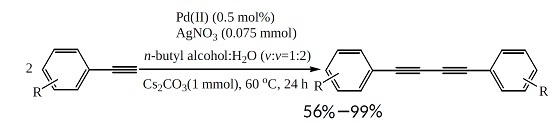
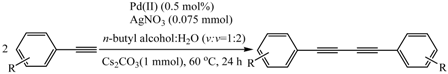
The Homocoupling Reaction of Aromatic Terminal Alkynes by a Highly Active Palladium(II)/AgNO3 Cocatalyst in Aqueous Media Under Aerobic Conditions
Abstract
:1. Introduction
2. Results and Discussion
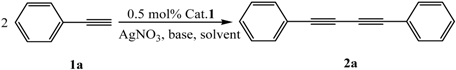
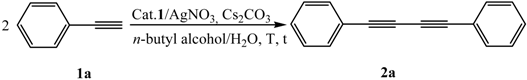
2.1. Optimization of the Homocoupling Reaction Conditions
2.2. Scope and Limitations of Substrates
3. Experimental Section
3.1. Reagents and Machine
3.2. General Experimental Procedure for the Homocoupling Reaction of Various Aromatic Alkynes
3.3. Analytical Data of Representative Products
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Glaser, C. Beitriige zur kenntnifs des acetenylbenzols. Ber. Dtsch. Chem. Ges. 1869, 2, 422–424. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.S.; Tykwinski, R.R. Synthesis of naturally occurring polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef] [PubMed]
- Stefani, H.A.; Costa, I.M.; Zeni, G. Synthesis of polyacetylenic montiporic acids A and B. Terahedron Lett. 1999, 40, 9215–9217. [Google Scholar] [CrossRef]
- Anderson, S.; Anderson, H.L. Synthesis of a water-soluble conjugated [3]rotaxane. Angew. Chem. Int. Ed. Engl. 1996, 35, 1956–1959. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, C.F. Synthesis and structure of a triptycene-based nanosized molecular cage. J. Org. Chem. 2007, 72, 9339–9341. [Google Scholar] [CrossRef] [PubMed]
- Sienmsen, P.; Livingston, R.C.; Diederich, F. Acetylenic coupling: A powerful tool in molecular construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Sindhu, K.S.; Gopinathan, A. Recent advances and applications of Glaser coupling employing greener protocols. RSC Adv. 2014, 4, 27867–27887. [Google Scholar] [CrossRef]
- Stefani, H.A.; Guarezemini, A.S.; Cella, R. Homocoupling reactions of alkynes, alkenes and alkyl compounds. Tetrahedron 2010, 66, 7871–7918. [Google Scholar] [CrossRef]
- Alonso, F.; Yus, M. Heterogeneous catalytic homocoupling of terminal alkynes. ACS Catal. 2012, 2, 1441–1451. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. Chemical from alkynes with palladium catalysts. Chem. Rev. 2014, 114, 1783–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Liang, Y.; Zhang, X.D. Amine and phosphine-free palladium(II)-catalyzed homocoupling reaction of terminal alkynes. Tetrahedron 2005, 61, 1903–1907. [Google Scholar] [CrossRef]
