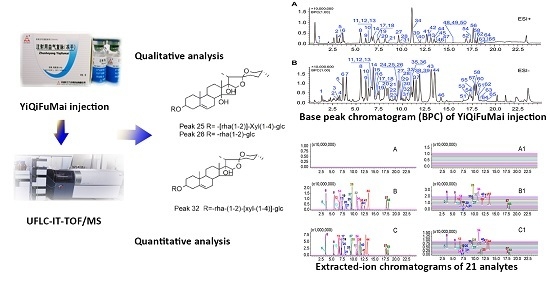
Simultaneous Qualitative and Quantitative Analysis of Multiple Chemical Constituents in YiQiFuMai Injection by Ultra-Fast Liquid Chromatography Coupled with Ion Trap Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Conditions
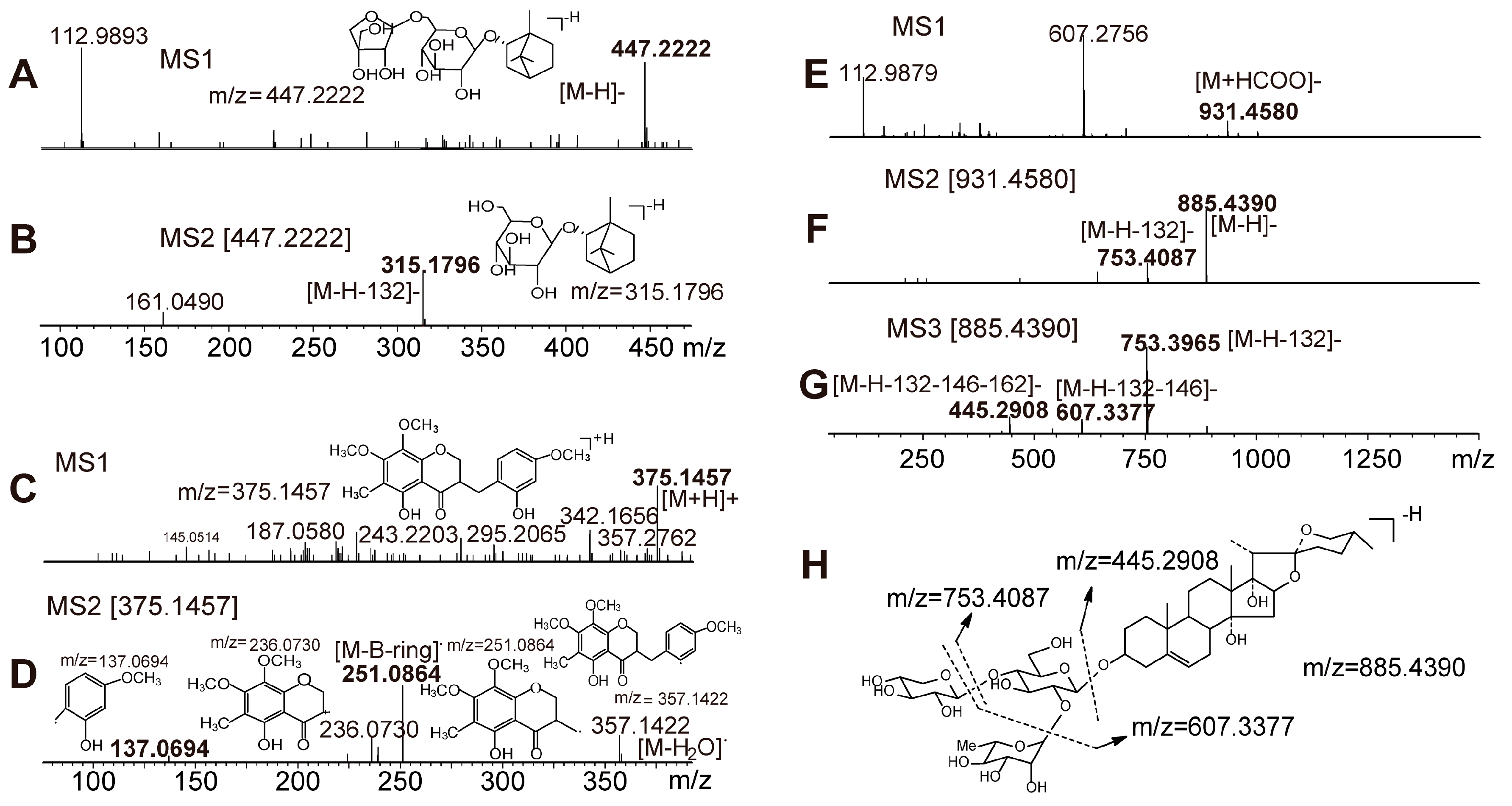
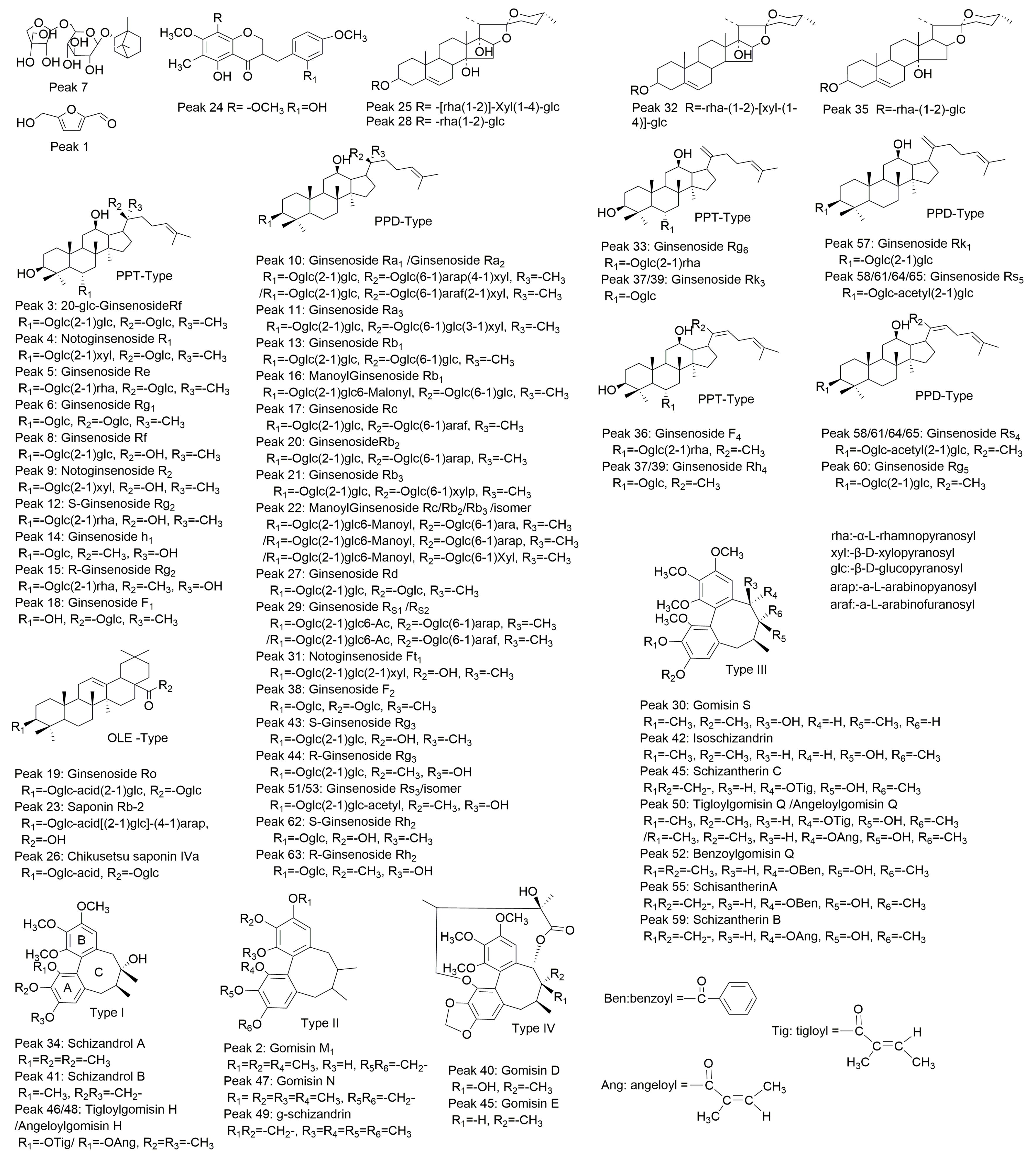
2.2. Identification of Chemical Constituents in YQFM
2.2.1. Identification of Compounds in O. japonicus
2.2.2. Identification of Compounds in P. ginseng
2.2.3. Identification of Compounds in S. chinensis
2.3. Quantitative Analysis of 21 Compounds in YQFM by UFLC-IT-TOF/MS
2.3.1. Method Validation
2.3.2. Method Application for Quantification of 10 Batches of YQFM
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Standard Solutions and Sample Preparation
3.2.1. Standard Solutions Preparation
3.2.2. Sample Solution Preparation
3.2.2.1. Sample Solutions for Qualitative Analysis
3.2.2.2. Sample Solutions for Quantitative Analysis
3.3. UFLC-IT-TOF/MS Analysis Conditions
3.3.1. Qualitative Analysis Conditions
3.3.2. Quantitative Analysis Conditions
3.4. Method Validation
3.4.1. Specificity
3.4.2. Linear Range and Sensitivity
3.4.3. Precision, Stability, Reproducibility and Accuracy
3.5. Application to Analysis of Samples of YQFM
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| YQFM | YiQiFuMai injection |
| UFLC-IT-TOF/MS | Ultra-fast liquid chromatography coupled with ion trap time-of-flight mass spectrometry |
| BPC | Base peak chromatogram |
| SPE | Solid phase extraction |
| TCM | Traditional Chinese Medicine |
| SMS | Sheng-mai San |
References
- Wang, Y.H.; Qiu, C.; Wang, D.W.; Hu, Z.F.; Yu, B.Y.; Zhu, D.N. Identification of multiple constituents in the traditional chinese medicine formula Sheng-Mai San and rat plasma after oral administration by HPLC-DAD-MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 1110–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Liu, C.H.; Zhang, J.Q.; Zhu, D.N.; Yu, B.Y. Protective effects and active ingredients of Yi-Qi-Fu-Mai sterile powder against myocardial oxidative damage in mice. J. Pharmacol. Sci. 2013, 122, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J. Shengmaisan regulates pacemaker potentials in interstitial cells of cajal in mice. J. Pharmacopunct. 2013, 16, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. Application of Shengmai decoction on deficiency of both qi and yin in heart failure. China Health Stand. Manag. 2015, 6, 69–70. [Google Scholar]
- Yang, Y.; Jiang, T.; Wang, F.; Xu, W.; Zhou, Y.B. Clinical study of YiQiFuMai injection (lyophilized) for treatment of chronic heart failure with qi and yin deficiency in 60 cases. Acta Chin. Med. Pharmacol. 2012, 40, 115–117. [Google Scholar]
