogenesis 2013, 34, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Zhuang, Y.; Wang, J.; Ye, J.; Zhang, S.; Wu, J.; Yu, K.; Han, Y. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS ONE 2012, 7, e46853. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, W.; Liu, J.; Xue, X.; Lu, Y.; Shan, H.; Yang, B. Antiarrhythmic properties of long-term treatment with matrine in arrhythmic rat induced by coronary ligation. Biol. Pharm. Bull. 2009, 32, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Hu, J.H.; Zhu, Q.G.; Li, F.Q.; Wang, J.; Sun, H.J. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int. Immunopharmacol. 2007, 7, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xia, B.; Zhang, L.; Zhou, F.; Zhang, Y.X.; Ye, M.; Hu, Z.G.; Li, J.; Li, J.; Wang, Z.L.; et al. Matrine improves 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Pharmacol. Res. 2006, 53, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Lin, X.T.; Zeng, K.L.; Zhang, L. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis. Int. 2004, 3, 69–72. [Google Scholar] [PubMed]
- Zhang, J.P.; Zhang, M.; Zhou, J.P.; Liu, F.T.; Zhou, B.; Xie, W.F.; Guo, C. Antifibrotic effects of matrine on in vitro and in vivo models of liver fibrosis in rats. Acta Pharmacol. Sin. 2001, 22, 183–186. [Google Scholar] [PubMed]
- Sun, M.; Cao, H.; Sun, L.; Dong, S.; Bian, Y.; Han, J.; Zhang, L.; Ren, S.; Hu, Y.; Liu, C.; et al. Antitumor activities of kushen: Literature review. Evid. Based Complement. Altern. Med. 2012, 2012, 373219. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.X.; Lin, C.L.; Liang, L.; Zhao, Y.Y.; Liu, J.; Cui, J.; Yang, Q.M.; Wang, Y.L.; Zhang, A.R. Enhancing effect of compound Kusheng injection in combination with chemotherapy for patients with advanced non-small cell lung cancer. Chin. J. Oncol. 2010, 32, 294–297. [Google Scholar]
- Chen, J.; Mei, Q.; Xu, Y.C.; Du, J.; Wei, Y.; Xu, Z.M. Effects of Matrine Injection on T-lymphocyte subsets of patients with malignant tumor after gamma knife radiosurgery. J. Chin. Integr. Med. 2006, 4, 78–79. [Google Scholar] [CrossRef]
- Lao, Y. Clinical study on effect of matrine injection to protect the liver function for patients with primary hepatic carcinoma after trans-artery chemo-embolization (TAE). J. Chin. Med. Mater. 2005, 28, 637–638. [Google Scholar]
- Lao, Y. Clinical study of matrine injection on preventing liver function damage of anti-tumor drugs during chemotherapy of breast cancer. J. Chin. Med. Mater. 2005, 28, 735–737. [Google Scholar]
- Hu, H.; Wang, S.; Zhang, C.; Wang, L.; Ding, L.; Zhang, J.; Wu, Q. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines. Bioorg. Med. Chem. Lett. 2010, 20, 7537–7539. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Hu, H.G.; Tian, Y.; Wang, S.Z.; Li, J.; Li, J.Z.; Deng, X.; Qian, H.; Qiu, L.; Hu, Z.L.; et al. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis. Hepatology 2014, 60, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Stryker, J.A. Science to practice: Why is the liver a radiosensitive organ? Radiology 2007, 242, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Julius, D.; Nathans, J. Signaling by sensory receptors. Cold Spring Harb. Perspect. Biol. 2012, 4, a005991. [Google Scholar] [CrossRef] [PubMed]