- Yang, F.; Cui, X.L.; Li, N.; Zhang, J.L.; Ren, G.R.; Wu, Y.J. Cyclopalladated ferrocenylimines: Efficient catalysts for homocoupling and Sonogashira reaction of terminal alkynes. Tetrahedron 2007, 63, 1963–1969. [Google Scholar] [CrossRef]
- Chen, S.N.; Wu, W.Y.; Tsai, F.Y. Homocoupling reaction of terminal alkynes catalyzed by a reusable cationic 2,2′-bipyridyl palladium(II)/CuI system in water. Green Chem. 2009, 11, 269–274. [Google Scholar] [CrossRef]
- Shi, M.; Qian, H.X. NHC-Pd(II) complex-Cu(I) cocatalyzed homocoupling reaction of terminal alkynes. Appl. Organomet. Chem. 2006, 20, 771–774. [Google Scholar] [CrossRef]
- Li, J.H.; Liang, Y.; Xie, Y.X. Efficient palladium-catalyzed homocoupling reaction and sonogashira cross-coupling reaction of terminal alkynes under aerobic conditions. J. Org. Chem. 2005, 70, 4393–4396. [Google Scholar] [CrossRef] [PubMed]
- Gil-Moltó, J.; Nájera, C. Palladium(II) chloride and a (dipyridin-2-ylmethyl)amine-derived palladium(II) chloride complex as highly efficient catalysts for the synthesis of alkynes in water or in NMP and of diynes in the absence of reoxidant. Eur. J. Org. Chem. 2005, 19, 4073–4081. [Google Scholar] [CrossRef]
- Wang, P.P.; Liu, X.Y.; Zhang, S.L. Ligand-free synthesis of 1,4-disubstituted-1,3-diynes by iron/copper cocatalyzed homocoupling of terminal alkynes. Chin. J. Chem. 2013, 31, 187–194. [Google Scholar] [CrossRef]
- Wu, K.Y.; Guo, J.; Wang, C.C. Gelation of metalloporphyrin-based conjugated microporous polymers by oxidative homocoupling of terminal alkynes. Chem. Mater. 2014, 26, 6241–6250. [Google Scholar] [CrossRef]
- Crowley, J.D.; Goldup, S.M.; Gowans, N.D.; Leigh, D.A.; Ronaldson, V.E.; Slawin, A.M.Z. An unusual nickel−copper-mediated alkyne homocoupling reaction for the active-template synthesis of [2]rotaxanes. J. Am. Chem. Soc. 2010, 132, 6243–6248. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.E.; Hirosawa, C.; Dalal, N.; Ramsey, C.; Steigman, A. Cobalt-catalyzed homocoupling of terminal alkynes: Synthesis of 1,3-diynes. Tetrahedron Lett. 2001, 42, 7733–7736. [Google Scholar] [CrossRef]