- Zhang, B.; Tian, F.L. Clinical efficacy of YiQiFuMai injection on hypertrophic obstructive cardiomyopathy. Chin. J. Evid. Based Cardiovasc. Med. 2014, 6, 314–316. [Google Scholar]
- Xing, L.; Jiang, M.; Dong, L.; Gao, J.; Hou, Y.; Bai, G.; Luo, G. Cardioprotective effects of the YiQiFuMai injection and isolated compounds on attenuating chronic heart failure via NF-κB inactivation and cytokine suppression. J. Ethnopharmacol. 2013, 148, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tan, Y.S.; Chen, H.L.; Yan, Y.; Zhai, K.F.; Li, D.P.; Kou, J.P.; Yu, B.Y. Identification of schisandrin as a vascular endothelium protective component in YiQiFuMai powder injection using huvecs binding and HPLC-DAD-Q-TOF-MS/MS analysis. J. Pharmacol. Sci. 2015, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zhang, J.Q.; Liu, C.H.; Zhu, D.N.; Yu, B.Y. Screening and identifying the myocardial-injury protective ingredients from Sheng-Mai-San. Pharm. Biol. 2013, 51, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Tang, H.T.; Liu, H.Q.; Liu, L.; Tang, Y. Simultaneous determination of nine compositions in Shengmai injection by HPLC. Chin. Tradit. Patent Med. 2013, 3, 508–512. [Google Scholar]
- Zhou, D.D.; Jiang, S.J.; Tong, L.; Yang, Y.W.; Wang, G.L.; Ye, Z.L.; Wang, Z.T.; Lin, R.C. Quantitative determination of eight major constituents in the traditional chinese medicinal Yi-Qi-Fu-Mai preparation by LC. Chromatographia 2009, 70, 969–974. [Google Scholar] [CrossRef]
- Han, X.; Li, D.; Zhou, D.; Lin, R.; Liu, L.; Ye, Z. Determination of total content of ten ginsenosides in YiQiFuMai lyophilized injection by near infrared spectroscopy. Zhongguo Zhong Yao Za Zhi 2011, 36, 1603–1605. [Google Scholar] [PubMed]
- Li, F.; Cheng, T.F.; Dong, X.; Li, P.; Yang, H. Global analysis of chemical constituents in Shengmai injection using high performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 117, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Nie, L.X.; Li, X.F.; Cheng, X.L.; Zhang, Y.Y.; Wang, G.L.; Lin, R.C. Simultaneous determination of ginsenosides and lignans in Sheng-Mai injection by ultra-performance liquid chromatography with diode array detection. Pharmazie 2012, 67, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Kang, L.; Wang, Y.; Zhao, X.; Liu, X.; Xu, L.; Li, Z. A metabonomic study of cardioprotection of ginsenosides, schizandrin, and ophiopogonin D against acute myocardial infarction in rats. