Sorption Kinetics on Open Carbon Nanohorn Aggregates: The Effect of Molecular Diameter
Abstract
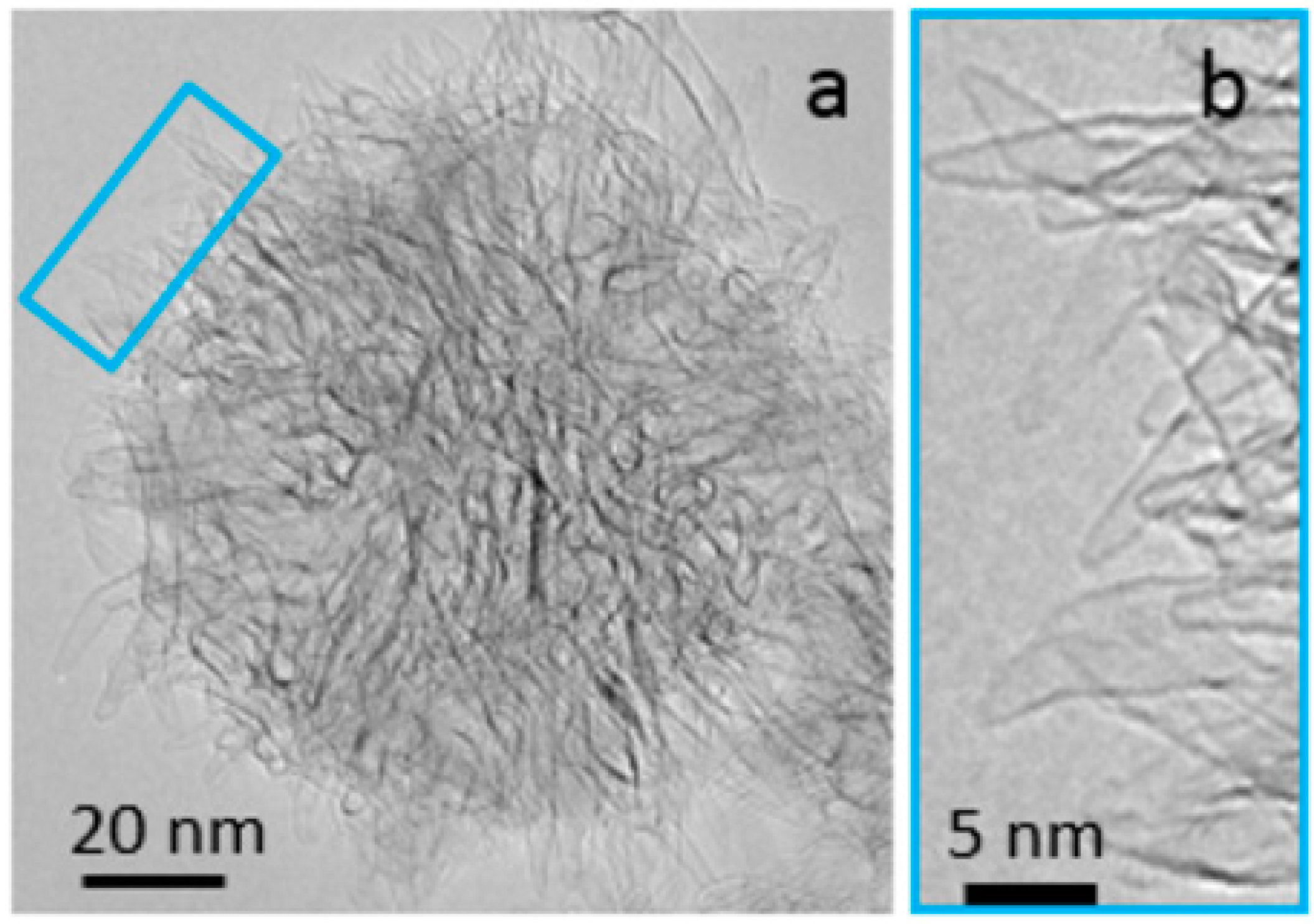
:1. Introduction
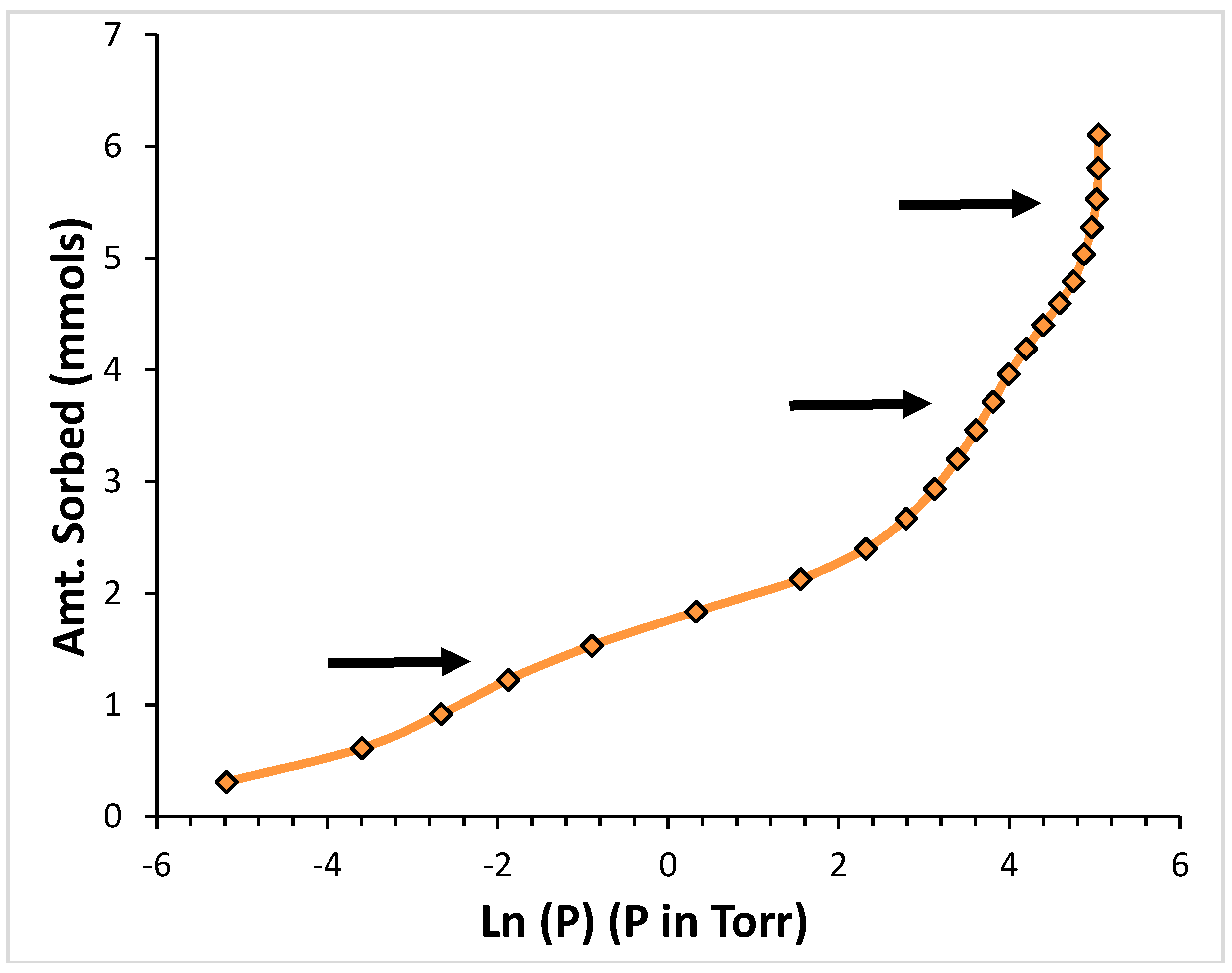
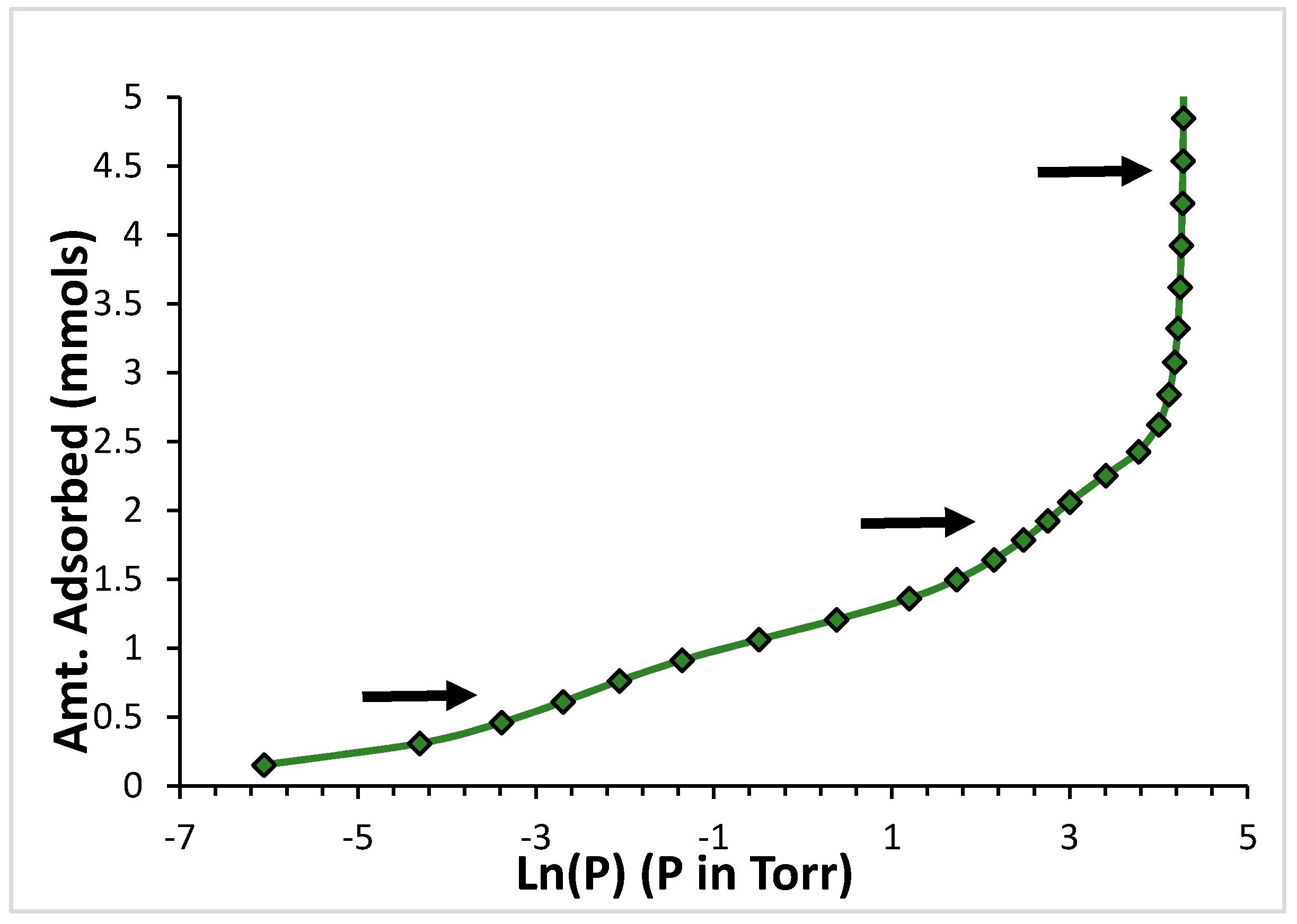
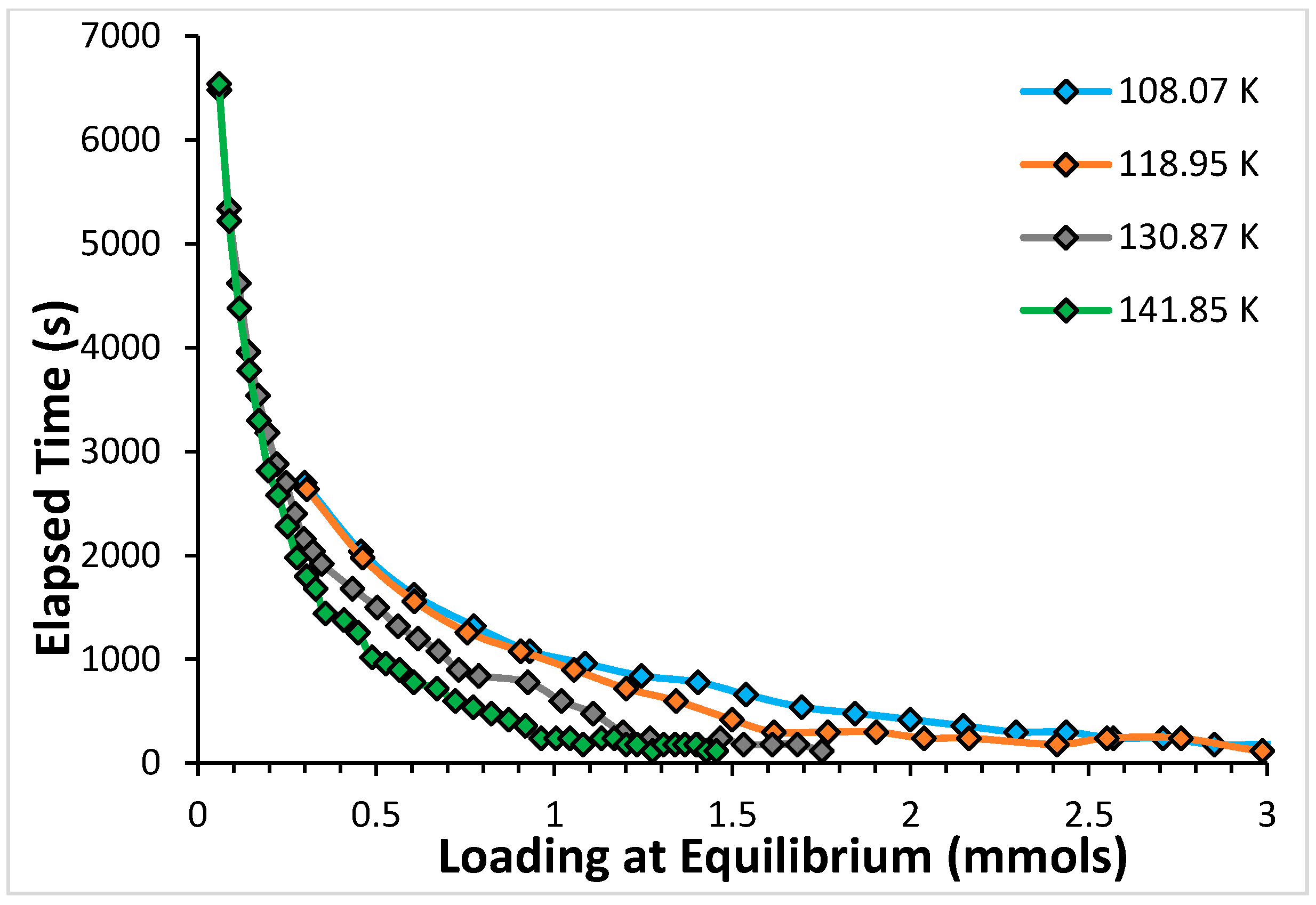
2. Results
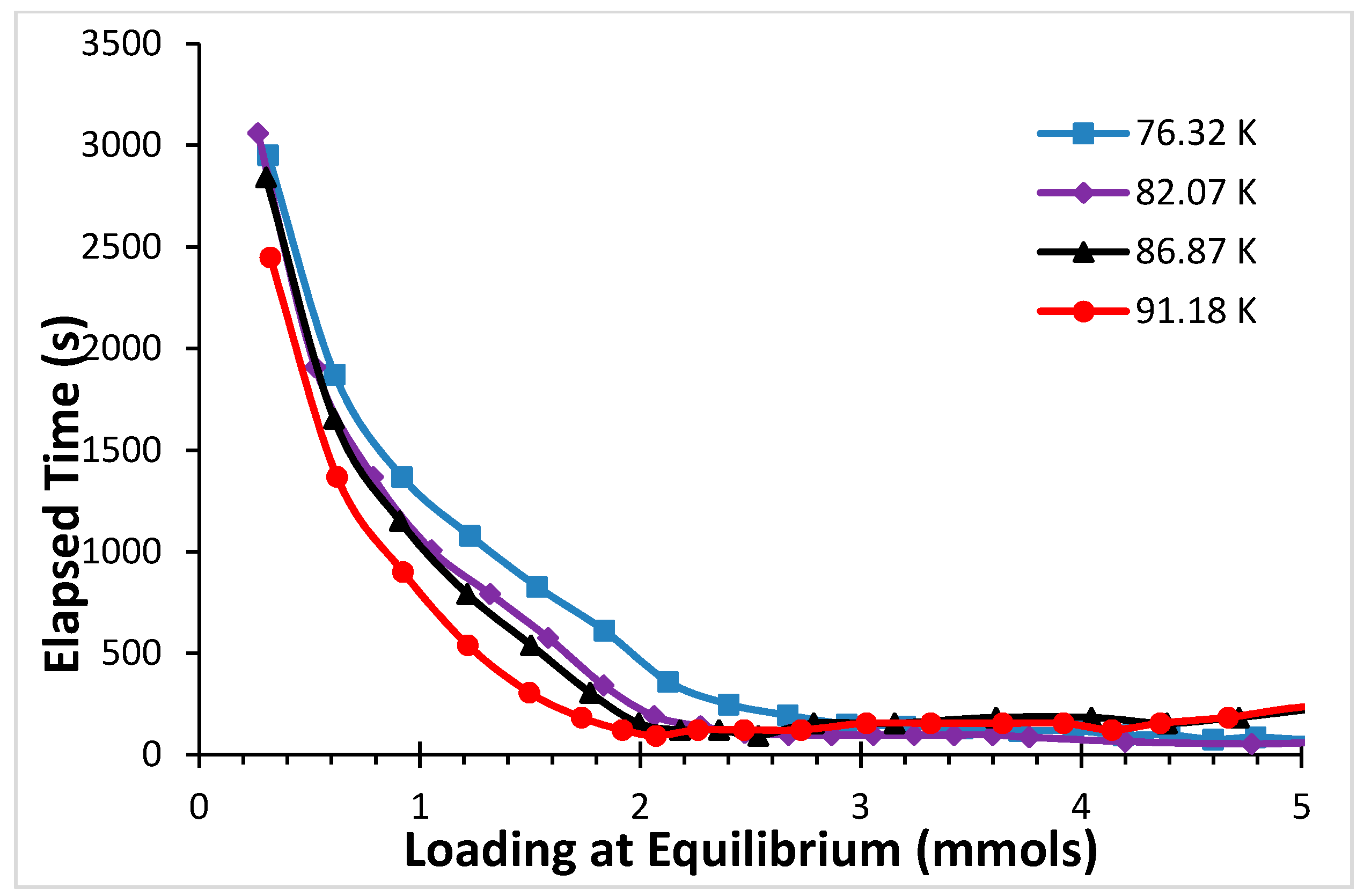
2.1. Argon
2.2. CF4
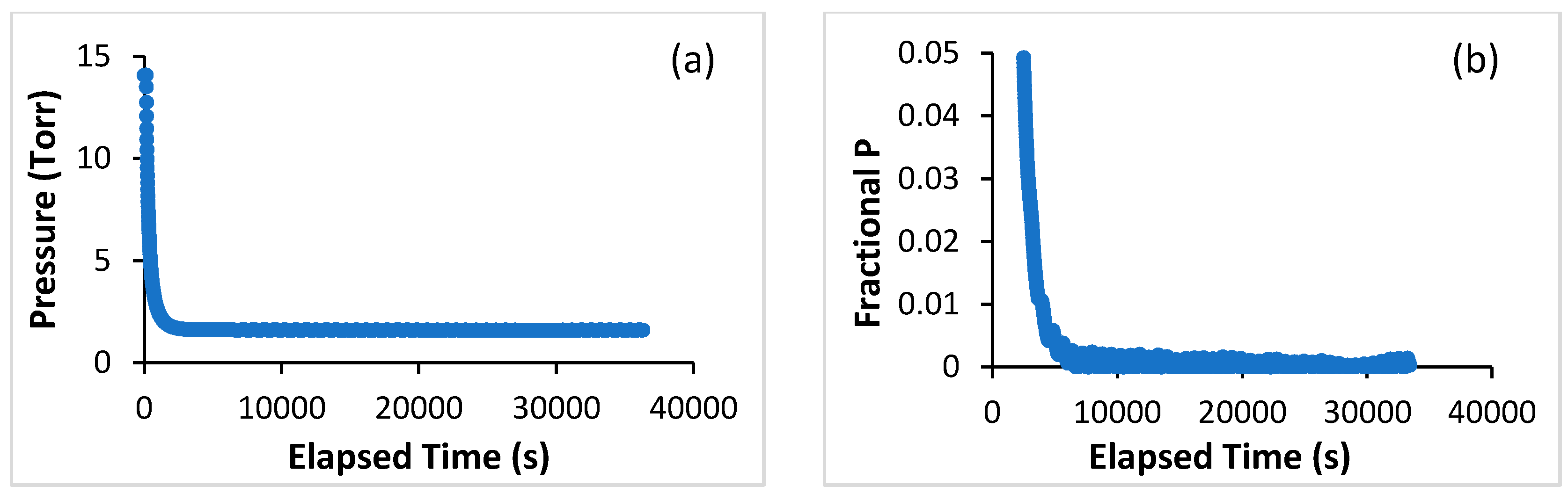
3. Materials and Methods
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taub, H.; Torzo, G.; Lauter, H.J.; Fain, S.C., Jr. Phase Transitions in Surface Films 2; NATO ASI Series B Physics; Plenum Press: New York, NY, USA, 1991; Volume 267. [Google Scholar]
- Wang, Z.; Wei, J.; Morse, P.; Das, J.G.; Vilches, O.E.; Cobden, D.H. Phase Transitions of Adsorbed Atoms on the Surface of a Carbon Nanotube. Science 2010, 327, 552–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, H.-C.; Vilches, O.E.; Wang, Z.; Frederickson, E.; Morse, P.; Roy, R.; Dubenko, B.; Cobden, D.H. Kr and 4He Adsorption on Individual Suspended Single-Walled Carbon Nanotubes. J. Low Temp. Phys. 2012, 169, 338–349. [Google Scholar] [CrossRef]
