Volatile Organic Compounds Emissions from Luculia pinceana Flower and Its Changes at Different Stages of Flower Development
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Scent Components
2.2. Dynamic Changes of Scent Emission in Different Development Floral Stages
3. Materials and Methods
3.1. Plant Materials
3.2. Method
3.3. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, W.; Barrett, S.C.H.; Wang, H.; Li, D.-Z. Reciprocal herkogamy promotes disassortative mating in a distylous species with intramorph compatibility. New Phytol. 2015, 206, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, Y.; Li, Z.H.; Wan, Y.M.; Liu, X.X.; Liang, N. A study on the breeding system of Luculia pinceana. Forest Res. 2009, 22, 373–378. [Google Scholar]
- Zhou, W.; Wang, H.; Li, D.Z.; Yang, J.B.; Zhou, W. Isolation and characterization of 13 microsatellite loci from Luculia pinceana (Rubiaceae), a typical distylous species. Hortscience 2010, 45, 840–841. [Google Scholar]
- Zhou, W.; Barrett, S.C.H.; Wang, H.; Li, D.Z. Loss of floral polymorphism in heterostylous Luculia pinceana (Rubiaceae): A molecular phylogeographic perspective. Mol. Ecol. 2012, 21, 4631–4645. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.M.; Li, Z.H.; Ma, H.; Liu, X.X. Microsporogenesis and male gametophyte development of long-style flower of Luculia pinciana (Rubiaceae). Acta Bot. Boreal-Occident. Sin. 2014, 34, 1570–1575. [Google Scholar]
- Murray, B.G. Heterostyly and pollen-tube interactions in Luculia gratissima (Rubiaceae). Ann. Bot. 1990, 65, 691–698. [Google Scholar]
- Kang, W.Y.; Du, Z.Z.; Yang, X.S.; Hao, X.J. A new triterpene from Luculia pinciana Hook. J. Asian Nat. Prod. Res. 2005, 7, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Rodiek, S.; Fujii, E.; Miyazaki, Y.; Park, B.J.; Ann, S.W. Physiological and psychological response to floral scent. Hortscience 2013, 48, 82–88. [Google Scholar]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotech. 2008, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 22 February 2016).