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Tian, Y.; Tang, Y.; Yan, J.; Yu, B. Antithrombotic activities of aqueous extract from Radix ophiopogon japonicus and its two constituents. Biol. Pharm. Bull. 2006, 29, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Jiang, F.; Wang, B.; Yu, Y.; Zhang, X.; Yin, Z.; Liu, C. Ophiopogonin D prevents H2O2-induced injury in primary human umbilical vein endothelial cells. J. Ethnopharmacol. 2010, 128, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Meng, C.; Zhang, X.M.; Yuan, C.H.; Wen, M.D.; Chen, Z.; Dong, D.C.; Gao, Y.H.; Liu, C.; Zhang, Z. Ophiopogonin D attenuates doxorubicin-induced autophagic cell death by relieving mitochondrial damage in vitro and in vivo. J. Pharmacol. Exp. Ther. 2015, 352, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Liang, Y.; Hao, H.; A, J.; Xie, L.; Gong, P.; Dai, C.; Liu, L.; Kang, A.; Zheng, X.; et al. Rapid identification of ophiopogonins and ophiopogonones in Ophiopogon japonicus extract with a practical technique of mass defect filtering based on high resolution mass spectrometry. J. Chromatogr. A 2012, 1227, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, Y.; Zhou, Y.; Guan, T.; Xing, L.; Rao, T.; Zhou, L.; Yu, X.; Wang, Q.; Xie, L.; et al. Experimental evidence for ion accumulation time affecting qualitative and quantitative analysis of ophiopogons in ophiopogon extract by hybrid ion trap time-of-flight mass spectrometry. Chromatographia 2013, 76, 949–958. [Google Scholar] [CrossRef]
- Xing, L.; Xie, L.; Liang, Y.; Xing, R.; Rao, T.; Zhou, L.; Wang, Q.; Fu, H.; Ye, W.; Wang, G. Evaluation of liquid chromatography-ion trap-time of flight hybrid mass spectrometry on the quantitative analysis for ginsenosides. Biomed. Chromatogr. 2014, 28, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hao, H.; Kang, A.; Xie, L.; Xie, T.; Zheng, X.; Dai, C.; Wan, L.; Sheng, L.; Wang, G. Qualitative and quantitative determination of complicated herbal components by liquid chromatography hybrid ion trap time-of-flight mass spectrometry and a relative exposure approach to herbal pharmacokinetics independent of standards. J. Chromatogr. A 2010, 1217, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhou, Y.Y.; Liu, Y.N.; Guan, T.Y.; Zheng, X.; Dai, C.; Xing, L.; Rao, T.; Xie, L.; Wang, G.J. Study on the plasma protein binding rate of Schisandra lignans based on the LC-IT-TOF/MS technique with relative quantitative analysis. Chin. J. Nat. Med. 2013, 11, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Zhang, J.; Qiu, X.H.; Bai, J.Q.; Gao, Y.H.; Xu, W. Application of ultra-high-performance liquid chromatography coupled with LTQ-orbitrap mass spectrometry for the qualitative and quantitative analysis of Polygonum multiflorum Thumb. and its