Abstract
Luculia plants are famed ornamental plants with sweetly fragrant flowers, of which L. pinceana Hooker, found primarily in Yunnan Province, China, has the widest distribution. Solid phase microextraction-gas chromatography-mass spectrometry (SPME-GC-MS) was employed to identify the volatile organic compounds (VOCs) emitted from different flower development stages of L. pinceana for the evaluation of floral volatile polymorphism. Peak areas were normalized as percentages and used to determine the relative amounts of the volatiles. The results showed that a total of 39 compounds were identified at four different stages of L. pinceana flower development, including 26 at the bud stage, 26 at the initial-flowering stage, 32 at the full-flowering stage, and 32 at the end-flowering stage. The most abundant compound was paeonol (51%–83%) followed by (E,E)-α-farnesene, cyclosativene, and δ-cadinene. All these volatile compounds create the unique fragrance of L. pinceana flower. Floral scent emission offered tendency of ascending first and descending in succession, meeting its peak level at the initial-flowering stage. The richest diversity of floral volatile was detected at the third and later periods of flower development. Principal component analysis (PCA) indicated that the composition and its relative content of floral scent differed throughout the whole flower development. The result has important implications for future floral fragrance breeding of Luculia. L. pinceana would be adequate for a beneficial houseplant and has a promising prospect for development as essential oil besides for a fragrant ornamental owing to the main compounds of floral scent with many medicinal properties.
1. Introduction
Luculia Sweet is a small genus of the family Rubiaceae, consisting of about five types of small trees or shrubs. Three species occur in China, amongst which Luculia pinceana Hooker, found primarily in Yunnan Province at altitudes between 600 and 3000 m has the widest distribution in Nature [1,2,3]. Luculia can be easily recognized by its compact and long-term blooming (usually April to November) inflorescences with white to pink, sweetly fragrant flowers with extremely long corolla tubes. On account of the above-mentioned advantages, Luculia plants have been widely introduced around the world. Studies in the past are often concentrated on its reproductive system, distyly, molecular biology and gametophyte development [1,2,3,4,5,6,7]. In addition, as a traditional medicinal and ornamental plant, research work is underway concerning its cultivation and breeding.
Floral fragrance is one of the most important characteristics of ornamental plants or cut flowers, because it may affect people’s health and mood [8]. Thus, the study of the composition of floral scent and the breeding of new ornamental plant cultivars with sweet-smelling or pleasant fragrance have become new trends of the development of plant breeding nowadays [9]. Although L. pinceana possess the features of long-term blooming, sweet fragrance and has great potential in serving as an indoor plant, research on its floral scent has not been carried out. In this work, the volatile component emissions from different flower development stages of L. pinceana were analyzed by solid phase microextraction coupled with gas chromatography-mass spectrometry in order to build a foundation for future work on Luculia floral fragrance breeding programs. Furthermore, the results could provide a reference for determining whether Luculia species are suitable for indoor cultivation and extraction of essential oil.
2. Results and Discussion
2.1. Identification of Scent Components
Thirty-nine VOCs were identified, representing more than 99% of the total emission of the flowers. These volatiles grouped by their biochemical synthesis pathways [10] were described in Table 1. A total of 18 same volatile compounds were shared at four different stages of flower development of L. pinceana. Within these compounds, the main aroma-active one was paeonol followed by (E,E)-α-farnesene, cyclosativene, and δ-cadinene. These compounds might dominate the flavor for L. pinceana. For instance, paeonol has a specific odour; (E,E)-α-farnesene has a woody and sweet odour; δ-Cadinene gives thyme, medicine and wood odour [11]. As one of these components, γ-muurolene has a smell of herb, wood and spice; methyl salicylate has a flavor with peppermint aroma. The two compounds ranked second in relative content of VOCs at the full-flowering stage and the end-flowering stage separately, so they could also influence the floral aroma.

Table 1.
Volatile compounds identified in four different stages of L. pinceana flower development using SPME-GC-MS. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flowering stage.
