Abstract
A novel metal-free organobase-catalyzed regioselective benzoylation of diols and carbohydrates has been developed. Treatment of diol and carbohydrate substrates with 1.1 equiv. of 1-benzoylimidazole and 0.2 equiv. of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in MeCN under mild conditions resulted in highly regioselective benzoylation for the primary hydroxyl group. Importantly, compared to most commonly used protecting bulky groups for primary hydroxyl groups, the benzoyl protective group offers a new protection strategy.
1. Introduction
Selective protection of diols and polyols remains as a frequently encountered problem in organic chemistry during the synthesis of natural products and new drug candidates, especially, in carbohydrate chemistry [1,2,3,4]. Regioselectivity is the major obstacle to the synthesis of novel medicinal agents from readily available monosaccharide derivatives [5,6,7,8]. More specifically, developing environment-friendly, convenient, efficient and highly regioselective carbohydrate protection methods remains as one of the most prominent challenges [9]. Over the last several decades, many protection methods have been developed, including the use of reagents such as organotin [10,11,12], organoboron [13,14], organosilicon [15,16,17], metal salts [18,19,20,21,22,23], organobase [24,25,26], and enzymes [27,28,29,30]. Although these reagents each have advantages, they also possess troublesome shortcomings including inherent toxicity, high cost and the necessity to pre-protect secondary hydroxyl groups. One of the most frequently used protection methods is benzoylation. Many regioselective benzoylation methods using the above discussed reagents [20,21,22,25,26,27], have been reported accordingly. The Mitsunobu reaction is widely used in substitution of primary or secondary alcohols with nucleophiles mediated by a redox combination of a trialkyl or triarylphosphine and a dialkyl azodicarboxylate [31]. It may lead to regioselective benzoylation in some cases, but has poor regioselectivity and is more suitable for protection of secondary hydroxyls [32]. The DCC-mediated reaction, popularly known as the Steglich Esterification, may also lead to moderate regioselectivities in the benzoylation of hydroxyl groups with an excess amount of alcohol [33]. Albert and co-wokers used DMAP and DCC as counterion