processed products. Molecules 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhu, D.; Qi, J.; Qin, M.; Yu, B. Characterization of homoisoflavonoids in different cultivation regions of Ophiopogon japonicus and related antioxidant activity. J. Pharm. Biomed. Anal. 2010, 52, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Zeng, K.W.; Zhou, Y.; Zhang, J.Y.; Che, Y.Y.; Tu, P.F. Cytotoxic steroidal saponins from Ophiopogon japonicus. Steroids 2013, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, M.; Parrilli, M.; Zhu, Y. Terpenoid glycosides from Ophiopogon japonicus roots. Phytochemistry 1990, 29, 1696–1699. [Google Scholar] [CrossRef]
- Ye, M.; Guo, D.; Ye, G.; Huang, C. Analysis of homoisoflavonoids in Ophiopogon japonicus by HPLC-DAD-ESI-MSn. J. Am. Soc. Mass Spectrom. 2005, 16, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, H.Y.; Zhang, H.; Wang, C.Z.; Li, P.; Yuan, C.S. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2012, 1230, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Y.; Liu, P.; Wang, H.Y.; Qi, L.W.; Wang, C.Z.; Li, P.; Yuan, C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1286, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Guan, T.; Zhou, Y.; Liu, Y.; Xing, L.; Zheng, X.; Dai, C.; Du, P.; Rao, T.; Zhou, L.; et al. Effect of mobile phase additives on qualitative and quantitative analysis of ginsenosides by liquid chromatography hybrid quadrupole-time of flight mass spectrometry. J. Chromatogr. A 2013, 1297, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Hao, H.; Wang, X.; Wu, X.; Wang, G.; Sang, G.; Liang, Y.; Xie, L.; Xia, C.; Yao, X. Diagnostic fragment-ion-based extension strategy for rapid screening and identification of serial components of homologous families contained in traditional chinese medicine prescription using high-resolution LC-ESI- IT-TOF/MS: Shengmai injection as an example. J. Mass Spectrom. 2009, 44, 230–244. [Google Scholar] [PubMed]
- Chu, C.; Xu, S.; Li, X.; Yan, J.; Liu, L. Profiling the ginsenosides of three ginseng products by LC-Q-TOF/MS. J. Food Sci. 2013, 78, C653–659. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Song, F.; Liu, Z.; Liu, S. Studies on lignan constituents from Schisandra chinensis (Turcz.) baill. fruits using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. J. Mass Spectrom. 2007, 42, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Sun, H.; Wei, W.; Han, Y.; Wang, P.; Dong, T.; Yan, G.; Wang, X. Rapid and global detection and characterization of the constituents in Shengmai San by ultra-performance liquid chromatography-high-definition mass spectrometry. J. Sep. Sci. 2011, 34, 3194–3199. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, D.F. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A 2009, 1216, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Che, Y.Y.; Zhang, L.; Zhang, J.Y.; Zhou, Y.; Jiang, Y.; Tu, P.F. Fingerprint analysis of Ophiopogonis radix by HPLC-UV-ELSD coupled with chemometrics methods. J. Chin. Pharm. Sci. 2013, 22, 55–63. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, X.L.; Li, M.H.; Iqbal, J.; Bourantas, C.V.; Li, J.J.; Su, X.Y.; Muramatsu, T.; Tian, N.L.; Chen, S.L. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J. Cardiovasc. Pharmacol. 2013, 62, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Shao, H.T.; Ge, W.P.; Liu, N.; Zhang, X.; Ma, C.M.; Qin, C.; Zhang, L.F. Ginsenoside-Rb1 and tetramethylpyrazine phosphate act synergistically to prevent dilated cardiomyopathy in CTNTR141W transgenic mice. J. Cardiovasc. Pharmacol. 2012, 59, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Wang, X.; Lau, W.; Wang, Y.; Xing, Y.; Zhang, X.; Ma, X.; Gao, F. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via AKT/GSK-3beta signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS ONE 2013, 8, e70956. [Google Scholar]
- Wang, Y.; Hu, Z.; Sun, B.; Xu, J.; Jiang, J.; Luo, M. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via AKT/endothelial nitric oxide synthase signaling and the B cell lymphoma/B cell lymphoma associated X protein pathway. Mol. Med. Rep. 2015, 11, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Ma, Z.; Yu, X.; Qu, S.; Sui, D. Effect of ginsenoside Rh1 on myocardial injury and heart function in isoproterenol-induced cardiotoxicity in rats. Toxicol. Mech. Methods 2012, 22, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Yi, F.; Shuang, L.; Chenjie, W.; Zheng, X.W. Chemical constituents from the fibrous root of Ophiopogon japonicus, and their effect on tube formation in human myocardial microvascular endothelial cells. Fitoterapia 2013, 85, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Thandavarayan, R.A.; Giridharan, V.V.; Arumugam, S.; Suzuki, K.; Ko, K.M.; Krishnamurthy, P.; Watanabe, K.; Konishi, T. Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PLoS ONE 2015, 10, e0119214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Ye, L.M.; Chen, X.B. A new C 27-steroidal glycoside from Ophiopogon japonicus. Chin. Chem. Lett. 2008, 19, 82–84. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds ophiogenin 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside, pennogenin-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyl-(1→4)-β-d-glucopyranoside in this paper and 10 batches of YQFM are available from the authors.