- Kang, W.Y.; Wang, J.M.; Ji, Z.Q. Flavonoids in Luculia pinceana. Chem. Nat. Compd. 2008, 44, 644–645. [Google Scholar] [CrossRef]
- Kim, H.K.; Tak, J.H.; Ahn, Y.J. Acaricidal activity of paeonia suffruticosa root bark-derived compounds against dermatophagoides farinae and dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2005, 52, 7857–7861. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.J.; Hong, C.; Liang, Y.C.; Lin, H.H.; Horng, J.C.; Kuo, Y.C.; Chen, C.W.; Tsai, F.Y.; Yen, S.C.; Chou, S.C. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-HBV agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Togar, B.; Stefano, A.D.; Taspınar, N.; Sozio, P. Protective effects of cyclosativene on H2O2-induced injury in cultured rat primary cerebral cortex cells. Cytotechnology 2014, 67, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Rasoarivelo, S.T.R.; Grougnet, R.; Verite, P.; Lecsoe, M.; Butel, M.J.; Tillequin, F.; Guillou, C.R.; Deguin, B. Chemical composition and antimicrobial activity of the essential oils of Anthospermum emirnense and Anthospermum perrieri (Rubiaceae). Chem. Biodivers. 2011, 8, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N.; Noletto, J.A.; Lawton, R.O. Chemical composition of the floral essential oil of Randia matudae from Monteverde, Costa Rica. Flavour Frag. J. 2006, 21, 244–246. [Google Scholar] [CrossRef]
- Kaiser, R. Scents from rain forests. Chimia 2000, 54, 346–363. [Google Scholar]
- Vieira, R.C.; Delprete, P.G.; Leitao, G.G.; Leitao, S.G. Anatomical and chemical analyses of leaf secretory cavities of Rustia formosa (Rubiaceae). Am. J. Bot. 2001, 88, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Nilsson, L.A.; Groth, I.; Bergstrom, G. Floral scents in butterfly-pollinated plants: Possible convergence in chemical composition. Bot. J. Linn. Soc. 2002, 140, 129–153. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.Y.; Lv, H. Constituents and bacteriostatic activity of volatile matter from four flower plant species. Indian J. Agric. Res. 2010, 44, 157–167. [Google Scholar]