to obtain direct regioselective acetylation of octyl β-d-glucopyranoside, but with unsatisfactory results [34]. Recently, a green regioselective benzoylation method using benzoate anion as a catalyst was developed by us [35]. However, the method is not suitable for monobenzoylation of the unprotected methyl d-pyranosides. We also developed a regioselective acetylation method conducted in water with tetramethyl-ammonium hydroxide (TMAH) as a catalyst [36]. Despite its high selectivity, its main drawback is that it cannot be applied on substrates with poor water solubility. Thus, we started to investigate organobase-(DBU)-catalyzed selective benzoylation of diols and carbohydrates in organic solvents such as acetonitrile, where 1-benzoylimidazole was used as the acylation reagent, as in our previous study. As expected, this method showed good regioselectivity for the primary hydroxyl group in most cases (Scheme 1). Compared to other methods, not only does this robust method show better regioselectivities, but the catalyst is metal-free, and the reagents used are of lower cost, and easily acquired. Particularly, in contrast to the most often used protecting bulky groups for primary hydroxyl groups such as silyl [37] or trityl groups [38] which are removed under acid conditions, removal of the benzoyl group can take place in the presence of base.
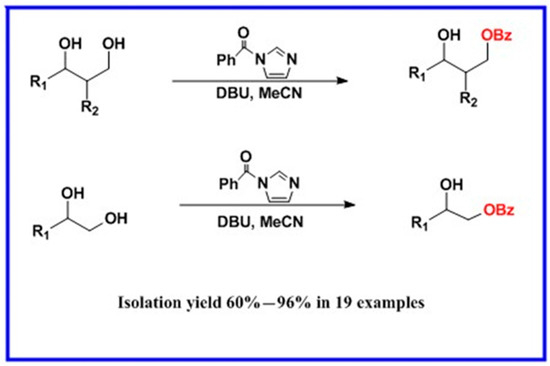
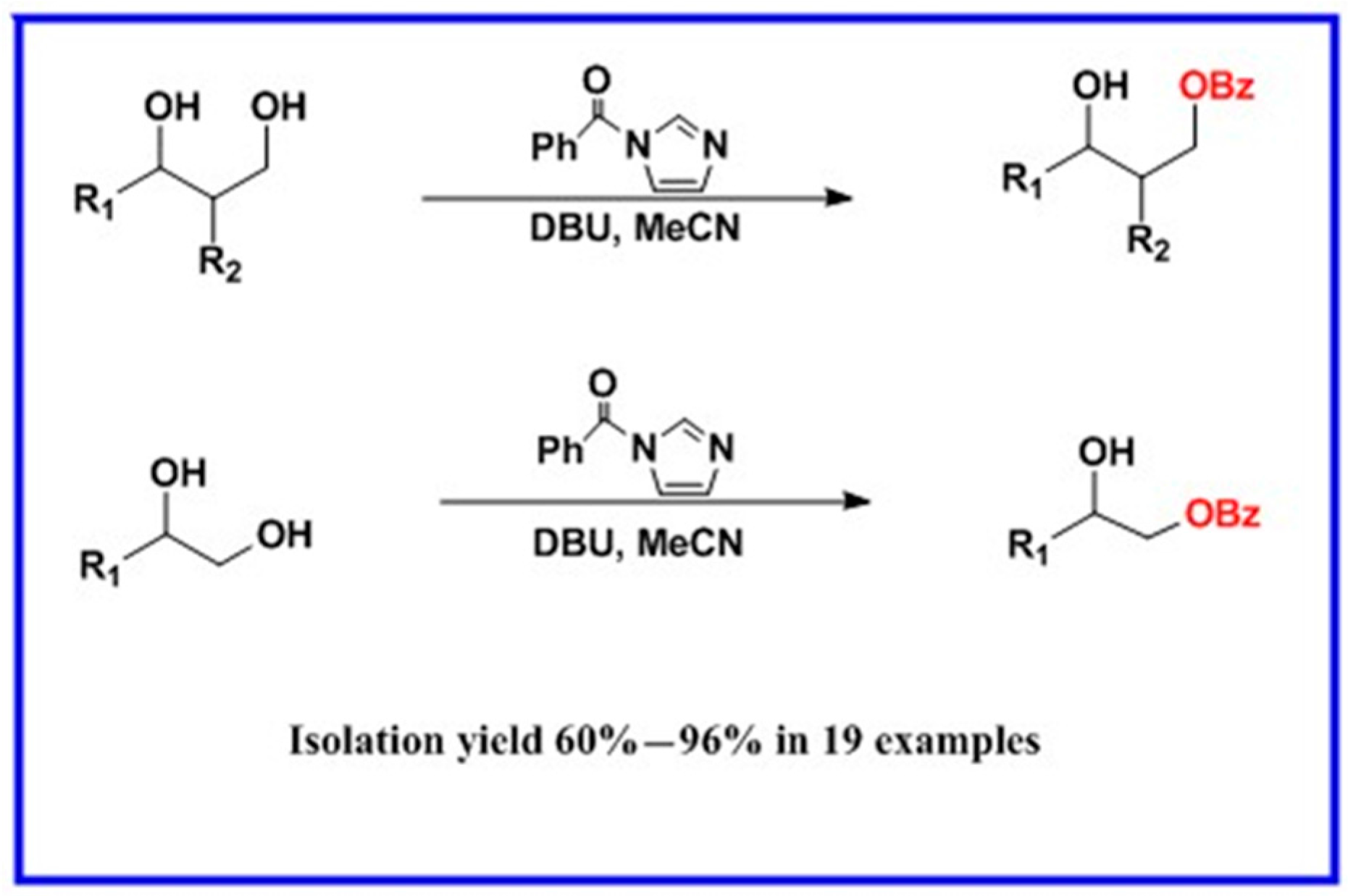
Scheme 1.
DBU-catalyzed benzoylation of 1,2 and 1,3-diols.
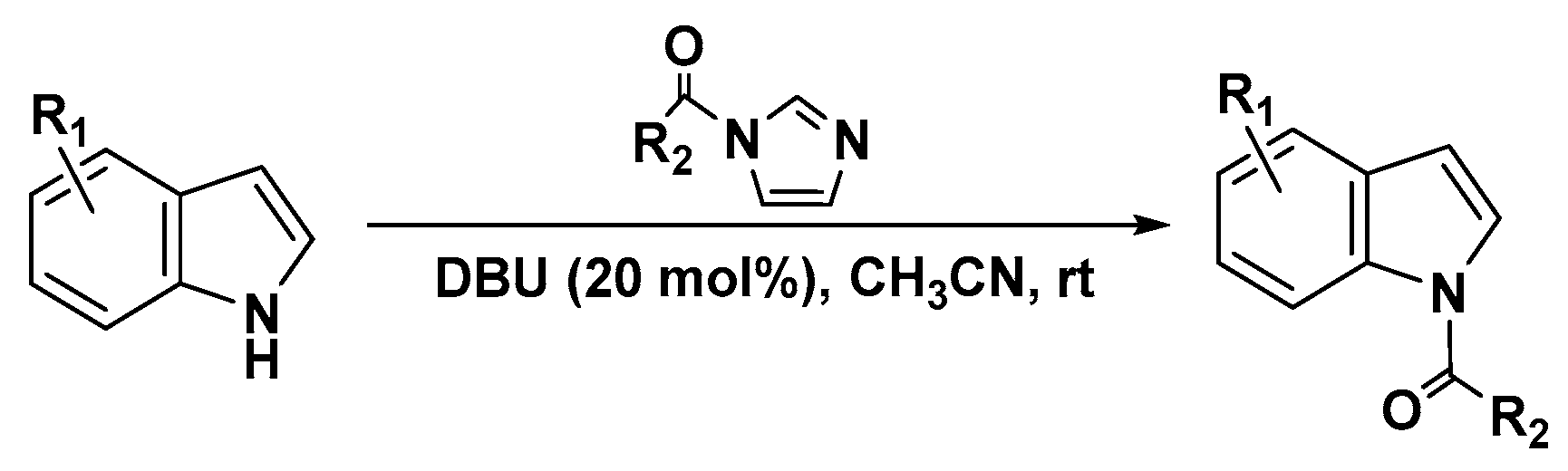
The carbonylimidazole derivatives are a class of highly acylation reagents, particularly in the preparation of esters and amides [39,40,41,42]. In several cases, they have shown superior selectivity for selective acylation reactions [43,44,45] and N-acetylation of oxindoles and indoles (Scheme 2) [46]. Inspired by these works, we decided to explore the possibility of replacing the acyl halide, which is toxic and unstable, with 1-benzoylimidazole as the benzoylation reagent with 1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) as the catalyst. DBU is often used as a organobase catalyst in many base-catalyzed reactions [47,48,49,50], having been recognized as an excellent nucleophilic catalyst, leading to rapid and efficient acetylation of non-nucleophilic azacycles, such as indoles, pyrroles, and oxazolidinones.