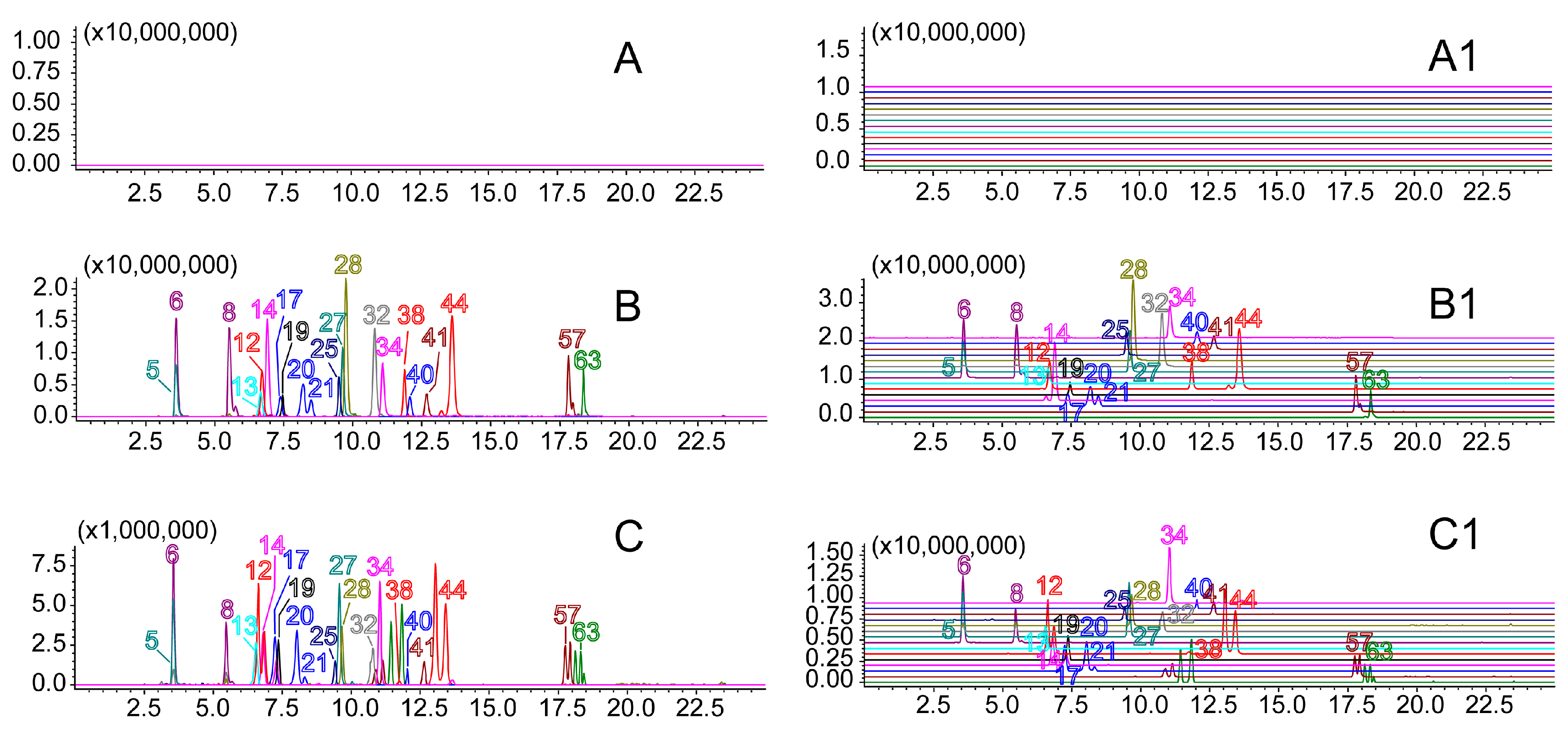
| Analyte | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | Y8 | Y9 | Y10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Peak 25 | 10.03 ± 0.12 | 10.54 ± 0.22 | 9.26 ± 0.40 | 8.35 ± 0.26 | 9.31 ± 0.33 | 9.22 ± 0.10 | 8.94 ± 0.20 | 9.81 ± 0.12 | 9.58 ± 0.21 | 9.93 ± 0.17 |
| Peak 28 | 340.79 ± 9.34 | 338.47 ± 5.92 | 335.26 ± 14.29 | 291.53 ± 1.52 | 309.36 ± 2.44 | 316.55 ± 5.08 | 300.84 ± 4.62 | 316.60 ± 6.92 | 309.69 ± 2.47 | 337.38 ± 0.42 |
| Peak 32 | 20.53 ± 0.74 | 20.16 ± 0.12 | 18.82 ± 0.10 | 14.27 ± 0.02 | 18.44 ± 0.86 | 18.80 ± 0.26 | 17.84 ± 0.20 | 20.36 ± 0.94 | 18.34 ± 0.78 | 21.90 ± 0.02 |
| Re | 239.22 ± 4.41 | 252.46 ± 2.39 | 260.48 ± 1.60 | 258.82 ± 3.84 | 269.16 ± 1.87 | 269.12 ± 5.50 | 264.25 ± 3.79 | 260.87 ± 0.35 | 260.10 ± 3.79 | 259.90 ± 3.60 |
| Rg1 | 1655.22 ± 71.14 | 1718.39 ± 70.12 | 1763.12 ± 59.12 | 1726.97 ± 10.05 | 1822.21 ± 2.40 | 1798.42 ± 35.13 | 1744.87 ± 17.27 | 1596.24 ± 10.16 | 1646.69 ± 17.24 | 1880.85 ± 6.02 |
| Rf | 681.40 ± 15.78 | 608.42 ± 3.51 | 603.54 ± 16.76 | 751.55 ± 8.44 | 792.20 ± 38.74 | 810.92 ± 22.34 | 734.34 ± 34.89 | 809.52 ± 32.91 | 734.60 ± 33.41 | 878.60 ± 23.79 |
| Rb1 | 1862.88 ± 34.38 | 1712.90 ± 25.32 | 1410.29 ± 65.97 | 1913.08 ± 93.66 | 2111.27 ± 80.02 | 2049.96 ± 3.82 | 1929.42 ± 71.45 | 2071.68 ± 29.32 | 2077.31 ± 77.55 | 2352.52 ± 37.43 |
| S-Rg2 | 20.45 ± 0.31 | 19.60 ± 0.29 | 18.65 ± 0.16 | 20.83 ± 0.06 | 20.30 ± 0.28 | 20.53 ± 0.10 | 21.30 ± 0.85 | 20.49 ± 0.22 | 20.17 ± 0.19 | 20.65 ± 0.11 |
| Ro | 1538.77 ± 69.92 | 1474.66 ± 4.35 | 1313.99 ± 40.31 | 1615.63 ± 40.01 | 1573.38 ± 18.22 | 1632.99 ± 35.10 | 1606.87 ± 25.73 | 1657.41 ± 57.58 | 1623.26 ± 15.37 | 1673.61 ± 43.11 |