- Doménech, A.; Leyva-Pérez, A.; Al-Resayes, S.I.; Corma, A. Electrochemical monitoring of the oxidative coupling of alkynes catalyzed by triphenylphosphine gold complexes. Electrochem. Commun. 2012, 19, 145–148. [Google Scholar] [CrossRef]
- Bharathi, P.; Periasamy, M. Direct Metalation of 1-alkynes using TiCl4/Et3N and the reactions of the organotitanium intermediates with electrophiles. Organometallics 2000, 19, 5511–5513. [Google Scholar] [CrossRef]
- Meng, X.; Li, C.; Han, B.; Wang, T.; Chen, B. Iron/copper promoted oxidative homo-coupling reaction of terminal alkynes using air as the oxidant. Tetrahedron 2010, 66, 4029–4031. [Google Scholar] [CrossRef]
- Atobe, S.; Sonoda, M.; Suzuki, Y.; Yamamoto, T.; Masuno, H.; Shinohara, H.; Ogawa, A. Palladium-catalyzed oxidative homocoupling reaction of terminal acetylenes using tran-bidentatable 1-(2-pyridylethynyl)-2-(2-thienylethynyl)benzene. Res. Chem. Intermed. 2013, 39, 359–370. [Google Scholar] [CrossRef]
- Chen, L.R.; Lemma, B.E.; Rich, J.S.; Mack, J. Freedom: A copper-free, oxidant-free and solvent-free palladium catalysed homocoupling reaction. Green Chem. 2014, 16, 1101–1103. [Google Scholar] [CrossRef]
- Feng, X.J.; Zhao, Z.R.; Yang, F.; Jin, T.N.; Ma, Y.J.; Bao, M. 1,3-Diynes synthesis by homocoupling of terminal alkynes using a Pd(PPh3)4/Ag2O simple catalyst system. J. Organomet. Chem. 2011, 696, 1479–1482. [Google Scholar] [CrossRef]
- Guo, M.P.; Zhang, Q. An inexpensive and highly stable palladium(II) complex for room temperature Suzuki coupling reactions under ambient atmosphere. Tetrahedron Lett. 2009, 50, 1965–1968. [Google Scholar] [CrossRef]
- Guo, M.P.; Ge, J.Y.; Zhu, Z.Y.; Wu, X.C. Efficient Synthesis of aromatic nitriles via cyanation of aryl bromides and K4[Fe(CN)6] catalyzed by a palladium(II) complex. Lett. Org. Chem. 2013, 10, 213–215. [Google Scholar] [CrossRef]
- Yin, K.; Li, C.J.; Li, J.; Jia, X.S. CuI-catalyzed homocoupling of terminal alkynes to 1,3-diynes. Appl. Organomet. Chem. 2011, 25, 16–20. [Google Scholar] [CrossRef]
- Wu, T.M.; Huang, S.H.; Tsai, F.Y. A reusable CuSO4·5H2O/cationic 2,2’-bipyridyl system catalyzed homocoupling of terminal alkynes in water. Appl. Organomet. Chem. 2011, 25, 395–399. [Google Scholar] [CrossRef]
- Shi, X.L.; Hu, Q.Q.; Wang, F.; Zhang, W.Q.; Duan, P.G. Application of the polyacrylonitrile fiber as a novel support for polymer-supported copper catalysts in terminal alkyne homocoupling reactions. J. Catal. 2016, 337, 233–239. [Google Scholar] [CrossRef]
