Two New Bioactive α-Pyrones from Hypericum japonicum
Abstract
:1. Introduction
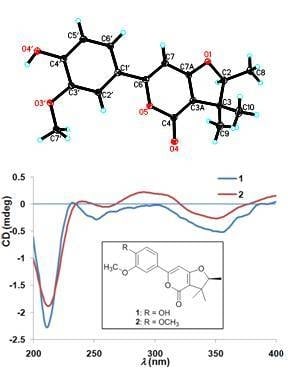
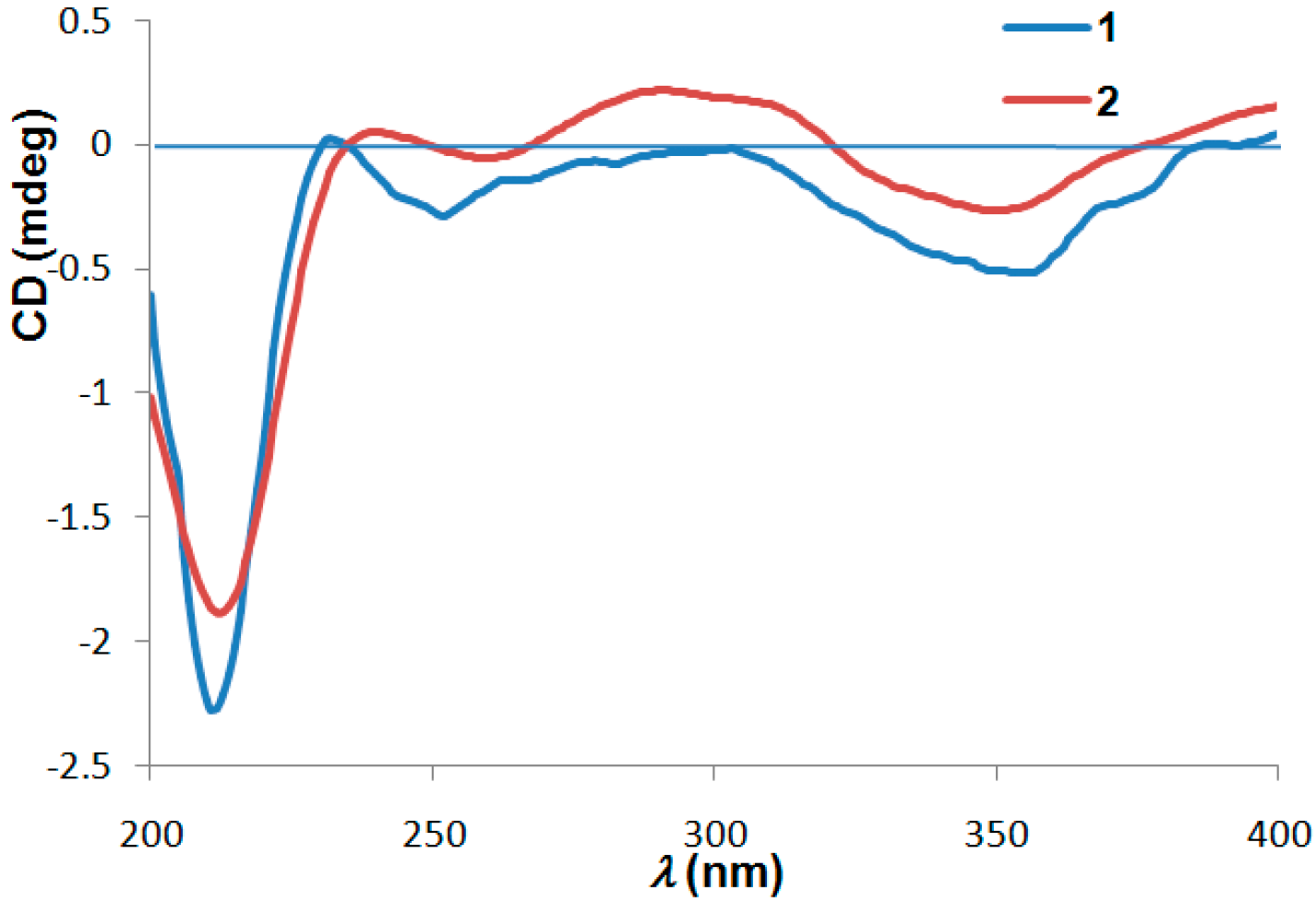
2. Results
3. Materials and Methods
3.1. General Experiments
3.2. Plant Material
3.3. Extraction and Isolation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| H. japonicum | Hypericum japonicum |
| ECD | electronic circular dichroism |
| HRESIMS | High-resolution electrospray ionization mass spectra |
| CC | column chromatography |
| HPLC | High Performance Liquid Chromatography |
| BACE1 | β-site amyloid precursor protein cleaving enzyme 1 |
| KSHV | Kaposi’s sarcoma associated herpes virus |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| CDV | cidofovir |
| EtOH | ethanol |
References
- Liu, L.S.; Liu, M.H.; He, J.Y. Hypericum japonicum Thunb. ex Murray: Phytochemistry, pharmacology, quality control and pharmacokinetics of an important herbal medicine. Molecules 2014, 19, 10733–10754. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Mao, Y.; Wang, N.L.; Yao, X.S. A new phloroglucinol diglycoside derivative from Hypericum japonicum Thunb. Molecules 2008, 13, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Wang, S.P.; Zhang, S.M.; Yang, J.S.; Xiao, P.G. Chromone glycosides and flavonoids from Hypericum japonicum. Phytochemistry 1998, 49, 1417–1420. [Google Scholar] [CrossRef]
- Wu, Q.L.; Wang, S.P.; Du, L.J.; Yang, J.S. Xanthones from Hypericum japonicum and H. henryi. Phytochemistry 1998, 49, 1395–1402. [Google Scholar] [CrossRef]
- Ishiguro, K.; Nagata, S.; Fukumoto, H.; Yamaki, M.; Isoi, K.; Oyama, Y. An isopentenylated flavonol from H. japonicum. Phytochemistry 1993, 32, 1583–1585. [Google Scholar] [CrossRef]
- Hu, L.H.; Khoo, C.W.; Vittal, J.J.; Sim, K.Y. Phloroglucinol derivatives from Hypericum japonicum. Phytochemistry 2000, 53, 705–709. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Yang, J.; Li, X.N.; Liu, J.; Sun, B.; Huang, S.X.; Li, D.; Yao, G.; Luo, Z.; et al. Bioactive acylphloroglucinols with adamantyl skeleton from Hypericum sampsonii. Org. Lett. 2014, 16, 