The idea that L. pinceana has medicinal properties goes back hundreds of years in China [12]. Some of volatile compounds from flowers are pharmacologically active compounds. For example, paeonol has several interesting biological activities, and it has been used as an anti-inflammatory, analgesic, antioxidant, antidiabetic, and acaricidal agent [13,14]; cyclosativene demonstrates strong anti-inflammatory, expectorant, antifungal effect [15]; γ-muurolene and δ-cadinene have antifungal properties. Despite the fact that essential oils are seldom encountered in the Rubiaceae [16], L. pinceana would have a lot of potential for essential oil extraction according to the L. pinceana solid phase microextraction results. Not only that, the essential oil of L. pinceana flowers might have special therapeutic qualities in view of the above active ingredients among the volatile compounds.
Benzenoids were the most abundant amongst floral scent compounds, which content reached at least 51%. The same scenario was noted in the floral essential oil of Randia matudae [17] compared to other species of different genera in the family Rubiaceae. In contrast, the quantity and amount of predominant compounds in the floral scent of Posoqueria latifolia [18], the leaf essential oil of Rustia formosa and the essential oil from aerial parts of Anthospermum emirnense and A. perrieri were sesquiterpenes [16,19]; the floral scent of Cephalanthus occidentalis, Warszewiczia coccinea and Gardenia jasminoides were monoterpenes [20,21]; the floral scent of Coffea Arabica were aliphatics [22]. It has been reported that the floral scent composition probably significantly varied amongst closely related species, and our results partly support this view [10].
2.2. Dynamic Changes of Scent Emission in Different Development Floral Stages
L. pinceana flowers were selected on the basis of their botanical characteristics to evaluate the dynamic changes and diversity of floral volatiles according to different development stages: bud stage, initial-flowering stage, full-flowering stage, and end-flowering stage (Figure 1). Table 1 and Figure 2 show the distinct changes in scent composition and concentration across flowering stages. Scent components were drastically emitted at the initial-flowering stage, and then declined gradually at the full-flowering stage. The amount of VOCs at the bud stage and the end-flowering stage was obviously lower than that at the initial-flowering stage. The emission pattern of L. pinceana flowering stages was different from Cananga odorata [23], Vanda Mimi Palmer [24], and Hosta flowers [25] of which the fragrance ingredients were drastically emitted at the full-flowering stage and decreased greatly afterwards. These results showed that the emissions at different flower stages evidently differed. Investigation of the spatial and temporal patterns of gene expression has provided new information on the factors regulating the emission of plant volatile compounds [26].

Figure 1.
The morphological characteristics of L. pinceana flower in four different stages. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flowering stage.
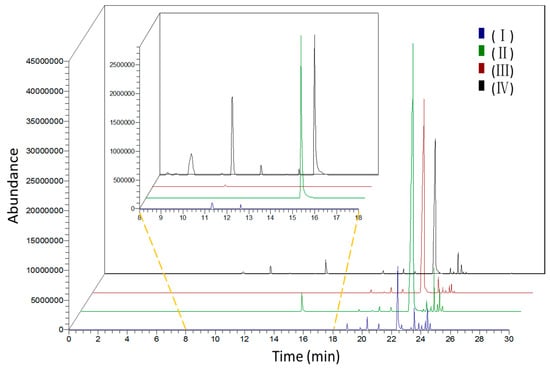
Figure 2.
Total ionic chromatogram of volatile components emitted from flowers of L. pinceana in different stages. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flowering stage.
As for the bud stage, 26 volatile compounds belonging to different chemical classes were identified: benzenoids (51.61%), sesquiterpenes (44.41%), aliphatics (2.46%), and monoterpenes (1.48%). The most abundant compound was paeonol, accounting for about 52% of the total GC peak area, followed by δ-cadinene (10.98%), cubebol (5.48%), isoledene (5.45%), and cyclosativene (4.96%). By contrast, relative content of (E)-β-ocimene (0.87%), (−)-β-cadinene (2.34%), cubebol (5.48%), and cubenol (1.02%) at the bud stage were higher than that at the other stages of flower development.