- Reddy, A.S.; Laali, K.K. Sonogashira cross-coupling in a designer ionic liquid (IL) without copper, external base, or additive, and with recycling and reuse of the IL. Tetrahedron Lett. 2015, 56, 4807–4810. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Dasgupta, P.; Ramakrishna, B.S.; Murty, V.N. Domino synthesis of 1,3-diynes from 1,1-dibromoalkenes: a Pd-catalyzed copper-free coupling method. Tetrahedron Lett. 2014, 55, 3529–3533. [Google Scholar] [CrossRef]
- Zhang, W.S.; Xu, W.J.; Zhang, F.; Qu, G.R. Synthsis of symmetrical 1,3-diynes via tandem reaction of (Z)-arylvinyl bromides in the presence of DBU and CuI. Chinese Chem. Lett. 2013, 24, 407–410. [Google Scholar] [CrossRef]
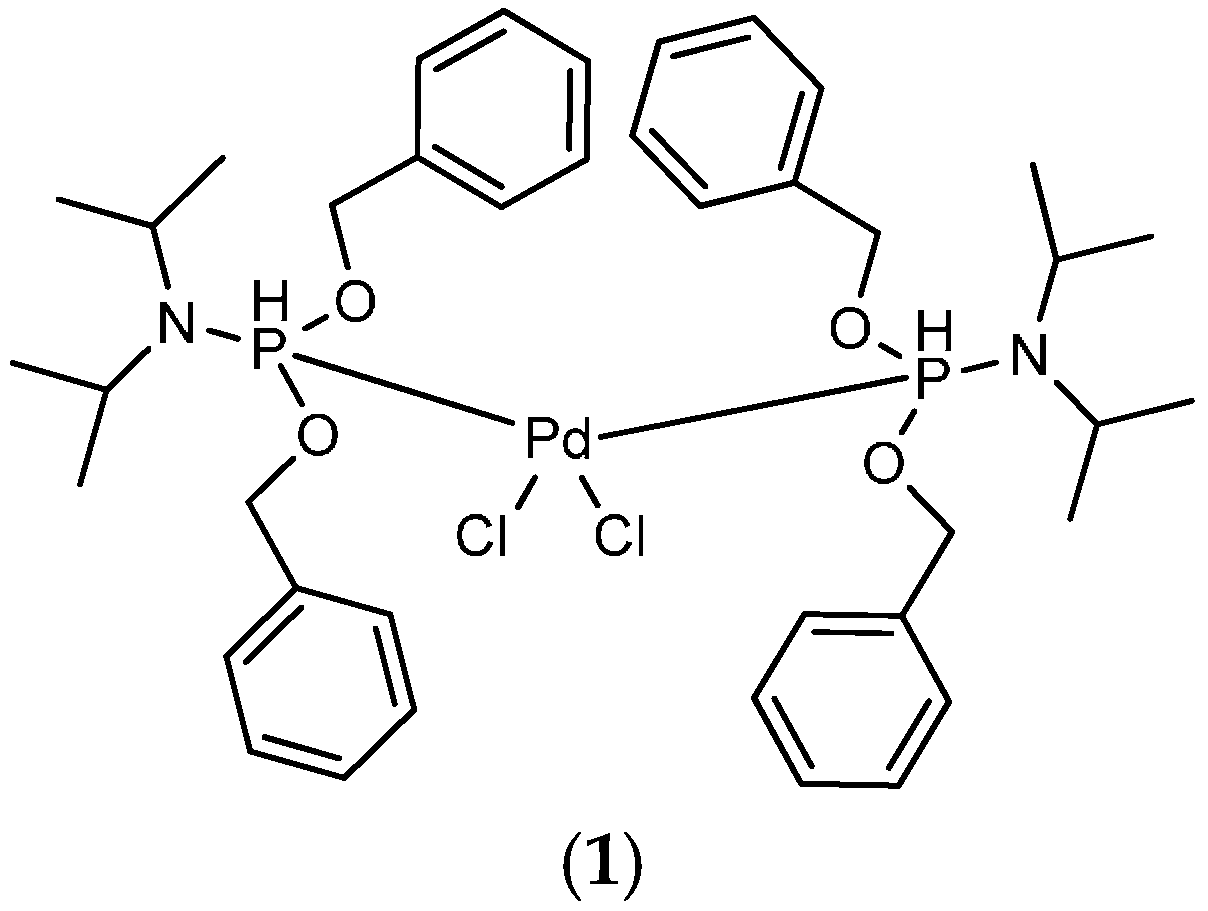
- Sample Availability: Samples of the compound (1) are available from the authors.
| Entry | Ase | Solvent | Yield c (%) |
|---|---|---|---|
| 1 b | NaOH | THF/H2O | 10 |
| 2 | NaOH | THF/H2O | 51 |
| 3 | KOH | THF/H2O | 45 |
| 4 | Na2CO3 | THF/H2O | 67 |
| 5 | K2CO3 | THF/H2O | 57 |
| 6 | NaHCO3 | THF/H2O | 63 |
| 7 | NaH2PO4 | THF/H2O | 71 |
| 8 | KHCO3 | THF/H2O | 64 |
| 9 | KH2PO4 | THF/H2O | 70 |
| 10 | K3PO4 | THF/H2O | 70 |
| 11 | Cs2CO3 | THF/H2O | 85 |
| 12 | NaF | THF/H2O | 47 |
| 13 | CH3COONa | THF/H2O | 53 |
| 14 | NEt3 | THF/H2O | 63 |
| 15 | Pyridine | THF/H2O | 54 |
| 16 | Cs2CO3 | DMSO/H2O | 47 |
| 17 | Cs2CO3 | N,N-Dimethylacetylamide/H2O | 39 |
| 18 | Cs2CO3 | PEG400/H2O | 59 |
| 19 | Cs2CO3 | Acetone/H2O | 88 |
| 20 | Cs2CO3 | 1,4-Dioxane/H2O | 79 |
| 21 | Cs2CO3 | Ethanol/H2O | 54 |
| 22 | Cs2CO3 | N-Butyl alcohol/H2O | 93 |
| 23 | Cs2CO3 | Methanol/H2O | 45 |
| Entry | n-Butyl alcohol/H2O (v/v) | Catalyst (mol %) | AgNO3 (mmol) | Time (h) | Temperature (°C) | Yield b (%) |
|---|---|---|---|---|---|---|
| 1 | 3:0 | 0.5 | 0.05 | 24 | 60 | 50 |
| 2 | 2:0.5 | 0.5 | 0.05 | 24 | 60 | 89 |
| 3 | 2:1 | 0.5 | 0.05 | 24 | 60 | 89 |
| 4 | 1.5:1.5 | 0.5 | 0.05 | 24 | 60 | 93 |
| 5 | 1:2 | 0.5 | 0.05 | 24 | 60 | 97 |
| 6 | 0:3 | 0.5 | 0.05 | 24 | 60 | 48 |
| 7 | 1:2 | 0 | 0.05 | 24 | 60 | trace |
| 8 | 1:2 | 0.25 | 0.05 | 24 | 60 | 65 |
| 9 | 1:2 | 1 | 0.05 | 24 | 60 | 96 |
| 10 | 1:2 | 1.5 | 0.05 | 24 | 60 | 90 |
| 11 | 1:2 | 0.5 | 0 | 24 | 60 | trace |
| 12 | 1:2 | 0.5 | 0.01 | 24 | 60 | 35 |