6322–6325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Liu, J.; Sun, B.; Wei, G.; Li, Y.; Zhang, J.; Yao, G.; Luo, Z.; Xue, Y.; et al. Hyperascyrones A–H, polyprenylated spirocyclic acylphloroglucinol derivatives from Hypericum ascyron Linn. Phytochemistry 2015, 115, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xue, Y.; Zhu, H.; Li, Y.; Sun, B.; Liu, J.; Yao, G.; Zhang, J.; Du, G.; Zhang, Y. Hyperattenins A–I, bioactive polyprenylated acylphloroglucinols from Hypericum attenuatum Choisy. RSC Adv. 2015, 5, 5277–5287. [Google Scholar] [CrossRef]
- Brachmann, A.O.; Brameyer, S.; Kresovic, D.; Hitkova, I.; Kopp, Y.; Manske, C.; Schubert, K.; Bode, H.B.; Heermann, R. Pyrones as bacterial signaling molecules. Nat. Chem. Biol. 2013, 9, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Nagata, S.; Fukumoto, H.; Yamaki, M.; Isoi, K.; Yamagata, Y. A 2-Pyrone derivative from Hypericum japonicum. Phytochemistry 1994, 37, 283–284. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s Sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Jeong, S.G.; Park, J.E.; Han, J.A.; Kang, H.R.; Lee, D.; Song, M.J. Antiviral activity of angelicin against gammaherpesviruses. Antivir. Res. 2013, 100, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Glesby, M.J.; Hoover, D.R.; Weng, S.; Graham, N.M.; Phair, J.P.; Detels, R.; Ho, M.; Saah, A.J. Use of antiherpes drugs and the risk of Kaposi’s sarcoma: Data from the Multicenter AIDS Cohort Study. J. Infect. Dis. 1996, 173, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.F.; Dunn, J.P.; Davis, J.L.; Duker, J.S.; Engstrom, R.E., Jr.; Friedberg, D.N.; Jaffe, G.J.; Kuppermann, B.D.; Polis, M.A.; Whitley, R.J.; et al. Use of the ganciclovir implant for the treatment of cytomegalovirus retinitis in the era of potent antiretroviral therapy: Recommendations of the International AIDS Society-USA panel. Am. J. Ophthalmol. 1999, 127, 329–339. [Google Scholar] [PubMed]
- Chen, J.; Jiang, L.; Lan, K.; Chen, X. Celecoxib inhibits the lytic activation of Kaposi’s Sarcoma-Associated Herpesvirus through down-regulation of RTA expression by inhibiting the activation of p38 MAPK. Viruses 2015, 7, 2268–2287. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound 1 is available from the authors.