- Herok, R.; Konopacka, M.; Polanska, J.; Swierniak, A.; Rogolinski, J.; Jaksik, R.; Hancock, R.; Rzeszowska-Wolny, J. Bystander effects induced by medium from irradiated cells: Similar transcriptome responses in irradiated and bystander K562 cells. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhang, E.; He, R.; Wang, X. Newly developed strategies for improving sensitivity to radiation by targeting signal pathways in cancer therapy. Cancer Sci. 2013, 104, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Naziroglu, M.; Tokat, S.; Demirci, S. Role of melatonin on electromagnetic radiation-induced oxidative stress and Ca2+ signaling molecular pathways in breast cancer. J. Recept. Signal Transduct. Res. 2012, 32, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahmed, M.M. A global perspective of radiation-induced signal transduction pathways in cancer therapeutics. Indian J. Exp. Biol. 2004, 42, 1153–1176. [Google Scholar] [PubMed]
- Dent, P.; Yacoub, A.; Fisher, P.B.; Hagan, M.P.; Grant, S. MAPK pathways in radiation responses. Oncogene 2003, 22, 5885–5896. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Gremy, O.; Benderitter, M. Reduction of peroxisome proliferation-activated receptor gamma expression by gamma-irradiation as a mechanism contributing to inflammatory response in rat colon: Modulation by the 5-aminosalicylic acid agonist. J. Pharmacol. Exp. Ther. 2008, 324, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Schmidberger, H.; Lakomek, M.; Witt, O.; Wuttke, W.; Jarry, H. Reduction of gamma-aminobutyric acid-ergic neurotransmission as a putative mechanism of radiation induced activation of the gonadotropin releasing-hormone-pulse generator leading to precocious puberty in female rats. Neurosci. Lett. 2001, 297, 45–48. [Google Scholar] [CrossRef]
- Olme, C.H.; Finnon, R.; Brown, N.; Kabacik, S.; Bouffler, S.D.; Badie, C. Live cell detection of chromosome 2 deletion and Sfpi1/PU1 loss in radiation-induced mouse acute myeloid leukaemia. Leuk. Res. 2013, 37, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.M.; Bedford, J.S.; Bielefeldt-Ohmann, H.; Ray, F.A.; Genik, P.C.; Ehrhart, E.J.; Fallgren, C.M.; Hailu, F.; Battaglia, C.L.; Charles, B.; et al. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon 56Fe ions. Radiat. Res. 2009, 172, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, A.I.; Tishkin, S.M.; Zelensky, S.N.; Ivanova, I.V.; Kizub, I.V.; Pavlova, A.A.; Moreland, R.S. Ionizing radiation alters myofilament calcium sensitivity in vascular smooth muscle: Potential role of protein kinase C. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R755–R762. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ivanov, V.N.; Gillespie, J.; Geard, C.R.; Amundson, S.A.; Brenner, D.J.; Yu, Z.; Lieberman, H.B.; Hei, T.K. Mechanism of radiation-induced bystander effect: Role of the cyclooxygenase-2 signaling pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 14641–14646. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Schneider, U. A method for determining weights for excess relative risk and excess absolute risk when applied in the calculation of lifetime risk of cancer from radiation exposure. Radiat. Environ. Biophys. 2013, 52, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Little, J.B. Radiation carcinogenesis. Carcinogenesis 2000, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gauger, M.A.; Sancar, A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 2005, 65, 6828–6834. [Google Scholar] [CrossRef] [PubMed]
- Rubin, N.H. Influence of the circadian rhythm in cell division on radiation-induced mitotic delay in vivo. Radiat. Res. 1982, 89, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Butler, E.B.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, M.W.; Hirst, D.G. Nicotinamide relaxes vascular smooth muscle by inhibiting myosin light chain kinase-dependent signaling pathways: Implications for anticancer efficacy. Oncol. Res. 2004, 14, 483–489. [Google Scholar] [PubMed]