- Stashenko, E.E.; Martinez, J.R.; Cardenas-Vargas, S.; Saavedra-Barrera, R.; Duran, D.C. Gc-ms study of compounds isolated from Coffea arabica flowers by different extraction techniques. J. Sep. Sci. 2013, 36, 2901–2914. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.W.; Hao, C.Y.; He, S.Z.; Wu, G.; Tan, L.H.; Xu, F.; Hu, R.S. Volatile organic compound emissions from different stages of Cananga odorata flower development. Molecules 2014, 19, 8965–8980. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Hairul, A.R.; Namasivayam, P.; Lian, G.E.C.; Abdullah, J.O. Terpenoid, benzenoid, and phenylpropanoid compounds in the floral scent of Vanda Mimi Palmer. J. Plant Biol. 2010, 53, 358–366. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, G.; Wang, S.; Lin, Q.; Zhang, J.; Li, X. Analysis of the variation in scent components of Hosta flowers by HS-SPME and GC–MS. Sci. Hortic. 2014, 175, 57–67. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, P.M. Misidentification of the bray-curtis similarity index. Mar. Ecol. Prog. Ser. 2008, 368, 309–310. [Google Scholar] [CrossRef]
- Al-Kateb, H.; Mottram, D.S. The relationship between growth stages and aroma composition of lemon basil Ocimum citriodorum vis. Food Chem. 2014, 152, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.C.F.; Raguso, R.A.; Kessler, A.; Parachnowitsch, A.L. Spatiotemporal floral scent variation of Penstemon digitalis. J. Chem. Ecol. 2015, 41, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

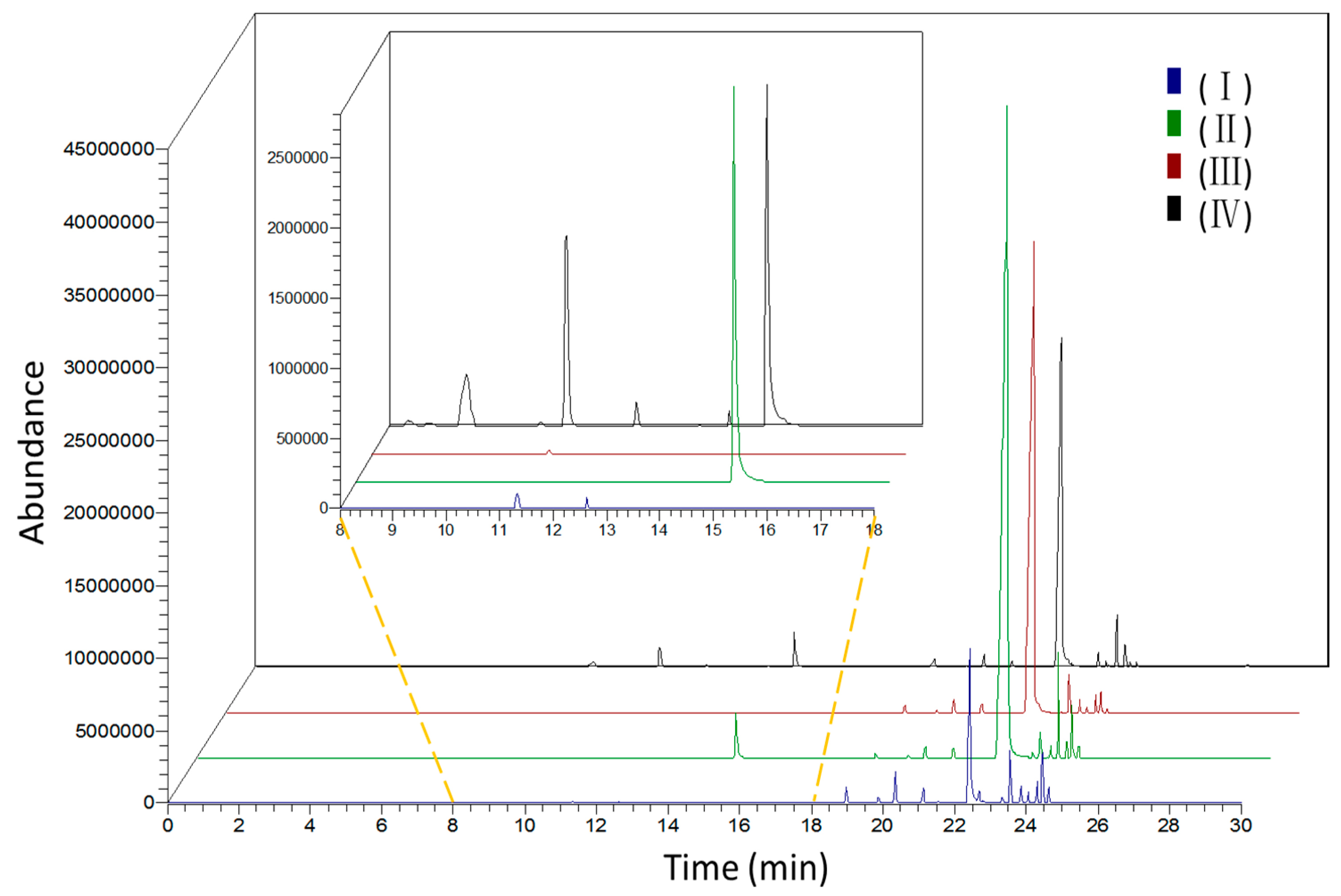
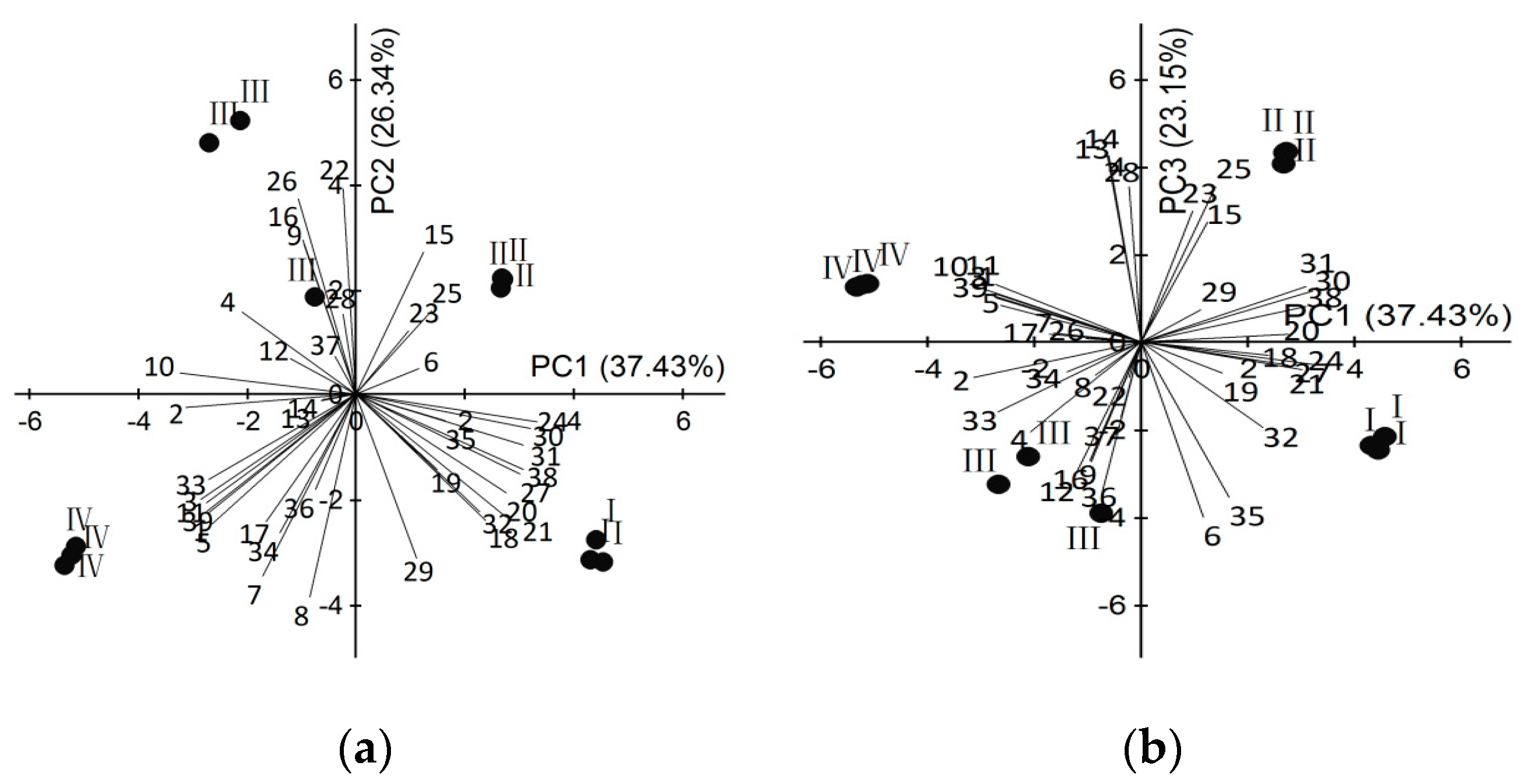
| Peak | RT | LRI | Compounds | CAS # | Relative Content(%) ± SD | ||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||