- Talapatra, S.; Krungleviciute, V.; Migone, A.D. Higher coverage gas adsorption on the surface of carbon nanotubes: Evidence for a possible new phase in the second layer. Phys. Rev. Lett. 2002, 89. [Google Scholar] [CrossRef] [PubMed]
- Krungleviciute, V.; Heroux, L.; Talapatra, S.; Migone, A.D. Gas adsorption on HiPco nanotubes: Surface area determinations, and neon second layer data. Nano Lett. 2004, 4, 1133–1137. [Google Scholar] [CrossRef]
- Calbi, M.M.; Gatica, S.M.; Bojan, M.J.; Cole, M.W. Phases of neon, xenon, and methane adsorbed on nanotube bundles. J. Chem. Phys. 2001, 115, 9975–9981. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbons; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Migone, A.D. Adsorption on carbon nanotubes: Experimental results. In Adsorption by Carbons; Bottani, E.J., Tascon, J.M.D., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nanoaggregates of single-walled graphitic carbon nano-horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar]
- Kasuya, D.; Yudasaka, M.; Takahashi, K.; Kokai, F.; Iijima, S. Selective production of single-wall carbon nanohorn aggregates and their formation mechanism. J. Phys. Chem. B 2002, 106, 4947–4951. [Google Scholar] [CrossRef]