As for the initial-flowering stage, 26 volatile compounds belonging to different chemical classes were identified: benzenoids (85.88%), sesquiterpenes (13.37%), aliphatics (0.53%), and monoterpenes (0.15%). The most abundant compound was paeonol, accounting for about 83% of the total GC peak area, followed by (E,E)-α-farnesene (3.89%), methyl salicylate (2.85%), and δ-cadinene (2.61%). On the other hand, relative content of nonanoic acid, ethyl ester (0.01%) and methyl salicylate (2.85%) in the initial-flowering stage were significantly lower than that in the end-flowering stage, but was higher than that in the bud stage and the full-flowering stage. Relative content of hexyl caprylate was significantly lower than that in the other flower development stages.
As for the full-flowering stage, 32 volatile compounds belonging to different chemical classes were identified: benzenoids (80.05%), sesquiterpenes (16.79%), monoterpenes (1.63%), and aliphatics (1.46%). The most abundant compound was paeonol, accounting for about 80% of the total GC peak area, followed by γ-muurolene (5.86%), (E,E)-α-farnesene (4.90%), and cyclosativene (2.07%). In addition, relative content of β-ylangene (0.09%) and γ-muurolene (5.86%) in the full-flowering stage were higher than that in the other stages of flower development.
As for the end-flowering stage, 32 volatile compounds belonging to different chemical classes were identified: benzenoids (75.38%), sesquiterpenes (13.91%), monoterpenes (7.91%), and aliphatics (2.81%). The most abundant compound was paeonol, accounting for about 70% of the total GC peak area, followed by methyl salicylate (4.81%), (E,E)-α-farnesene (4.75%), and 3-carene (3.73%). By contrast, relative content of monoterpenes in the end-flowering stage were significantly higher than that in the other stages of flower development, including (1S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (0.45%), α-pinene (2.11%), 3-carene (3.73%), and α-santoline alcohol (0.32%). Relative content of nonanoic acid, ethyl ester (0.02%) and benzyl benzoate (0.78%) in the end-flowering stage were significantly higher than that in the other stages of flower development.
The Bray-Curtis similarity index is a statistic used for comparing the similarity of two samples [27]. The mean Bray-Curtis similarity index was 74.57% ± 11.53% (range: 61.81%~90.23%, n = 12, Table 2). The initial-flowering stage was more similar to the full-flowering stage (BCS = 90.23%) than to the end-flowering stage (BCS = 83.59%), and was largely dissimilar to the bud stage (BCS = 62.77%). Across all the flower-life stages, the bud stage was distinctly dissimilar to the full-flowering stage (BCS = 61.81%). Variations of the volatile compositions were apparently involved in the maturity stages of flower. The same phenomena are also observed in other plants, such as the flowers of Ocimum citriodorum [28], Penstemon digitalis [29], and Cananga odorata [23]. In this study, the highest diversity of floral volatiles was detected at the third and later periods of the flower development. Meanwhile, the richness of volatile compounds showed an unimodal pattern between the number of VOCs and times of flower development.

Table 2.
The Bray-Curtis similarity index (%) among different stages of L. pinceana flower development. (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flowering stage.
To identify which volatiles contributed the most to the differences among the four flower stages and to display the differences in a more visually appealing manner, the data on 39 volatile compounds identified in L. pinceana at a full life-flower scale were analyzed by using principal component analysis (PCA). The first three components of PCA explained 37.43%, 26.34%, and 23.15% of the variation, explaining ~87% of combined variance (Figure 3). Hereinto, volatiles that had high positive scores on PC 1 included (−)-β-cadinene, δ-cadinene, cadine-1,4-diene, cubenol, cubebol, isoledene, caryophyllene and α-cubebene, which were highly positively related to the bud stage and the initial-flowering stage. Volatiles with high positive scores on PC 2 comprised β-ylangene, γ-muurolene, unknown-1, unknown-2 and perilla alcohol, which were positively correlated with the full-flowering stage and initial-flowering stage. Volatiles with high positive scores on PC 3 included cis-verbenol, α-acorenol, (3E,5Z)-1,3,5-undecatriene and cedrol, which were negatively correlated with the full-flowering stage. The remaining 22 volatiles were composed of common components, megastigma-4,6(E),8(E)-triene, cyclosativene and 4-epi-cubebol. The principal component plots did not overlap amongst the four flower developmental stages indicated that the composition and its relative content of floral scent differed throughout the whole floral development, and the initial-flowering stage was recommended the best harvesting time when the high level of VOCs and essential oil are a concern.