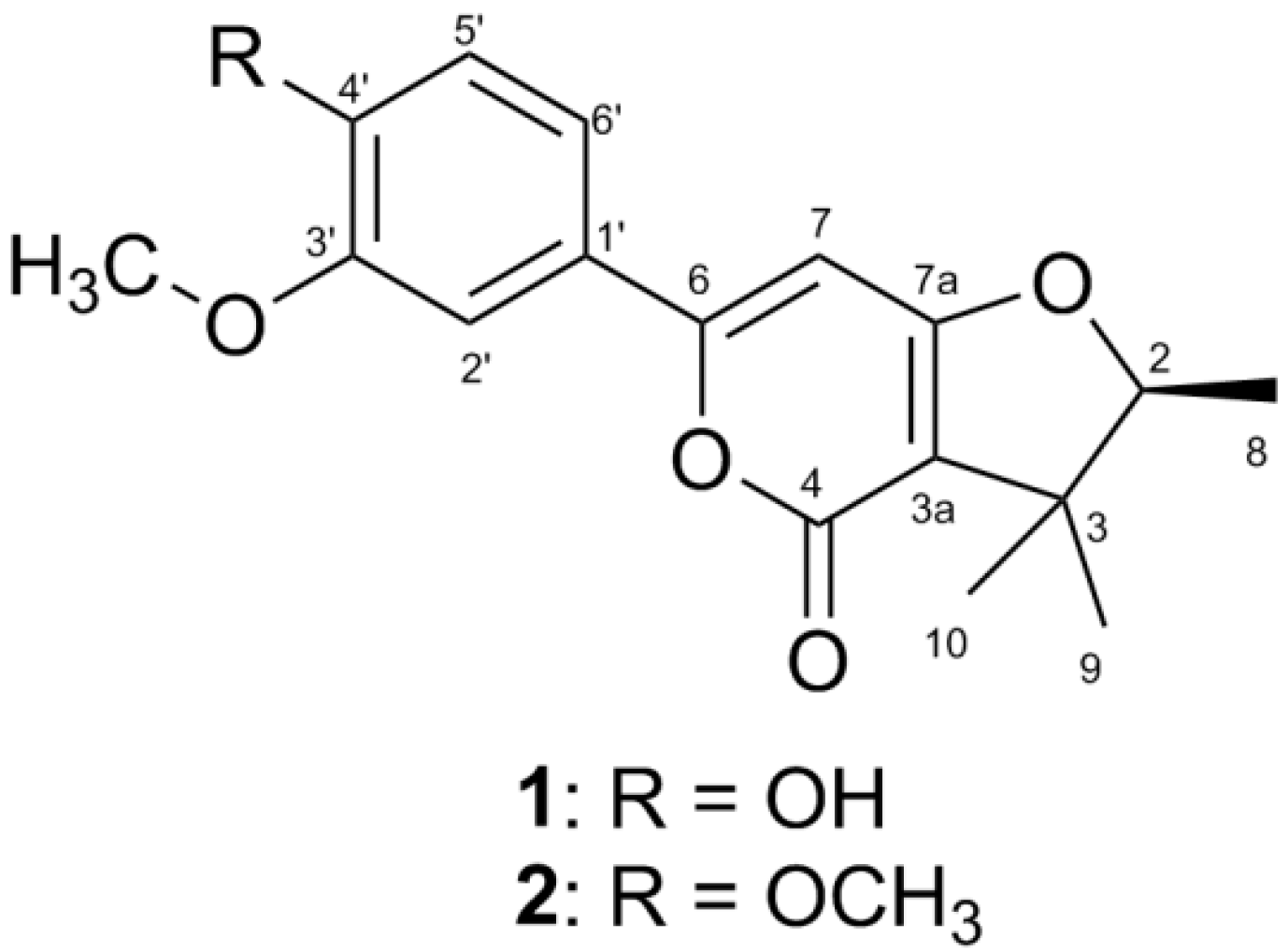
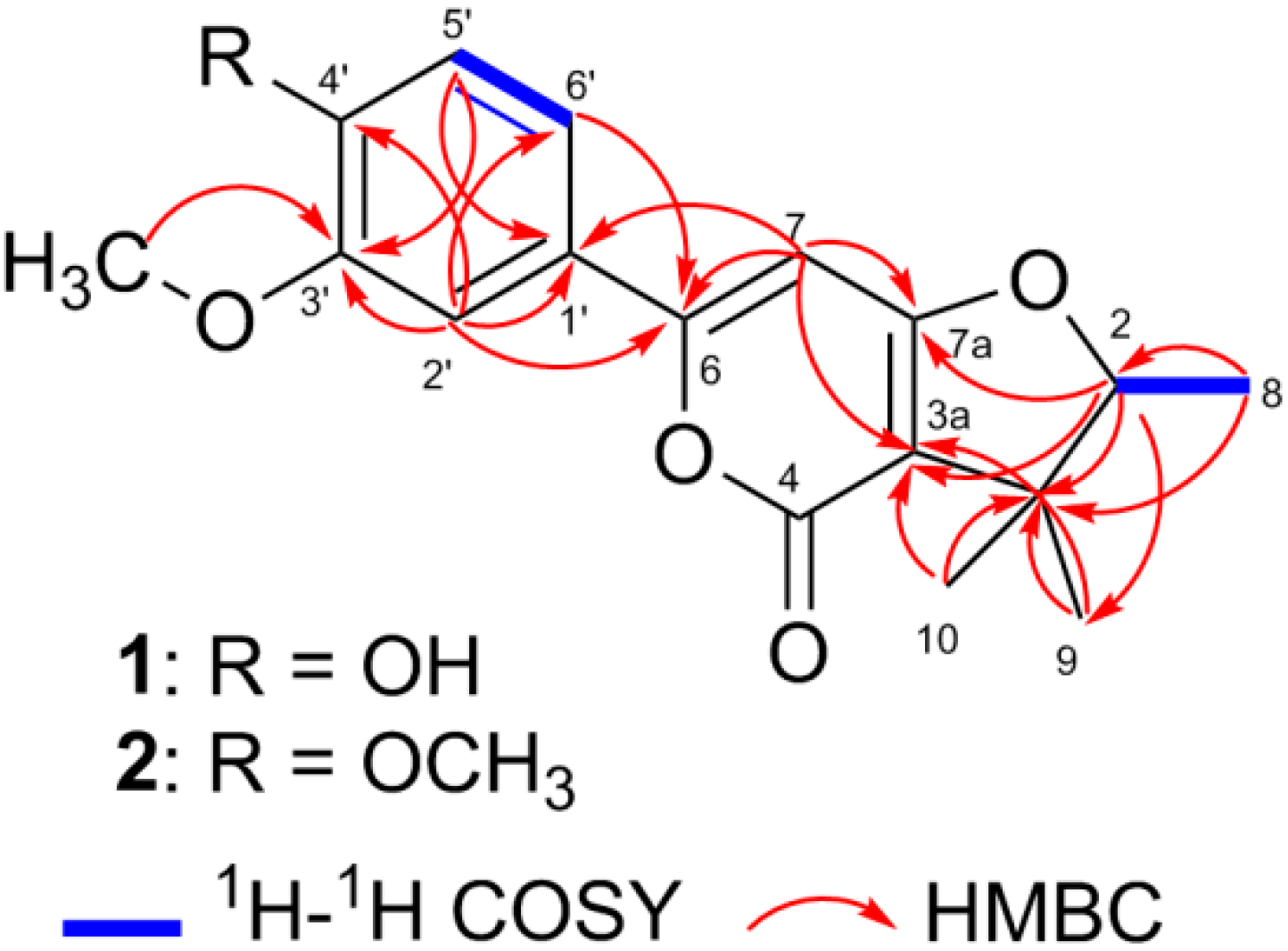
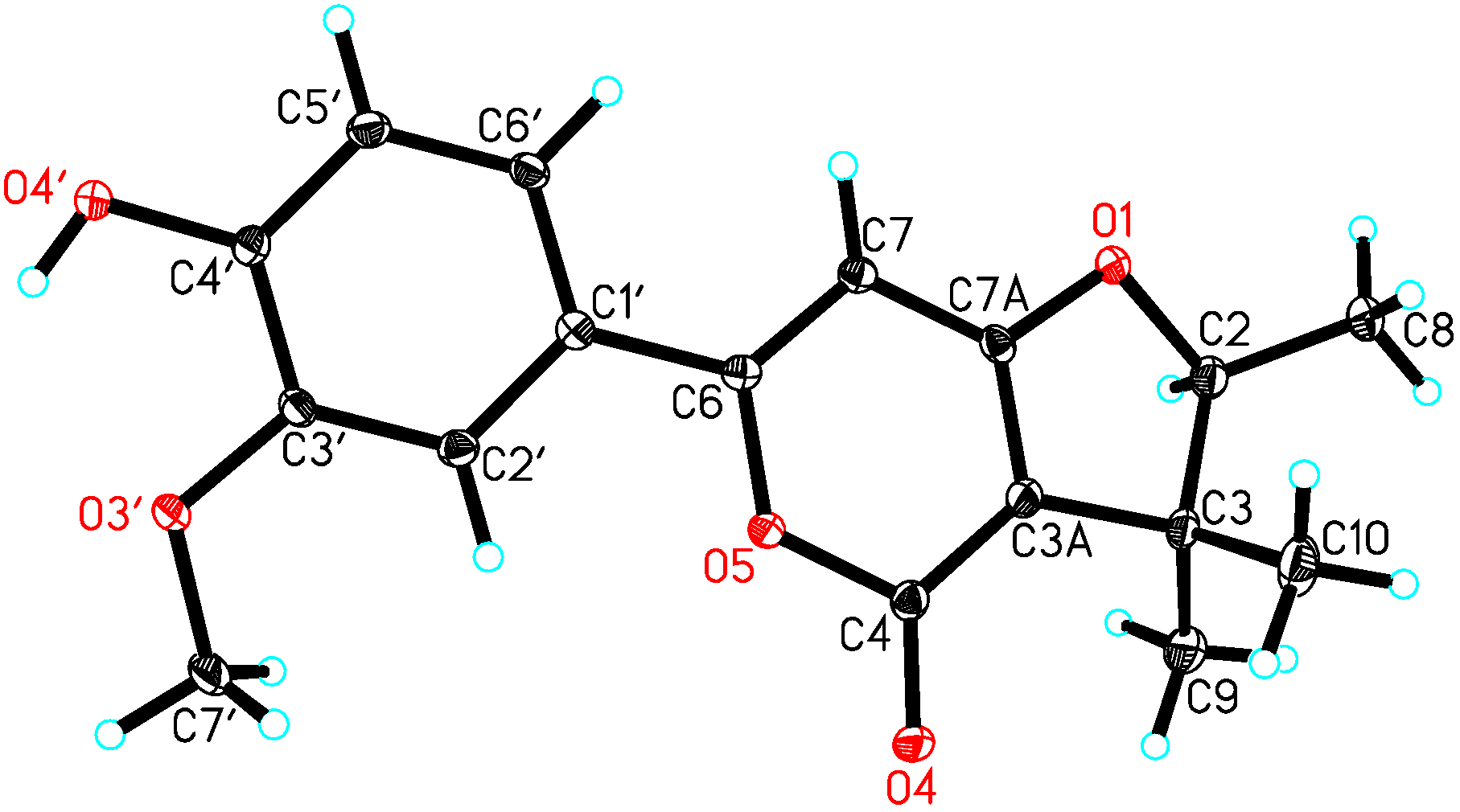

| NO. | 1 a | 2 b | ||
|---|---|---|---|---|
| δH (ppm) | δC (ppm) | δH (ppm) | δC (ppm) | |
| 2 | 4.61q (J = 6.6 Hz) | 91.4 | 4.64q (J = 6.6 Hz) | 93.9 |
| 3 | 42.1 | 43.9 | ||
| 4 | 159.7 | 163.5 | ||
| 6 | 162.7 | 164.8 | ||
| 7 | 6.94s | 91.6 | 6.77s | 93.7 |
| 8 | 1.34d (J = 6.6 Hz) | 14.4 | 1.42d (J = 6.6 Hz) | 14.9 |
| 9 | 1.30s | 25.1 | 1.40s | 25.9 |
| 10 | 1.11s | 20.1 | 1.21s | 20.6 |
| 1′ | 122.4 | 125.5 | ||
| 2′ | 7.35s | 109.4 | 7.43d (J = 2.1 Hz) | 110.2 |
| 3′ | 149.7 | 150.9 | ||
| 4′ | 147.9 | 153.5 | ||
| 5′ | 6.87d (J = 8.0 Hz) | 115.7 | 7.05d (J = 8.6 Hz) | 112.9 |
| 6′ | 7.33d (J = 8.0 Hz) | 119.4 | 7.50dd (J = 8.5, 2.2 Hz) | 120.8 |
| 3a | 107.2 | 109.6 | ||
| 7a | 169.7 | 172.8 | ||
| 3′-OCH3 | 3.83s | 55.8 | 3.90s | 56.7 |
| 4′-OCH3 | 3.89s | 56.6 | ||