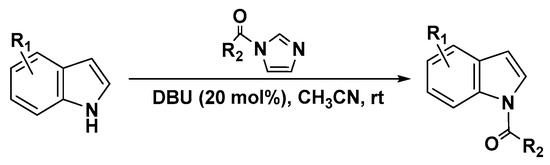
Scheme 2.
N-acetylation of heterocycles with carbonyl-azoles.
2. Results and Discussion
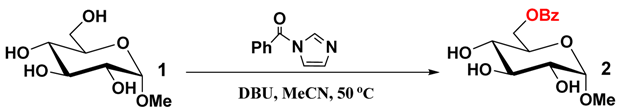
After a series of optimization studies on selective benzoylation of methyl α-d-glucopyranoside (1, Table 1), we determined the best condition to be the use of 0.2 equiv. of DBU and 1.1 equiv. of 1-benzoylimidazole in acetonitrile with a small amount of DMF (MeCN/DMF: 20/1) at 50 °C for 8 h (Entry 1). Under this condition, methyl glucoside 2 with a benzoylated primary hydroxyl group was isolated in 70% yield. The yield of 2 was only increased to 72% when 1.3 equiv. of 1-benzoylimidazole was used (Entry 2). Without the addition of DMF the yield decreased to 53%, which is likely due to the low solubility of polyols in pure acetonitrile (Entry 3). If the reaction was performed at room temperature, the yield of 2 decreased to only 21% (Entry 4). Increasing the reaction temperature to 70 °C or prolonging the reaction time to 12 h. failed to improve the yield of 2 (Entries 5–6). No reaction occurred in the absence of DBU or with Et3N instead of DBU (Entries 7–8). Low yields were obtained with DMAP, DIPEA or DBN instead of DBU (Entries 9, 10 and 11). The yield of 2 increased with the amount of DBU used up to 0.4 equiv (Entries 12, 13 and 14).

Table 1.
Optimization studies on selective benzoylation of methyl α-d-glucopyranoside (1) a.

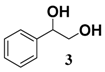
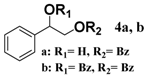
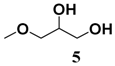
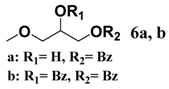
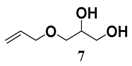
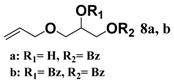
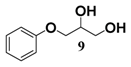
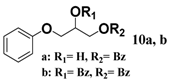
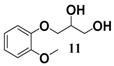
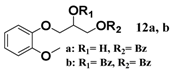
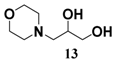
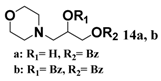
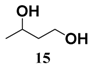
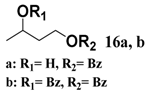
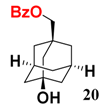
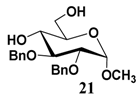
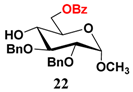
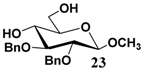
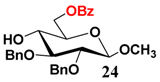
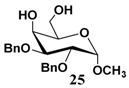
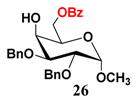
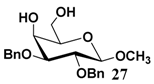
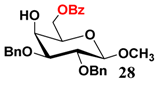
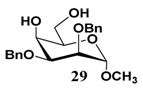
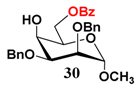
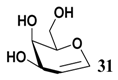
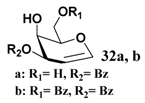
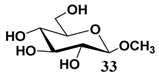
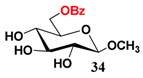
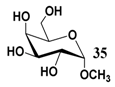
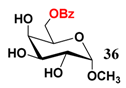
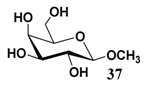
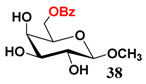
In light of these results, a wide range of substrates was further selectively benzoylated under the optimized conditions (Table 2), including 1,2-diols 3, 5, 7, 9, 11 and 13 (Entries 1–6), 1,3-diols 15, 17 and 19 (Entries 7–9), methyl d-pyranoside 1,3-diols 23, 25, 27 and 29 (Entries 11–14), and carbohydrate polyols 31, 33, 35 and 37 (Entries 15–18). The addition of DMF is not necessary for diols due to their good solubility in acetonitrile. All of the substrates contain one primary hydroxyl group with one or several secondary hydroxyl groups. Good yields (60%–96%) where the primary hydroxyl group was selective protected were obtained in most cases. For non-carbohydrate diols, the only by-products were a small amount of overbenzoylated compounds (Entries 1–7). Particularly, excellent selectivities (87%–96%) were shown due to striking steric effects for the benzoylation of 1,3-diols 17 and 19 (Entries 8 and 9), containing a tertiary hydroxyl group, and the five carbohydrate diols 21, 23, 25, 27 and 29 (Entries 10–14).

Table 2.
1-Benzoylimidazole used for selective benzoylation of diols and carbohydrates.