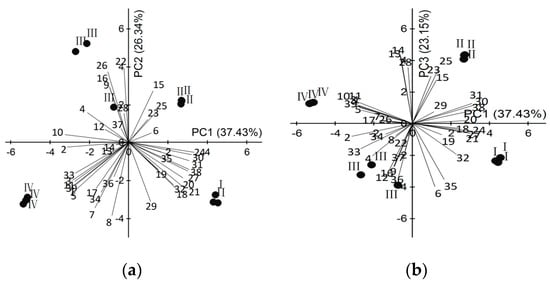
Figure 3.
Principal component plot (PC1 vs. PC2 plots (a) and PC1 vs. PC3 plots (b)) for L. pinceana at different stages of growth, showing correlations with volatiles (numbers correspond to those in Table 1). (I) bud stage; (II) initial-flowering stage; (III) full-flowering stage; and (IV) end-flowering stage.
3. Materials and Methods
3.1. Plant Materials
Fifteen fresh early flowering inflorescences from three L. pinceana plants (separation between plants more than 100 m, five inflorescences per plant) were collected from the Nujiang Lisu Autonomous Prefecture, Yunnan Province (25°58′N; 98°48′E) during their flourishing florescence on 10 November 2014, and inserted into deionized water before transported to the Research Institute of Resources Insects, Chinese Academy of Forestry (RIRICAF) in Kunming. Subsequently, the inflorescences were preserved at room temperature (25 ± 1 °C). The flowers were classified into four groups [23] according to their botanical characteristics (Figure 1): (I) bud stage: buds complete closed, two or three days before full bloom; (II) initial-flowering stage: semi-open petal, one day before full bloom; (III) full-flowering stage: completely open petals, observable pistils and stamens; and (IV) end-flowering stage: petals and calyxes withered, five or six day after full bloom.
3.2. Method
Volatile compounds of a complete flower were trapped immediately into a 20 mL capped solid-phase microextraction vial at 8:00–11:00 a.m. Three replicate experiments (five flowers from five inflorescences per replicate) were conducted using different plants randomly and the results were means of three tests (fifteen flowers).
SPME analysis was performed by 100 μm polydimethylsiloxane (PDMS) SPME fiber which was highly sensitive in the analysis of scent components equipped with a manual SPME holder. After the equilibration time, the fiber was exposed to the headspace of the capped vial to absorb volatile compounds for 40 min at room temperature (25 ± 1 °C). The fiber was conditioned in the GC injection port for 1 h at 250 °C before it was used for the first time. In addition, the empty capped vial was used as the blank control. A gas chromatograph-mass spectrometer (TRACE GC Ultra/ITQ900, Thermo Fisher Scientific, Waltham, MA, USA) coupled with a DB-5MS capillary column (5% diphenyl cross-linked 95% dimethylpolysiloxane, 30 m × 0.25 mm i.d., 0.25 μm film thickness. Agilent J & W Scientific, Folsom, CA, USA) were used for the GC-MS analysis. Follow the SPME analysis, the fiber with volatile compounds was exposed in the GC injector port for desorption at 260 °C for 1 min in the splitless mode. Helium was used as a carrier gas at a constant flow-rate of 1 mL/min. The oven temperature was programmed at 40 °C for 2 min, increasing 6 °C/min to 130 °C and then increasing 15 °C/min to 280 °C for 5 min. The split and splitless injection port were held at 260 °C and 200 °C in split mode at a split ratio of 1:10. The temperature of the transfer line and the ion source were 200 °C and 250 °C. The ionization potential of mass selective detector was 70 eV and the scan range was 50–650 amu.
3.3. Data Analysis
Identification of the volatile compounds was based on comparison of their mass spectra with NIST08 database through Software TF Xcalibur 2.1.0 (Thermo Fisher Scientific). Volatile compounds were identified on the basis of their linear retention index (LRI) and by comparing their mass spectra with a computerized MS-database using NIST 2008 library and published data (Pherobase, http://www.pherobase.com/; NIST, http://webbook.nist.gov/chemistry/; The LRI and Odour Database, http://www.odour.org.uk).