| 13 | 1:2 | 0.5 | 0.025 | 24 | 60 | 62 |
| 14 | 1:2 | 0.5 | 0.075 | 24 | 60 | 99 |
| 15 | 1:2 | 0.5 | 0.1 | 24 | 60 | 86 |
| 16 | 1:2 | 0.5 | 0.075 | 4 | 60 | 13 |
| 17 | 1:2 | 0.5 | 0.075 | 12 | 60 | 72 |
| 18 | 1:2 | 0.5 | 0.075 | 21 | 60 | 76 |
| 19 | 1:2 | 0.5 | 0.075 | 30 | 60 | 86 |
| 20 | 1:2 | 0.5 | 0.075 | 24 | 40 | 56 |
| 21 | 1:2 | 0.5 | 0.075 | 24 | 80 | 92 |
| Entry | Alkyne | Product | Yield b (%) |
|---|---|---|---|
| 1 |  | 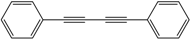 | 99 |
| 2 | 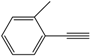 |  | 86 |
| 3 | 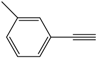 |  | 84 |
| 4 | 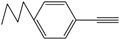 | 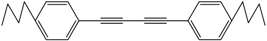 | 88 |
| 5 | 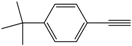 |  | 82 |
| 6 |  | 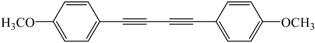 | 83 |
| 7 | 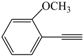 | 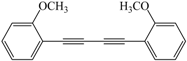 | 93 |
| 8 | 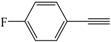 | 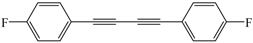 | 56 |
| 9 | 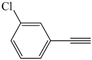 |  | 78 |
| 10 | 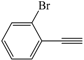 |  | 82 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Chen, B.; Lv, M.; Zhou, X.; Wen, Y.; Shen, X. The Homocoupling Reaction of Aromatic Terminal Alkynes by a Highly Active Palladium(II)/AgNO3 Cocatalyst in Aqueous Media Under Aerobic Conditions. Molecules 2016, 21, 606. https://doi.org/10.3390/molecules21050606
Guo M, Chen B, Lv M, Zhou X, Wen Y, Shen X. The Homocoupling Reaction of Aromatic Terminal Alkynes by a Highly Active Palladium(II)/AgNO3 Cocatalyst in Aqueous Media Under Aerobic Conditions. Molecules. 2016; 21(5):606. https://doi.org/10.3390/molecules21050606
Chicago/Turabian StyleGuo, Mengping, Bo Chen, Meiyun Lv, Xiuling Zhou, Yongju Wen, and Xiuli Shen. 2016. "The Homocoupling Reaction of Aromatic Terminal Alkynes by a Highly Active Palladium(II)/AgNO3 Cocatalyst in Aqueous Media Under Aerobic Conditions" Molecules 21, no. 5: 606. https://doi.org/10.3390/molecules21050606
APA StyleGuo, M., Chen, B., Lv, M., Zhou, X., Wen, Y., & Shen, X. (2016). The Homocoupling Reaction of Aromatic Terminal Alkynes by a Highly Active Palladium(II)/AgNO3 Cocatalyst in Aqueous Media Under Aerobic Conditions. Molecules, 21(5), 606. https://doi.org/10.3390/molecules21050606