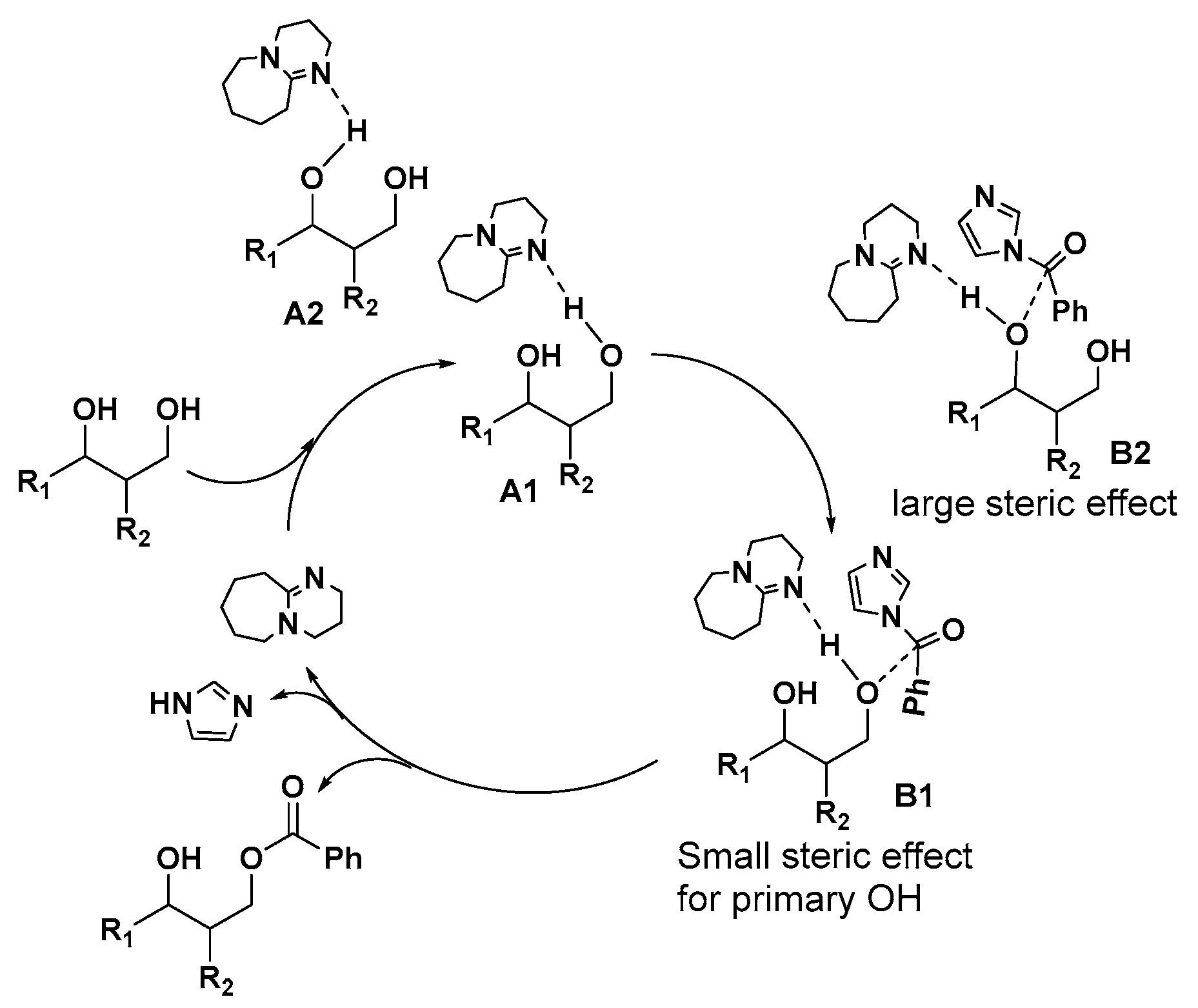
Evidently, the benzoylation occcurs via by transacylation of imidazolides under basic condition [51,52,53]. Based on the traditional base-catalysis principle, this transacylation is usually proposed to start from a deprotonation of hydroxyl groups in the presence of strong base such as DBU. Recently, it was proposed that the hydrogen bond between the hydroxyl group and the base play a key role in the transacylation reaction [54,55,56]. Thus, a mechanism based on hydrogen bond principle for this selective benzoylation is proposed in Figure 1. H-bond complexes A1 and A2 can be formed between the hydroxyl group of substrate and DBU, following the formation of the transition state intermediates B1 and B2 with the approach of 1-benzoylimidazole. It is clear that the formation of intermediate B1 is more favoured due to the less steric hindrance of A1. In the transition state (TS) B, the deprotonation of the hydroxyl group by DBU and the attack of the hydroxyl group on 1-Benzoylimidazole occur at the same time. Consequently, the markedly less steric hindrance in A1 and B1 than in A2 and B2 explained the good selectivity for benzoylation of the primary hydroxyl group.
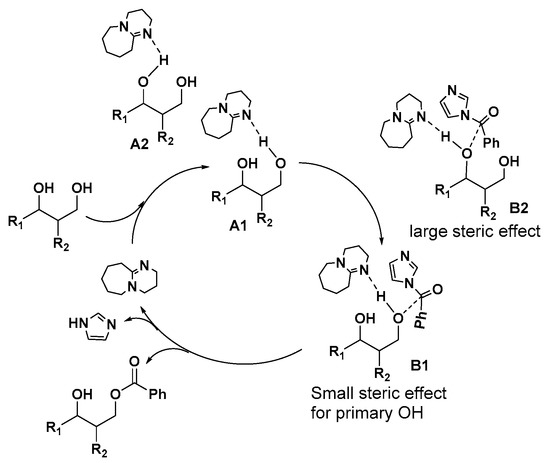
Figure 1.