| Monoterpenes | 1.48 ± 0.69b | 0.15 ± 0.02b | 1.63 ± 0.83b | 7.91 ± 1.15a | |||||
| 1 | 8.37 | 904 | (1S)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene | 7785-26-4 | 0b | 0b | 0b | 0.45 ± 0.08a | |
| 2 | 8.75 | 918 | Santolina triene | 2153-66-4 | 0a | 0a | 0.32 ± 0.24a | 0.34 ± 0.04a | |
| 3 | 9.46 | 943 | α-Pinene | 80-56-8 | 0b | 0.05 ± 0.01b | 0.26 ± 0.15b | 2.11 ± 0.24a | |
| 4 | 10.84 | 994 | Limonene | 138-86-3 | 0a | 0a | 0.60 ± 0.43a | 0.22 ± 0.03a | |
| 5 | 11.33 | 1012 | 3-Carene | 13466-78-9 | 0.47 ± 0.48b | 0.02 ± 0.00b | 0.18 ± 0.12b | 3.73 ± 0.61a | |
| 6 | 11.94 | 1034 | (Z)-Verbenol | 1845-30-3 | 0.05 ± 0.03a | 0a | 0.04 ± 0.02a | 0a | |
| 7 | 12.37 | 1049 | γ-Terpiene | 99-85-4 | 0.03 ± 0.02a | 0b | 0b | 0.03 ± 0.00a | |
| 8 | 12.64 | 1059 | (E)-β-Ocimene | 3779-61-1 | 0.87 ± 0.43a | 0.02 ± 0.00b | 0.09 ± 0.07b | 0.61 ± 0.07ab | |
| 10 | 13.83 | 1102 | α-Campholenal | 91819-58-8 | 0b | 0.02 ± 0.00b | 0.07 ± 0.03a | 0.08 ± 0.01a | |
| 11 | 14.36 | 1123 | α-Santoline alcohol | 90823-36-2 | 0.02 ± 0.01b | 0.02 ± 0.00b | 0.04 ± 0.03b | 0.32 ± 0.06a | |
| 15 | 18.31 | 1295 | Perilla alcohol | 536-59-4 | 0.04 ± 0.02a | 0.03 ± 0.00a | 0.05 ± 0.02a | 0.02 ± 0.00a | |
| Aliphatics | 2.46 ± 1.31a | 0.53 ± 0.08a | 1.46 ± 0.48a | 2.81 ± 0.33a | |||||
| 12 | 14.84 | 1142 | (3 E,5Z)-1,3,5-Undecatriene | 51447-08-6 | 0.04 ± 0.02a | 0a | 0.06 ± 0.04a | 0.03 ± 0.00a | |
| 14 | 17.39 | 1251 | Nonanoic acid,ethyl ester | 123-29-5 | 0c | 0.01 ± 0.00b | 0c | 0.02 ± 0.00a | |
| 17 | 18.98 | 1318 | Megastigma-4,6(E),8(E)-triene | 51468-86-1 | 2.13 ± 1.28a | 0.48 ± 0.07a | 1.18 ± 0.53a | 2.49 ± 0.31a | |
| 33 | 25.40 | 1577 | Hexyl caprylate | 1117-55-1 | 0.08 ± 0.05ab | 0b | 0.08 ± 0.02ab | 0.10 ± 0.01a | |
| 34 | 25.62 | 1592 | Pentanoic acid, 2,2,4-trimethyl- 3-carboxyisopropyl, isobutyl ester | 959016-51-4 | 0.22 ± 0.13a | 0.04 ± 0.00a | 0.14 ± 0.02a | 0.17 ± 0.02a | |