LRI were calculated by the use of a series of n-alkane standards (C6–C19) (Accu Standard, New Haven, CT, USA). It is defined as Equation (1):
Peak areas were normalized as percentage and used to determine the relative amounts of the volatiles. The normalization method from the Equation (2):
The data were expressed as mean ± standard deviation (SD) of triplicate measurements. One-way analysis of variance (ANOVA) with Tukey’s test in SPSS software was used to assess differences in aroma compounds among four different stages of L. pinceana flower development. Volatile compounds identified at a full life-flower scale were analyzed by using principal component analysis (PCA) and Bray-Curtis similarity (BCS). PCA and BCS were carried out using PC-ORD for Windows (Version 5.0; MjM Software, Gleneden Beach, OR, USA).
4. Conclusions
The present investigation showed that 39 VOCs were identified in all flower-life stages of L. pinceana, amongst them, 26 at bud stage, 26 at initial-flowering stage, 32 at full-flowering stage, and 32 at end-flowering stage. Benzenoids were the most abundant amongst floral scent compounds. The amount of floral scent emission offered tendency of ascending first and descending in succession, reaching peak level at initial-flowering stage. The highest diversity of floral volatile was detected at the third and later periods of flower developmental stage. The odour evidently differed in composition and its relative content in the four flower developmental stages. The second flower developmental stage was recommended as the best harvesting time if a high level of VOCs and essential oil are of interest. L. pinceana could serve as beneficial houseplant in the future, besides as fragrant ornamental according to the VOCs emitted from the flowers. Furthermore, L. pinceana has a promising prospects for development as an essential oil source owing to the many medicinal properties of the main compounds of the floral scent.
Acknowledgments
We thank Wenwen Zhang, Yanlong Yang and Shuaifeng Li for assistance in data analysis. This work was financially supported by Special Fund for Forest Scientific Research in the Public Welfare (201404705), Technology Innovation Talent Project of Yunnan Province and Applied Basic Research Project of Yunnan Province (2010ZC089).
Author Contributions
Y.-Y.L., H.M., and Z.-H.L. conceived and designed the experiments; Y.-Y.L. and T.-Q.L. performed the experiment; Y.-Y.L. and Y.-M.W. analyzed the data; X.-X.L. and Z.-H.S. contributed materials and performed research; Y.-Y.L. and H.M. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, W.; Barrett, S.C.H.; Wang, H.; Li, D.-Z. Reciprocal herkogamy promotes disassortative mating in a distylous species with intramorph compatibility. New Phytol. 2015, 206, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, Y.; Li, Z.H.; Wan, Y.M.; Liu, X.X.; Liang, N. A study on the breeding system of Luculia pinceana. Forest Res. 2009, 22, 373–378. [Google Scholar]
- Zhou, W.; Wang, H.; Li, D.Z.; Yang, J.B.; Zhou, W. Isolation and characterization of 13 microsatellite loci from Luculia pinceana (Rubiaceae), a typical distylous species. Hortscience 2010, 45, 840–841. [Google Scholar]
- Zhou, W.; Barrett, S.C.H.; Wang, H.; Li, D.Z. Loss of floral polymorphism in heterostylous Luculia pinceana (Rubiaceae): A molecular phylogeographic perspective. Mol. Ecol. 2012, 21, 4631–4645. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.M.; Li, Z.H.; Ma, H.; Liu, X.X. Microsporogenesis and male gametophyte development of long-style flower of Luculia pinciana (Rubiaceae). Acta Bot. Boreal-Occident. Sin. 2014, 34, 1570–1575. [Google Scholar]
- Murray, B.G. Heterostyly and pollen-tube interactions in Luculia gratissima (Rubiaceae). Ann. Bot. 1990, 65, 691–698. [Google Scholar]
- Kang, W.Y.; Du, Z.Z.; Yang, X.S.; Hao, X.J. A new triterpene from Luculia pinciana Hook. J. Asian Nat. Prod. Res. 2005, 7, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Rodiek, S.; Fujii, E.; Miyazaki, Y.; Park, B.J.; Ann, S.W. Physiological and psychological response to floral scent. Hortscience 2013, 48, 82–88. [Google Scholar]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotech. 2008, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 22 February 2016).