Proposed mechanism for selective benzoylation of primary OH using 1-benzoylimidazole as acylation reagent catalyzed by DBU.
3. Materials and Methods
3.1. General Information
All commercially available starting materials and solvents were of reagent grade and dried prior to use. Chemical reactions were monitored with thin-layer chromatography using precoated silica gel 60 (0.25 mm thickness) plates. Flash column chromatography was performed on silica gel 60 (0.040–0.063 mm). 1H- and 13C-NMR spectra were recorded with a Bruker AVANCE III instrument operating at 500 and 125 MHz, and using the residual signals from DMSO-d6 (1H: δ = 2.50 ppm), CD3OD (1H: δ = 3.34 ppm) and CDCl3 (1H: δ = 7.27 ppm) as internal standard.
3.2. General Procedure for Selective Benzoylation of Diols (a)
To a solution of substrate (100 mg) in 2.5 mL MeCN (dry) was added DBU (0.2 equiv.), and the mixture was allowed to stir at 50 °C for 10 min. 1-Benzoylimidazole (1.1 equiv.) in MeCN (dry, 0.5 mL) was added to the reaction mixture in two portions and it was allowed to stir at 50 °C for 8 h. MeCN was removed under reduced pressure and the resulting mixture was purified by flash column chromatography (ethyl acetate/petroleum ether = 1:6 to 2:1) to afford benzoylated products.
3.3. General Procedure for Selective Benzoylation of Polyols (b)
To a solution of substrate (100 mg) in 2.5 mL MeCN (dry) and 0.15 mL DMF (dry) was added DBU (0.2 equiv.), and the mixture was allowed to stir at 50 °C for 10 min. 1-Benzoylimidazole (1.1 equiv.) in 0.5 mL MeCN (dry) was added to the reaction mixture in two portions and it was allowed to stir at 50 °C for 8 h. MeCN was removed under reduced pressure and the resulting mixture was purified by flash column chromatography (ethyl acetate/petroleum ether = 1:1 to 10:1) to afford benzoylated products.
3.4 Product Characterization
3-(Allyloxy)propane-1,2-diyl dibenzoate (8b). Colorless oil, yield: 76%, Rf = 0.2 (ethyl acetate/petroleum ether = 1:3), 1H-NMR (CDCl3) δ 8.09–7.99 (m, 4H, ArH), 7.59–7.52 (m, 2H, ArH), 7.47–7.39 (m, 4H, ArH), 5.94–5.83 (m, 1H, -CH=CH2), 5.65–5.56 (m, 1H, -CH-), 5.33–5.15 (m, 2H, -CH=CH2), 4.72–4.58 (m, 2H, -CH2COOPh), 4.09–4.04 (m, 2H, -CH2CH=CH2), 3.84–3.76 (m, 2H, -CH2OCH2CH=CH2). 13C-NMR (CDCl3) δ 166.7, 166.3, 150.1, 148.0, 133.2, 129.8, 128.5, 122.6, 121.1, 115.8, 115.5, 112.1, 71.6, 68.6, 65.7, 55.9. HRMS m/z calcd for C20H20O5 [M + Na]+: 363.1203. Found 363.1207.
2-Hydroxy-3-(2-methoxyphenoxy)propyl benzoate (12a). Colorless oil, yield: 75%, Rf = 0.2 (ethyl acetate/petroleum ether = 1:2), 1H-NMR (CDCl3) δ 8.12–7.22 (m, 2H, ArH), 7.64–7.58 (m, 1H, ArH), 7.51–7.45 (m, 2H, ArH), 7.07–6.99 (m, 2H, ArH), 6.98–6.91 (m, 2H, ArH), 4.61–4.51 (m, 2H, PhCO2CH2-), 4.46–4.38 (m, 1H, -HCOH-), 4.28–4.12 (m, 2H, -PhOCH2-), 3.90 (s, 3H, -OCH3). 13C-NMR (CDCl3) δ 165.9, 150.3, 148.0, 133.3, 133.2, 129.9, 128.5, 122.6, 121.0, 115.8, 112.5, 70.7, 68.2, 63.4, 55.9. HRMS m/z calcd for C17H18O5 [M + Na]+: 325.1046. Found 325.1051.
3-(2-Methoxyphenoxy)propane-1,2-diyl dibenzoate (12b). Colorless oil, yield: 15%, Rf = 0.2 (ethyl acetate/petroleum ether = 1:3), 1H-NMR (CDCl3) δ 8.11–8.05 (m, 4H, ArH), 7.63–7.57 (m, 2H, ArH), 7.50–7.43 (m, 4H, ArH), 7.09–6.99 (m, 2H, ArH), 6.96–6.93 (m, 2H, ArH), 5.87–5.80 (m, 1H, -CH2CHCH2-),δ 4.88 (dd, J = 12.0, 3.8 Hz, 1H, PhCO2CH2-), 4.80 (dd, J = 12.0, 6.0 Hz, 1H, PhCO2CH2-), 4.46 (d, J = 5.4 Hz, 2H, -PhOCH2-), 3.84 (s, 3H, -OCH3). 13C-NMR (CDCl3) δ 166.3, 165.9, 150.2, 148.0, 134.3, 133.3, 133.2, 133.1, 129.8, 129.7, 128.5, 128.4, 122.7, 121.1, 117.5, 112.1, 71.1, 68.3, 63.6, 55.9. HRMS m/z calcd for C24H22O6 [M + Na]+: 429.1309. Found 429.1312.