- Azami, T.; Kasuya, D.; Yuge, R.; Yudasaka, M.; Iijima, S.; Yoshitake, T.; Kubo, Y. Large-Scale Production of Single-Wall Carbon Nanohorns with High Purity. J. Phys. Chem. C 2008, 112, 1330–1334. [Google Scholar] [CrossRef]
- Yudasaka, M.; Iijima, S.; Crespi, V.H. Single-wall carbon nanohorns and nanocones. In Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Topics Applied Physic; Springer-Verlag Berlin: Heidelberg, Germany, 2008; Volume 111, pp. 605–629. [Google Scholar]
- Yudasaka, M.; Zhang, M.; Matsumura, S.; Yuge, R.; Ichihashi, T.; Irie, H.; Shiba, K.; Iijima, S. Not nanocarbon but dispersant induced abnormality in lysosome macrophages in vivo. Nanotechnology 2015, 26. Number 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yamaguchi, T.; Iijima, S.; Yudasaka, M. Individual single-wall carbon nanohorns separated from aggregates. J. Phys. Chem. C 2009, 113, 11184–11186. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present, and future. Phys. B 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Murata, K.; Kaneko, K.; Steele, W.A.; Kokai, F.; Takahashi, K.; Kasuya, D.; Yudasaka, M.; Iijima, S. Porosity Evaluation of Intrinsic Intraparticle Nanopores of Single Wall Carbon Nanohorn. Nano Lett. 2001, 1, 197–199. [Google Scholar] [CrossRef]
- Ohba, T.; Murata, K.; Kaneko, K.; Steele, W.A.; Kokai, F.; Takahashi, K.; Kasuya, D.; Yudasaka, M.; Iijima, S. N2 Adsorption in an internal Nanopore Space of Single-Walled Carbon Nanohorn: GCMC Simulation and Experiment. Nano Lett. 2001, 1, 371–371. [Google Scholar] [CrossRef]
- Krungleviciute, V.; Calbi, M.M.; Wagner, J.A.; Migone, A.D.; Yudasaka, M.; Iijima, S. Probing the Structure of Carbon Nanohorn Aggregates by Adsorbing Gases of Different Sizes. J. Phys. Chem. C 2008, 112, 5742–5746. [Google Scholar] [CrossRef]
- Talapatra, S.; Zambano, A.J.; Weber, S.E.; Migone, A.D. Gases do not adsorb on the interstitial channels of closed-ended single-walled carbon nanotube bundles. Phys. Rev. Lett. 2000, 85, 138–141. [Google Scholar] [CrossRef] [PubMed]
- LaBrosse, M.R.; Johnson, J.K. Defect and Nondefect Interstitial Channel Availability in Carbon Nanotube Bundles: Comparison of Modeling with Experiments. J. Phys. Chem. C 2010, 114, 7602–7610. [Google Scholar] [CrossRef]