| Benzenoids | 51.61 ± 22.32a | 85.88 ± 2.45a | 80.05 ± 6.24a | 75.38 ± 3.47a | |||||
| 13 | 15.08 | 1151 | Methyl salicylate | 119-36-8 | 0c | 2.85 ± 0.45b | 0c | 4.81 ± 0.46a | |
| 23 | 22.43 | 1433 | Paeonol | 552-41-0 | 51.58 ± 22.36a | 83.03 ± 2.91a | 79.72 ± 6.14a | 69.75 ± 4.00a | |
| 37 | 26.18 | 1639 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-(1-methylpropyl) | 14035-34-8 | 0a | 0a | 0.30 ± 0.36a | 0.04 ± 0.00a | |
| 39 | 27.75 | 1791 | Benzyl benzoate | 120-51-4 | 0.03 ± 0.04b | 0b | 0.03 ± 0.01b | 0.78 ± 0.07a | |
| Sesquiterpenes | 44.41 ± 21.11a | 13.37 ± 2.34a | 16.79 ± 5.16a | 13.91 ± 1.99a | |||||
| 18 | 19.87 | 1346 | α-Cubebene | 17699-14-8 | 2.21 ± 1.42a | 0.32 ± 0.06a | 0.28 ± 0.26a | 0.52 ± 0.07a | |
| 19 | 20.35 | 1362 | Cyclosativene | 22469-52-9 | 4.96 ± 2.66a | 1.04 ± 0.15a | 2.07 ± 1.17a | 1.87 ± 0.16a | |
| 20 | 21.12 | 1386 | Isoledene | 95910-36-4 | 5.45 ± 3.16a | 0.96 ± 0.20a | 0.57 ± 0.73a | 1.06 ± 0.12a | |
| 21 | 21.53 | 1399 | Caryophyllene | 87-44-5 | 1.34 ± 0.76a | 0.17 ± 0.03a | 0.15 ± 0.08a | 0.23 ± 0.01a | |
| 22 | 21.89 | 1413 | β-Ylangene | 20479-06-5 | 0b | 0.02 ± 0.00b | 0.09 ± 0.03a | 0b | |
| 24 | 23.37 | 1467 | (-)-β-Cadinene | 523-47-7 | 2.34 ± 1.35a | 0.38 ± 0.06ab | 0.58 ± 0.20ab | 0.12 ± 0.01b | |
| 26 | 23.59 | 1476 | γ-Muurolene | 30021-74-0 | 0b | 1.35 ± 0.22b | 5.86 ± 1.95a | 1.46 ± 0.14b | |
| 27 | 23.85 | 1485 | Cubebol | 23445-02-5 | 5.48 ± 3.14a | 0.77 ± 0.11a | 0.56 ± 0.63a | 0.46 ± 0.08a | |
| 28 | 24.06 | 1493 | (E,E)-α-Farnesene | 502-61-4 | 2.46 ± 1.47a | 3.89 ± 1.10a | 4.90 ± 4.38a | 4.75 ± 0.98a | |
| 29 | 24.30 | 1503 | 4- epi-Cubebol | 23445-02-5 | 4.76 ± 2.65a | 0.91 ± 0.15a | 0.73 ± 0.82a | 2.29 ± 0.30a | |
| 30 | 24.45 | 1513 | δ-Cadinene | 483-76-1 | 10.98 ± 6.25a | 2.61 ± 0.10ab | 0.64 ± 0.75b | 0.60 ± 0.08b | |
| 31 | 24.62 | 1525 | Cadine-1,4-diene | 16728-99-7 | 3.19 ± 1.84a | 0.77 ± 0.11a | 0.25 ± 0.31a | 0.42 ± 0.03a | |