4. Conclusions
In conclusion, a simple and efficient method for regioselective benzoylation of diols and carbohydrates has been developed, where the primary hydroxyl groups can be selectively benzoylated by using 1-benzoylimidazole as an acylation reagent catalyzed by DBU. In comparison to other methods, not only does this robust method appear to have provided a new protection strategy for the primary hydroxyl group, but also the used reagents are associated with lower cost, metal-free, and displayed better regioselectivity.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/5/641/s1.
Acknowledgments
We thank National Natural Science Foundation of China (Nos. 31270861 and 21272083), and Agricultural Science and Technology Innovation Project of Shaanxi Province (2012 NKC01-12) for financial support.
Author Contributions
Yuchao Lu designed the experiments, analyzed the data, and drafted the manuscript. Chenxi Hou, Jingli Ren, Xiaoting Xin and Hengfu Xu performed data acquisition and analysis. Yuxin Pei assisted with the revision of the manuscript. Hai Dong and Zhichao Pei directed the entire research study and drafted the manuscript. All authors read and approved the final manuscript for submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.C.; Lee, J.C.; Luo, S.-Y.; Kulkarni, S.S.; Huang, Y.W.; Lee, C.C.; Chang, K.L.; Hung, S.C. Regioselective one-pot protection of carbohydrates. Nature 2007, 446, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.A.; Longcore, K.E.; Miller, S.J.; Wender, P.A. An Approach to the Site-Selective Diversification of Apoptolidin A with Peptide-Based Catalysts. Nat. Prod. J. 2009, 72, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Dai, Y.W.; Li, W.; Laval, S.; Xu, P.; Yu, B. Effective Synthesis of α-d-GlcN-(1→4)-d-GlcA/L-IdoA Glycosidic Linkage under Gold(I) Catalysis. Asian J. Org. Chem. 2015, 8, 756–762. [Google Scholar] [CrossRef]
- Lou, Q.H.; Meng, X.B.; Lao, Z.Q.; Xuan, L.L.; Bai, J.Y.; Hou, Q.; Hu, G.Y.; Luo, R.N.; Tao, L.J.; Li, Z.J. Design, Synthesis and Antifibrotic Activities of Carbohydrate- Modified 1-(Substituted aryl) -5-trifluoromethyl-2(1H) Pyridones. Molecules 2012, 17, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, R.U.; Driguez, H. Chemical synthesis of 2-acetamido-2-deoxy-4-O-(α-l-fucopyranosyl)-3-O-(β-d-galactopyranosyl)-d-glucose. Lewis a blood-group antigenic determinant. J. Am. Chem. Soc. 1975, 97, 4063–4069. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Mitchell, H.J. Adventures in Carbohydrate Chemistry: New Synthetic Technologies, Chemical Synthesis, Molecular Design, and Chemical Biology A list of abbreviations can be found at the end of this article. Telemachos Charalambous was an inspiring teacher at the Pancyprian Gymnasium, Nicosia, Cyprus. Angew. Chem. Int. Ed. 2001, 40, 1576–1624. [Google Scholar]
- Guo, J.; Ye, X.S. Protecting Groups in Carbohydrate Chemistry: Influence on Stereoselectivity of Glycosylations. Molecules 2010, 15, 7235–7265. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.H.; Hoang, K.L.M.; He, J.; Tan, Y.J.; Liu, X.W. Reversing the Stereoselectivity of a Palladium-Catalyzed O-Glycosylation through an Inner-Sphere or Outer-Sphere Pathway. Angew. Chem. Int. Ed. 2015, 54, 604–607. [Google Scholar]
- David, S.; Hanessian, S. Regioselective manipulation of hydroxyl groups via organotin derivatives. Tetrahedron 1985, 41, 643–663. [Google Scholar] [CrossRef]
- Muramatsu, W.; Tanigawa, S.; Takemoto, Y.; Yoshimatsu, H.; Onomura, O. Organotin-Catalyzed Highly Regioselective Thiocarbonylation of Nonprotected Carbohydrates and Synthesis of Deoxy Carbohydrates in a Minimum Number of Steps. Chem. Eur. J. 2012, 18, 4850–4853. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhou, Y.X.; Pan, X.; Cui, F.; Liu, W.; Liu, J.; Ramström, O. Stereoelectronic Control in Regioselective Carbohydrate Protection. J. Org. Chem. 2012, 77, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.F.; Lu, Y.C.; Zhou, Y.X.; Ren, B.; Pei, Y.X.; Dong, H.; Pei, Z.C. Regioselective Benzylation of Diols and Polyols by Catalytic Amounts of an Organotin Reagent. Adv. Synth. Catal. 2014, 356, 1735–1740. [Google Scholar] [CrossRef]
- Lee, D.; Taylor, M.S. Borinic Acid-Catalyzed Regioselective Acylation of Carbohydrate Derivatives. J. Am. Chem. Soc. 2011, 133, 3724–3727. [Google Scholar] [CrossRef] [PubMed]
- Gouliaras, C.; Lee, D.; Chan, L.; Taylor, M.S. Regioselective Activation of Glycosyl Acceptors by a Diarylborinic Acid-Derived Catalyst. J. Am. Chem. Soc. 2011, 133, 13926–13929. [Google Scholar] [CrossRef] [PubMed]