| Compounds | CC50 | IC50 | Selective Index (CC50/IC50) |
|---|---|---|---|
| CDV | >1 | <0.004 | >250 |
| 1 | >200 | 85.34 | >2.34 |
| 2 | >200 | 29.46 | >6.79 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Wang, Z.; Zhang, J.; Lu, Y.; Wang, K.; Xue, Y.; Zhang, Y.; Zhang, Y. Two New Bioactive α-Pyrones from Hypericum japonicum. Molecules 2016, 21, 515. https://doi.org/10.3390/molecules21040515
Hu L, Wang Z, Zhang J, Lu Y, Wang K, Xue Y, Zhang Y, Zhang Y. Two New Bioactive α-Pyrones from Hypericum japonicum. Molecules. 2016; 21(4):515. https://doi.org/10.3390/molecules21040515
Chicago/Turabian StyleHu, Linzhen, Zhenzhen Wang, Jinwen Zhang, Yuanyuan Lu, Kaiping Wang, Yongbo Xue, Yu Zhang, and Yonghui Zhang. 2016. "Two New Bioactive α-Pyrones from Hypericum japonicum" Molecules 21, no. 4: 515. https://doi.org/10.3390/molecules21040515
APA StyleHu, L., Wang, Z., Zhang, J., Lu, Y., Wang, K., Xue, Y., Zhang, Y., & Zhang, Y. (2016). Two New Bioactive α-Pyrones from Hypericum japonicum. Molecules, 21(4), 515. https://doi.org/10.3390/molecules21040515