| 35 | 25.69 | 1597 | Cedrol | 77-53-2 | 0.11 ± 0.06a | 0a | 0.05 ± 0.03a | 0a | |
| 36 | 25.81 | 1606 | α-Acorenol | 28296-85-7 | 0.12 ± 0.07a | 0a | 0.07 ± 0.03a | 0.05 ± 0.01a | |
| 38 | 26.33 | 1652 | Cubenol | 21284-22-0 | 1.02 ± 0.58a | 0.18 ± 0.04ab | 0b | 0.07 ± 0.01b | |
| Unknowns | 0.03 ± 0.02ab | 0.07 ± 0.01a | 0.08 ± 0.03a | 0b | |||||
| 9 | 12.95 | 1070 | Unknown-1 | - | 0b | 0b | 0.02 ± 0.01a | 0b | |
| 16 | 18.60 | 1306 | Unknown-2 | - | 0b | 0b | 0.05 ± 0.02a | 0b | |
| 25 | 23.44 | 1470 | Unknown-3 | - | 0b | 0.07 ± 0.01a | 0b | 0b | |
| 32 | 25.27 | 1568 | Unknown-4 | - | 0.03 ± 0.02a | 0b | 0b | 0b | |
| I | II | III | IV | |
|---|---|---|---|---|
| I | 100 | |||
| II | 62.77 | 100 | ||
| III | 61.81 | 90.23 | 100 | |
| IV | 65.32 | 83.59 | 83.71 | 100 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ma, H.; Wan, Y.; Li, T.; Liu, X.; Sun, Z.; Li, Z. Volatile Organic Compounds Emissions from Luculia pinceana Flower and Its Changes at Different Stages of Flower Development. Molecules 2016, 21, 531. https://doi.org/10.3390/molecules21040531
Li Y, Ma H, Wan Y, Li T, Liu X, Sun Z, Li Z. Volatile Organic Compounds Emissions from Luculia pinceana Flower and Its Changes at Different Stages of Flower Development. Molecules. 2016; 21(4):531. https://doi.org/10.3390/molecules21040531
Chicago/Turabian StyleLi, Yuying, Hong Ma, Youming Wan, Taiqiang Li, Xiuxian Liu, Zhenghai Sun, and Zhenghong Li. 2016. "Volatile Organic Compounds Emissions from Luculia pinceana Flower and Its Changes at Different Stages of Flower Development" Molecules 21, no. 4: 531. https://doi.org/10.3390/molecules21040531
APA StyleLi, Y., Ma, H., Wan, Y., Li, T., Liu, X., Sun, Z., & Li, Z. (2016). Volatile Organic Compounds Emissions from Luculia pinceana Flower and Its Changes at Different Stages of Flower Development. Molecules, 21(4), 531. https://doi.org/10.3390/molecules21040531