- Tacke, R.; Bertermann, R.; Burschka, C.; Dragota, S. A Zwitterionic Spirocyclic Pentacoordinate Silicon Compound Synthesized in Water by Si_O and Si_C Bond Cleavage. Angew. Chem. Int. Ed. 2005, 44, 5292–5295. [Google Scholar] [CrossRef] [PubMed]
- Ager, B.J.; Bourque, L.E.; Buchner, K.M.; Woerpel, K.A. Silylene Transfer to Allylic Sulfides: Formation of Substituted Silacyclobutanes. J. Org. Chem. 2010, 75, 5729–5732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Ramstrom, O.; Dong, H. Organosilicon-mediated regioselective acetylation of carbohydrates. Chem. Commun. 2012, 48, 5370–5372. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Y.; Bin, H.C.; Yang, J.S. Tuning Effect of Silyl Protecting Groups on the Glycosylation Reactivity of Arabinofuranosyl Thioglycosides. Org. Lett. 2013, 15, 2834–2837. [Google Scholar] [CrossRef] [PubMed]
- Bourdreux, Y.; Lemetais, A.; Urban, D.; Beau, J.M. Iron(III) chloride-tandem catalysis for a one-pot regioselective protection of glycopyranosides. Chem. Commun. 2011, 47, 2146–2148. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maki, T.; Murakami, S.; Onomura, O. Copper Ion-Induced Activation and Asymmetric Benzoylation of 1,2-Diols: Kinetic Chiral Molecular Recognition. J. Am. Chem. Soc. 2003, 8, 2052–2053. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; She, J.; Zhang, L.H.; Ye, X.S. Silver(I) oxide mediated selective monoprotection of diols in pyranosides. J. Org. Chem. 2004, 69, 5774–5777. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.S.; Kluger, R. Magnesium ion enhances lanthanum-promoted monobenzoylation of a monosaccharide in water. Org. Biomol. Chem. 2010, 8, 2006–2008. [Google Scholar] [CrossRef] [PubMed]
- Felix, B.; Michael, H.; Thomas, Z. Synthesis and Spectroscopic Evaluation of Two Novel Glycosylated Zinc(II)-Phthalocyanines. Molecules 2015, 20, 18367–18386. [Google Scholar]
- Kurahashi, T.; Mizutani, T.; Yoshida, J.I. Functionalized DMAP catalysts for regioselective acetylation of carbohydrates. Tetrahedron 2002, 58, 8669–8677. [Google Scholar] [CrossRef]
- Kim, S.; Chang, H.; Kim, J.W. Selective Benzoylation of Diols with 1-(Benzoyloxy) benzotriazole. J. Org. Chem. 1985, 50, 1751–1752. [Google Scholar] [CrossRef]
- Pautard, A.M.; Evans, S.A., Jr. Chemoselective benzoylations of 1,2-diols. Reactivity comparisons of reagents. Triphenylphosphine-benzoyl peroxide and triphenylphosphine-diethyl azodicarboxylate-benzoic acid. J. Org. Chem. 1988, 10, 2300–2303. [Google Scholar] [CrossRef]
- Ciuffreda, P.; Alessandrini, L.; Terraneo, G.; Santaniello, E. Lipase-catalyzed selective benzoylation of 1,2-diols with vinyl benzoate in organic solvents. Tetrahedron Asymmetry 2003, 20, 3197–3201. [Google Scholar] [CrossRef]
- Wang, Y.F.; Lalonde, J.J.; Momongan, M.; Bergbreiter, D.E.; Wong, C.H. Lipase-Catalyzed Irreversible Transesterifications Using Enol Esters as Acylating Reagents: Preparative Enantio- and Regioselective Syntheses of Alcohols, Glycerol Derivatives, Sugars, and Organometallics. J. Am. Chem. Soc. 1988, 110, 7200–7205. [Google Scholar] [CrossRef]
- Roux, M.C.; Seyden-Penne, J.; Wartski, L.; Posner, G.H.; Nierlich, M.; Vigner, D.; Lance, M. Scope of [l + 2 + 3] Annulations Using Aminonitriles as Michael Donors. J. Org. Chem. 1993, 58, 3969–3973. [Google Scholar] [CrossRef]
- Danieli, B.; Luisetti, M.; Sampognaro, G.; Carrea, G.; Riva, S. Regioselective acylation of polyhydroxylated natural compounds catalyzed by Candida Antarctica lipase B (Novozym 435) in organic solvents. J. Mol. Catal. B Enzym. 1997, 3, 193–201. [Google Scholar] [CrossRef]
- Kumara Swamy, K.C.; Bhuvan Kumar, N.N.; Balaraman, E.; Pavan Kumar, K.V.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar] [CrossRef] [PubMed]
- Mikhai, K.; Barbara, G.; Aleksander, Z. From methyl d-glucopyranoside to methyl d-allopyranoside via the Mitsunobu reaction. Carbohydr. Res. 1999, 320, 244–249. [Google Scholar]
- Bernhard, N.; Wolfgang, S. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. Ed. 1978, 17, 522–524. [Google Scholar]
- Kattnig, E.; Albert, M. Counterion-Directed Regioselective Acetylation of Octyl β-d-Glucopyranoside. Org. Lett. 2004, 6, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Ren, B.; Ge, J.T.; Pei, Z.C.; Dong, H. A green and convenient method for regioselective mono and multiple benzoylation of diols and polyols. Tetrahedron 2016, 72, 1005–1010. [Google Scholar] [CrossRef]