- Munshi, A.; Ramesh, R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Ullrich, R.K.; Dent, P.; Grant, S.; Mikkelsen, R.B.; Valerie, K. Signal transduction and cellular radiation responses. Radiat. Res. 2000, 153, 245–257. [Google Scholar] [CrossRef]
- Zhao, B.X.; Chen, H.Z.; Du, X.D.; Luo, J.; He, J.P.; Wang, R.H.; Wang, Y.; Wu, R.; Hou, R.R.; Hong, M.; et al. Orphan receptor TR3 enhances p53 transactivation and represses DNA double-strand break repair in hepatoma cells under ionizing radiation. Mol. Endocrinol. 2011, 25, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Ameri, K.; Harris, A.L. Activating transcription factor 4. Int. J. Biochem. Cell Biol. 2008, 40, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Sonntag, W.E.; Mitschelen, M.; Yan, H.; Lee, Y.W. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int. J. Radiat. Biol. 2010, 86, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Di Giacomo, V.; Rapino, M.; Zara, S.; Rana, R.A. Ionizing radiation induces apoptotic signal through protein kinase Cdelta (delta) and survival signal through Akt and cyclic-nucleotide response element-binding protein (CREB) in Jurkat T cells. Biol. Bull. 2009, 217, 202–212. [Google Scholar] [PubMed]
- Chappell, J.; Dalton, S. Roles for MYC in the Establishment and Maintenance of Pluripotency. Cold Spring Harb. Perspect. Med. 2013, 3, a014381. [Google Scholar] [CrossRef] [PubMed]
- Borovitskaya, A.E.; Evtushenko, V.I.; Sabol, S.L. Gamma-radiation-induced cell death in the fetal rat brain possesses molecular characteristics of apoptosis and is associated with specific messenger RNA elevations. Brain Res. Mol. Brain Res. 1996, 35, 19–30. [Google Scholar] [CrossRef]
- Wilson, R.E.; Taylor, S.L.; Atherton, G.T.; Johnston, D.; Waters, C.M.; Norton, J.D. Early response gene signalling cascades activated by ionising radiation in primary human B cells. Oncogene 1993, 8, 3229–3237. [Google Scholar] [PubMed]
- Liebermann, D.A.; Hoffman, B. Gadd45 in the response of hematopoietic cells to genotoxic stress. Blood Cells Mol. Dis. 2007, 39, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.B.; McLeland, C.B.; Blakely, W.F. Real-time quantitative RT-PCR assay of GADD45 gene expression changes as a biomarker for radiation biodosimetry. Int. J. Radiat. Biol. 2002, 78, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Daino, K.; Ichimura, S.; Nenoi, M. Early induction of CDKN1A (p21) and GADD45 mRNA by a low dose of ionizing radiation is due to their dose-dependent post-transcriptional regulation. Radiat. Res. 2002, 157, 478–482. [Google Scholar] [CrossRef]
- Li, X.; Li, H.P.; Amsler, K.; Hyink, D.; Wilson, P.D.; Burrow, C.R. PRKX, a phylogenetically and functionally distinct cAMP-dependent protein kinase, activates renal epithelial cell migration and morphogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 9260–9265. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.; Bae, J.H.; Kim, M.J.; Hwang, J.Y.; Kim, S.J.; Yoon, M.S.; Lee, M.K.; Kim, D.W.; Chung, B.S.; Kang, C.D.; et al. Radiosensitization by targeting radioresistance-related genes with protein kinase A inhibitor in radioresistant cancer cells. Exp. Mol. Med. 2005, 37, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Ohnishi, K.; Asahi, M.; Wang, X.; Takahashi, A.; Ohnishi, T. Effects of protein kinase inhibitors on radiation-induced WAF1 accumulation in human cultured melanoma cells. Br. J. Dermatol. 1999, 141, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Elfenbein, A.; Simons, M. Non-canonical fibroblast growth factor signalling in angiogenesis. Cardiovasc. Res. 2008, 78, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Bull, H.A.; Leslie, T.A.; Chopra, S.; Dowd, P.M. Expression of nerve growth factor receptors in cutaneous inflammation. Br. J. Dermatol. 1998, 139, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Weinkauf, B.; Rukwied, R.; Quiding, H.; Dahllund, L.; Johansson, P.; Schmelz, M. Local gene expression changes after UV-irradiation of human skin. PLoS ONE 2012, 7, e39411. [Google Scholar] [CrossRef] [PubMed]