- Krungleviciute, V.; Ziegler, C.A.; Banjara, S.R.; Yudasaka, M.; Iijima, S.; Migone, A.D. Neon and CO2 Adsorption on Open Carbon Nanohorns. Langmuir 2013, 29, 9388–9397. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.J.; Thomas, K.M. Adsorption and Desorption Kinetics of n-Octane and n-Nonane Vapors on Activated Carbon. Langmuir 1999, 15, 6908–6914. [Google Scholar] [CrossRef]
- Berenguer-Murcia, A.; Fletcher, A.J.; Garcia-Martinez, J.; Cazorla-Amoros, D.; Linares-Solano, A.; Thomas, K.M. Probe Molecule Kinetic Studies of Adsorption on MCM-42. J. Phys. Chem. B 2003, 102, 1012–1020. [Google Scholar] [CrossRef]
- Bekyarova, E.; Murata, K.; Yudasaka, M.; Kasuya, D.; iijima, S.; Tanaka, H.; Kahoh, H.; Kaneko, K. Single-Wall Nanostructured Carbon for Methane Storage. J. Phys. Chem. B 2002, 107, 4681–4684. [Google Scholar] [CrossRef]
- Utsumi, S.; Miyawaki, J.; Tanaka, H.; Hattori, Y.; Itoi, T.; Ichikuni, N.; Kanoh, H.; Yudasaka, M.; Iijima, S.; Kaneko, K. Opening Mechanism of Internal Nanoporosity of Single-Wall Carbon Nanohorn. J. Phys. Chem. B 2005, 109, 14319–14324. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Hirahara, K.; Yudasaka, M.; Iijima, S.; Kasuya, D.; Kaneko, K. Nanowindow-Induced Molecular Sieving Effect in a Single-Wall Carbon Nanohorn. J. Phys. Chem. B 2002, 106, 12668–12669. [Google Scholar] [CrossRef]
- Bekyarova, E.; Kaneko, K.; Yudasaka, M.; Kasuya, D.; Iijima, S.; Huidobro, A.; Rodriguez-Reinoso, F. Controlled Opening of Single-Wall Carbon Nanohorn by Heat treatment in Carbon Dioxide. J. Phys. Chem B 2003, 107, 4479–4484. [Google Scholar] [CrossRef]
- Yang, C.-M.; Noguchi, H.; Murata, K.; Yudasaka, M.; Hashimoto, A.; Iijima, S.; Kaneko, K. Highly Ultramicroporous Single-Walled Carbon Nanohorn Assemblies. Adv. Mater. 2005, 17, 866–870. [Google Scholar] [CrossRef]
- Kittel, C.; Kroemer, H. Thermal Physics, 2nd ed.; W. H. Freeman and Company: New York, NY, USA, 1980; Chapter 5; p. 121. [Google Scholar]
- Vilches, O.E. Phase-transitions in mono-molecular layer films physisorbed on crystalline surfaces. Ann. Rev. Phys. Chem. 1980, 31, 463–490. [Google Scholar] [CrossRef]