- Lu, Y.C.; Wei, P.; Pei, Y.X.; Xu, H.F.; Xin, X.T.; Pei, Z.C. Regioselective acetylation of carbohydrates and diols catalyzed by tetramethyl-ammonium hydroxide in water. Green Chem. 2014, 16, 4510–4514. [Google Scholar] [CrossRef]
- Chung, M.K.; Orlova, G.; Goddard, J.D.; Schlaf, M.; Harris, R.; Beveridge, T.J.; White, G.; Hallett, F.R. Regioselective Silylation of Sugars through Palladium Nanoparticle-Catalyzed Silane Alcoholysis. J. Am. Chem. Soc. 2002, 124, 10508–10518. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.B.; Hynd, G.; Wright, J.M.; Sharma, A. On the Selective Deprotection of Trityl Ethers. J. Org. Chem. 2000, 1, 263–265. [Google Scholar] [CrossRef]
- Heller, S.T.; Fu, T.; Sarpong, R. Dual Brønsted Acid/Nucleophilic Activation of Carbonylimidazole Derivatives. Org. Lett. 2012, 14, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Carey, F.A.; Hodgson, K.O. Efficient syntheses of methyl 2-O-benzoyl-4,6-O-benzylidene-α-d-glucopyra -noside and methyl 2-O-benzoyl-4,6-O-benzylidene-α-d-ribo-hexopyranosid-3-ulose. Carbohydr. Res. 1970, 12, 463–465. [Google Scholar] [CrossRef]
- Chittenden, G.J.F. An improved preparation of benzyl 3-O-benzoyl-4,6-O-benzylidene-β-d-galactopyra -noside. Carbohydr. Res. 1971, 16, 495–496. [Google Scholar] [CrossRef]
- Holder, N.L.; Fraser-Reid, B. An Improved Synthesis of Methyl 2-O-Benzoyl-4,6-O-benzylidene-α-d-altropyranoside. Synthesis 1972, 2. [Google Scholar] [CrossRef]
- Wang, Z.Z. Convenient Preparation of Mono-2 or Mono-3-O-benzoyl-β-cyclodextrin Dependent the Solvent Equilibrium. Chem. Sci. Trans. 2012, 1, 53–56. [Google Scholar] [CrossRef]
- Grzyb, J.A.; Shen, M.; Yoshina-Ishii, C.; Chi, W.; Brown, R.S.; Batey, R.A. Carbamoylimidazolium and thiocarbamoylimidazolium salts: Novel reagents for the synthesis of ureas, thioureas, carbamates, thiocarbamates and amides. Tetrahedron 2005, 61, 7153–7175. [Google Scholar] [CrossRef]
- Heller, S.T.; Sarpong, R. Chemoselective Esterification and Amidation of Carboxylic Acids with Imidazole Carbamates and Ureas. Org. Lett. 2010, 12, 4572–4575. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.T.; Schultz, E.E.; Sarpong, R. Chemoselective N-Acylation of Indoles and Oxazolidinones with Carbonylazoles. Angew. Chem. Int. Ed. 2012, 51, 8304–8308. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.V.; Mereu, A. Superior amine catalysts for the Baylis–Hillman reaction: The use of DBU and its implications. Chem. Commun. 1999, 22, 2311–2312. [Google Scholar] [CrossRef]
- Shieh, W.-C.; Dell, S.; Repič, O. Nucleophilic Catalysis with 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) for the Esterification of Carboxylic Acids with Dimethyl Carbonate. J. Org. Chem. 2002, 67, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Larrivée-Aboussafy, C.; Jones, B.P.; Price, K.E.; Hardink, M.A.; McLaughlin, R.W.; Lillie, B.M.; Hawkins, J.M.; Vaidyanathan, R. DBU Catalysis of N,N’-Carbonyldiimidazole-Mediated Amidations. Org. Lett. 2010, 12, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Carafa, M.; Mesto, E.; Quaranta, E. DBU-Promoted Nucleophilic Activation of Carbonic Acid Diesters. Eur. J. Org. Chem. 2011, 13, 2458–2465. [Google Scholar] [CrossRef]
- Staab, H.A. New Methods of Preparative Organic Chmistry IV. Syntheses Using Heterocyclic Amides. Angew. Chem. Int. Ed. 1962, 1, 351–367. [Google Scholar] [CrossRef]
- Box, L.L.; Box, V.G.S.; Roberts, E.V.E. The selective 2-benzoylation of methyl-4,-6-O-benzylidene-α-d-glucopyranoside. Carbohydr. Res. 1979, 69. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.Z.; Yang, Z.W.; Liu, X.Y.; Wang, Qi.W. Selective acylation of ribonucleotides and ribonucleosides with acylimidazoles. Acta Chim. Sin. 1988, 6, 244–250. [Google Scholar]
- Zhou, Y.X.; Rahm, M.; Wu, B.; Zhang, X.L.; Ren, B.; Dong, H. H-Bonding Activation in Highly Regioselective Acetylation of Diols. J. Org. Chem. 2013, 78, 11618–11622. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Rahm, M.; Zhang, X.L.; Zhou, Y.X.; Dong, H. Regioselective Acetylation of Diols and Polyols by Acetate Catalysis: Mechanism and Application. J. Org. Chem. 2014, 79, 8134–8142. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wang, M.Y.; Liu, J.Y.; Ge, J.T.; Zhang, X.L.; Dong, H. Zemplén transesterification: A name reaction that has misled us for 90 years. Green Chem. 2015, 17, 1390–1394. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 21–30, 34, 36, 38 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).