| Rh1 | 473.68 ± 10.32 | 446.74 ± 2.02 | 388.87 ± 0.19 | 452.80 ± 3.69 | 427.09 ± 2.40 | 430.71 ± 9.74 | 429.85 ± 18.48 | 434.27 ± 4.07 | 417.29 ± 2.54 | 435.27 ± 1.74 |
| Rc | 84.83 ± 2.88 | 82.08 ± 2.00 | 80.59 ± 2.03 | 75.90 ± 0.13 | 79.84 ± 2.22 | 79.33 ± 0.53 | 78.03 ± 2.13 | 81.99 ± 3.66 | 76.47 ± 0.82 | 80.25 ± 1.38 |
| Rb2 | 477.20 ± 2.00 | 459.21 ± 1.74 | 419.09 ± 9.67 | 478.33 ± 3.28 | 495.10 ± 3.62 | 509.51 ± 21.34 | 483.88 ± 13.24 | 516.15 ± 15.09 | 483.72 ± 7.12 | 521.33 ± 3.29 |
| Rb3 | 98.25 ± 4.30 | 98.91 ± 0.80 | 89.71 ± 3.43 | 97.38 ± 0.58 | 103.03 ± 3.93 | 107.85 ± 0.86 | 101.33 ± 2.40 | 103.45 ± 4.03 | 97.51 ± 1.00 | 106.48 ± 2.91 |
| Rd | 672.80 ± 2.28 | 634.78 ± 3.03 | 601.17 ± 6.81 | 750.13 ± 29.17 | 760.54 ± 5.51 | 766.45 ± 1.01 | 727.08 ± 20.25 | 732.32 ± 23.51 | 728.89 ± 14.60 | 777.63 ± 6.88 |
| F2 | 2.10 ± 0.06 | 2.12 ± 0.07 | 2.19 ± 0.05 | 2.55 ± 0.07 | 2.68 ± 0.01 | 2.78 ± 0.02 | 2.46 ± 0.07 | 2.62 ± 0.11 | 2.52 ± 0.08 | 2.77 ± 0.02 |
| R-Rg3 | 143.24 ± 3.05 | 125.54 ± 1.43 | 101.18 ± 4.62 | 142.83 ± 4.49 | 143.42 ± 2.03 | 144.29 ± 3.34 | 143.62 ± 2.96 | 155.16 ± 2.29 | 144.93 ± 1.40 | 151.46 ± 1.20 |
| Rk1 | 84.54 ± 1.54 | 72.62 ± 1.72 | 59.09 ± 3.16 | 86.94 ± 1.27 | 88.67 ± 1.98 | 88.81 ± 0.71 | 86.57 ± 1.10 | 94.12 ± 0.75 | 89.53 ± 4.26 | 94.05 ± 3.23 |
| R-Rh2 | 0.94 ± 0.04 | 0.64 ± 0.02 | 0.71 ± 0.02 | 0.87 ± 0.01 | 0.98 ± 0.02 | 0.99 ± 0.01 | 0.92 ± 0.04 | 1.06 ± 0.04 | 1.04 ± 0.03 | 0.93 ± 0.02 |
| Schizandrol A | 129.08 ± 1.90 | 132.32 ± 1.70 | 123.19 ± 4.82 | 153.32 ± 2.64 | 155.45 ± 2.93 | 150.29 ± 2.66 | 142.81 ± 3.76 | 137.67 ± 4.66 | 142.85 ± 1.92 | 147.75 ± 1.46 |