- Krungleviciute, V.; Lask, K.; Migone, A.D.; Lee, J.-Y.; Li, J. Kinetics and equilibrium of gas adsorption on RPM1-Co and Cu-BTC metal-organic frameworks: Potential for gas separation applications. AIChE J. 2008, 54, 918–923. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Nakamura, M.; Iijima, S.; Yudasaka, M. Double oxidation with oxygen and hydrogen peroxide for hole-forming in sungle wall carbon nanohorns. Appl. Phys. A 2010, 100, 379–383. [Google Scholar] [CrossRef]
- Urita, K.; Seki, S.; Utsumi, S.; Noguchi, D.; Kanoh, H.; Tanaka, H.; Hattori, Y.; Ochiai, Y.; Aoki, N.; Yudasaka, M.; et al. Effects of Gas Adsorption on the Electrical Conductivity of Single-wWall Carbon Nanohors. Nano Lett. 2006, 6, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.A.; Migone, A.D.; Calbi, M.M.; Petucci, J.; Yudasaka, M.; Iijima, S. Ethane Adsorption on Aggregates of Dahlia-Like Nanohorns: Experiments and Computer Simulations. Phys. Chem. Chem. Phys. 2016. submitted. [Google Scholar]
- Rawat, D.S.; Krungleviciute, V.; Heroux, L.; Bulut, M.; Calbi, M.M.; Migone, A.D. Dependence of Single-Walled Carbon Nanotube Adsorption Kinetics on Temperature and Binding Energy. Langmuir 2008, 24, 13465–13469. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, B.A.; Khanal, P.; Calbi, M.M.; Yudasaka, M.; Iijima, S.; Migone, A.D. Sorption Kinetics on Open Carbon Nanohorn Aggregates: The Effect of Molecular Diameter. Molecules 2016, 21, 521. https://doi.org/10.3390/molecules21040521
Russell BA, Khanal P, Calbi MM, Yudasaka M, Iijima S, Migone AD. Sorption Kinetics on Open Carbon Nanohorn Aggregates: The Effect of Molecular Diameter. Molecules. 2016; 21(4):521. https://doi.org/10.3390/molecules21040521
Chicago/Turabian StyleRussell, Brice A., Pravin Khanal, Maria M. Calbi, Masako Yudasaka, Sumio Iijima, and Aldo D. Migone. 2016. "Sorption Kinetics on Open Carbon Nanohorn Aggregates: The Effect of Molecular Diameter" Molecules 21, no. 4: 521. https://doi.org/10.3390/molecules21040521
APA StyleRussell, B. A., Khanal, P., Calbi, M. M., Yudasaka, M., Iijima, S., & Migone, A. D. (2016). Sorption Kinetics on Open Carbon Nanohorn Aggregates: The Effect of Molecular Diameter. Molecules, 21(4), 521. https://doi.org/10.3390/molecules21040521