- Mayahara, K.; Yamaguchi, A.; Sakaguchi, M.; Igarashi, Y.; Shimizu, N. Effect of Ga-Al-As laser irradiation on COX-2 and cPLA2-alpha expression in compressed human periodontal ligament cells. Lasers Surg. Med. 2010, 42, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Degousee, N.; Ghomashchi, F.; Stefanski, E.; Singer, A.; Smart, B.P.; Borregaard, N.; Reithmeier, R.; Lindsay, T.F.; Lichtenberger, C.; Reinisch, W.; et al. Groups IV, V, and X phospholipases A2s in human neutrophils: Role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J. Biol. Chem. 2002, 277, 5061–5073. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.H.; Ma, C.H.; Yue, Z.Q.; Yao, X.; Mao, C.M. Protective Effect of Naringenin against Lipopolysaccharide-Induced Injury in Normal Human Bronchial Epithelium via Suppression of MAPK Signaling. Inflammation 2015, 38, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Martinel Lamas, D.J.; Carabajal, E.; Prestifilippo, J.P.; Rossi, L.; Elverdin, J.C.; Merani, S.; Bergoc, R.M.; Rivera, E.S.; Medina, V.A. Protection of radiation-induced damage to the hematopoietic system, small intestine and salivary glands in rats by JNJ7777120 compound, a histamine H4 ligand. PLoS ONE 2013, 8, e69106. [Google Scholar]
- Che, J.; Lu, Y.W.; Sun, K.K.; Feng, C.; Dong, A.J.; Jiao, Y. Overexpression of TOB1 confers radioprotection to bronchial epithelial cells through the MAPK/ERK pathway. Oncol. Rep. 2013, 30, 637–642. [Google Scholar] [PubMed]
- Arany, P.R.; Flanders, K.C.; DeGraff, W.; Cook, J.; Mitchell, J.B.; Roberts, A.B. Absence of Smad3 confers radioprotection through modulation of ERK-MAPK in primary dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, R.; Burns, T.M.; Kiang, J.G. Ciprofloxacin enhances stress erythropoiesis in spleen and increases survival after whole-body irradiation combined with skin-wound trauma. PLoS ONE 2014, 9, e90448. [Google Scholar] [CrossRef] [PubMed]
- Toth, L.A. Defining the moribund condition as an experimental endpoint for animal research. ILAR J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 2000, 41, 72–79. [Google Scholar] [CrossRef]
- Hussein, M.R.; Abu-Dief, E.E.; Abou El-Ghait, A.T.; Adly, M.A.; Abdelraheem, M.H. Morphological evaluation of the radioprotective effects of melatonin against X-ray-induced early and acute testis damage in Albino rats: An animal model. Int. J. Exp. Pathol. 2006, 87, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Gong, X.; Liu, Y.; Feng, H.; Qiu, L.; Hu, Z.; Zhang, J. A transcript profiling approach reveals the zinc finger transcription factor ZNF191 is a pleiotropic factor. BMC Genom. 2009, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
| GOId | Name | Hits | Percent | Enrichment Test p Value |
|---|---|---|---|---|
| GO:0003674 | molecular_function | 550 | 3.7% | 1.0 |
| GO:0005215 | transporter activity | 65 | 6.07% | 0.0094 |
| GO:0045499 | chemorepellent activity | 2 | 40.0% | 0.0331 |
| GO:0005575 | cellular_component | 555 | 3.78% | 1.0 |
| GO:0044456 | synapse part | 24 | 6.72% | 0.0376 |
| GO:0008150 | biological_process | 518 | 3.76% | 1.0 |
| GO:0016265 | death | 75 | 5.69% | 0.0219 |
| GO:0032501 | multicellular organismal process | 275 | 5.1% | 0.0054 |
| GO:0032502 | developmental process | 204 | 5.02% | 0.0324 |
| GO:0040007 | growth | 36 | 6.04% | 0.0484 |
| GO:0040011 | locomotion | 54 | 6.41% | 0.0068 |
| GO:0048511 | rhythmic process | 26 | 11.02% | 0.0001 |