| Gomisin D | 4.39 ± 0.13 | 4.90 ± 0.14 | 4.98 ± 0.02 | 5.56 ± 0.27 | 6.03 ± 0.29 | 5.67 ± 0.05 | 5.84 ± 0.04 | 5.77 ± 0.21 | 6.10 ± 0.04 | 5.69 ± 0.14 |
| Schizandrol B | 5.83 ± 0.20 | 6.52 ± 0.28 | 6.00 ± 0.25 | 7.03 ± 0.18 | 6.37 ± 0.10 | 6.82 ± 0.21 | 6.68 ± 0.32 | 6.55 ± 0.18 | 6.42 ± 0.22 | 6.31 ± 0.10 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ju, A.; Zhou, D.; Li, D.; Kou, J.; Yu, B.; Qi, J. Simultaneous Qualitative and Quantitative Analysis of Multiple Chemical Constituents in YiQiFuMai Injection by Ultra-Fast Liquid Chromatography Coupled with Ion Trap Time-of-Flight Mass Spectrometry. Molecules 2016, 21, 640. https://doi.org/10.3390/molecules21050640
Liu C, Ju A, Zhou D, Li D, Kou J, Yu B, Qi J. Simultaneous Qualitative and Quantitative Analysis of Multiple Chemical Constituents in YiQiFuMai Injection by Ultra-Fast Liquid Chromatography Coupled with Ion Trap Time-of-Flight Mass Spectrometry. Molecules. 2016; 21(5):640. https://doi.org/10.3390/molecules21050640
Chicago/Turabian StyleLiu, Chunhua, Aichun Ju, Dazheng Zhou, Dekun Li, Junping Kou, Boyang Yu, and Jin Qi. 2016. "Simultaneous Qualitative and Quantitative Analysis of Multiple Chemical Constituents in YiQiFuMai Injection by Ultra-Fast Liquid Chromatography Coupled with Ion Trap Time-of-Flight Mass Spectrometry" Molecules 21, no. 5: 640. https://doi.org/10.3390/molecules21050640
APA StyleLiu, C., Ju, A., Zhou, D., Li, D., Kou, J., Yu, B., & Qi, J. (2016). Simultaneous Qualitative and Quantitative Analysis of Multiple Chemical Constituents in YiQiFuMai Injection by Ultra-Fast Liquid Chromatography Coupled with Ion Trap Time-of-Flight Mass Spectrometry. Molecules, 21(5), 640. https://doi.org/10.3390/molecules21050640