- Kang, W.Y.; Wang, J.M.; Ji, Z.Q. Flavonoids in Luculia pinceana. Chem. Nat. Compd. 2008, 44, 644–645. [Google Scholar] [CrossRef]
- Kim, H.K.; Tak, J.H.; Ahn, Y.J. Acaricidal activity of paeonia suffruticosa root bark-derived compounds against dermatophagoides farinae and dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2005, 52, 7857–7861. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.J.; Hong, C.; Liang, Y.C.; Lin, H.H.; Horng, J.C.; Kuo, Y.C.; Chen, C.W.; Tsai, F.Y.; Yen, S.C.; Chou, S.C. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-HBV agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Togar, B.; Stefano, A.D.; Taspınar, N.; Sozio, P. Protective effects of cyclosativene on H2O2-induced injury in cultured rat primary cerebral cortex cells. Cytotechnology 2014, 67, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Rasoarivelo, S.T.R.; Grougnet, R.; Verite, P.; Lecsoe, M.; Butel, M.J.; Tillequin, F.; Guillou, C.R.; Deguin, B. Chemical composition and antimicrobial activity of the essential oils of Anthospermum emirnense and Anthospermum perrieri (Rubiaceae). Chem. Biodivers. 2011, 8, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N.; Noletto, J.A.; Lawton, R.O. Chemical composition of the floral essential oil of Randia matudae from Monteverde, Costa Rica. Flavour Frag. J. 2006, 21, 244–246. [Google Scholar] [CrossRef]
- Kaiser, R. Scents from rain forests. Chimia 2000, 54, 346–363. [Google Scholar]
- Vieira, R.C.; Delprete, P.G.; Leitao, G.G.; Leitao, S.G. Anatomical and chemical analyses of leaf secretory cavities of Rustia formosa (Rubiaceae). Am. J. Bot. 2001, 88, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Nilsson, L.A.; Groth, I.; Bergstrom, G. Floral scents in butterfly-pollinated plants: Possible convergence in chemical composition. Bot. J. Linn. Soc. 2002, 140, 129–153. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.Y.; Lv, H. Constituents and bacteriostatic activity of volatile matter from four flower plant species. Indian J. Agric. Res. 2010, 44, 157–167. [Google Scholar]
- Stashenko, E.E.; Martinez, J.R.; Cardenas-Vargas, S.; Saavedra-Barrera, R.; Duran, D.C. Gc-ms study of compounds isolated from Coffea arabica flowers by different extraction techniques. J. Sep. Sci. 2013, 36, 2901–2914. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.W.; Hao, C.Y.; He, S.Z.; Wu, G.; Tan, L.H.; Xu, F.; Hu, R.S. Volatile organic compound emissions from different stages of Cananga odorata flower development. Molecules 2014, 19, 8965–8980. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Hairul, A.R.; Namasivayam, P.; Lian, G.E.C.; Abdullah, J.O. Terpenoid, benzenoid, and phenylpropanoid compounds in the floral scent of Vanda Mimi Palmer. J. Plant Biol. 2010, 53, 358–366. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, G.; Wang, S.; Lin, Q.; Zhang, J.; Li, X. Analysis of the variation in scent components of Hosta flowers by HS-SPME and GC–MS. Sci. Hortic. 2014, 175, 57–67. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, P.M. Misidentification of the bray-curtis similarity index. Mar. Ecol. Prog. Ser. 2008, 368, 309–310. [Google Scholar] [CrossRef]
- Al-Kateb, H.; Mottram, D.S. The relationship between growth stages and aroma composition of lemon basil Ocimum citriodorum vis. Food Chem. 2014, 152, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.C.F.; Raguso, R.A.; Kessler, A.; Parachnowitsch, A.L. Spatiotemporal floral scent variation of Penstemon digitalis. J. Chem. Ecol. 2015, 41, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).