| GO:0050896 | response to stimulus | 236 | 4.96% | 0.0344 |
| GO:0051179 | localization | 174 | 5.12% | 0.0281 |
| Name | Hits | Percent | Enrichment Test p Value |
|---|---|---|---|
| Olfactory transduction | 58 | 5.69% | 0.0 |
| Metabolic pathways | 38 | 3.15% | 0.0091 |
| Neuroactive ligand-receptor interaction | 18 | 5.54% | 0.0003 |
| Pathways in cancer | 16 | 4.79% | 0.0024 |
| MAPK signaling pathway | 13 | 4.73% | 0.0065 |
| Calcium signaling pathway | 13 | 6.81% | 0.0003 |
| Vascular smooth muscle contraction | 12 | 9.38% | 0.0 |
| Cytokine-cytokine receptor interaction | 12 | 4.88% | 0.0070 |
| Purine metabolism | 10 | 5.99% | 0.0035 |
| PPAR signaling pathway | 9 | 12.0% | 0.0001 |
| GnRH signaling pathway | 8 | 8.08% | 0.0016 |
| Gap junction | 8 | 9.2% | 0.0007 |
| Arachidonic acid metabolism | 7 | 9.46% | 0.0013 |
| Adipocytokine signaling pathway | 7 | 10.45% | 0.0008 |
| RNA degradation | 6 | 9.84% | 0.0024 |
| Type II diabetes mellitus | 5 | 9.43% | 0.0065 |
| Fatty acid metabolism | 5 | 11.11% | 0.0034 |
| Acute myeloid leukemia | 5 | 8.47% | 0.0098 |
| Galactose metabolism | 4 | 16.67% | 0.0024 |
| Circadian rhythm-mammal | 4 | 30.77% | 0.0003 |
| Bladder cancer | 4 | 10.81% | 0.0095 |
| GenBank | Symbol | Description | Fold Change TBI (6 Gy) | Fold Change MASM + TBI |
|---|---|---|---|---|
| NM_130817 | Fgf3 | fibroblast growth factor 3 | 2.11 | 1.55 |
| NM_012603 | Myc | myelocytomatosis oncogene | 3.41 | 2.54 |
| NM_031598 | Pla2g2a/sPLA2 | phospholipase A2, group IIA | 5.65 | 2.12 |
| NM_001008321 | Gadd45b | growth arrest and DNA-damage-inducible, β | 3.0 | 1.53 |
| XM_001073032 | Map3k10 | mitogen activated protein kinase kinase kinase 10 | 2.13 | 1.02 |
| XM_344798 | RGD1306565/Ask1/Map3k5 | similar to apoptosis signal-regulating kinase 1 | 0.42 | 0.76 |
| NM_001033963 | Prkx/PKA | protein kinase, X-linked | 1.58 | 1.34 |
| NM_019294 | Cacna1e | calcium channel, voltage-dependent, R type, alpha 1E subunit | 2.14 | 1.08 |
| NM_053873 | Cacna1s/CACN | calcium channel, voltage-dependent, L type, alpha 1S subunit | 3.30 | 1.13 |
| NM_021589 | Ntrk1 | neurotrophic tyrosine kinase, receptor, type 1 | 0.29 | 0.61 |
| NM_022702 | Taok2 | TAO kinase 2 | 1.66 | 1.0 |
| NM_024403 | Atf4/CREB | activating transcription factor 4 | 1.54 | 1.13 |
| NM_024388 | Nr4a1/Nur77 | nuclear receptor subfamily 4, group A, member 1 | 13.56 | 6.78 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, J.; Lu, Y.; Qiu, L.; Xu, W.; Lu, B.; Hu, Z.; Chu, Z.; Chai, Y.; Zhang, J. MASM, a Matrine Derivative, Offers Radioprotection by Modulating Lethal Total-Body Irradiation-Induced Multiple Signaling Pathways in Wistar Rats. Molecules 2016, 21, 649. https://doi.org/10.3390/molecules21050649
Li J, Xu J, Lu Y, Qiu L, Xu W, Lu B, Hu Z, Chu Z, Chai Y, Zhang J. MASM, a Matrine Derivative, Offers Radioprotection by Modulating Lethal Total-Body Irradiation-Induced Multiple Signaling Pathways in Wistar Rats. Molecules. 2016; 21(5):649. https://doi.org/10.3390/molecules21050649
Chicago/Turabian StyleLi, Jianzhong, Jing Xu, Yiming Lu, Lei Qiu, Weiheng Xu, Bin Lu, Zhenlin Hu, Zhiyong Chu, Yifeng Chai, and Junping Zhang. 2016. "MASM, a Matrine Derivative, Offers Radioprotection by Modulating Lethal Total-Body Irradiation-Induced Multiple Signaling Pathways in Wistar Rats" Molecules 21, no. 5: 649. https://doi.org/10.3390/molecules21050649
APA StyleLi, J., Xu, J., Lu, Y., Qiu, L., Xu, W., Lu, B., Hu, Z., Chu, Z., Chai, Y., & Zhang, J. (2016). MASM, a Matrine Derivative, Offers Radioprotection by Modulating Lethal Total-Body Irradiation-Induced Multiple Signaling Pathways in Wistar Rats. Molecules, 21(5), 649. https://doi.org/10.3390/molecules21050649





